Efficient Catalytic Degradation of Methyl Orange by Various ZnO-Doped Lignin-Based Carbons
Abstract
1. Introduction
2. Characterization and Analysis
2.1. Micromorphology and Microstructure of Various Prepared Catalysts
2.2. Optical Properties of Various Prepared Catalysts
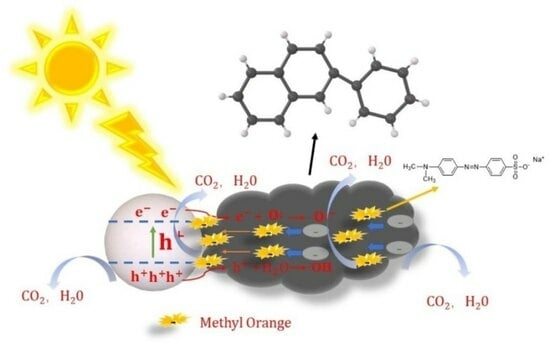
2.3. Photocatalytic Activity of Various Prepared Catalysts
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Preparation of Various ZnO-Doped Lignin-Based Carbons
3.3. Characterization Methods
3.4. Photocatalytic Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Qiu, X.; Zhong, R.; Fu, F.; Qian, Y.; Yang, D. One-pot in-situ preparation of a lignin-based carbon/ZnO nanocomposite with excellent photocatalytic performance. Mater. Chem. Phys. 2017, 199, 193–202. [Google Scholar] [CrossRef]
- Abbas, S.; Hsieh, L.-H.C.; Techato, K. Supply chain integrated decision model in order to synergize the energy system of textile industry from its resource waste. Energy 2021, 229, 120754. [Google Scholar] [CrossRef]
- Lassesson, H.; Malovanyy, A.; Andersson, A. Optimizing resource flow of industrial processes, with a case study of zero liquid discharge at a copper smelting plant. J. Clean. Prod. 2021, 286, 125452. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, D.; Qiu, X.; Qian, Y.; Wang, H.; Yi, C.; Zhang, D. Fabricating ZnO/lignin-derived flower-like carbon composite with excellent photocatalytic activity and recyclability. Carbon 2020, 162, 256–266. [Google Scholar] [CrossRef]
- Tang, D.; Lu, G.; Shen, Z.; Hu, Y.; Yao, L.; Li, B.; Zhao, G.; Peng, B.; Huang, X. A review on photo-, electro- and photoelectro- catalytic strategies for selective oxidation of alcohols. J. Energy Chem. 2022, 77, 80–118. [Google Scholar] [CrossRef]
- Li, T.; Pan, H.; Xu, L.; Shen, Y.; Li, K. Preparation and properties of lignin-based carbon/ZnO photocatalytic materials. J. Porous Mater. 2022, 29, 1883–1893. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, S.; Lee, H.; Kurisingal, J.F.; Han, S.H.; Kim, Y.H.; An, K. Complete utilization of waste lignin: Preparation of lignin-derived carbon supports and conversion of lignin-derived guaiacol to nylon precursors. Catal. Sci. Technol. 2022, 12, 5021–5031. [Google Scholar] [CrossRef]
- Wang, H.; Yi, G.; Zu, X.; Jiang, X.; Zhang, Z.; Luo, H. A highly sensitive and self-powered ultraviolet photodetector composed of titanium dioxide nanorods and polyaniline nanowires. Mater. Lett. 2015, 138, 204–207. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Han, D.; Ma, C.; Wei, M.; Huo, P.; Yan, Y. Biomass derived the V-doped carbon/Bi2O3 composite for efficient photocatalysts. Environ. Res. 2020, 182, 108998. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B Environ. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, W.; Zhou, H. Lignosulfonate-controlled BiOBr/C hollow microsphere photocatalyst for efficient removal of tetracycline and Cr(VI) under visible light. Chem. Eng. J. 2023, 453, 139819. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Usgodaarachchi, L.; Jayanetti, M.; Liyanaarachchi, C.; Kandanapitiye, M.; Vigneswaran, S. Efficient Visible-Light Photocatalysis and Antibacterial Activity of TiO2-Fe3C-Fe-Fe3O4/Graphitic Carbon Composites Fabricated by Catalytic Graphitization of Sucrose Using Natural Ilmenite. ACS Omega 2022, 7, 25403–25421. [Google Scholar] [CrossRef] [PubMed]
- Mendis, A.; Thambiliyagodage, C.; Ekanayake, G.; Liyanaarachchi, H.; Jayanetti, M.; Vigneswaran, S. Fabrication of Naturally Derived Chitosan and Ilmenite Sand-Based TiO2/Fe2O3/Fe-N-Doped Graphitic Carbon Composite for Photocatalytic Degradation of Methylene Blue under Sunlight. Molecules 2023, 28, 3154. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-F.; Sun, G.-H.; Li, Y.; Cheng, J.-Y.; Chen, J.-P.; Song, G.; Kong, Q.-Q.; Xie, L.-J.; Chen, C.-M. Pre-oxidation of lignin precursors for hard carbon anode with boosted lithium-ion storage capacity. Carbon 2021, 178, 243–255. [Google Scholar] [CrossRef]
