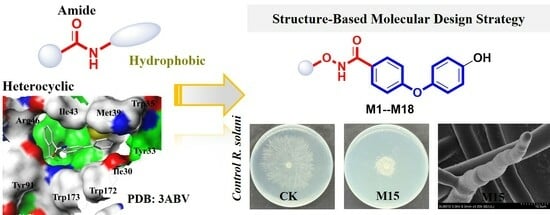
Design, Synthesis, and Antifungal Activity of N-(alkoxy)-Diphenyl Ether Carboxamide Derivates as Novel Succinate Dehydrogenase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
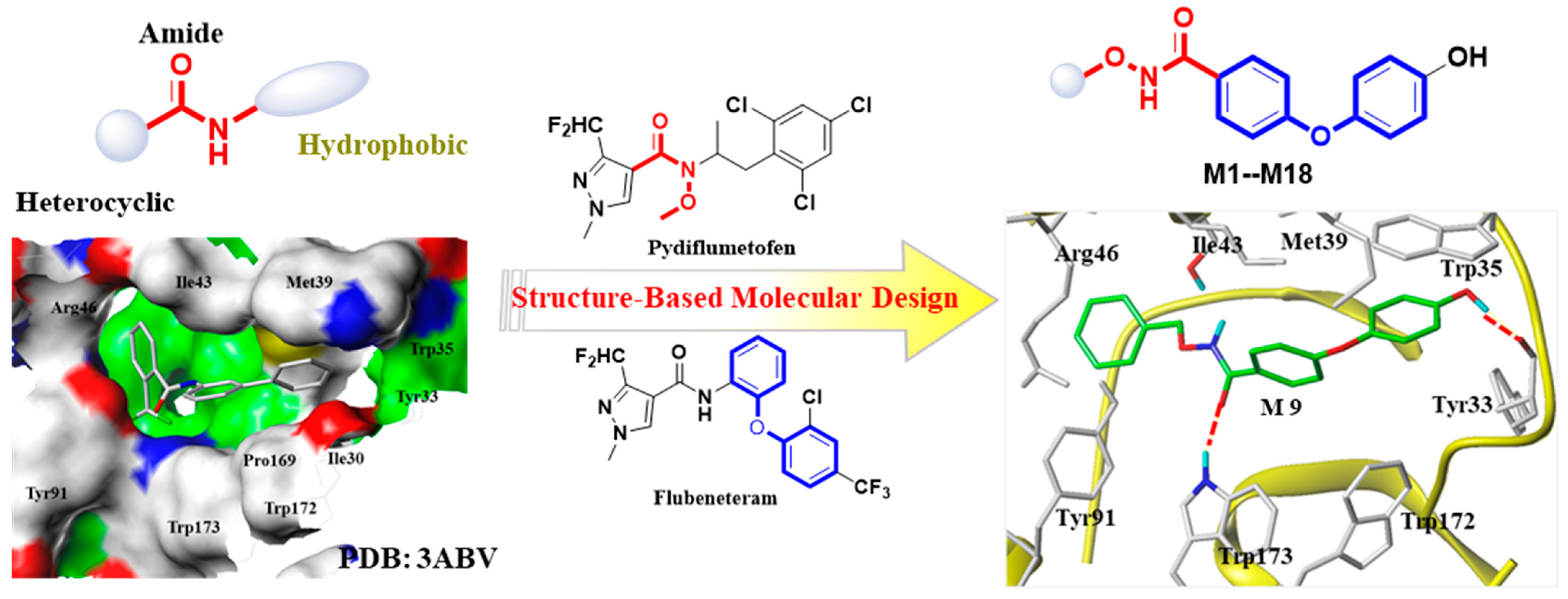
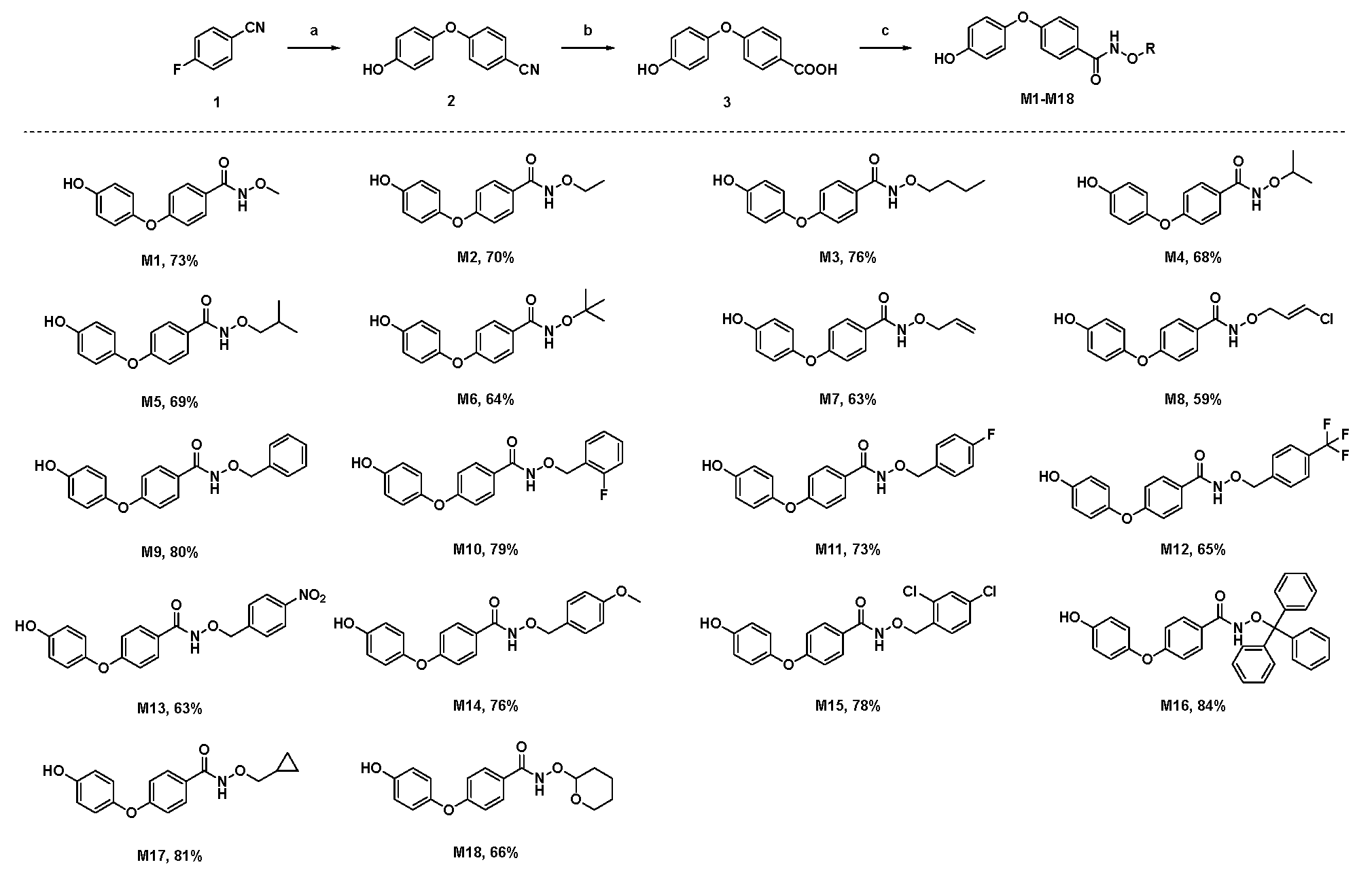
2.1. Molecular Design and Synthesis
2.2. Antifungal Activity and Structure–Activity Relationship
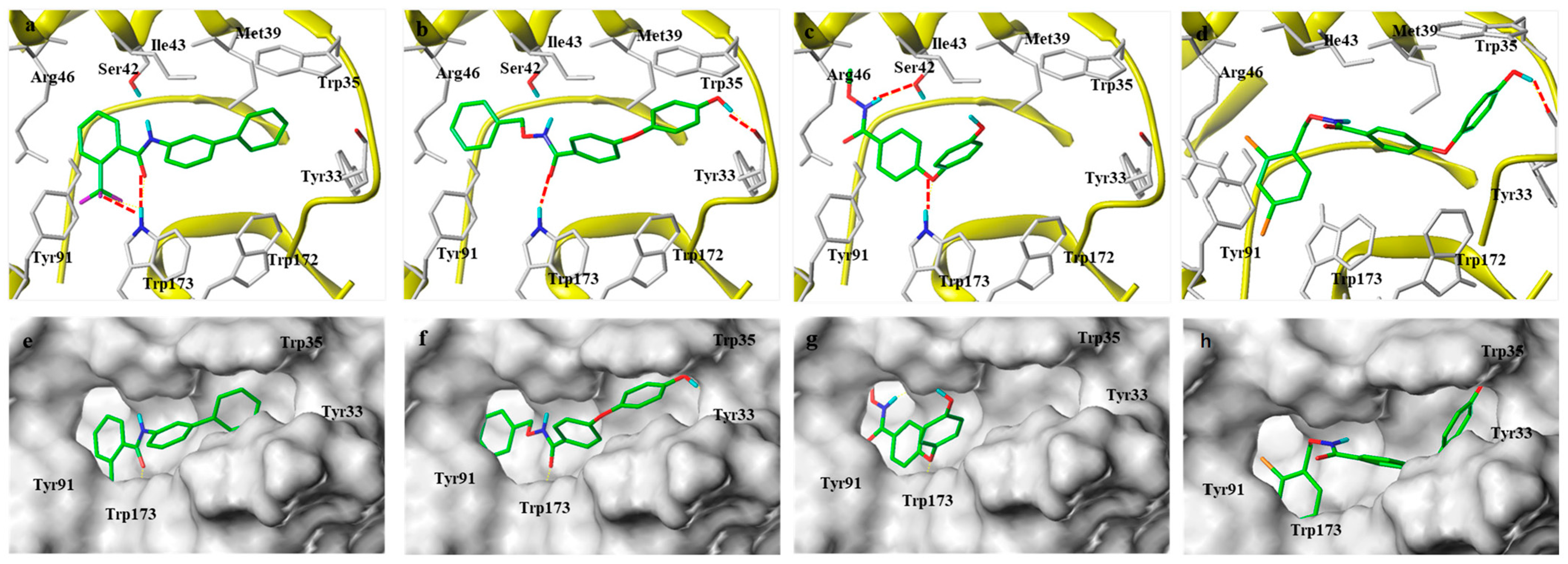
2.3. Molecular Docking Models of the Flutolanil Derivative, M1, M9, and M15 with Porcine SDH (PDB ID 3ABV)
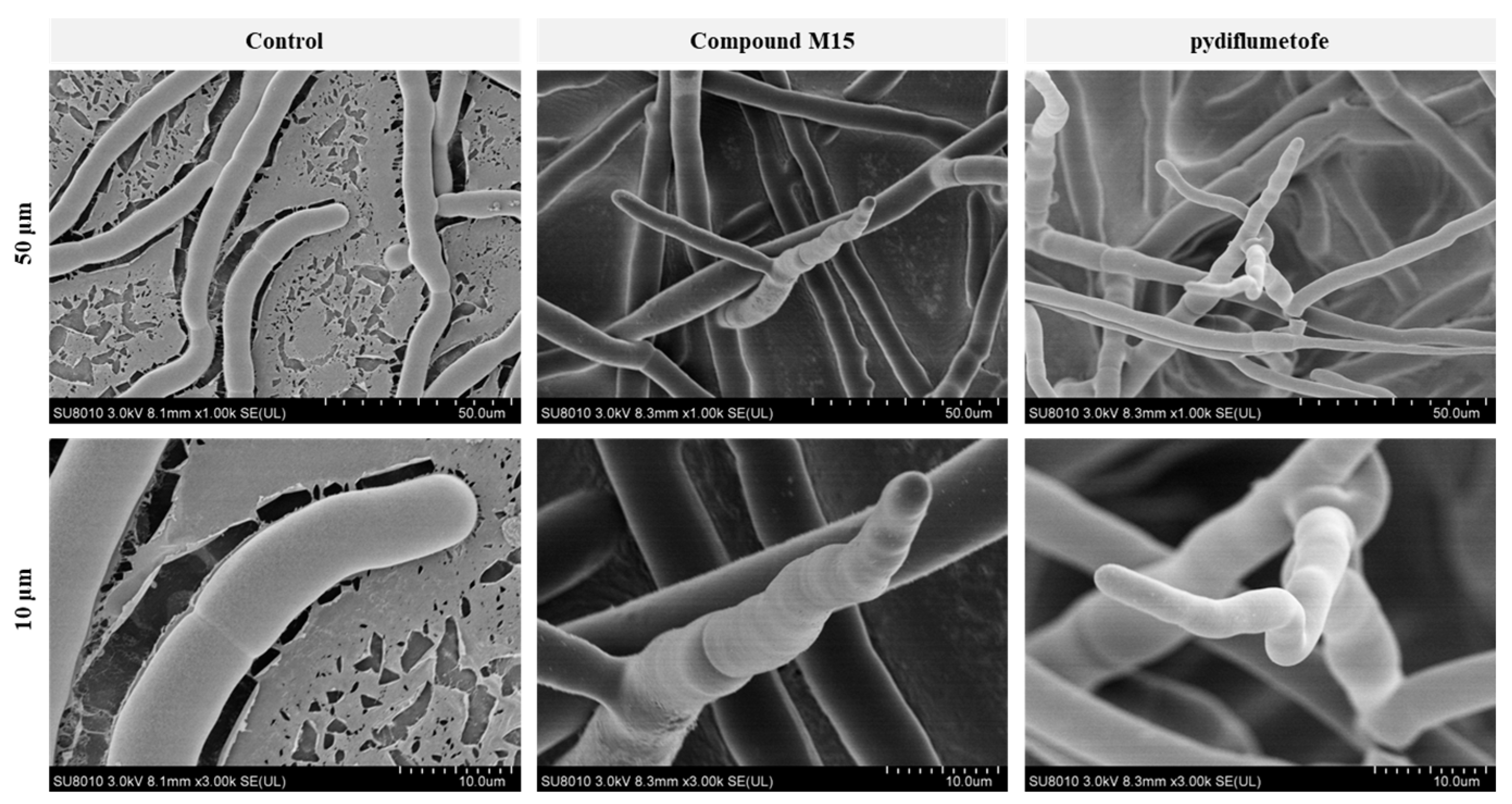
2.4. SEM Analysis
2.5. In Vitro SDH Inhibitory Activity Assay
2.6. In Vivo Antifungal Activity
3. Materials and Methods
3.1. Chemical Reagents and Instruments
3.2. General Synthesis Method of Intermediate 2
3.3. General Synthesis Method of Intermediate 3
3.4. General Synthesis Method of Title Compounds M1–M18
3.5. Antifungal Activity Screening
3.6. In Vitro SDH Inhibitory Activity Assay
3.7. Antifungal Activity In Vivo
3.8. Molecular Docking
3.9. Scanning Electron Microscopy (SEM) Observations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hu, Y.; Wang, W.; Yan, W.; Ye, Y. The progress towards novel herbicide modes of action and targeted herbicide development. Agronomy 2022, 12, 2792. [Google Scholar] [CrossRef]
- van Montfort, R.L.M.; Workman, P. Structure-based drug design: Aiming for a perfect fit. Essays Biochem. 