The Promoting Role of HK II in Tumor Development and the Research Progress of Its Inhibitors
Abstract
1. Introduction
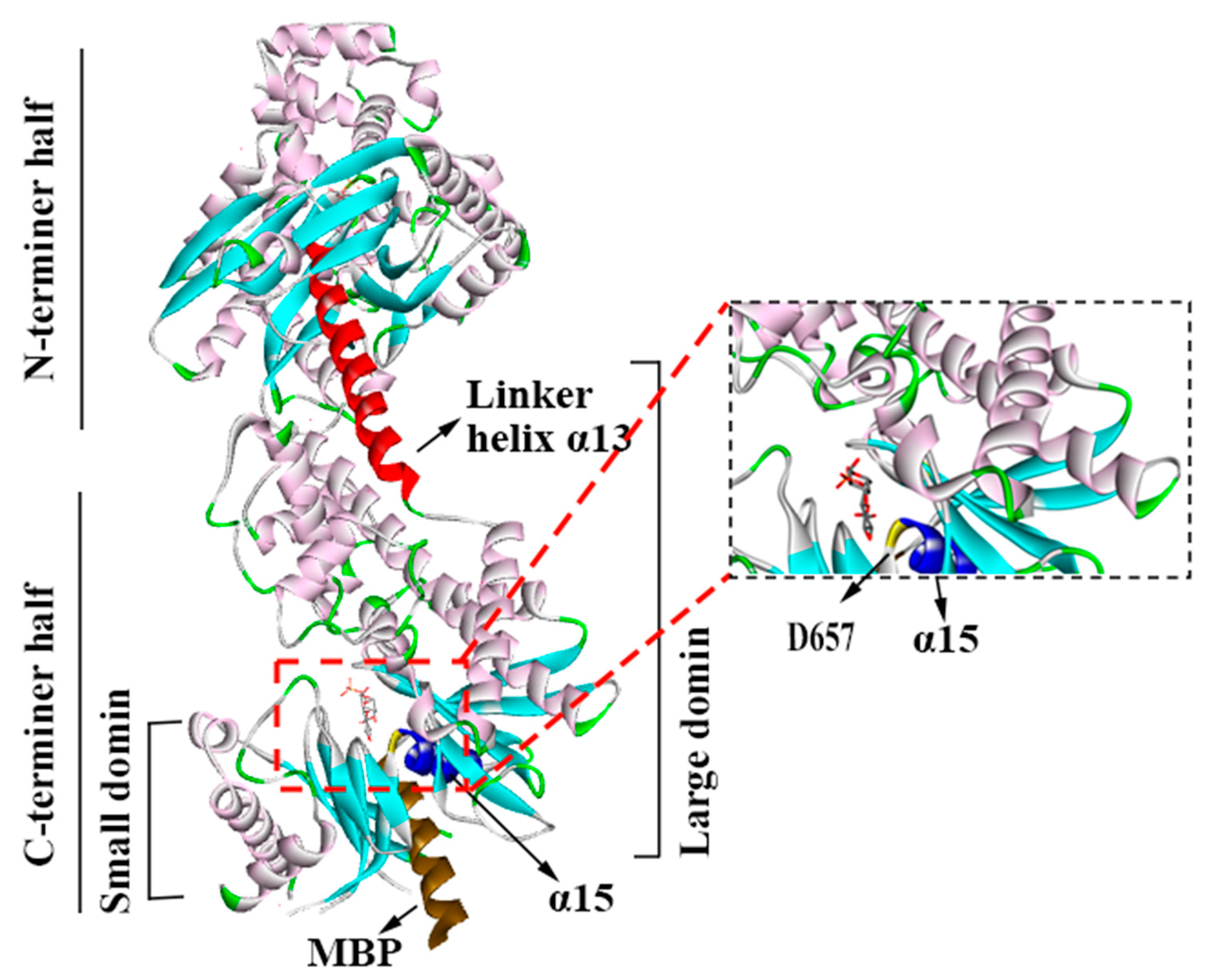
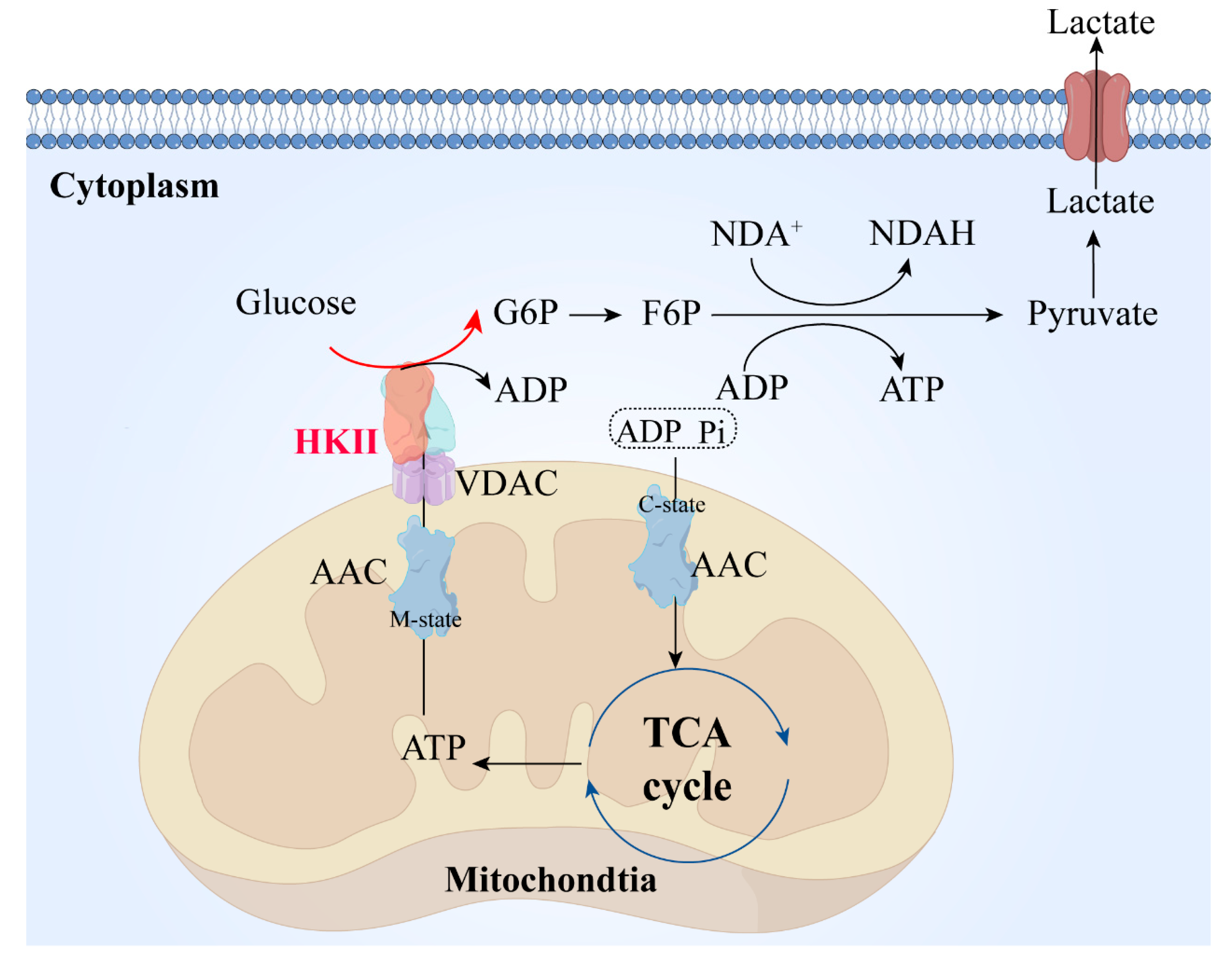
2. Structural Features and Functional Implications of Hexokinase II
3. The Significance of HK II in Tumor Development and Progression
3.1. Upward Adjustment of HK Pathways
3.1.1. Proteins and Transcription Factors
3.1.2. Signaling Pathways
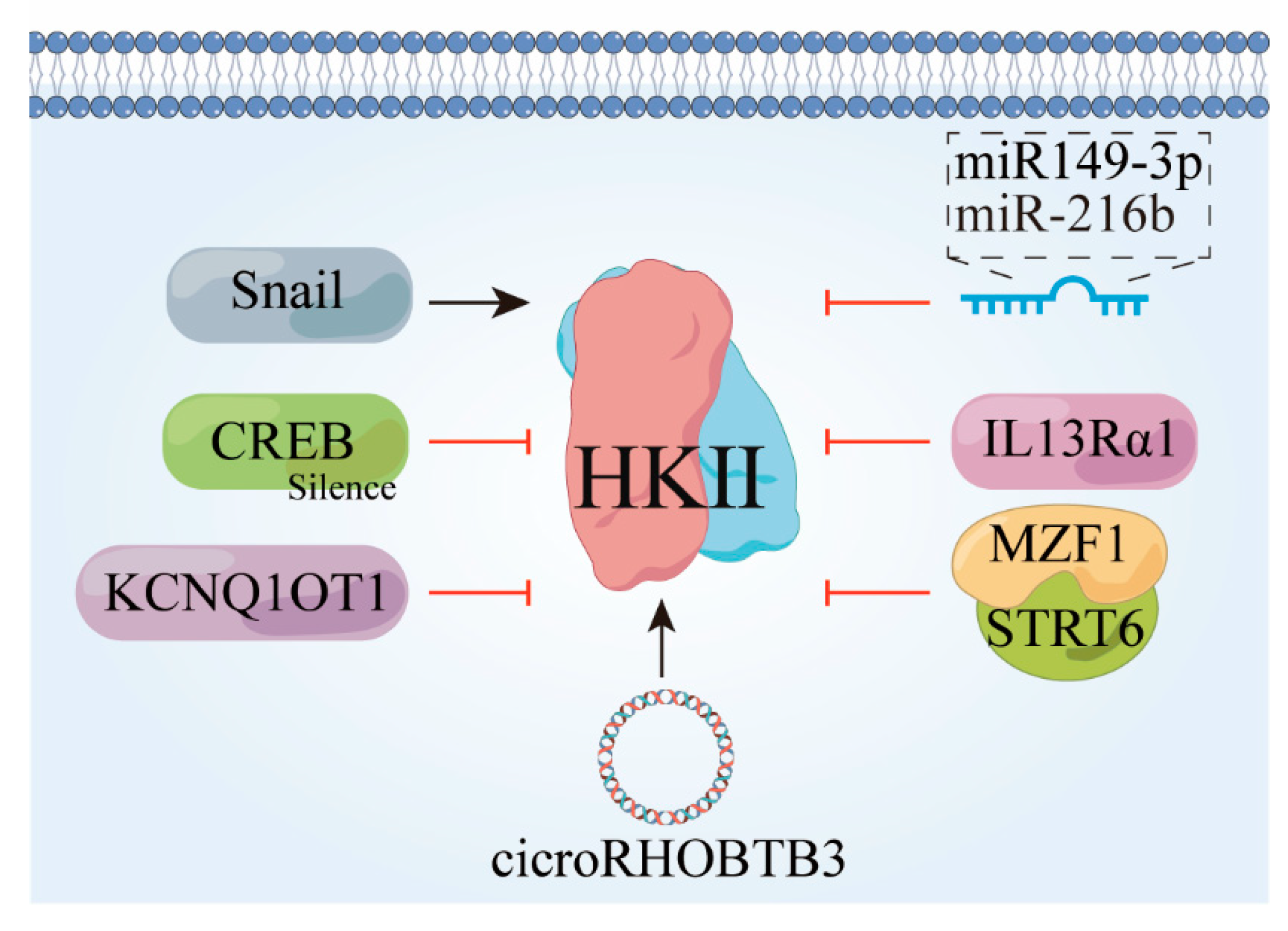
3.2. HK II Undergoes Downward Adjustment
3.3. HK Promotes Fructose Utilization
4. The Association between HK II and Tumor Drug Resistance
4.1. Mechanisms of Drug Resistance
HK II Mediates Drug Resistance
4.2. Drug Synergism
4.2.1. Sorafenib
4.2.2. Rapamycin
4.2.3. Drugs Related to the Tumor Microenvironment
4.2.4. Oncolytic Virus Therapy
4.2.5. HDACis
4.2.6. Alkylating Agents
4.2.7. Other Antitumor Drugs
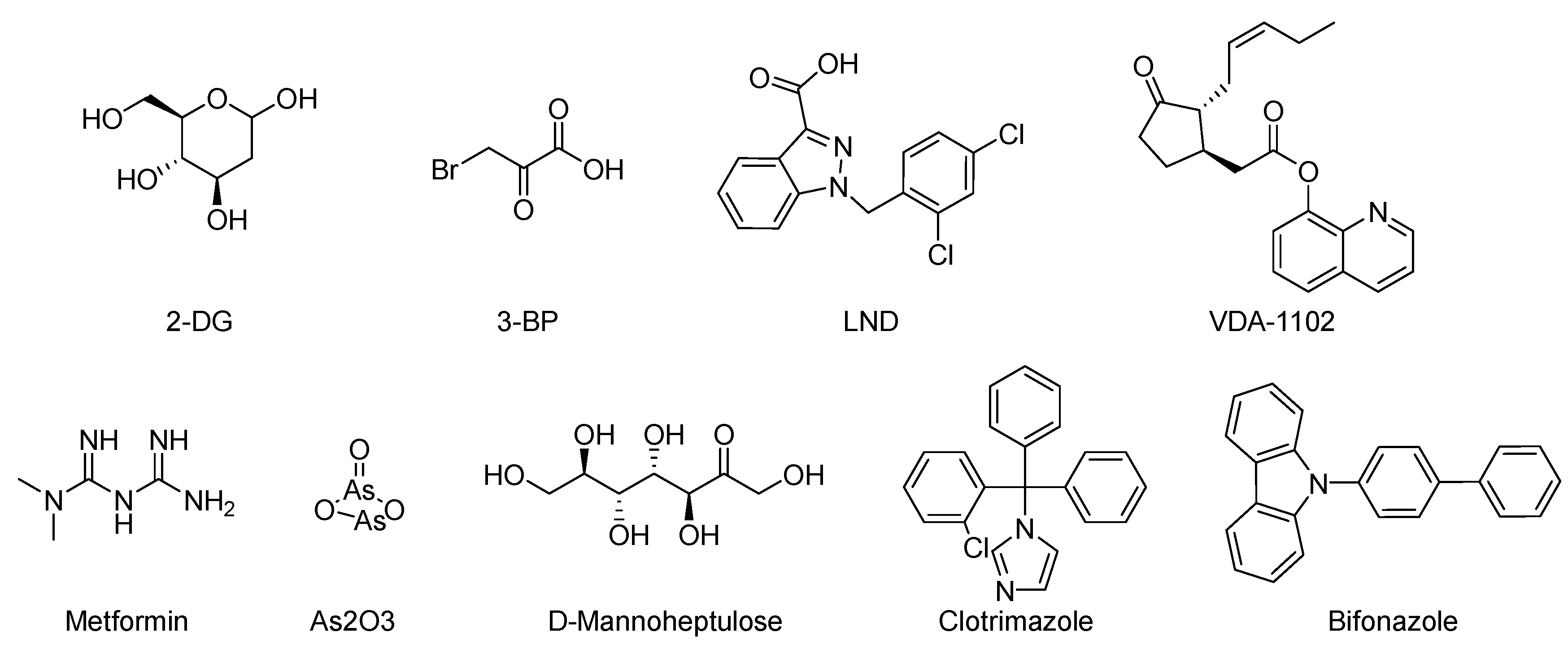
5. HK II Inhibitors
5.1. Natural Compounds
5.2. Targeted Drugs
5.3. Residue Targets
5.4. Lead Compounds
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, R.S.; Zhang, S.W.; Sun, K.X.; Chen, R.; Wang, S.M.; Li, L.; Zeng, H.M.; Wei, W.W.; He, J. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi 2023, 45, 212–220. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S.V.U.M. Dual or multi-targeting inhibitors: The next generation anticancer agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.-g.; Sun, Y.; Sheng, W.-b.; Liao, D.-f. Designing multi-targeted agents: An emerging anticancer drug discovery paradigm. Eur. J. Med. Chem. 2017, 136, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kinarivala, N.; Sharma, S. Multi-Targeting Anticancer Agents: Rational Approaches, Synthetic Routes and Structure Activity Relationship. Anti-Cancer Agents Med. Chem. 2019, 19, 842–874. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Wang, J.; Tao, M.; Wang, T.; Wang, Z.; Xiao, J.; Ding, S.; Chen, R. Knockdown of hexokinase 2 (HK2) inhibits breast cancer cell proliferation and reduces their resistance to fluorouracil. Chin. J. Cell. Mol. Immunol. 2021, 37, 722–727. [Google Scholar]
- Huang, Y.; Ouyang, F.; Yang, F.; Zhang, N.; Zhao, W.; Xu, H.; Yang, X. The expression of Hexokinase 2 and its hub genes are correlated with the prognosis in glioma. BMC Cancer 2022, 22, 900. [Google Scholar] [CrossRef]
- Li, R.; Mei, S.; Ding, Q.; Wang, Q.; Yu, L.; Zi, F. A pan-cancer analysis of the role of hexokinase II (HK2) in human tumors. Sci. Rep. 2022, 12, 18807. [Google Scholar] [CrossRef]
- Mazure, N.M. VDAC in cancer. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.H.; Ferreira, J.C.; Nedyalkova, L.; Zhu, H.; Carrasco-López, C.; Kirmizialtin, S.; Rabeh, W.M. The catalytic inactivation of the N-half of human hexokinase 2 and structural and biochemical characterization of its mitochondrial conformation. Biosci. Rep. 2018, 38, BSR20171666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yip, Y.M.; Li, L. In silico construction of HK2-VDAC1 complex and investigating the HK2 binding-induced molecular gating mechanism of VDAC1. Mitochondrion 2016, 30, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr. 2007, 39, 222. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; King, M.S.; Zögg, T.; Aleksandrova, A.A.; Pardon, E.; Crichton, P.G.; Steyaert, J.; Kunji, E.R. The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier. Cell 2019, 176, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pasch, S.; Schamberger, T.; Maneck, M.; Möhlendick, B.; Schumacher, S.; Brockhoff, G.; Knoefel, W.T.; Izbicki, J.; Polzer, B.; et al. Diagnostic pathology of early systemic cancer: ERBB2 gene amplification in single disseminated cancer cells determines patient survival in operable esophageal cancer. Int. J. Cancer 2018, 142, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, X.; Jin, H.; Ren, S.; Liu, Z.; Fang, X.; Zhang, G. Overexpression of ErbB2 renders breast cancer cells susceptible to 3-BrPA through the increased dissociation of hexokinase II from mitochondrial outer membrane. Oncol. Lett. 2016, 11, 1567–1573. [Google Scholar] [CrossRef]
- Myant, K.B.; Cammareri, P.; Hodder, M.C.; Wills, J.; Von Kriegsheim, A.; Győrffy, B.; Rashid, M.; Polo, S.; Maspero, E.; Vaughan, L.; et al. HUWE1 is a critical colonic tumour suppressor gene that prevents MYC signalling, DNA damage accumulation and tumour initiation. EMBO Mol. Med. 2017, 9, 181–197. [Google Scholar] [CrossRef]
- Yang, D.; Cheng, D.; Tu, Q.; Yang, H.; Sun, B.; Yan, L.; Dai, H.; Luo, J.; Mao, B.; Cao, Y.; et al. HUWE1 controls the development of non-small cell lung cancer through down-regulation of p53. Theranostics 2018, 8, 3517–3529. [Google Scholar] [CrossRef]
- Gong, X.; Du, D.; Deng, Y.; Zhou, Y.; Sun, L.; Yuan, S. The structure and regulation of the E3 ubiquitin ligase HUWE1 and its biological functions in cancer. Investig. New Drugs 2020, 38, 515–524. [Google Scholar] [CrossRef]
- Lee, H.-J.; Li, C.-F.; Ruan, D.; He, J.; Montal, E.D.; Lorenz, S.; Girnun, G.D.; Chan, C.-H. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat. Commun. 2019, 10, 2625. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Liu, H. TRIM59 knockdown blocks cisplatin resistance in A549/DDP cells through regulating PTEN/AKT/HK2. Gene 2020, 747, 144553. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Lignitto, L.; LeBoeuf, S.E.; Homer, H.; Jiang, S.; Askenazi, M.; Karakousi, T.R.; Pass, H.I.; Bhutkar, A.J.; Tsirigos, A.; Ueberheide, B.; et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell 2019, 178, 316–329.e18. [Google Scholar] [CrossRef] [PubMed]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ren, C.; Qiao, P.; Han, X.; Wang, L.; Lv, S.; Sun, Y.; Liu, Z.; Du, Y.; Yu, Z. PIM2-mediated phosphorylation of hexokinase 2 is critical for tumor growth and paclitaxel resistance in breast cancer. Oncogene 2018, 37, 5997–6009. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Ma, Y.; Cao, L.; Zhan, S.; Xu, Y.; Fu, F.; Liu, C.; Zhang, G.; Wang, Z.; Wang, R.; et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019, 10, 308. [Google Scholar] [CrossRef]
- Li, M.; Jin, R.; Wang, W.; Zhang, T.; Sang, J.; Li, N.; Han, Q.; Zhao, W.; Li, C.; Liu, Z. STAT3 regulates glycolysis via targeting hexokinase 2 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 24777–24784. [Google Scholar] [CrossRef]
- Sheng, T.; Mao, X.-B.; Zhang, S.-H. CaMKKβ regulates proliferation, apoptosis, and glycolysis of hepatocellular carcinoma via PI3K/AKT pathway. Ann. Palliat. Med. 2020, 9, 3857–3869. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J. Wnt/β-Catenin Signaling and Obesity. Front. Physiol. 2018, 9, 792. [Google Scholar] [CrossRef]