- Donaldson, L. Environmentally friendly hybrid semiconductor being developed. Mater. Today 2019, 23, 5–6. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Vogel-Heuser, B.; Wang, L. Industry 4.0 and Industry 5.0—Inception, conception and perception. J. Manuf. Syst. 2021, 61, 530–535. [Google Scholar]
- Zhang, L.; You, T.; Zhou, T.; Zhou, X.; Xu, F. Interconnected Hierarchical Porous Carbon from Lignin-Derived Byproducts of Bioethanol Production for Ultra-High Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 13918–13925. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kim, T.-H.; Liu, K.; Ma, M.-G.; Choi, S.-E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, T.; Yan, M.; Miao, C.; Zhou, X.; Tong, G. High-value utilization of bamboo pulp black liquor lignin: Preparation of silicon-carbide derived materials and its application. Int. J. Biol. Macromol. 2022, 217, 66–76. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Shi, K.; Zhang, F.; Xiao, S.; Huang, L.; Zhu, H. Lignin-derived porous carbon for zinc-ion hybrid capacitor. Ind. Crops Prod. 2022, 187, 115519. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-based electrodes for energy storage application. Ind. Crops Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Argyropoulos, D.S.; Crestini, C. A Perspective on Lignin Refining, Functionalization, and Utilization. ACS Sustain. Chem. Eng. 2016, 4, 5089. [Google Scholar] [CrossRef]
- Mohamed, W.A.A.; Handal, H.T.; Ibrahem, I.A.; Galal, H.R.; Mousa, H.A.; Labib, A.A. Recycling for solar photocatalytic activity of Dianix blue dye and real industrial wastewater treatment process by zinc oxide quantum dots synthesized by solvothermal method. J. Hazard. Mater. 2021, 404, 123962. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, E.; Brech, Y.L.; Gadiou, R.; Bertaud, F.; Leclerc, S.; Vidal, L.; Meins, J.-M.L.; Dufour, A. Depolymerization of Technical Lignins in Supercritical Ethanol: Effects of Lignin Structure and Catalyst. Energy Fuels 2021, 35, 17769–17783. [Google Scholar] [CrossRef]
- Correa, C.A.; de Santi, C.R.; Leclerc, A. Green-PVC with full recycled industrial waste and renewably sourced content. J. Clean. Prod. 2019, 229, 1397–1411. [Google Scholar] [CrossRef]
- Li, H.; Shi, F.; An, Q.; Zhai, S.; Wang, K.; Tong, Y. Three-dimensional hierarchical porous carbon derived from lignin for supercapacitors: Insight into the hydrothermal carbonization and activation. Int. J. Biol. Macromol. 2021, 166, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.; Hecht, P.; Sommertune, J.; Ek, M.; Sedin, M.; Sjöholm, E. Carbon Fibers from Lignin–Cellulose Precursors: Effect of Carbonization Conditions. ACS Sustain. Chem. Eng. 2020, 8, 6826–6833. [Google Scholar] [CrossRef]
- Stevens, J.C.; Shi, J. Biocatalysis in ionic liquids for lignin valorization: Opportunities and recent developments. Biotechnol. Adv. 2019, 37, 107418. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, X.; Zhao, X.; Wang, D.; Yin, R.; Li, X.; An, J. Facile preparation of well-dispersed ZnO/cyclized polyacrylonitrile nanocomposites with highly enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2017, 204, 304–315. [Google Scholar] [CrossRef]
- Akhavan, O. Graphene Nanomesh by ZnO Nanorod Photocatalysts. ACS Nano 2010, 4, 4174–4180. [Google Scholar] [CrossRef]
- Park, M.; Lee, A.; Rho, H.K.; Lee, S.-K.; Bae, S.; Jeon, D.-Y.; Lee, D.S.; Kim, T.-W.; Im, Y.-H.; Lee, S.H. Large area thermal light emission from autonomously formed suspended graphene arrays. Carbon 2018, 136, 217–223. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Zi, T.-Q.; Zhao, X.-R.; Liu, C.; Ren, Q.; Fang, J.-B.; Li, W.-M.; Li, A.-D. Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition. Sci. Rep. 2020, 10, 13437. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, B.; Wang, Z.; Lv, X.; Zhang, C.; Chen, B.; San, H.; Hofmann, W. Face-to-face intercrossed ZnO nanorod arrays with extensive NR-NR homojunctions for a highly sensitive and self-powered ultraviolet photodetector. Nano Energy 2019, 65, 104042. [Google Scholar] [CrossRef]
