New Charged Cholinesterase Inhibitors: Design, Synthesis, and Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of New 1,2,3-Triazolo-Stilbenes 10–22
2.2. Photocyclization of 1,2,3-Triazolo-Stilbenes 10–22 to the Targeted Final Photoproducts 23–35
2.3. Synthesis of the Triazolium Salts 44–51
2.4. Spectroscopic Characterization in Biorelevant Medium and DNA-Binding Ability of Triazolium Salts
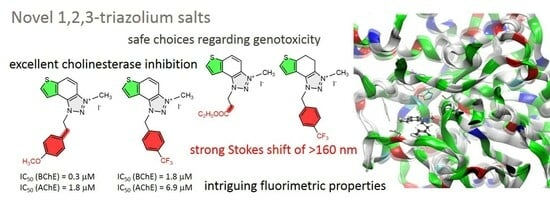
2.5. ChEs Inhibition
2.6. Molecular Docking into Cholinesterases
2.7. Genotoxicity
3. Materials and Methods
3.1. General Procedure
3.2. Synthesis of Triazole Aldehydes 1–9

- 1-(furan-2-ylmethyl)-1H-1,2,3-triazol-4-carbaldehyde (1): 99.6 mg, 61% of the isolated yield; yellow oil; Rf (DCM) = 0.23; 1H NMR (CDCl3, 600 MHz) δ/ppm: 10.13 (s, 1H), 8.10 (s, 1H), 7.46 (d, J = 1.9 Hz, 1H), 6.53 (d, J = 3.3 Hz, 1H), 6.42 (dd, J = 3.3, 1.9 Hz, 1H), 5.61 (s, 2H).
- 1-(3-fluorobenzyl)-1H-1,2,3-triazole-4-carbaldehyde (2): 143.6 mg, 76% of the isolated yield; yellow oil; Rf (DCM) = 0.22; 1H NMR (CDCl3, 600 MHz) δ/ppm: 10.14 (s, 1H), 8.03 (s, 1H), 7.41–7.34 (m, 1H), 7.11–7.07 (m, 2H), 7.00 (d, J = 9.1 Hz, 1H), 5.59 (s, 2H).

- 1-(3-methoxybenzyl)-1H-1,2,3-triazole-4-carbaldehyde (3): 83.0 mg, 42% of the isolated yield; yellow oil; Rf (DCM) = 0.19; 1H NMR (CDCl3, 600 MHz) δ/ppm: 10.13 (s, 1H), 8.00 (s, 1H), 7.32 (t, J = 8.1 Hz, 1H), 6.92 (d, J = 8.5 Hz, 1H), 6.88 (d, J = 7.8 Hz, 1H), 6.82 (s, 1H), 5.55 (s, 2H), 3.80 (s, 1H).
- 1-(3-chlorobenzyl)-1H-1,2,3-triazole-4-carbaldehyde (4): 145.1 mg, 71% of the isolated yield; yellow oil; Rf (DCM) = 0.16; 1H NMR (CDCl3, 600 MHz) δ/ppm: 10.14 (s, 1H), 8.04 (s, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.35 (t, J = 7.9 Hz, 1H), 7.30 (s, 1H), 7.19 (d, J = 7, 3 Hz, 1H), 5.57 (s, 2H).

- 1-(4-methoxybenzyl)-1H-1,2,3-triazole-4-carbaldehyde (5): 70 mg, 36% of isolated yield; yellow oil; Rf (DCM/EtOAc (2%)) = 0.33; 1H NMR (CDCl3, 300 MHz) δ/ppm: 10.10 (s, 1H), 8.08 (s, 1H), 7.26 (d, J = 8.6 Hz, 2H), 6.91 (d, J = 8.7 Hz, 2H), 5.52 (s, 2H), 3.81 (s, 3H).
- 1-(4-(dimethylamino)benzyl)-1H-1,2,3-triazole-4-carbaldehyde (6): 139.4 mg, 73% of the isolated yield; yellow oil; Rf (DCM) = 0.11; 1H NMR (CDCl3, 600 MHz) δ/ppm: 10.10 (s, 1H), 7.91 (s, 1H), 7.19 (d, J = 7.2 Hz, 2H), 6.70 (d, J = 6.7 Hz, 2H), 5.46 (s, 2H), 2.97 (s, 6H).