- Juan, J.; Muraguchi, T.; Iezza, G.; Sears, R.C.; McMahon, M. Diminished WNT → β-catenin → c-MYC signaling is a barrier for malignant progression of BRAFV600E-induced lung tumors. Genes Dev. 2014, 28, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chu, T.-Y.; Ding, D.-C. WNT/β-Catenin signaling pathway regulates non-tumorigenesis of human embryonic stem cells co-cultured with human umbilical cord mesenchymal stem cells. Sci. Rep. 2017, 7, 41913. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, K.; Wang, G.; Zhang, D.; Shi, C.; Ding, Y.; Hong, D.; Zhang, D.; He, H.; Sun, L.; et al. Lanatoside C inhibits cell proliferation and induces apoptosis through attenuating Wnt/β-catenin/c-Myc signaling pathway in human gastric cancer cell. Biochem. Pharmacol. 2018, 150, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fu, X.; Li, R. CNN1 regulates the DKK1/Wnt/β-catenin/c-myc signaling pathway by activating TIMP2 to inhibit the invasion, migration and EMT of lung squamous cell carcinoma cells. Exp. Ther. Med. 2021, 22, 855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.; Rychahou, P.; Harris, J.W.; Zaytseva, Y.Y.; Liu, J.; Wang, C.; Weiss, H.L.; Liu, C.; Lee, E.Y.; et al. Deptor Is a Novel Target of Wnt/β-Catenin/c-Myc and Contributes to Colorectal Cancer Cell Growth. Cancer Res. 2018, 78, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Seo, E.; Kim, J.W.; Nam, S.A.; Lee, J.Y.; Jun, J.; Oh, S.; Park, M.; Jho, E.H.; Yoo, K.H.; et al. TAZ/Wnt-β-catenin/c-MYC axis regulates cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2020, 117, 29001–29012. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zuo, X.; Sun, X.; Tian, X.; Teng, Y. Hexokinase 2 Promotes Cell Proliferation and Tumor Formation through the Wnt/β-catenin Pathway-mediated Cyclin D1/c-myc Upregulation in Epithelial Ovarian Cancer. J. Cancer 2022, 13, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, X.; Niu, C.; Wang, X.; Li, W.; Liu, M.; Wang, Y.; Huang, S.; Chen, X.; Li, X.; et al. aFGF alleviates diabetic endothelial dysfunction by decreasing oxidative stress via Wnt/β-catenin-mediated upregulation of HXK2. Redox Biol. 2021, 39, 101811. [Google Scholar] [CrossRef]
- Cui, N.; Li, L.; Feng, Q.; Ma, H.M.; Lei, D.; Zheng, P.S. Hexokinase 2 Promotes Cell Growth and Tumor Formation Through the Raf/MEK/ERK Signaling Pathway in Cervical Cancer. Front. Oncol. 2020, 10, 581208. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, F.; Cai, Y.; Sheng, H.; Zheng, R.; Yin, X.; Lu, Z.; Su, L.; Chen, X.; Zeng, C.; et al. The EGFR-p38 MAPK axis up-regulates PD-L1 through miR-675-5p and down-regulates HLA-ABC via hexokinase-2 in hepatocellular carcinoma cells. Cancer Commun. 2021, 41, 62–78. [Google Scholar] [CrossRef]
- Yu, C.; Xue, J.; Zhu, W.; Jiao, Y.; Zhang, S.; Cao, J. Warburg meets non-coding RNAs: The emerging role of ncRNA in regulating the glucose metabolism of cancer cells. Tumour Biol. 2015, 36, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Feng, S.; Chen, Z.; Wen, G.; Zu, X.; Zhong, J. Non-coding RNAs: Key regulators of aerobic glycolysis in breast cancer. Life Sci. 2020, 250, 117579. [Google Scholar] [CrossRef]
- Gao, S.-J.; Ren, S.-N.; Liu, Y.-T.; Yan, H.W.; Chen, X.-B. Targeting EGFR sensitizes 5-Fu-resistant colon cancer cells through modification of the lncRNA-FGD5-AS1-miR-330-3p-Hexokinase 2 axis. Mol. Ther. Oncolytics 2021, 23, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shan, G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021, 505, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liao, W.-L.; Lu, Q.-J.; Zhang, P.; Zhu, J.; Jiang, G.-N. Retraction Notice to: Hypoxic tumor-derived exosomal circular RNA SETDB1 promotes invasive growth and EMT via the miR-7/Sp1 axis in lung adenocarcinoma. Mol. Ther. Nucleic Acids 2022, 28, 596. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, X.; Liu, L.; Hu, X.; Liu, J.; Yang, J.; Yang, J.; Lu, L.; Zhang, Z.; Ma, S.; et al. Emerging Epigenetic Regulation of Circular RNAs in Human Cancer. Mol. Ther. Nucleic Acids 2019, 16, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ni, L.; Zhao, F.; Dai, X.; Tao, J.; Pan, J.; Shi, A.; Shen, Z.; Su, C.; Zhang, Y. Overexpression of hsa_circ_0002874 promotes resistance of non-small cell lung cancer to paclitaxel by modulating miR-1273f/MDM2/p53 pathway. Aging 2021, 13, 5986–6009. [Google Scholar] [CrossRef]
- Ding, L.; Xie, Z. CircWHSC1 regulates malignancy and glycolysis by the miR-212-5p/AKT3 pathway in triple-negative breast cancer. Exp. Mol. Pathol. 2021, 123, 104704. [Google Scholar] [CrossRef]
- Sui, C.; Qu, W.; Lian, Y.; Feng, C.; Zhan, Y. Hsa_circ_0069094 knockdown inhibits cell proliferation, migration, invasion and glycolysis, while induces cell apoptosis by miR-661/HMGA1 axis in breast cancer. Anticancer Drugs 2021, 32, 829–841. [Google Scholar] [CrossRef]
- Yalan, S.; Yanfang, L.; He, C.; Yujie, T. Circular RNA circRHOBTB3 inhibits ovarian cancer progression through PI3K/AKT signaling pathway. Panminerva Med. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Yu, H.; Luo, H.; Liu, X. Knockdown of circ_0102273 inhibits the proliferation, metastasis and glycolysis of breast cancer through miR-1236-3p/PFKFB3 axis. Anti-Cancer Drugs 2022, 33, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, M.; Dong, Y.; Xu, B.; Chen, J.; Ding, Y.; Qiu, S.; Li, L.; Karamfilova Zaharieva, E.; Zhou, X.; et al. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sheikh, T.; Sen, E. SIRT6 regulated nucleosomal occupancy affects Hexokinase 2 expression. Exp. Cell Res. 2017, 357, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.; Gupta, P.; Gowda, P.; Patrick, S.; Sen, E. Hexokinase 2 and nuclear factor erythroid 2-related factor 2 transcriptionally coactivate xanthine oxidoreductase expression in stressed glioma cells. J. Biol. Chem. 2018, 293, 4767–4777. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Konno, Y.; Ihira, K.; Kobayashi, N.; Yue, J.; Watari, H. Long non-coding RNA DLEU2 drives EMT and glycolysis in endometrial cancer through HK2 by competitively binding with miR-455 and by modulating the EZH2/miR-181a pathway. J. Exp. Clin. Cancer Res. 2021, 40, 216. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Chen, M.; Jiang, R.; Guan, H.; Huang, Y.; Su, H.; Hu, Q.; Han, X.; Xiao, J. Involvement of EZH2 in aerobic glycolysis of prostate cancer through miR-181b/HK2 axis. Oncol. Rep. 2017, 37, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, X.; Li, L.; Gao, Z.; Su, X.; Ji, M.; Liu, J. N6-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020, 11, 911. [Google Scholar] [CrossRef]