2017, 61, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.W.; Rees, D.C. The rise of fragment-based drug discovery. Nat. Chem. 2009, 1, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, H.; Jiang, L.N.; Ge, J.M.; Yang, W.C.; Zhu, X.L.; Yang, G.F. Structure-based discovery of potential fungicides as succinate ubiquinone oxidoreductase inhibitors. J. Agric. Food Chem. 2017, 65, 1021–1029. [Google Scholar] [CrossRef]
- Zhu, X.L.; Xiong, L.; Li, H.; Song, X.Y.; Liu, J.J.; Yang, G.F. Computational and Experimental Insight into the Molecular Mechanism of Carboxamide Inhibitors of Succinate--Ubquinone Oxidoreductase. Chemmedchem 2014, 9, 1512–1521. [Google Scholar] [CrossRef]
- Iverson, T.M.; Luna-Chavez, C.; Cecchini, G.; Rees, D.C. Structure of the Escherichia coli fumarate reductase respiratory complex. Science 1999, 284, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhai, Y.; Lou, J.; Liu, M.; Pang, X.; Sun, F. Thiabendazole inhibits ubiquinone reduction activity of mitochondrial respiratory complex II via a water molecule mediated binding feature. Protein Cell 2011, 2, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Veloukas, T.; Markoglou, A.N.; Karaoglanidis, G.S. Differential Effect of SdhB Gene Mutations on the Sensitivity to SDHI Fungicides in Botrytis cinerea. Plant Dis. 2013, 97, 118–122. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Dong, Y.; Wang, Y.X.; Zhu, X.L. Design, synthesis, and fungicidal evaluation of novel N-methoxy pyrazole-4-carboxamides as potent succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2023, 71, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.B.; Xiu, Q.; Li, H.R.; Li, T.; Wang, J.X.; Zhou, M.G. Pharmacological