- 1-allyl-1H-1,2,3-triazole-4-carbaldehyde (7): 80 mg, 70% of the isolated yield; yellow oil; Rf (DCM/EtOAc (2%)) = 0.47; 1H NMR (CDCl3, 300 MHz) δ/ppm: 10.14 (s, 1H), 6.10 –6.01 (m, 1H), 5.45 (d, J = 10.3 Hz, 1H), 5.37 (d, J = 17.9 Hz, 1H), 5.07 (d, J = 6.2 Hz, 2H).
- 1-(but-3-en-1-yl)-1H-1,2,3-triazol-4-carbaldehyde (8): 72.5 mg, 58% of the isolated yield; yellow oil; Rf (DCM) = 0.35; 1H NMR (CDCl3, 600 MHz) δ/ppm: 10.15 (s, 1H), 8.07 (s, 1H), 6.07–6.02 (m, 1H), 5.45 (d, J = 9.4 Hz, 1H), 5.36 (d, J = 18.2 Hz, 1H), 5.07 (d, J = 7.7 Hz, 2H).

- 1-(pent-4-en-1-yl)-1H-1,2,3-triazole-4-carbaldehyde (9): 95 mg, 27% of the isolated yield; yellow oil; Rf (DCM/EtOAc (2%)) = 0.47; 1H NMR (CDCl3, 300 MHz) δ/ppm: 10.14 (s, 1H), 5.82–5.73 (m, 1H), 5.09–5.06 (m, 2H), 4.44 (t, J = 4.4 Hz, 2H), 3.87–3.82 (m, 2H), 3.65–3.60 (m, 2H).
3.3. Synthesis of Triazolostilbenes 10–22 by Wittig Reaction

- 1-(furan-2-ylmethyl)-4-(2-(thiophen-2-yl)vinyl)-1H-1,2,3-triazole (10). Compound 10 was synthesized by a Wittig reaction from 1-(furan-2-ylmethyl)-1H-1,2,3-triazole-4-carbaldehyde (1) (99.6 mg, 0.56 mmol) and triphenylphosphonium salt (246.8 mg, 0.56 mmol) with sodium in 10% excess (14.2 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 3 cm; h = 17 cm; PE/E system (50%)). 67.4 mg, 68% of the isolated mixture of isomers; yellow oil; Rf (PE/E (20%)) = 0.51.
- 1-(3-fluorobenzyl)-4-(2-(thiophen-2-yl)vinyl)-1H-1,2,3-triazole (11). Compound 11 was synthesized from 1-(3-fluorobenzyl)-1H-1,2,3-triazole-4-carbaldehyde (2) (143.6 mg, 0.62 mmol) and triphenylphosphonium salt (270.4 mg, 0.62 mmol) with sodium in 10% excess (15.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 3 cm; h = 18.5 cm; system PE/E (50%)). 88.3 mg, 50% isolated mixture of isomers; yellow oil; Rf (PE/E (20%)) = 0.53.