- Sun, R.F.; Zhao, C.Y.; Chen, S.; Yu, W.; Zhou, M.M.; Gao, C.R. Androgen Receptor Stimulates Hexokinase 2 and Induces Glycolysis by PKA/CREB Signaling in Hepatocellular Carcinoma. Dig. Dis. Sci. 2021, 66, 802–813. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Q.; Lv, Z.; Ling, Y.; Hou, X.; Chen, Z.; Dinglin, X.; Ma, S.; Li, D.; Wu, Y.; et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol. Cancer 2019, 18, 181. [Google Scholar] [CrossRef]
- Tang, J.; Pan, H.; Wang, W.; Qi, C.; Gu, C.; Shang, A.; Zhu, J. MiR-495-3p and miR-143-3p co-target CDK1 to inhibit the development of cervical cancer. Clin. Transl. Oncol. 2021, 23, 2323–2334. [Google Scholar] [CrossRef]
- Peng, J.; Wu, H.J.; Zhang, H.F.; Fang, S.Q.; Zeng, R. miR-143-3p inhibits proliferation and invasion of hepatocellular carcinoma cells by regulating its target gene FGF1. Clin. Transl. Oncol. 2021, 23, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, Y.; Li, H.; Hu, Q.; Chen, X.; He, Y.; Xue, C.; Ren, F.; Ren, Z.; Li, J.; et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol. Cancer 2019, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiang, X.-L.; Cai, P.; Jiang, Y.-L.; Zhu, Z.-W.; Hu, F.-L.; Wang, J. CircRNA-ACAP2 contributes to the invasion, migration, and anti-apoptosis of neuroblastoma cells through targeting the miRNA-143-3p-hexokinase 2 axis. Transl. Pediatr. 2021, 10, 3237–3247. [Google Scholar] [CrossRef]
- Deng, M.; Tang, H.; Zhou, Y.; Zhou, M.; Xiong, W.; Zheng, Y.; Ye, Q.; Zeng, X.; Liao, Q.; Guo, X.; et al. miR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J. Cell Sci. 2011, 124, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ye, P.; Ye, Y.; Han, B. MicroRNA-216b targets HK2 to potentiate autophagy and apoptosis of breast cancer cells via the mTOR signaling pathway. Int. J. Biol. Sci. 2021, 17, 2970–2983. [Google Scholar] [CrossRef] [PubMed]
- Sadrkhanloo, M.; Entezari, M.; Orouei, S.; Ghollasi, M.; Fathi, N.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Saebfar, H.; Hashemi, M.; et al. STAT3-EMT axis in tumors: Modulation of cancer metastasis, stemness and therapy response. Pharmacol. Res. 2022, 182, 106311. [Google Scholar] [CrossRef] [PubMed]
- Blaha, C.S.; Ramakrishnan, G.; Jeon, S.-M.; Nogueira, V.; Rho, H.; Kang, S.; Bhaskar, P.; Terry, A.R.; Aissa, A.F.; Frolov, M.V.; et al. A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis. Nat. Commun. 2022, 13, 899. [Google Scholar] [CrossRef]
- Shangguan, X.; He, J.; Ma, Z.; Zhang, W.; Ji, Y.; Shen, K.; Yue, Z.; Li, W.; Xin, Z.; Zheng, Q.; et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat. Commun. 2021, 12, 1812. [Google Scholar] [CrossRef]
- Amsalem, Z.; Arif, T.; Shteinfer-Kuzmine, A.; Chalifa-Caspi, V.; Shoshan-Barmatz, V. The Mitochondrial Protein VDAC1 at the Crossroads of Cancer Cell Metabolism: The Epigenetic Link. Cancers 2020, 12, 1031. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Stowe, D.F.; Tajkhorshid, E.; Kwok, W.M.; Camara, A.K. Knockout of VDAC1 in H9c2 Cells Promotes Oxidative Stress-Induced Cell Apoptosis through Decreased Mitochondrial Hexokinase II Binding and Enhanced Glycolytic Stress. Cell. Physiol. Biochem. 2020, 54, 853–874. [Google Scholar]
- Feng, T.; Wang, J.; Cheng, K.; Lu, Q.; Zhao, R.; Wang, S.; Zhang, Q.; Ge, L.; Pan, J.; Song, G.; et al. IL13Rα1 prevents a castration resistant phenotype of prostate cancer by targeting hexokinase 2 for ubiquitin-mediated degradation. Cancer Biol. Med. 2021, 19, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, M.; Wang, C.; Sun, D.; Liu, P.; Zhong, X.; Yu, W. Long noncoding RNA KCNQ1OT1 promotes colorectal carcinogenesis by enhancing aerobic glycolysis via hexokinase-2. Aging 2020, 12, 11685–11697. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.J.; Taylor, S.; Nahiyaan, N.; Song, J.; Murphy, C.J.; Dantas, E.; Cheng, S.; Hsu, T.-W.; Ramsamooj, S.; Grover, R.; et al. GLUT5 (SLC2A5) enables fructose-mediated proliferation independent of ketohexokinase. Cancer Metab. 2021, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.D.; Lu, C.; Tutnauer, J.; Hartman, T.E.; Hwang, S.-K.; Murphy, C.J.; Pauli, C.; Morris, R.; Taylor, S.; Bosch, K.; et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019, 363, 1345–1349. [Google Scholar] [CrossRef]
- Wang, J.; Shao, F.; Yang, Y.; Wang, W.; Yang, X.; Li, R.; Cheng, H.; Sun, S.; Feng, X.; Gao, Y.; et al. A non-metabolic function of hexokinase 2 in small cell lung cancer: Promotes cancer cell stemness by increasing USP11-mediated CD133 stability. Cancer Commun. 2022, 42, 1008–1027. [Google Scholar] [CrossRef]
- Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Leung, T.H.Y.; Han, C.Y.; Tsang, B.K.; Cheung, A.N.Y.; Ngan, H.Y.S.; Chan, K.K.L. Hexokinase 2 Regulates Ovarian Cancer Cell Migration, Invasion and Stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 Signaling Cascades. Cancers 2019, 11, 813. [Google Scholar] [CrossRef]
- Aleksakhina, S.N.; Kashyap, A.; Imyanitov, E.N. Mechanisms of acquired tumor drug resistance. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1872, 188310. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Levchenko, A. Epigenetics as a mediator of plasticity in cancer. Science 2023, 379, eaaw3835. [Google Scholar] [CrossRef]
- Chiappa, M.; Guffanti, F.; Bertoni, F.; Colombo, I.; Damia, G. Overcoming PARPi resistance: Preclinical and clinical evidence in ovarian cancer. Drug Resist. Updates 2021, 55, 100744. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
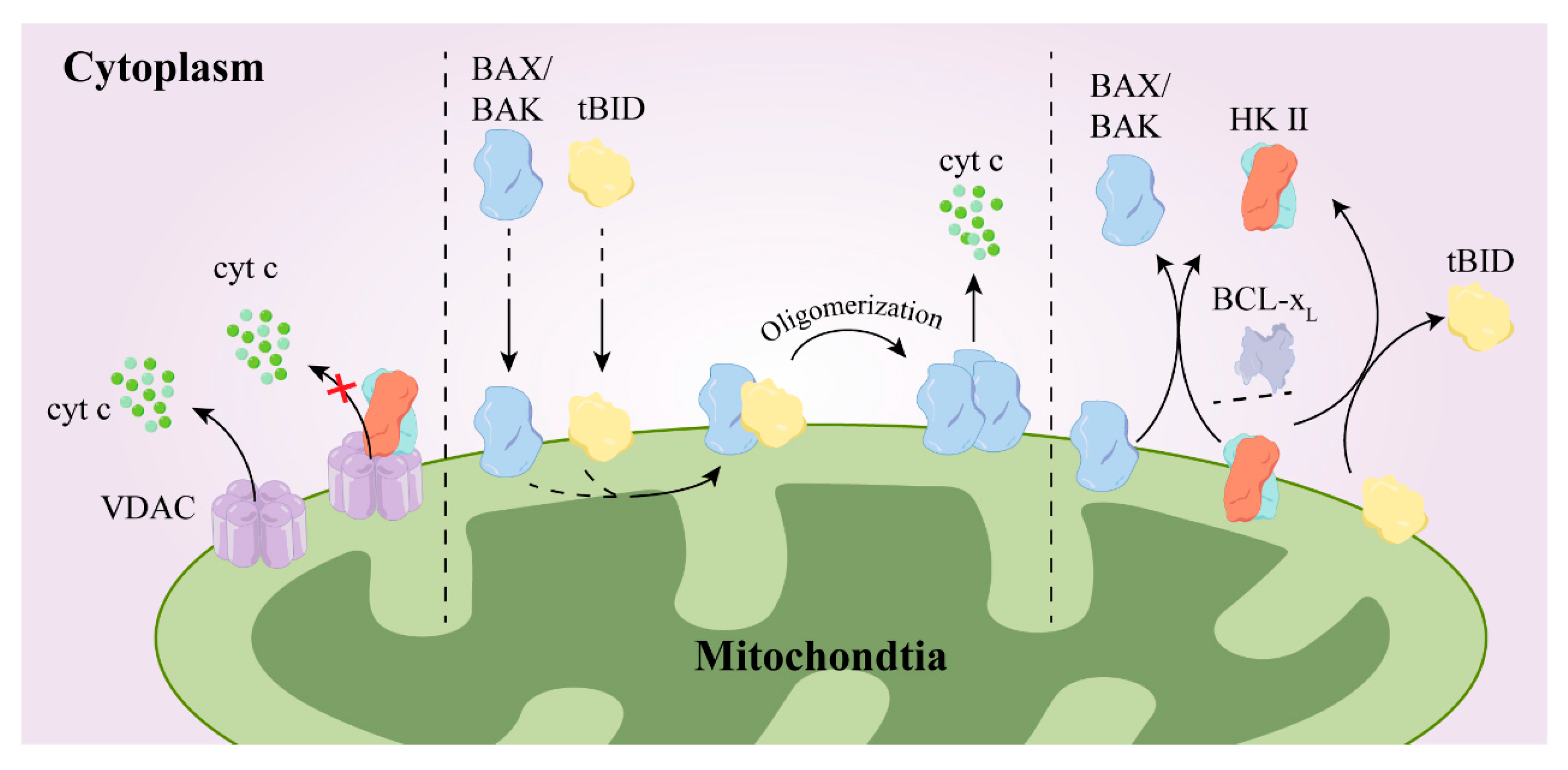
- Schoeniger, A.; Wolf, P.; Edlich, F. How Do Hexokinases Inhibit Receptor-Mediated Apoptosis? Biology 2022, 11, 412. [Google Scholar] [CrossRef]
- Sattler, M.; Liang, H.; Nettesheim, D.; Meadows, R.P.; Harlan, J.E.; Eberstadt, M.; Yoon, H.S.; Shuker, S.B.; Chang, B.S.; Minn, A.J.; et al. Structure of Bcl-xL-Bak Peptide Complex: Recognition Between Regulators of Apoptosis. Science 1997, 275, 983–986. [Google Scholar] [CrossRef]
- Lauterwasser, J.; Fimm-Todt, F.; Oelgeklaus, A.; Schreiner, A.; Funk, K.; Falquez-Medina, H.; Klesse, R.; Jahreis, G.; Zerbes, R.M.; O’Neill, K.; et al. Hexokinases inhibit death receptor–dependent apoptosis on the mitochondria. Proc. Natl. Acad. Sci. USA 2021, 118, e2021175118. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Arbel, N.; Calo, D.; Arzoine, L.; Israelson, A.; Keinan, N.; Ben-Romano, R.; Friedman, O.; Shoshan-Barmatz, V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009, 122, 1906–1916. [Google Scholar] [CrossRef]
- Thomas, G.E.; Egan, G.; García-Prat, L.; Botham, A.; Voisin, V.; Patel, P.S.; Hoff, F.W.; Chin, J.; Nachmias, B.; Kaufmann, K.B.; et al. The metabolic enzyme hexokinase 2 localizes to the nucleus in AML and normal haematopoietic stem and progenitor cells to maintain stemness. Nat. Cell Biol. 2022, 24, 872–884. [Google Scholar] [CrossRef]