Characteristics and Control Efficacy of a Novel SDHI Fungicide Pydiflumetofen Against. Plant Dis. 2019, 103, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wei, L.; Zhao, W.; Wang, B.; Zheng, H.; Zhang, P.; Lou, T.; Duan, Y.; Hou, Y.; Zhou, M.; et al. Resistance risk assessment for a novel succinate dehydrogenase inhibitor pydiflumetofen in Fusarium asiaticum. Pest Manag. Sci. 2020, 77, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Mu, W.; Bi, Y.; Zhang, Y.; Zhang, S.; Song, J.; Liu, X. Heterokaryotic state of a point mutation (H249Y) in SDHB protein drives the evolution of thifluzamide resistance in Rhizoctonia solani. Pest Manag. Sci. 2020, 77, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xiong, H.; Yang, J.F.; Zhu, X.L.; Qu, R.Y.; Yang, G.F. Diaryl ether: A privileged scaffold for drug and agrochemical discovery. J. Agric. Food Chem. 2020, 68, 9839–9877. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect. Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.X.; Zhu, X.L.; Yang, G.F. Discovery of a fungicide candidate targeting succinate dehydrogenase via computational substitution optimization. J. Agric. Food Chem. 2021, 69, 13227–13234. [Google Scholar] [CrossRef]
- Inaoka, D.K.; Shiba, T.; Sato, D.; Balogun, E.O.; Sasaki, I.; Nagahama, M.; Oda, M.; Matsuoka, S.; Ohmori, J.; Honma, L.; et al. Structural insights into the molecular design of flutolanil derivatives targeted for fumarate respiration of parasite mitochondria. Int. J. Mol. Sci. 2015, 16, 15287–15308. [Google Scholar] [CrossRef]