- 1-(3-methoxybenzyl)-4-(2-(thiophen-2-yl)vinyl)-1H-1,2,3-triazole (12). Compound 12 was synthesized by a Wittig reaction from 1-(3-methoxybenzyl)-1H-1,2,3-triazole-4-carbaldehyde (3) (83.0 mg, 0.28 mmol) and triphenylphosphonium salt (122.5 mg, 0.28 mmol) with sodium in 10% excess (7.1 mg) and 30 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 1.5 cm; h = 14 cm; system PE/E (50%)). 63.6 mg, 77% isolated mixture of isomers; yellow oil; Rf (PE/E (20%)) = 0.45.
- 1-(3-chlorobenzyl)-4-(2-(thiophen-2-yl)vinyl)-1H-1,2,3-triazole (13). Compound 13 was synthesized from 1-(3-chlorobenzyl)-1H-1,2,3-triazole-4-carbaldehyde (4) (72.6 mg, 0.29 mmol) and triphenylphosphonium salt (127.3 mg, 0.29 mmol) with sodium in 10% excess (7.3 mg) and 30 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 3 cm; h = 18.5 cm; system PE/E (50%)). 109.8 mg, 99% of isolated mixture of isomers; yellow oil; Rf (PE/E (20%)) = 0.49.

- N,N-dimethyl-4-((4-(2-(thiophen-2-yl)vinyl)-1H-1,2,3-triazol-1-yl)methyl)aniline (14). Compound 14 was synthesized by a Wittig reaction from 1-(4-(dimethylamino)benzyl)-1H-1,2,3-triazole-4-carbaldehyde (6) (69.7 mg, 0.30 mmol) and triphenylphosphonium salt (132.9 mg, 0.30 mmol) with sodium in 10% excess (7.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 1.5 cm; h = 10 cm; system PE/E (60%)). 19.7 mg, 21% isolated mixture of isomers; yellow oil; Rf (PE/E (90%)) = 0.25.
- 1-(but-3-en-1-yl)-4-(2-(thiophen-2-yl)vinyl)-1H-1,2,3-triazole (15). Compound 15 was synthesized from 1-(but-3-en-1-yl)-1H-1,2,3-triazole-4-carbaldehyde (8) (72.5 mg, 0.48 mmol) and triphenylphosphonium salt (210.5 mg, 0.48 mmol) with sodium in 10% excess (12.1 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 2 cm; h = 10 cm; PE/E system (80%)). 80.7 mg, 73% of the isolated mixture of isomers; yellow oil; Rf (PE/E (90%)) = 0.81.

- N,N-dimethyl-4-((4-(2-(5-methylthiophen-2-yl)vinyl)-1H-1,2,3-triazol-1-yl)methyl)aniline (16). Compound 16 was synthesized from 1-(4-(dimethylamino)benzyl)-1H-1,2,3-triazole-4-carbaldehyde (6) (69.7 mg, 0.30 mmol) and triphenylphosphonium salt (140.2 mg, 0.30 mmol) with sodium in 10% excess (7.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 1.5 cm; h = 10 cm; system PE/E (70%)). 46.0 mg, 33% of the isolated mixture of isomers; yellow oil; Rf (PE/E (70%)) = 0.12.
- 4-((4-(3-methoxystyryl)-1H-1,2,3-triazol-1-yl)methyl)-N,N-dimethylaniline (17). Compound 17 was synthesized from 1-(4-(dimethylamino)benzyl)-1H-1,2,3-triazole-4-carbaldehyde (6) (69.7 mg, 0.30 mmol) and triphenylphosphonium salt (140.2 mg, 0.30 mmol) with sodium in 10% excess (7.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 2 cm; h = 10 cm; PE/E system (80%)). 53.6 mg, 53% of the isolated mixture of isomers; yellow oil; Rf (PE/E (90%)) = 0.40.