- Zhu, Q.-Q.; Ma, C.; Wang, Q.; Song, Y.; Lv, T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biol. 2016, 37, 185–197. [Google Scholar] [CrossRef]
- Deng, J.-J.; Zhang, W.; Xu, X.-M.; Zhang, F.; Tao, W.-P.; Ye, J.-J.; Ge, W. Twist mediates an aggressive phenotype in human colorectal cancer cells. Int. J. Oncol. 2016, 48, 1117–1124. [Google Scholar] [CrossRef]
- Zhang, B.; Chan, S.-H.; Liu, X.-Q.; Shi, Y.-Y.; Dong, Z.-X.; Shao, X.-R.; Zheng, L.-Y.; Mai, Z.-Y.; Fang, T.-L.; Deng, L.-Z.; et al. Targeting hexokinase 2 increases the sensitivity of oxaliplatin by Twist1 in colorectal cancer. J. Cell. Mol. Med. 2021, 25, 8836–8849. [Google Scholar] [CrossRef]
- Han, C.Y.; Patten, D.A.; Lee, S.G.; Parks, R.J.; Chan, D.W.; Harper, M.-E.; Tsang, B.K. p53 Promotes chemoresponsiveness by regulating hexokinase II gene transcription and metabolic reprogramming in epithelial ovarian cancer. Mol. Carcinog. 2019, 58, 2161–2174. [Google Scholar] [CrossRef]
- Zeh, H.J.; Bahary, N.; Boone, B.A.; Singhi, A.D.; Miller-Ocuin, J.L.; Normolle, D.P.; Zureikat, A.H.; Hogg, M.E.; Bartlett, D.L.; Lee, K.K.; et al. A Randomized Phase II Preoperative Study of Autophagy Inhibition with High-Dose Hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in Pancreatic Cancer Patients. Clin. Cancer Res. 2020, 26, 3126–3134. [Google Scholar] [CrossRef]
- Xia, J.; He, Y.; Meng, B.; Chen, S.; Zhang, J.; Wu, X.; Zhu, Y.; Shen, Y.; Feng, X.; Guan, Y.; et al. NEK2 induces autophagy-mediated bortezomib resistance by stabilizing Beclin-1 in multiple myeloma. Mol. Oncol. 2020, 14, 763–778. [Google Scholar] [CrossRef]
- Liu, X.; Miao, W.; Huang, M.; Li, L.; Dai, X.; Wang, Y. Elevated Hexokinase II Expression Confers Acquired Resistance to 4-Hydroxytamoxifen in Breast Cancer Cells. Mol. Cell. Proteom. 2019, 18, 2273–2284. [Google Scholar] [CrossRef]
- Lin, C.; Chen, H.; Han, R.; Li, L.; Lu, C.; Hao, S.; Wang, Y.; He, Y. Hexokinases II-mediated glycolysis governs susceptibility to crizotinib in ALK-positive non-small cell lung cancer. Thorac. Cancer 2021, 12, 3184–3193. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.; Hu, X.; Zhang, S.; Jiang, Y.; Yao, G.; Hu, K.; Xu, X.; Liang, B.; Wu, Q.; et al. Disrupting Circadian Rhythm via the PER1–HK2 Axis Reverses Trastuzumab Resistance in Gastric Cancer. Cancer Res. 2022, 82, 1503–1517. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Yu, S.J.; Na, J.; Kim, K.; Cho, Y.Y.; Lee, Y.B.; Cho, E.J.; Lee, J.-H.; Kim, Y.J.; Youn, H.; et al. Hexokinase-II Inhibition Synergistically Augments the Anti-tumor Efficacy of Sorafenib in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1292. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Peng, S.; Liu, X. The combination of the glycolysis inhibitor 2-DG and sorafenib can be effective. OncoTargets Ther. 2019, 12, 5359–5373. [Google Scholar] [CrossRef]
- Huang, J.P.; Zhang, J.L.; Hua, Y.; Huang, P.; Ge, C.L. Inhibition of Sirolimus on the growth of pancreatic carcinoma and its effect on the expression of glucose transporter and hexokinase Ⅱ. Zhonghua Yi Xue Za Zhi 2016, 96, 438–441. [Google Scholar]
- Gan, L.; Ren, Y.; Lu, J.; Ma, J.; Shen, X.; Zhuang, Z. Synergistic Effect of 3-Bromopyruvate in Combination with Rapamycin Impacted Neuroblastoma Metabolism by Inhibiting Autophagy. OncoTargets Ther. 2020, 13, 11125–11137. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, J.; Lubet, R.A.; Komas, S.M.; Kalyanaraman, B.; Wang, Y.; You, M. Enhanced Antitumor Activity of 3-Bromopyruvate in Combination with Rapamycin In Vivo and In Vitro. Cancer Prev. Res. 2015, 8, 318–326. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, H.-L.; Li, D.-D.; Yang, K.-L.; Tang, J.; Li, X.; Ji, J.; Yu, Y.; Wu, R.-Y.; Ravichandran, S.; et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2). Autophagy 2018, 14, 671–684. [Google Scholar] [CrossRef]
- Ikeda, S.; Abe, F.; Matsuda, Y.; Kitadate, A.; Takahashi, N.; Tagawa, H. Hypoxia-inducible hexokinase-2 enhances anti-apoptotic function via activating autophagy in multiple myeloma. Cancer Sci. 2020, 111, 4088–4101. [Google Scholar] [CrossRef]
- Goetze, K.; Walenta, S.; Ksiazkiewicz, M.; Kunz-Schughart, L.A.; Mueller-Klieser, W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 2011, 39, 453–463. [Google Scholar] [CrossRef]
- Baumann, F.; Leukel, P.; Doerfelt, A.; Beier, C.P.; Dettmer, K.; Oefner, P.J.; Kastenberger, M.; Kreutz, M.; Nickl-Jockschat, T.; Bogdahn, U.; et al. Lactate promotes glioma migration by TGF-β2–dependent regulation of matrix metalloproteinase-2. Neuro-Oncology 2009, 11, 368–380. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Gu, Y.; Cang, W.; Sun, P.; Xiang, Y. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int. J. Mol. Sci. 2022, 23, 11943. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs 2018, 27, 963–970. [Google Scholar] [CrossRef]
- Cho, E.J.; Yu, S.J.; Kim, K.; Cho, H.; Cho, Y.Y.; Lee, Y.B.; Lee, J.-H.; Kim, Y.J.; Youn, H.; Yoon, J.-H. Carbonic anhydrase-IX inhibition enhances the efficacy of hexokinase II inhibitor for hepatocellular carcinoma in a murine model. J. Bioenerg. Biomembr. 2019, 51, 121–129. [Google Scholar] [CrossRef]
- Yu, S.-j.; Yoon, J.-h.; Lee, J.-h.; Myung, S.-j.; Jang, E.-s.; Kwak, M.-s.; Cho, E.-j.; Jang, J.-j.; Kim, Y.-j.; Lee, H.-s. Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol. Sin. 2011, 32, 912–920. [Google Scholar] [CrossRef]
- Martuza, R.L.; Malick, A.; Markert, J.M.; Ruffner, K.L.; Coen, D.M. Experimental Therapy of Human Glioma by Means of a Genetically Engineered Virus Mutant. Science 1991, 252, 854–856. [Google Scholar] [CrossRef]
- Al-Ziaydi, A.G.; Al-Shammari, A.M.; Hamzah, M.I.; Kadhim, H.S.; Jabir, M.S. Hexokinase inhibition using D-Mannoheptulose enhances oncolytic newcastle disease virus-mediated killing of breast cancer cells. Cancer Cell Int. 2020, 20, 420. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, W.; Lin, Y.; Hu, J.; Liu, X.; Xu, W.; Liu, Y.; Hu, C.; He, S.; Gong, S.; et al. Lonidamine potentiates the oncolytic efficiency of M1 virus independent of hexokinase 2 but via inhibition of antiviral immunity. Cancer Cell Int. 2020, 20, 532. [Google Scholar] [CrossRef]
- McDonald, A.J.; Curt, K.M.; Patel, R.P.; Kozlowski, H.; Sackett, D.L.; Robey, R.W.; Gottesman, M.M.; Bates, S.E. Targeting mitochondrial hexokinases increases efficacy of histone deacetylase inhibitors in solid tumor models. Exp. Cell Res. 2019, 375, 106–112. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, B.; Zhang, M.; Liu, Z.; Huang, Y.; Zhao, X.; Hu, H.; Tan, M.; Xu, J.-Y. An Integrative Proteome-Based Pharmacologic Characterization and Therapeutic Strategy Exploration of SAHA in Solid Malignancies. J. Proteome Res. 2022, 21, 953–964. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mansouri, S.; Burrell, K.; Li, M.; Mamatjan, Y.; Liu, J.; Nejad, R.; Kumar, S.; Jalali, S.; Singh, S.K.; et al. Ketoconazole and Posaconazole Selectively Target HK2-expressing Glioblastoma Cells. Clin. Cancer Res. 2019, 25, 844–855. [Google Scholar] [CrossRef]