- Lakouraj, M.M.; Mokhtary, M. Synthesis of poly(ether amide)s from p-xylylene glycol and bis(ether nitrile)s. Polym. Int. 2009, 58, 1167–1172. [Google Scholar] [CrossRef]
- Mao, X.; Wu, Z.; Bi, C.; Wang, J.; Zhao, F.; Gao, J.; Hou, Y.; Zhou, M. Molecular and biochemical characterization of pydiflumetofen-resistant mutants of didymella bryoniae. J. Agric. Food Chem. 2020, 68, 9120–9130. [Google Scholar] [CrossRef] [PubMed]
- Yankovskaya, V.; Horsefield, R.; Tornroth, S.; Luna-Chavez, C.; Miyoshi, H.; Leger, C.; Byrne, B.; Cecchini, G.; Iwata, S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 2003, 299, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, Y.; Liu, Q.; Li, A.; Wang, W.; Gu, W. Design, synthesis, and antifungal activity of novel thiophene/furan-1,3,4-oxadiazole carboxamides as potent succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2021, 69, 13373–13385. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, S.; Wang, J.; Wu, F.; Wang, T.; Xu, G. Bioactivity-Guided Synthesis Accelerates the Discovery of 3-(Iso)quinolinyl-4-chromenones as Potent Fungicide Candidates. J. Agric. Food Chem. 2021, 69, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Xu, S.; Zhou, X.Y.; Zhao, R.; Lin, Y.; Cao, J.; Zang, W.D.; Tao, H.; Xu, W.; Li, M.Q.; et al. Low chorionic villous succinate accumulation associates with recurrent spontaneous abortion risk. Nat. Commun. 