- (Z)-1-allyl-4-(4-chlorostyryl)-1H-1,2,3-triazole (cis-18): 25 mg, 11% isolated yield; yellow oil; Rf (PE/E (50%)) = 0.43; 1H NMR (CDCl3, 300 MHz) δ/ppm: 7.31 (s, 4H), 7.10 (s, 1H), 6.70 (d, J = 11.2 Hz, 1H), 6.64 (d, J = 11.2 Hz, 1H), 5.96–5.90 (m, 1H), 5.29 (d, J = 10.1 Hz, 1H), 5.20 (d, J = 16.4 Hz, 1H), 4.87 (d, J = 7.9 Hz, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 144.3, 136.0, 133.4, 131.1, 130.0, 129.7, 128.8, 121.5, 120.6, 119.9, 52.4, 30.2.
- 4-((4-(3-methoxystyryl)-1H-1,2,3-triazol-1-yl)methyl)-N,N-dimethylaniline (cis-19): 13 mg, 61% isolated yield; yellow oil; Rf (PE/E (50%)) = 0.51; 1H NMR (CDCl3, 300 MHz) δ/ppm: 7.28 (d, J = 10.2 Hz, 4H), 7.07 (s, 1H), 6.71 (d, J = 12.4 Hz, 1H) 6.63 (d, J = 12.4 Hz, 1H), 5.75–5.62 (m, 1H), 5.09–5.01 (m, 2H), 4.30 (t, J = 6.2 Hz, 1H), 2.57 (dd, J = 13.2, 6.9 Hz, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 143.9, 136.1, 133.4, 133.1, 129.9, 129.8, 128.8, 121.6, 120.7, 118.5, 49.5, 34.3.

- 4-(4-chlorostyryl)-1-(pent-4-en-1-yl)-1H-1,2,3-triazole (20). Compound 20 was synthesized from 1-(pent-4-en-1-yl)-1H-1,2,3-triazole-4-carbaldehyde (9) (50 mg, 0.30 mmol) and triphenylphosphonium salt (140.2 mg, 0.30 mmol) with sodium in 10% excess (7.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 1.5 cm; h = 10 cm; system PE/E (70%)). 46.0 mg, 33% of the isolated mixture of isomers; yellow oil; Rf (PE/E (70%)) = 0.55.
- 4-(4-chlorostyryl)-1-(4-methoxybenzyl)-1H-1,2,3-triazole (21). Compound 21 was synthesized according to the general procedure from 1-(4-(dimethylamino)benzyl)-1H-1,2,3-triazole-4-carbaldehyde (6) (69.7 mg, 0.30 mmol) and triphenylphosphonium salt (140.2 mg, 0.30 mmol) with sodium in 10% excess (7.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 2 cm; h = 10 cm; PE/E system (80%)). 62.6 mg, 64% of the isolated mixture of isomers; yellow oil; Rf (PE/E (90%)) = 0.40.

- 4-((4-(4-chlorostyryl)-1H-1,2,3-triazol-1-yl)methyl)-N,N-dimethylaniline (22). Compound 22 was synthesized from 1-(4-(dimethylamino)benzyl)-1H-1,2,3-triazole-4-carbaldehyde (6) (69.7 mg, 0.30 mmol) and triphenylphosphonium salt (140.2 mg, 0.30 mmol) with sodium in 10% excess (7.6 mg) and 40 mL of NaOEt. The obtained product was purified by column chromatography (ϕ = 2 cm; h = 10 cm; PE/E system (80%)). 25.2 mg, 25% of the isolated mixture of isomers; yellow oil; Rf (PE/E (90%)) = 0.25.
3.4. Photochemical Synthesis of Naphthotriazoles 23–34