- Kirstein, A.; Schilling, D.; Combs, S.E.; Schmid, T.E. Lomeguatrib Increases the Radiosensitivity of MGMT Unmethylated Human Glioblastoma Multiforme Cell Lines. Int. J. Mol. Sci. 2021, 22, 6781. [Google Scholar] [CrossRef]
- Phillips, W.P., Jr.; Willson, J.K.; Markowitz, S.D.; Zborowska, E.; Zaidi, N.H.; Liu, L.; Gordon, N.H.; Gerson, S.L. O6-methylguanine-DNA methyltransferase (MGMT) transfectants of a 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU)-sensitive colon cancer cell line selectively repopulate heterogenous MGMT+/MGMT- xenografts after BCNU and O6-benzylguanine plus BCNU. Cancer Res. 1997, 57, 4817–4823. [Google Scholar]
- Sun, X.; Fan, T.; Sun, G.; Zhou, Y.; Huang, Y.; Zhang, N.; Zhao, L.; Zhong, R.; Peng, Y. 2-Deoxy-D-glucose increases the sensitivity of glioblastoma cells to BCNU through the regulation of glycolysis, ROS and ERS pathways: In vitro and in vivo validation. Biochem. Pharmacol. 2022, 199, 115029. [Google Scholar] [CrossRef]
- Sun, X.; Sun, G.; Huang, Y.; Hao, Y.; Tang, X.; Zhang, N.; Zhao, L.; Zhong, R.; Peng, Y. 3-Bromopyruvate regulates the status of glycolysis and BCNU sensitivity in human hepatocellular carcinoma cells. Biochem. Pharmacol. 2020, 177, 113988. [Google Scholar] [CrossRef]
- Sun, X.; Sun, G.; Huang, Y.; Zhang, S.; Tang, X.; Zhang, N.; Zhao, L.; Zhong, R.; Peng, Y. Glycolytic inhibition by 3-bromopyruvate increases the cytotoxic effects of chloroethylnitrosoureas to human glioma cells and the DNA interstrand cross-links formation. Toxicology 2020, 435, 152413. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, T.; Doh, H.M.; Trinh, K.R.; Catapang, A.; Lee, J.T.; Braas, D.; Bayley, N.A.; Yamada, R.E.; Vasuthasawat, A.; et al. An HK2 Antisense Oligonucleotide Induces Synthetic Lethality in HK1−HK2+ Multiple Myeloma. Cancer Res. 2019, 79, 2748–2760. [Google Scholar] [CrossRef]
- Marini, C.; Salani, B.; Massollo, M.; Amaro, A.; Esposito, A.I.; Maria Orengo, A.; Capitanio, S.; Emionite, L.; Riondato, M.; Bottoni, G.; et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 2013, 12, 3490–3499. [Google Scholar] [CrossRef]
- Zhang, H.-n.; Yang, L.; Ling, J.-y.; Czajkowsky, D.M.; Wang, J.-F.; Zhang, X.-W.; Zhou, Y.-M.; Ge, F.; Yang, M.-k.; Xiong, Q.; et al. Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc. Natl. Acad. Sci. USA 2015, 112, 15084–15089. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Goldberg, A.F.; Lin, Z.; Ko, Y.-H.; Flomenberg, N.; Wang, C.; Pavlides, S.; Pestell, R.G.; Howell, A.; Sotgia, F.; et al. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol. Ther. 2011, 12, 924–938. [Google Scholar] [CrossRef]
- Yang, X.; Sun, D.; Tian, Y.; Ling, S.; Wang, L. Metformin sensitizes hepatocellular carcinoma to arsenic trioxide-induced apoptosis by downregulating Bcl2 expression. Tumor Biol. 2015, 36, 2957–2964. [Google Scholar] [CrossRef]
- Ling, S.; Xie, H.; Yang, F.; Shan, Q.; Dai, H.; Zhuo, J.; Wei, X.; Song, P.; Zhou, L.; Xu, X.; et al. Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: Roles of p38 MAPK, ERK3, and mTORC1. J. Hematol. Oncol. 2017, 10, 59. [Google Scholar] [CrossRef]
- Rocca, A.; Cortesi, P.; Cortesi, L.; Gianni, L.; Matteucci, F.; Fantini, L.; Maestri, A.; Giunchi, D.C.; Cavanna, L.; Ciani, R.; et al. Phase II study of liposomal doxorubicin, docetaxel and trastuzumab in combination with metformin as neoadjuvant therapy for HER2-positive breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920985632. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Liu, X.; Mao, K.; Zhong, D.; Marcus, A.I.; Khuri, F.R.; Sun, S.Y.; He, Y.; Zhou, W. Inhibition of IGF1R enhances 2-deoxyglucose in the treatment of non-small cell lung cancer. Lung Cancer 2018, 123, 36–43. [Google Scholar] [CrossRef]
- Zhong, D.; Xiong, L.; Liu, T.; Liu, X.; Liu, X.; Chen, J.; Sun, S.Y.; Khuri, F.R.; Zong, Y.; Zhou, Q.; et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J. Biol. Chem. 2009, 284, 23225–23333. [Google Scholar] [CrossRef]
- Maher, J.C.; Wangpaichitr, M.; Savaraj, N.; Kurtoglu, M.; Lampidis, T.J. Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-d-glucose. Mol. Cancer Ther. 2007, 6, 732–741. [Google Scholar] [CrossRef]
- Hellemann, E.; Walker, J.L.; Lesko, M.A.; Chandrashekarappa, D.G.; Schmidt, M.C.; O’Donnell, A.F.; Durrant, J.D. Novel mutation in hexokinase 2 confers resistance to 2-deoxyglucose by altering protein dynamics. PLoS Comput. Biol. 2022, 18, e1009929. [Google Scholar] [CrossRef]
- El Sayed, S.M. Enhancing anticancer effects, decreasing risks and solving practical problems facing 3-bromopyruvate in clinical oncology: 10 years of research experience. Int. J. Nanomed. 2018, 13, 4699–4709. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Baghdadi, H.; Zolaly, M.; Almaramhy, H.H.; Ayat, M.; Donki, J.G. The promising anticancer drug 3-bromopyruvate is metabolized through glutathione conjugation which affects chemoresistance and clinical practice: An evidence-based view. Med. Hypotheses 2017, 100, 67–77. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Lu, W.; Huang, P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 553–560. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, K.J.; Kim, J.-A.; Kim, H.M.; Lee, J.D.; Lee, J.M.; Kim, S.J.; Park, J.H. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells. J. Bioenerg. Biomembr. 2008, 40, 607–618. [Google Scholar] [CrossRef]
- Forster, R.; Campana, A.; D’Onofrio, E.; Henderson, L.; Mosesso, P.; Barcellona, P.S. Lonidamine: A non-mutagenic antitumor agent. Carcinogenesis 1990, 11, 1509–1515. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers 2020, 12, 3332. [Google Scholar] [CrossRef]
- Salani, B.; Marini, C.; Rio, A.D.; Ravera, S.; Massollo, M.; Orengo, A.M.; Amaro, A.; Passalacqua, M.; Maffioli, S.; Pfeffer, U.; et al. Metformin Impairs Glucose Consumption and Survival in Calu-1 Cells by Direct Inhibition of Hexokinase-II. Sci. Rep. 2013, 3, 2070. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhang, Y.; Wu, C.; Yang, K.; Gao, S.; Zheng, M.; Li, X.; Li, H.; Chen, L. Structure based discovery of novel hexokinase 2 inhibitors. Bioorganic Chem. 2020, 96, 103609. [Google Scholar] [CrossRef]
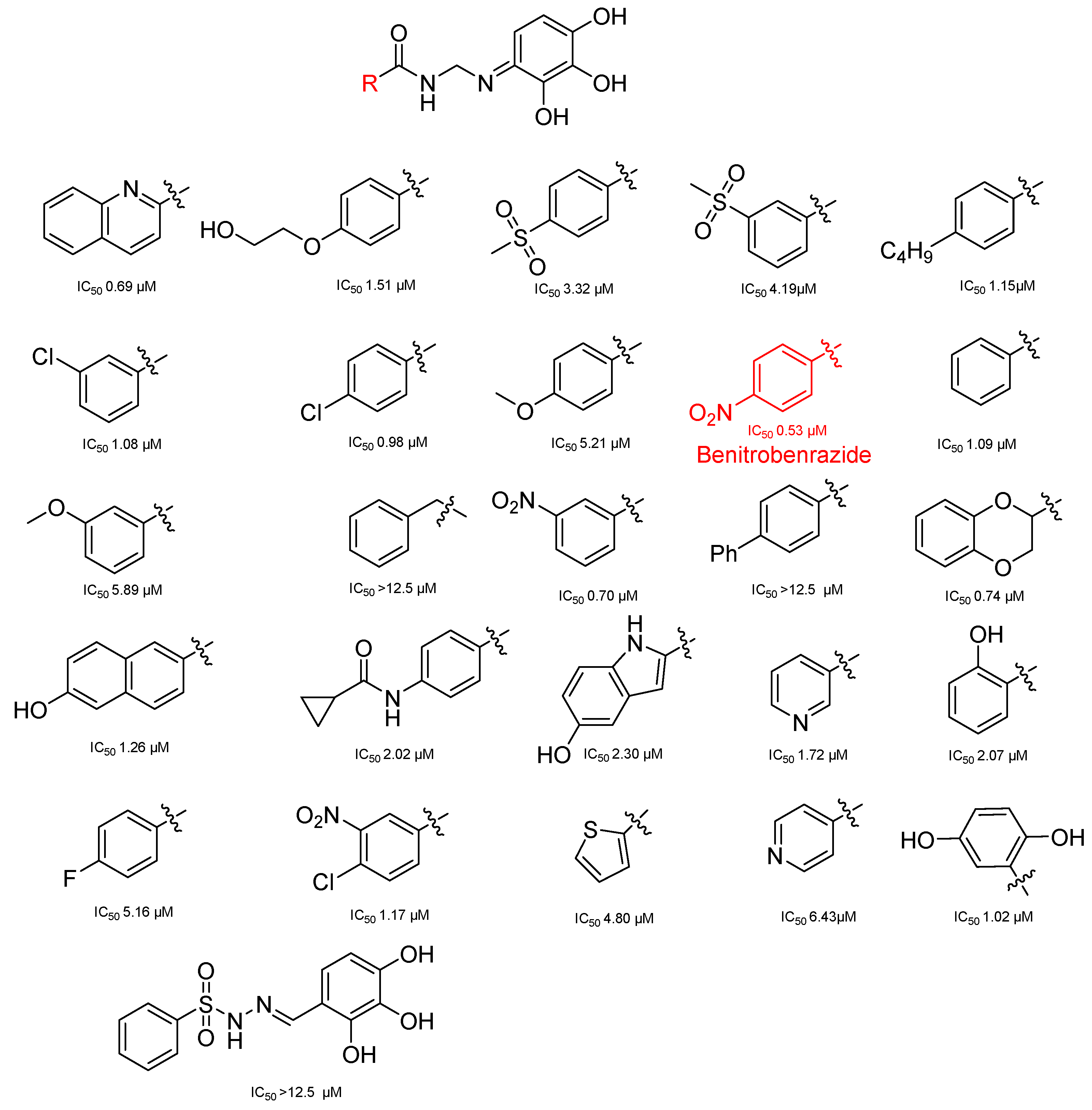