2021, 12, 3428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Han, L.; Jin, F.; Jiao, J.; Chen, M.; Yang, C.; Xue, W. Novel pyrazole-4-acetohydrazide derivatives potentially targeting fungal succinate dehydrogenase: Design, synthesis, three-dimensional quantitative structure-activity relationship, and molecular docking. J. Agric. Food Chem. 2021, 69, 9557–9570. [Google Scholar] [CrossRef] [PubMed]


| No. | R | Inhibition Rate ± Standard Deviation (SD) %, 50 μg/mL | |||
|---|---|---|---|---|---|
| R. solani | S. sclerotiorum | B. cinerea | F. graminearum | ||
| M1 | methyl | 13.09 ± 3.93 n | 18.10 ± 1.10 j | 32.17 ± 1.98 h | 2.92 ± 2.62 l |
| M2 | ethyl | 3.11 ± 1.67 q | 21.63 ± 1.77 i | 11.14 ± 0.74 l | 11.31 ± 1.20 j |
| M3 | butyl | 25.78 ± 1.40 j | 35.67 ± 2.90 f | 37.65 ± 0.99 g | 25.91 ± 1.65 fg |
| M4 | isopropyl | 1.86 ± 0.96 q | 16.85 ± 2.75 j | 1.20 ± 2.72 o | 2.92 ± 3.58 l |
| M5 | isobutyl | 34.47 ± 1.40 h | 36.52 ± 3.64 f | 47.89 ± 1.36 f | 27.37 ± 1.65 f |
| M6 | tert-butyl | 2.17 ± 1.02 q | 21.91 ± 2.54 i | 2.11 ± 1.36 o | 14.6 ± 2.77 i |
| M7 | allyl | 28.88 ± 2.18 i | 25.00 ± 6.28 h | 4.52 ± 2.11 n | 10.58 ± 2.15 j |
| M8 | (E)-3-chloroallyl | 25.47 ± 1.18 j | 27.25 ± 2.48 g | 11.75 ± 2.11 kl | 25.18 ± 2.15 g |
| M9 | benzyl | 56.83 ± 1.40 d | 57.02 ± 1.77 e | 62.35 ± 2.11 e | 43.80 ± 1.13 e |
| M10 | 2-fluorobenzyl | 52.80 ± 2.81 e | 64.04 ± 0.87 d | 62.65 ± 0.93 e | 43.43 ± 2.56 e |
| M11 | 4-fluorobenzyl | 47.83 ± 1.67 f | 69.10 ± 2.04 c | 64.46 ± 0.93 d | 51.09 ± 2.26 d |
| M12 | 4-(trifluoromethyl)benzyl | 38.82 ± 1.40 g | 16.57 ± 3.49 j | 1.20 ± 1.48 o | 6.93 ± 2.30 k |
| M13 | 4-nitrobenzyl | 14.91 ± 2.26 m | 25.56 ± 1.27 gh | 18.67 ± 1.14 j | 20.80 ± 2.15 h |