- 1-(furan-2-ylmethyl)-1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazole (23): 10.3 mg, 31% of the isolated compound; yellow oil; Rf (PE/E (30%)) = 0.54; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.97 (d, J = 8.9 Hz, 1H), 7.82–7.80 (m, 2H), 7.66 (d, J = 5.5 Hz, 1H), 7.36 (d, J = 1.8 Hz, 1H), 6.34 (d, J = 3.3 Hz, 1H), 6.32 (dd, J = 3.3, 1.8 Hz, 1H), 6.08 (s, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 148.1, 144.5, 143.1, 140.2, 128.6, 127.7, 122.8, 120.3, 119.2, 116.0, 110.9, 109.3, 46.4. MS (ESI) m/z (%, fragment): 256 (100); HRMS (m/z) for C13H10N3OS: [M + H]+calcd = 255.0466, and [M + H]+measured = 255.0463.
- 1-(3-fluorobenzyl)-1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazole (24): 28.2 mg, 64% of the isolated compound; yellow oil; Rf (PE/E (30%)) = 0.51; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.00 (d, J = 9.0 Hz, 1H), 7.82 (d, J = 8.9 Hz, 1H), 7.56 (d, J = 5.5 Hz, 1H), 7.46 (d, J = 5.5 Hz, 1H), 7.33–7.27 (m, 1H), 7.02–6.93 (m, 2H), 6.86 (d, J = 9.3 Hz, 1H), 6.10 (s, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 163.1 (d, JC-F = 247 Hz), 144.7, 140.3, 137.7, 130.8, 128.0, 122.5, 119.9, 119.4, 116.1, 115.6, 115.3, 113.7 (d, JC-F = 22.5 Hz), 52.5; MS (ESI) m/z (%, fragment): 284 (50), 122 (100); HRMS (m/z) for C15H11FN3S: [M + H]+calcd = 283.0579, and [M + H]+measured = 283.0581.
- 1-(3-methoxybenzyl)-1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazole (25): 9.5 mg, 30% of the isolated compound; yellow oil; Rf (PE/E (30%)) = 0.45; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.99 (d, J = 9.0 Hz, 1H), 7.80 (d, J = 8.8 Hz, 1H), 7.54 (d, J = 5.5 Hz, 1H), 7.50 (d, J = 5.5 Hz, 1H), 7.23 (t, J = 2.1 Hz, 1H), 6.81 (d, J = 8.3 Hz, 1H), 6.78 (d, J = 7.7 Hz, 1H), 6.71 (s, 1H), 6.08 (s, 2H), 3.70 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 160.2, 144.6, 140.1, 136.7, 130.2, 128.7, 127.7, 122.7, 120.3, 119.2, 118.8, 116.1, 113.6, 112.4, 55.2, 53.0.
- 1-(3-chlorobenzyl)-1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazole (26): 21.7 mg, 59% of the isolated compound; yellow oil; Rf (PE/E (30%)) = 0.42; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.00 (d, J = 9.0 Hz, 1H), 7.82 (d, J = 8.9 Hz, 1H), 7.58 (d, J = 5.5 Hz, 1H), 7.47 (d, J = 5.5 Hz, 1H), 7.26–7.25 (m, 1H), 7.24 (t, J = 8.0 Hz, 1H), 7.21 (s, 1H), 7.03 (d, J = 7.4 Hz, 1H), 6.08 (s, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 144.7, 140.3, 137.1, 135.2, 130.4, 128.7, 128.6, 128.1, 126.7, 124.7, 122.5, 119.8, 119.4, 116.1, 52.4; MS (ESI) m/z (%, fragment): 300/302 (100); HRMS (m/z) for C15H11ClN3S: [M + H]+calcd = 299.0284, and [M + H]+measured = 299.0288.