- Juszczak, K.; Kubicka, A.; Kitel, R.; Dzido, G.; Łabieniec-Watała, M.; Zawadzki, S.; Marczak, A.; Walczak, K.; Matczak, K.; Tomczyk, M.D. Hexokinase 2 Inhibition and Biological Effects of BNBZ and Its Derivatives: The Influence of the Number and Arrangement of Hydroxyl Groups. Int. J. Mol. Sci. 2022, 23, 2616. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, C.; Yang, K.; Yang, Y.; Liu, Y.; Gao, S.; Wang, Q.; Li, C.; Chen, L.; Li, H. Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol. Res. 2021, 164, 105367. [Google Scholar] [CrossRef] [PubMed]
- Behar, V.; Hamo, R.Y.; Dor-On, E.; Becker, O.M. Abstract 1725: A bi-functional mechanism of action: Activating the NLRP3 inflammasome and triggering apoptosis in cancer via a HK2-VDAC modulator. Cancer Res. 2018, 78 (Suppl. S13), 1725. [Google Scholar] [CrossRef]
- Sheng, H.; Tang, W. Glycolysis Inhibitors for Anticancer Therapy: A Review of Recent Patents. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.B.; Hay, N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 2006, 25, 4683–4696. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhou, Y.; Tam, K.Y. The development of small-molecule inhibitors targeting hexokinase 2. Drug Discov. Today 2022, 27, 2574–2585. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L. Voltage dependent anion channels (VDACs): A brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancer. J. Bioenerg. Biomembr. 2008, 40, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.S.S.; Boechat, F.C.; Brasil, A.A.; Neto, J.R.M.; Ribeiro, G.D.; Paranhos, L.H.; Neves de Souza, N.; Vieira, T.; Outeiro, T.F.; Neves, B.C.; et al. Hexokinase 2: The preferential target of trehalose-6-phosphate over hexokinase 1. J. Cell. Biochem. 2022, 123, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, H.L.; Yang, Y.J.; Wang, L.; Lee, S.C. Overcome Cancer Cell Drug Resistance Using Natural Products. Evid.-Based Complement. Altern. Med. 2015, 2015, 767136. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Liu, W.-B.; Zhou, Z.-S.; Zhang, R.; Li, J.-L.; Zhou, K.-C. Epigallocatechin gallate inhibits human tongue carcinoma cells via HK2-mediated glycolysis. Oncol. Rep. 2015, 33, 1533–1539. [Google Scholar] [CrossRef]
- Khan, A.; Mohammad, T.; Shamsi, A.; Hussain, A.; Alajmi, M.F.; Husain, S.A.; Iqbal, M.A.; Hassan, M.I. Identification of plant-based hexokinase 2 inhibitors: Combined molecular docking and dynamics simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 10319–10331. [Google Scholar] [CrossRef]
- Wu, Z.; Han, X.; Tan, G.; Zhu, Q.; Chen, H.; Xia, Y.; Gong, J.; Wang, Z.; Wang, Y.; Yan, J. Dioscin Inhibited Glycolysis and Induced Cell Apoptosis in Colorectal Cancer via Promoting c-myc Ubiquitination and Subsequent Hexokinase-2 Suppression. OncoTargets Ther. 2020, 13, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wu, Y.; Cai, F.; Li, Z.; Su, S.; Wang, J.; Cao, J.; Ma, L. Matrine Promotes Human Myeloid Leukemia Cells Apoptosis Through Warburg Effect Mediated by Hexokinase 2. Front. Pharmacol. 2019, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, G.; Mani, S. Molecular docking and simulation studies of phytocompounds derived from Centella asiatica and Andrographis paniculata against hexokinase II as mitocan agents. Mitochondrion 2021, 61, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.-j.; Bai, H.-h.; Liu, L.-w.; Chen, H.-y.; Shi, Q.; Chang, L.-s.; Ding, M.-m.; Shi, Q.; Zhou, M.-x.; Chen, W.-l.; et al. Asiatic Acid Interferes with Invasion and Proliferation of Breast Cancer Cells by Inhibiting WAVE3 Activation through PI3K/AKT Signaling Pathway. BioMed Res. Int. 2020, 2020, 1874387. [Google Scholar] [CrossRef] [PubMed]
- Jiaqi, L.; Siqing, H.; Qin, W.; Di, Z.; Bei, Z.; Jialin, Y. Andrographolide promoted ferroptosis to repress the development of non-small cell lung cancer through activation of the mitochondrial dysfunction. Phytomedicine 2023, 109, 154601. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Hu, J.-h.; Bi, R.; Liu, S.-q.; Wang, C.-x. Andrographolide Inhibits Proliferation and Promotes Apoptosis in Bladder Cancer Cells by Interfering with NF- κ B and PI3K/AKT Signaling In Vitro and In Vivo. Chin. J. Integr. Med. 2022, 28, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cui, L.; Yang, J.; Vong, C.T.; Hu, Y.; Xiao, J.; Chan, G.; He, Z.; Zhong, Z. Anticancer effects of asiatic acid against doxorubicin-resistant breast cancer cells via an AMPK-dependent pathway in vitro. Phytomedicine 2021, 92, 153737. [Google Scholar] [CrossRef]
- Miao, G.; Han, J.; Zhang, J.; Wu, Y.; Tong, G. Targeting Pyruvate Kinase M2 and Hexokinase II, Pachymic Acid Impairs Glucose Metabolism and Induces Mitochondrial Apoptosis. Biol. Pharm. Bull. 2019, 42, 123–129. [Google Scholar] [CrossRef]
- Fang, C.; Liu, Y.; Chen, L.; Luo, Y.; Cui, Y.; Zhang, N.; Liu, P.; Zhou, M.; Xie, Y. α-Hederin inhibits the growth of lung cancer A549 cells in vitro and in vivo by decreasing SIRT6 dependent glycolysis. Pharm. Biol. 2021, 59, 11–20. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, Z.; Peng, C.; Yi, W. HK2 is associated with the Warburg effect and proliferation in liver cancer: Targets for effective therapy with glycyrrhizin Corrigendum in /10.3892/mmr.2021.12143. Mol. Med. Rep. 2021, 23, 343. [Google Scholar] [CrossRef]
- Bao, F.; Yang, K.; Wu, C.; Gao, S.; Wang, P.; Chen, L.; Li, H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018, 125, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-J.; Zheng, H.-B.; Peng, A.-H.; Ma, J.-H.; Lu, D.-D.; Li, X.; Zhang, H.-Y.; Xie, W.-D. Strepantibins A–C: Hexokinase II Inhibitors from a Mud Dauber Wasp Associated Streptomyces sp. J. Nat. Prod. 2019, 82, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yi, M.; Tan, Y.; Li, X.; Li, G.; Zeng, Z.; Xiong, W.; Xiang, B. Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. J. Exp. Clin. Cancer Res. 2021, 40, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, Y.; Zhang, L.; Cai, H.; Ji, Z.; Gu, L.; Yang, S. Wogonin inhibits the growth of HT144 melanoma via regulating hedgehog signaling-mediated inflammation and glycolysis. Int. Immunopharmacol. 2021, 101, 108222. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Jiang, Y.; Zhang, F.; Jin, H.; Lu, C.; Wu, X.; Lu, Y.; Zheng, S. Curcumin regulates cell fate and metabolism by inhibiting hedgehog signaling in hepatic stellate cells. Lab. Investig. 2015, 95, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, J.; Wang, F.; Lu, Y.; Wen, C.; Bian, Q.; Wu, H. Deoxyelephantopin suppresses hepatic stellate cells activation associated with inhibition of aerobic glycolysis via hedgehog pathway. Biochem. Biophys. Res. Commun. 2019, 516, 1222–1228. [Google Scholar] [CrossRef]
- Sucu, B.O.; Ipek, O.S.; Kurtulus, S.O.; Yazici, B.E.; Karakas, N.; Guzel, M. Synthesis of novel methyl jasmonate derivatives and evaluation of their biological activity in various cancer cell lines. Bioorganic Chem. 2019, 91, 103146. [Google Scholar] [CrossRef]