| M14 | 4-methoxybenzyl | 18.01 ± 2.04 l | 17.13 ± 1.97 j | 2.41 ± 1.62 o | 2.92 ± 2.26 l |
| M15 | 2,4-dichlorobenzyl | 74.22 ± 1.40 c | 63.20 ± 3.09 d | 68.07 ± 0.93 c | 68.98 ± 0.89 c |
| M16 | tri-phenylmethyl | 23.60 ± 2.89 k | 22.19 ± 3.09 i | 8.13 ± 2.11 m | 20.44 ± 2.26 h |
| M17 | cyclopropylmethyl | 6.83 ± 1.67 p | 27.25 ± 3.60 g | 13.25 ± 3.02 k | 19.34 ± 1.65 h |
| M18 | tetrahydro-2H-pyran-2-yl | 8.70 ± 1.67 o | 21.63 ± 1.77 i | 29.82 ± 1.78 i | 14.96 ± 2.15 i |
| Flubeneteram | 91.30 ± 0.96 b | 83.99 ± 0.92 b | 74.10 ± 1.48 b | 85.40 ± 1.79 b | |
| pydiflumetofe | 100 a | 100 a | 100 a | 100 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Hu, Y.; Chen, W.; He, X.; Zhang, E.; Hu, M.; Zhang, P.; Yan, W.; Ye, Y. Design, Synthesis, and Antifungal Activity of N-(alkoxy)-Diphenyl Ether Carboxamide Derivates as Novel Succinate Dehydrogenase Inhibitors. Molecules 2024, 29, 83. https://doi.org/10.3390/molecules29010083
He B, Hu Y, Chen W, He X, Zhang E, Hu M, Zhang P, Yan W, Ye Y. Design, Synthesis, and Antifungal Activity of N-(alkoxy)-Diphenyl Ether Carboxamide Derivates as Novel Succinate Dehydrogenase Inhibitors. Molecules. 2024; 29(1):83. https://doi.org/10.3390/molecules29010083
Chicago/Turabian StyleHe, Bo, Yanhao Hu, Wang Chen, Xu He, Enpei Zhang, Mengxu Hu, Pu Zhang, Wei Yan, and Yonghao Ye. 2024. "Design, Synthesis, and Antifungal Activity of N-(alkoxy)-Diphenyl Ether Carboxamide Derivates as Novel Succinate Dehydrogenase Inhibitors" Molecules 29, no. 1: 83. https://doi.org/10.3390/molecules29010083