- 4-((1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazol-1-yl)methyl)-N,N-dimethylaniline (27): 6.4 mg, 33% of the isolated compound; yellow oil; Rf (PE/E (50%)) = 0.24; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.97 (d, J = 8.8 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.60 (d, J = 5.5 Hz, 1H), 7.56 (d, J = 5.5 Hz, 1H), 7.11 (d, J = 8.8 Hz, 2H), 6.64 (d, J = 8.7 Hz, 2H), 6.01 (s, 2H), 2.88 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 150.4, 144.7, 139.9, 129.4, 128.6, 127.8, 127.5, 126.6, 122.9, 122.5, 120.6, 119.0, 116.0, 112.6, 52.9, 40.4; MS (ESI) m/z (%, fragment): 309 (100); HRMS (m/z) for C17H17N4S: [M + H]+calcd = 308.1096, and [M + H]+measured = 308.1100.
- 1-(but-3-en-1-yl)-1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazole (28): 24 mg, 81% isolated compound; yellow oil; Rf (PE/E (30%)) = 0.48; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.96 (d, J = 8.9 Hz, 1H), 7.80 (d, J = 8.9 Hz, 1H), 7.74 (d, J = 5.5 Hz, 1H), 7.70 (d, J = 5.5 Hz, 1H), 5.90–5.83 (m, 1H), 5.15–5.08 (m, 2H), 4.96 (t, J = 7.4 Hz, 2H), 2.85–2.80 (q, J =14.5, 7.2 Hz, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 144.4, 139.9, 133.1, 128.5, 128.0, 122.6, 119.6, 119.0, 118.4, 116.2, 49.0, 34.3; MS (ESI) m/z (%, fragment): 230 (40), 122 (100); HRMS (m/z) for C12H12N3S: [M + H]+calcd = 229.0674, and [M + H]+measured = 229.0676.
- N,N-dimethyl-4-((7-methyl-1H-thieno[2′,3′:3,4]benzo[1,2-d][1,2,3]triazol-1-yl)methyl)aniline (29): 12.6 mg, 36% of the isolated compound; yellow oil; Rf (PE/E (50%)) = 0.29; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.88 (d, J = 8.8 Hz, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.27 (s, 1H), 7.11 (d, J = 8.8 Hz, 2H), 6.64 (d, J = 8.6 Hz, 2H), 5.96 (s, 2H), 2.88 (s, 3H), 2.61 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 150.4, 144.7, 142.4, 139.1, 129.4, 128.2, 127.8, 123.2, 118.7, 118.4, 115.0, 112.6, 52.7, 40.4, 16.3.
- 4-((8-methoxy-1H-naphtho[1,2-d][1,2,3]triazol-1-yl)methyl)-N,N-dimethylaniline (30): 7.2 mg, 20% of the isolated compound; yellow oil; Rf (PE/E (50%)) = 0.35; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.86 (d, J = 8.5 Hz, 1H), 8.84 (d, J = 8.5 Hz, 1H), 7.62 (d, J = 9.0 Hz, 1H), 7.50 (d, J = 2.4 Hz, 1H), 7.17 (dd, J = 8.9, 2.6 Hz, 1H), 7.06 (d, J = 8.7 Hz, 2H), 6.64 (d, J = 8.3 Hz, 2H), 6.16 (s, 2H), 3.79 (s, 3H), 2.89 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 158.5, 150.4, 145.3, 130.5, 129.2, 128.9, 127.7, 127.3, 126.0, 121, 0, 117.7, 115.5, 112.8, 103.7, 55.6, 53.9, 40.5; MS (ESI) m/z (%, fragment): 333 (20), 134 (100); HRMS (m/z) for C20H21N4O: [M + H]+calcd = 332.1637, and [M + H]+measured = 332.1635.