- Huang, L.; He, C.; Zheng, S.; Wu, C.; Ren, M.; Shan, Y. AKT1/HK2 Axis-mediated Glucose Metabolism: A Novel Therapeutic Target of Sulforaphane in Bladder Cancer. Mol. Nutr. Food Res. 2022, 66, 2100738. [Google Scholar] [CrossRef]
- Kujundžić, R.N.; Stepanić, V.; Milković, L.; Gašparović, A.Č.; Tomljanović, M.; Trošelj, K.G. Curcumin and its Potential for Systemic Targeting of Inflamm-Aging and Metabolic Reprogramming in Cancer. Int. J. Mol. Sci. 2019, 20, 1180. [Google Scholar] [CrossRef]
- Wu, H.; Pan, L.; Gao, C.; Xu, H.; Li, Y.; Zhang, L.; Ma, L.; Meng, L.; Sun, X.; Qin, H. Quercetin Inhibits the Proliferation of Glycolysis-Addicted HCC Cells by Reducing Hexokinase 2 and Akt-mTOR Pathway. Molecules 2019, 24, 1993. [Google Scholar] [CrossRef]
- Shin, N.; Lee, H.J.; Sim, D.Y.; Im, E.; Park, J.E.; Park, W.Y.; Cho, A.R.; Shim, B.S.; Kim, S.-H. Apoptotic effect of compound K in hepatocellular carcinoma cells via inhibition of glycolysis and Akt/mTOR/c-Myc signaling. Phytother. Res. 2021, 35, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anti-Cancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Woldetsadik, A.D.; Vogel, M.C.; Rabeh, W.M.; Magzoub, M. Hexokinase II–derived cell-penetrating peptide targets mitochondria and triggers apoptosis in cancer cells. FASEB J. 2017, 31, 2168–2184. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Saleh, H.O.; Magzoub, M. Hexokinase II-Derived Cell-Penetrating Peptide Mediates Delivery of MicroRNA Mimic for Cancer-Selective Cytotoxicity. Biochemistry 2020, 59, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Q.; Zhang, M.; Cao, C.; Liu, X.; Zhang, M.; Li, G.; Xu, C.; Zhang, X. Enhanced antitumor effects of follicle-stimulating hormone receptor-mediated hexokinase-2 depletion on ovarian cancer mediated by a shift in glucose metabolism. J. Nanobiotechnology 2020, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Yoong, S.L.; Wong, B.S.; Zhou, Q.L.; Chin, C.F.; Li, J.; Venkatesan, T.; Ho, H.K.; Yu, V.; Ang, W.H.; Pastorin, G. Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials 2014, 35, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Russier, J.; Venturelli, E.; Fabbro, C.; Petronilli, V.; Bernardi, P.; Da Ros, T.; Prato, M.; Bianco, A. Peptide-based carbon nanotubes for mitochondrial targeting. Nanoscale 2013, 5, 9110–9117. [Google Scholar] [CrossRef]
- Jean, S.R.; Ahmed, M.; Lei, E.K.; Wisnovsky, S.P.; Kelley, S.O. Peptide-Mediated Delivery of Chemical Probes and Therapeutics to Mitochondria. Acc. Chem. Res. 2016, 49, 1893–1902. [Google Scholar] [CrossRef]
- Yoong, S.L.; Lau, W.L.; Liu, A.Y.; Prendergast, D.A.; Ho, H.K.; Yu, V.C.K.; Lee, C.; Ang, W.H.; Pastorin, G. Mitochondria-acting hexokinase II peptides carried by short-length carbon nanotubes with increased cellular uptake, endosomal evasion, and enhanced bioactivity against cancer cells. Nanoscale 2015, 7, 13907–13917. [Google Scholar] [CrossRef]
- Glenister, A.; Chen, C.K.J.; Paterson, D.J.; Renfrew, A.K.; Simone, M.I.; Hambley, T.W. Warburg Effect Targeting Co(III) Cytotoxin Chaperone Complexes. J. Med. Chem. 2021, 64, 2678–2690. [Google Scholar] [CrossRef]
- Chen, E.; Wang, T.; Zhang, J.; Zhou, X.; Niu, Y.; Liu, F.; Zhong, Y.; Huang, D.; Chen, W. Mitochondrial Targeting and pH-Responsive Nanogels for Co-Delivery of Lonidamine and Paclitaxel to Conquer Drug Resistance. Front. Bioeng. Biotechnol. 2021, 9, 787320. [Google Scholar] [CrossRef]
- Tsai, H.J. Functional Organization and Evolution of Mammalian Hexokinases: Mutations That Caused the Loss of Catalytic Activity in N-terminal Halves of Type I and Type III Isozymes. Arch. Biochem. Biophys. 1999, 369, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Khrbtli, A.R.; Shetler, C.L.; Mansoor, S.; Ali, L.; Sensoy, O.; Rabeh, W.M. Linker residues regulate the activity and stability of hexokinase 2, a promising anticancer target. J. Biol. Chem. 2021, 296, 100071. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Pan, P.; Lv, R.; Ma, C.; Wu, E.; Guo, R.; Zhao, Z.; Song, H.; Zhou, J.; Liu, Y.; et al. High-throughput glycolytic inhibitor discovery targeting glioblastoma by graphite dots–assisted LDI mass spectrometry. Sci. Adv. 2022, 8, eabl4923. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, M.; Wu, S.; Gao, S.; Yang, M.; Li, Z.; Min, Q.; Sun, W.; Chen, L.; Xiang, G.; et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017, 36, 58. [Google Scholar] [CrossRef] [PubMed]
- Noser, A.A.; Abdelmonsef, A.H.; El-Naggar, M.; Salem, M.M. New Amino Acid Schiff Bases as Anticancer Agents via Potential Mitochondrial Complex I-Associated Hexokinase Inhibition and Targeting AMP-Protein Kinases/mTOR Signaling Pathway. Molecules 2021, 26, 5332. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Vidalain, P.-O.; Jacquemin, C.; Aublin-Gex, A.; Olmstead, K.; Panthu, B.; Rautureau, G.J.P.; André, P.; Nyczka, P.; Hütt, M.-T.; et al. A hexokinase isoenzyme switch in human liver cancer cells promotes lipogenesis and enhances innate immunity. Commun. Biol. 2021, 4, 217. [Google Scholar] [CrossRef]
- Dey, P.; Kimmelman, A.C.; DePinho, R.A. Metabolic Codependencies in the Tumor Microenvironment. Cancer Discov. 2021, 11, 1067–1081. [Google Scholar] [CrossRef]
- Tang, S.-J.; Fan, K.-H.; You, G.-R.; Huang, S.-F.; Kang, C.-J.; Huang, Y.-F.; Huang, Y.-C.; Chang, J.T.; Cheng, A.-J. Tumor Suppressor miRNA-503 Inhibits Cell Invasion in Head and Neck Cancer through the Wnt Signaling Pathway via the WNT3A/MMP Molecular Axis. Int. J. Mol. Sci. 2022, 23, 15900. [Google Scholar] [CrossRef]
- Zheng, W.; Lin, P.; Ma, Y.; Shao, X.; Chen, H.; Chen, D.; Liu, X.; Li, X.; Ye, H. Psoralen promotes the expression of cyclin D1 in chondrocytes via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2017, 40, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Sato, H.; Takahashi, K.; Fujiya, M. Tumor-Progressive Mechanisms Mediating miRNA–Protein Interaction. Int. J. Mol. Sci. 2021, 22, 12303. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Pan, S.; Shang, S.; Li, C. Interaction between the Wnt/β-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J. Periodontal Res. 2018, 53, 842–852. [Google Scholar] [CrossRef] [PubMed]
 , cicroRNA;
, cicroRNA;  , miRNA;
, miRNA;  , phosphorylation;
, phosphorylation;  , ubiquitination;
, ubiquitination;  , active.
, active.
 , cicroRNA;
, cicroRNA;  , miRNA;
, miRNA;  , phosphorylation;
, phosphorylation;  , ubiquitination;
, ubiquitination;  , active.
, active.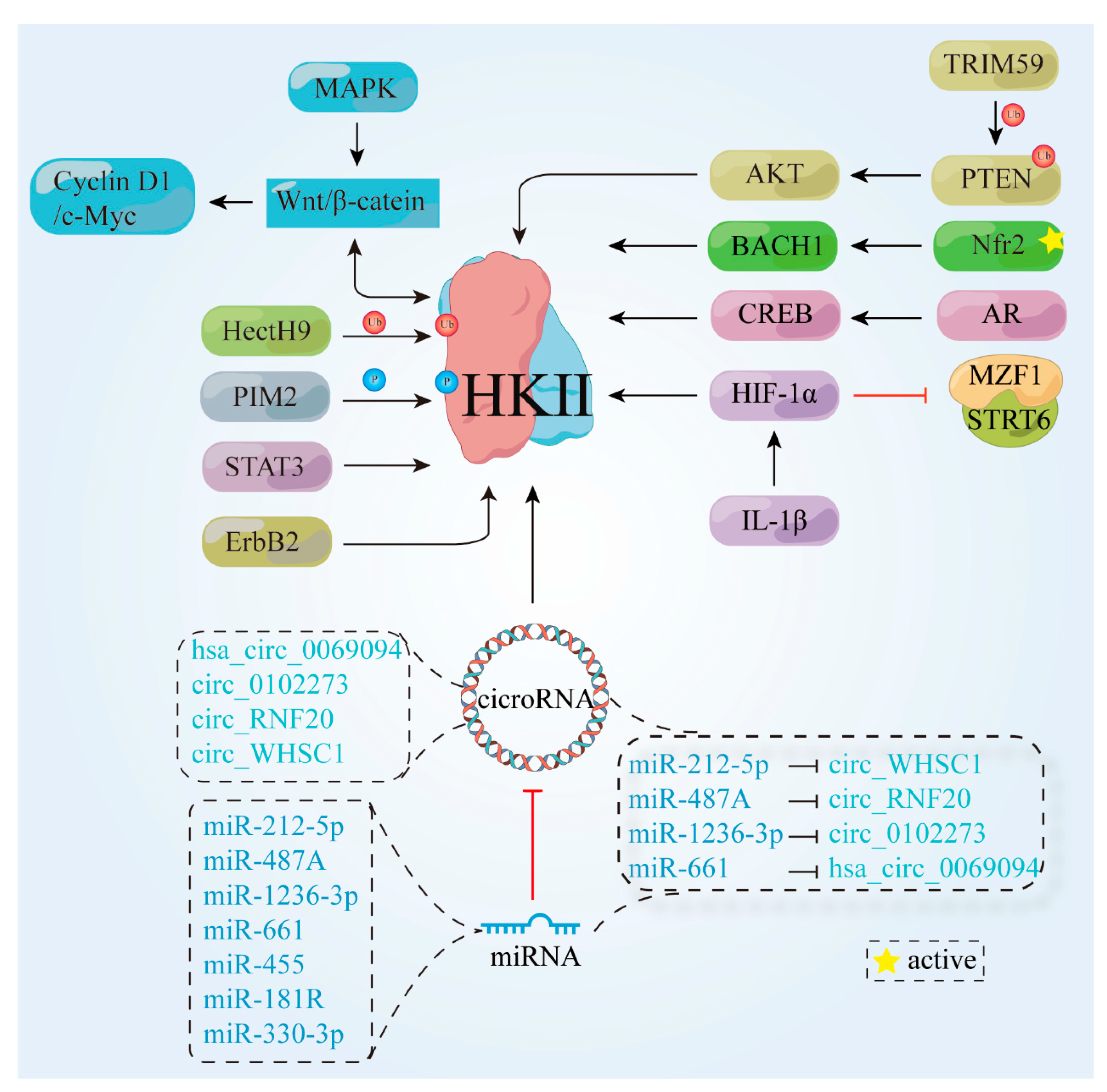
 , protein;
, protein;  , cicro RNA;
, cicro RNA;  , miRNA.
, miRNA.
 , protein;
, protein;  , cicro RNA;
, cicro RNA;  , miRNA.