- Jiang, X.; Kong, D.; Luo, B.; Wang, M.; Zhang, D.; Pu, X. Preparation of magnetically retrievable flower-like AgBr/BiOBr/NiFe2O4 direct Z-scheme heterojunction photocatalyst with enhanced visible-light photoactivity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127880. [Google Scholar] [CrossRef]
- Mao, L.; Cai, X.-Y.; Zhu, M.-S. Hierarchically 1D CdS decorated on 2D perovskite-type La2Ti2O7 nanosheet hybrids with enhanced photocatalytic performance. Rare Met. 2021, 40, 1067–1076. [Google Scholar] [CrossRef]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J.-J. Facile microwave-assisted green synthesis of Ag-ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation. Appl. Catal. B Environ. 2017, 203, 416–427. [Google Scholar] [CrossRef]
- El-Sayed, F.; Ganesh, V.; Hussien, M.S.A.; AlAbdulaal, T.H.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S.; Shakir, M.; Bitla, Y. Facile synthesis of Y2O3/CuO nanocomposites for photodegradation of dyes/mixed dyes under UV- and visible light irradiation. J. Mater. Res. Technol. 2022, 19, 4867–4880. [Google Scholar] [CrossRef]
- Bartlam, C.; Morsch, S.; Heard, K.W.J.; Quayle, P.; Yeates, S.G.; Vijayaraghavan, A. Nanoscale infrared identification and mapping of chemical functional groups on graphene. Carbon 2018, 139, 317–324. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Z.; Wang, P.; Chen, F.; Luo, X. Near-infrared light-triggered mild-temperature photothermal effect of nanodiamond with functional groups. Diam. Relat. Mater. 2022, 123, 108831. [Google Scholar] [CrossRef]
- Aljaafari, A. Size Dependent Photocatalytic Activity of ZnO Nanosheets for Degradation of Methyl Red. Front. Mater. 2020, 7, 562693. [Google Scholar] [CrossRef]
- Weng, B.; Yang, M.-Q.; Zhang, N.; Xu, Y.-J. Toward the enhanced photoactivity and photostability of ZnO nanospheres via intimate surface coating with reduced graphene oxide. J. Mater. Chem. A 2014, 2, 9380–9389. [Google Scholar] [CrossRef]
- Zhao, X.; Su, S.; Wu, G.; Li, C.; Qin, Z.; Lou, X.; Zhou, J. Facile synthesis of the flower-like ternary heterostructure of Ag/ZnO encapsulating carbon spheres with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 406, 254–264. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: The effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef]
- Yuan, M.; Zhou, W.-H.; Kou, D.-X.; Zhou, Z.-J.; Meng, Y.-N.; Wu, S.-X. Cu2ZnSnS4 decorated CdS nanorods for enhanced visible-light-driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2018, 43, 20408–20416. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W.; Hou, B. Highly Efficient Photocatalytic Performance of Graphene–ZnO Quasi-Shell–Core Composite Material. ACS Appl. Mater. Interfaces 2013, 5, 12361–12368. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Yang, Y.; Wei, W. Efficient Catalytic Degradation of Methyl Orange by Various ZnO-Doped Lignin-Based Carbons. Molecules 2024, 29, 1817. https://doi.org/10.3390/molecules29081817
Tang Z, Yang Y, Wei W. Efficient Catalytic Degradation of Methyl Orange by Various ZnO-Doped Lignin-Based Carbons. Molecules. 2024; 29(8):1817. https://doi.org/10.3390/molecules29081817
Chicago/Turabian StyleTang, Zhihao, Yonggang Yang, and Weiqi Wei. 2024. "Efficient Catalytic Degradation of Methyl Orange by Various ZnO-Doped Lignin-Based Carbons" Molecules 29, no. 8: 1817. https://doi.org/10.3390/molecules29081817
APA StyleTang, Z., Yang, Y., & Wei, W. (2024). Efficient Catalytic Degradation of Methyl Orange by Various ZnO-Doped Lignin-Based Carbons. Molecules, 29(8), 1817. https://doi.org/10.3390/molecules29081817