- 1-allyl-8-chloro-1H-naphtho[1,2-d][1,2,3]triazole (31): 5.1 mg, 17% of the isolated compound; yellow oil; Rf (PE/E (30%)) = 0.60; 1H NMR (CDCl3, 300 MHz) δ/ppm: 8.14 (d, J = 1.8 Hz, 1H), 7.94 (d, J = 9.3 Hz, 1H), 7.89 (d, J = 8.5 Hz, 1H), 7.63 (d, J = 8.9 Hz, 1H), 7.53 (dd, J = 9.1, 2.5 Hz, 1H), 6.17–6.10 (m, 1H), 5.05–5.01 (dt, J = 17.2, 2.5 Hz, 1H), 5.6–5.58 (m, 2H), 5.32 (d, J = 11.3 Hz, 1H), 5.03 (d, J = 17.3 Hz, 1H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 158.6, 143.9, 132.0, 130.3, 129.7, 127.6, 126.6, 124.9, 120.9, 119.5, 117.9, 117.5, 51.6, 28.7.
- 1-(but-3-en-1-yl)-8-chloro-1H-naphtho[1,2-d][1,2,3]triazole (32): 5.4 mg, 18% of the isolated compound; Rf (PE/E (30%)) = 0.51; 1H NMR (CDCl3, 300 MHz) δ/ppm: 8.15 (d, J = 2.5 Hz, 1H), 7.95 (d, J = 8.6 Hz, 1H), 7.90 (d, J = 8.6 Hz, 1H), 7.63 (d, J = 8.8 Hz, 1H), 7.53 (dd, J = 9.3, 2.4 Hz, 1H), 6.20–6.05 (m, 1H), 5.60–5.59 (m, 2H), 5.43 (t, J = 15.3 Hz, 1H), 5.33–5.22 (m, 2H), 5.02 (d, J = 16.8 Hz, 1H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 132.0, 130.3, 129.8, 129.7, 126.6, 124.9, 120.9, 119.5, 117.9, 117.5, 51.6, 30.9, 28.6, 21.6.
- 8-chloro-1-(4-methoxybenzyl)-1H-naphtho[1,2-d][1,2,3]triazole (33): 5.7 mg; 19% of the isolated compound; Rf (PE/E(30%)) = 0.49; 1H NMR (CDCl3, 300 MHz) δ/ppm: 8.14 (s, 1H), 8.02 (d, J = 8.6 Hz, 1H), 7.90 (d, J = 8.6 Hz, 1H), 7.67 (d, J = 8.8 Hz, 1H), 7.52 (d, J = 8.6 Hz, 1H), 7.15 (d, J = 8.6 Hz, 1H), 6.84 (d, J = 8.6 Hz, 1H), 6.17 (s, 2H), 3.75 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 158.6, 131.9, 130.2, 129.6, 126.9, 126.8, 126.5, 125.1, 124.8, 121.1, 119.5, 117.4, 113.6, 54.2, 52.7, 28.7.
- 4-((8-chloro-1H-naphtho[1,2-d][1,2,3]triazol-1-yl)methyl)-N,N-dimethylaniline (34): 12.2 mg, 40% of the isolated compound; Rf (PE/E (50%)) = 0.75; 1H NMR (CDCl3, 300 MHz) δ/ppm: 8.24 (s, 1H), 8.01 (d, J = 9.4 Hz, 1H), 7.88 (d, J = 8.6 Hz, 1H), 7.65 (d, J = 8.8 Hz, 1H), 7.51 (d, J = 8.6 Hz, 1H), 7.12 (d, J = 8.6 Hz, 2H), 6.66 (d, J = 8.6 Hz, 2H), 6.12 (s, 2H), 2.90 (s, 6H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 150.4, 145.2, 132.9, 131.2, 130.5, 128.6, 127.8, 127.4, 125.7, 122.3; 120.7, 118.4, 112.7, 53.9, 40.4.
3.5. General Methylation Procedure for the Synthesis of 44–51

- 1-(4-methoxyphenethyl)-3-methyl-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 44: 30 mg, 50% isolated yield; yellow powder; Rf (DCM (100%)) = 0.13; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.32 (d, J = 9.3 Hz, 1H), 8.23 (d, J = 9.1 Hz, 1H), 8.03 (d, J = 5.6 Hz, 1H), 7.96 (d, J = 5.2 Hz, 1H), 7.06 (d, J = 8.4, 2H), 6.79 (d, J = 8.4 Hz, 2H), 5.37 (t, J = 7.4 Hz, 2H), 4.81 (s, 3H), 3.76 (s, 3H), 3.50 (t, J = 7.4 Hz, 2H).; 13C NMR (CDCl3, 150 MHz) δ/ppm: 159.2, 142.9, 134.7, 133.0, 130.8, 129.8, 127.1, 126.8, 122.7, 120.1, 114.6, 109.2, 53.3, 53.2, 40.1, 34.2; MS (ESI) m/z (%, fragment): 324 (100); HRMS (m/z) for C18H18N3OS: [M + H]+calcd = 323.1092, and [M + H]+measured = 323.1089.
- 1-(3-ethoxy-3-oxopropyl)-3-methyl-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 45: 30 mg, 48% isolated yield; yellow powder; Rf (DCM (100%)) = 0.15; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.31 (d, J = 9.2 Hz, 1H), 8.19 (d, J = 5.3 Hz, 1H), 8.11 (d, J = 9.2 Hz, 1H), 8.05 (d, J = 5.8 Hz, 1H), 5.52 (t, J = 6.2 Hz, 1H), 4.78 (s, 3H), 4.15 (q, 2H), 3.48 (t, J = 5.8 Hz, 2H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 169.8, 142.9, 134.7, 133.0, 131.4, 127.1, 123.1, 120.8, 108.6, 61.7, 49.2, 39.9, 32.7, 14.1; MS (ESI) m/z (%, fragment): 290 (100), 214 (30); HRMS (m/z) for C14H16N3O2S: [M + H]+calcd = 289.0885, and [M + H]+measured = 289.0889.