, miRNA.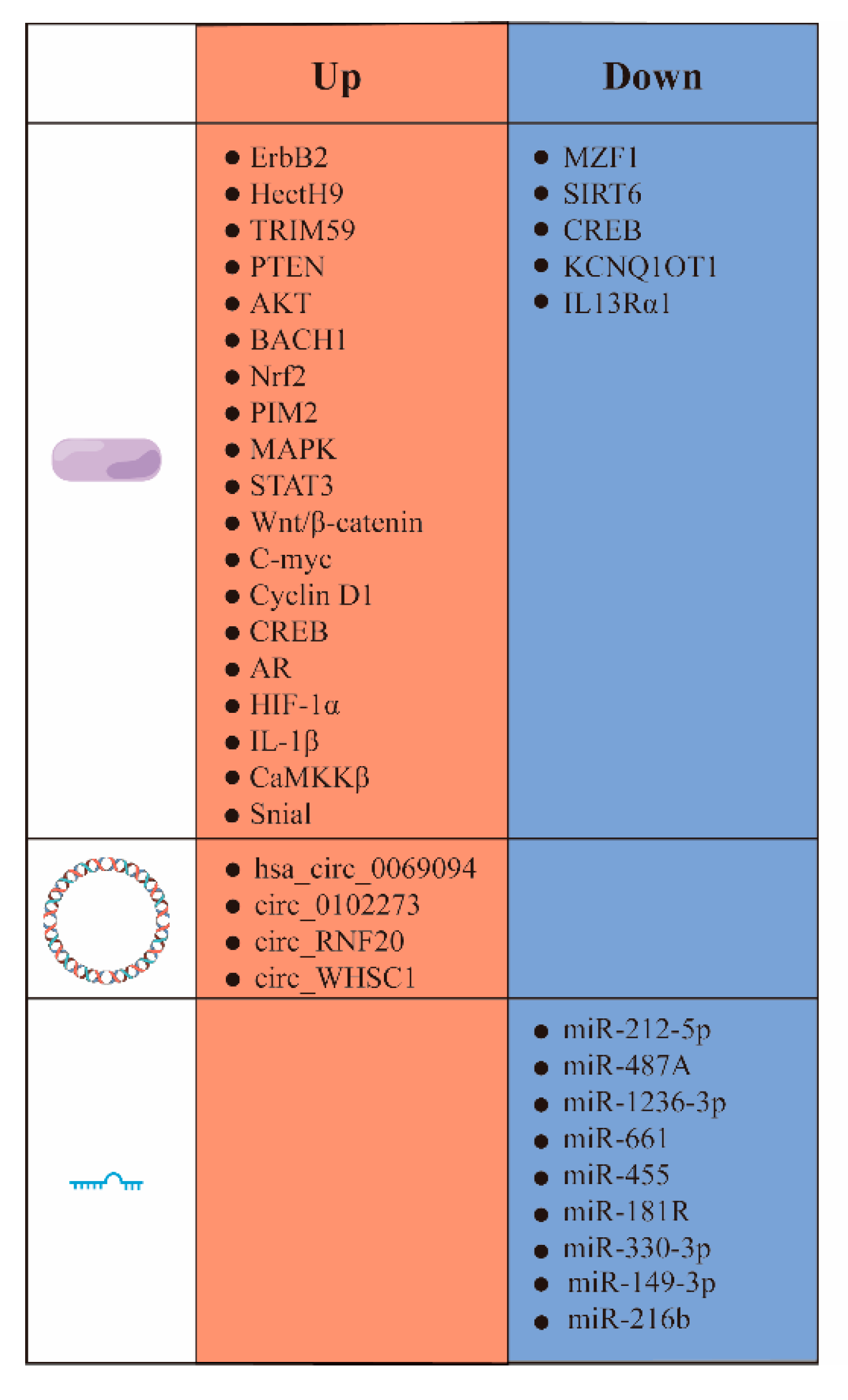
| Number | Antitumor Drug | Mechanism | HK II Inhibitors | Cell Types | Reference |
|---|---|---|---|---|---|
| 1 | 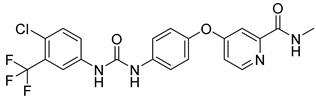 | Inhibits growth factors and multiple kinases | 3-BrPA 2-DG | Hepatocellular carcinoma (HCC); Hep3B Huh7 | [97,98] |
| 2 | 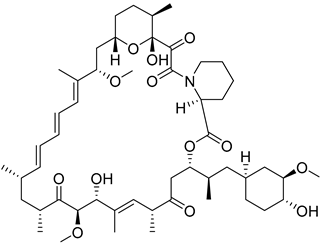 | Inhibition of the mTOR pathway | 3-BrPA | Human neuroblastoma (NB) cell NSCLC | [100,101] |
| 3 | 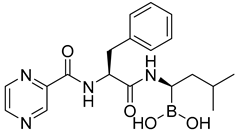 | Inhibition of 26S proteasome chymotrypsin-like activity | 3-BrPA | KMS-12-PE, KMS-11, H929, RPMI-8226, U266, MM.1S | [103] |
| 4 | 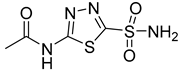 | Inhibition of carbonic anhydrase and regulation of microenvironmental pH | 3-BrPA | Huh-7 | [108] |
| 5 | 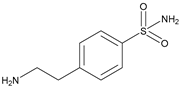 | Inhibition of carbonic anhydrase and regulation of microenvironmental pH | 3-BrPA | Huh-7, HepG2 | [109] |
| 6 | NDV | Direct killing of cancer cells and activation of the immune system | D-mannoheptulose | [111] | |
| 7 | M1 virus | 2-DG | [112] | ||
| 8 | 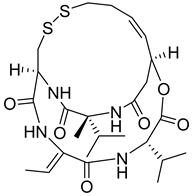 | Inhibition of histone deacetylase activity | Clotrimazole, Bifoncarbazole | HCT-116, A549, 786-0, IGROV1, MDA-MB-231 | [113] |
| 9 | 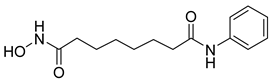 | Inhibition of histone deacetylase activity | 2-DG | H226 | [114] |
| 10 |  | Cross-linking reactions occur with DNA to form DNA–DNA or DNA–protein crosslinks, which disrupt normal DNA replication and transcription | 2-DG, 3-BP | SF763, SF126 SMMC-221, HepG2 SF763, SF126 | [118,119,120] |
| 11 | 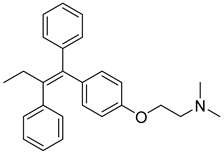 | Competition with estrogen for estrogen receptors | As2O3 | MCF7 | [124] |
| 12 | 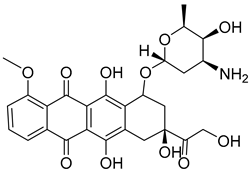 | Inhibition of nucleic acid synthesis | Metformin | [127] | |
| 13 |  | Enhancement of microtubule protein polymerization and inhibition of microtubule depolymerization that lead to the formation of stable, nonfunctional microtubule bundles and disruption of tumor cell mitosis | Metformin | [127] | |
| 14 | Tralizumab | Inhibition of cancer cell growth by blocking the formation of HER2 receptor dimers | Metformin | NCI-N87, SNU216 | [96,127] |
| 15 | 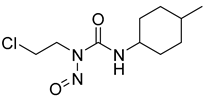 | Destruction of DNA function | 2-DG, 3-BrPA | [118,119,120] |
| Number | Name | Compound | Machine | IC50 for HK II | IC50 for Cells | References |
|---|---|---|---|---|---|---|
| 1 | G6P | 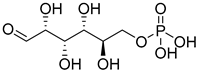 | 0.2 mM | [144] | ||
| 2 | T6P | 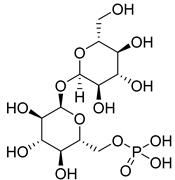 | G6P analogs | [147] | ||
| 3 | Epigallocatechin gallate | 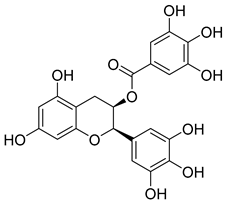 | Bind HK Ⅱ pocket | [149,150] | ||
| 4 | Quercitrin | 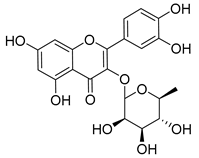 | [150] | |||
| 5 | Dioscin | 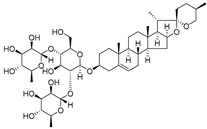 | Targeted HK Ⅱ-VDAC1 binding | 5 µM for HT29, HCT116, SW480 | [151] | |
| 6 | Matrine |  | ~0.5 mg/mL for K562, HL-60 | [152] | ||
| 7 | Asiaticacid | 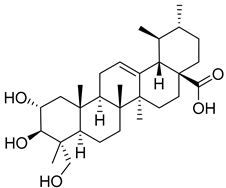 | [153] | |||
| 8 | Andrographolide | 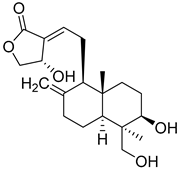 | [153] | |||
| 9 | Bayogenin | 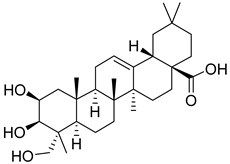 | [153] | |||
| 10 | Pachymic Acid | 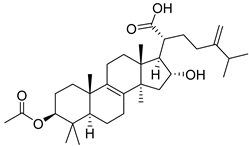 | Promotion of HK II dissociation from mitochondria and cty-c (etc.) | 5.01 µM | SK-BR-3 (5 µM) | [158] |
| 11 | α-Hederin | 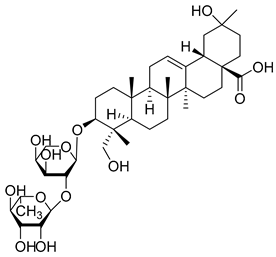 | 13.75 µM for NSCLC A549; 17.75 µM for NCI-H460; 18.04 µM for NCI-H292 | [159] | ||
| 12 | Triptolide | 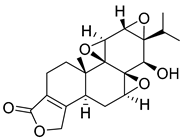 | [163] | |||
| 13 | Wogonin | 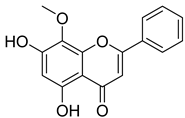 | [164] | |||
| 14 | Curcumin | 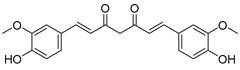 | Induction of dissociation of HK II from mitochondria by phosphorylation of HK II via AKT | [169] | ||
| 15 | Methyl jasmonate | 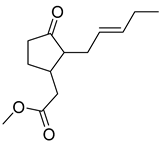 | 7.47 μM | 4.17 mM for SKOV-3; 6.383 mM for A549 | [167] | |
| 16 | Methyl jasmonate derivative | 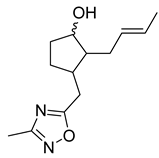 | 0.27 μM | 1.772 mM for SKOV-3; 2.45 mM for A549 | [167] | |
| 17 | Sulforaphane | 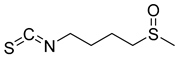 | 4.25 mM for A549, 1.772 mM for SK-OV3 | [168] | ||
| 18 | Ketoconazole | 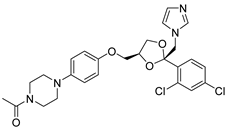 | [115] | |||
| 19 | Posaconazole | 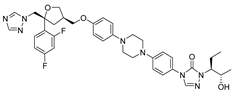 | [115] | |||
| 21 | Glycyrrhetinic acid | 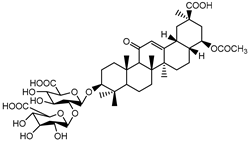 | 100 µg/mL | [160] | ||
| 22 | Steroids | 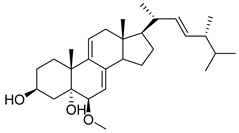 | 2.06 μM | 5.05 µM for SW1990, 22.59 µM for Vero | [161] | |
| 23 | Strepantibins A | 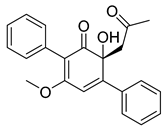 | 9.8 µM | [162] | ||
| 24 | Strepantibins B | 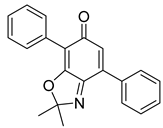 | 34.6 µM | [162] | ||
| 25 | Strepantibins C | 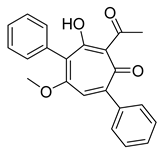 | 31.2 μM | [162] | ||
| 26 | Compound 27 | 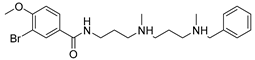 | 11.31 μM | [185] | ||
| 27 | 11 | 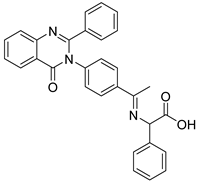 | 0.135 mM for MCF-7; 0.163 mM for MDA-231; 0.156 mM for PCL | [187] | ||
| 28 | 20 |  | 0.195 mM for MCF-7; 0.166 mM for MDA-231; 0.218 mM for PCL | [187] | ||
| 29 | Benserazide | 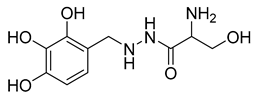 | 5.52 ± 0.17 mM | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Lu, Y.; Taledaohan, A.; Qiao, S.; Li, Q.; Wang, Y. The Promoting Role of HK II in Tumor Development and the Research Progress of Its Inhibitors. Molecules 2024, 29, 75. https://doi.org/10.3390/molecules29010075
Liu B, Lu Y, Taledaohan A, Qiao S, Li Q, Wang Y. The Promoting Role of HK II in Tumor Development and the Research Progress of Its Inhibitors. Molecules. 2024; 29(1):75. https://doi.org/10.3390/molecules29010075
Chicago/Turabian StyleLiu, Bingru, Yu Lu, Ayijiang Taledaohan, Shi Qiao, Qingyan Li, and Yuji Wang. 2024. "The Promoting Role of HK II in Tumor Development and the Research Progress of Its Inhibitors" Molecules 29, no. 1: 75. https://doi.org/10.3390/molecules29010075
APA StyleLiu, B., Lu, Y., Taledaohan, A., Qiao, S., Li, Q., & Wang, Y. (2024). The Promoting Role of HK II in Tumor Development and the Research Progress of Its Inhibitors. Molecules, 29(1), 75. https://doi.org/10.3390/molecules29010075