- 3-methyl-1-(4-(trifluoromethyl)benzyl)-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 46: 11 mg, 53% isolated yield; yellow powder; Rf (DCM (100%)) = 0.18; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.34 (d, J = 9.4 Hz, 1H), 8.06 (d, J = 9.4 Hz, 1H), 7.97 (d, J = 5.9 Hz, 1H), 7.84 (d, J = 5.1 Hz, 1H), 7.69 (d, J = 8.1 Hz, 2H), 6.64 (d, J = 8.1 Hz, 2H), 6.57 (s, 2H), 4.84 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 143.3, 135.1, 134.6, 133.3, 132.2, 131.2, 130.4, 128.5, 127.3, 126.7, 124.1, 122.8, 120.5, 108.4, 56.7, 39.9 (the characteristic CF3 coupling is not detected due to an insufficient number of scans); MS (ESI) m/z (%, fragment): 348 (100); HRMS (m/z) for C17H13F3N3S: [M + H]+calcd = 347.0704, and [M + H]+measured = 347.0708.
- 3-methyl-1-(2-morpholinoethyl)-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 47: 36 mg, 99% isolated yield; yellow powder; Rf (DCM (100%)) = 0.11; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.35 (d, J = 8.8 Hz, 1H), 8.17 (d, J = 9.1 Hz, 1H), 8.11 (d, J = 5.9 Hz, 1H), 8.08 (d, J = 5.4 Hz, 1H), 5.36 (t, J = 6.3 Hz, 2H), 4.85 (s, 3H), 3.59 (t, J = 4.8 Hz, 4H), 3.21 (t, J = 6.5 Hz, 2H), 2.57 (t, J = 5.2 Hz, 4H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 143.0, 134.7, 133.2, 131.0, 127.0, 122.9, 120.3, 108.5, 66.7, 56.1, 53.5, 51.5, 39.9; MS (ESI) m/z (%, fragment): 303 (100); HRMS (m/z) for C15H19N4OS: [M + H]+calcd = 302.1201, and [M + H]+measured = 302.1205.

- 1-(4-hydroxybutyl)-3-methyl-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 48: 3 mg, 10% isolated yield; yellow powder; Rf (DCM (100%)) = 0.07; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.33 (d, J = 8.9 Hz, 1H), 8.13 (d, J = 5.2 Hz, 1H), 8.08 (d, J = 6.2 Hz, 1H), 8.01 (d, J = 8.9 Hz, 1H), 5.30 (t, J = 8.0 Hz, 2H), 4.83 (s, 3H), 3.78 (q, 2H), 2.44 (t, J = 7.9 Hz, 2H), 2.36–2.33 (m, 2H), 1.92–1.88 (m, 2H); MS (ESI) m/z (%, fragment): 262 (100), 216 (30); HRMS (m/z) for C13H16N3OS: [M + H]+calcd = 261.0936, and [M + H]+measured = 261.0937.
- 1-(4-fluorobenzyl)-3-methyl-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 49: 16 mg, 25% isolated yield; yellow powder; Rf (DCM (100%)) = 0.22; 1H NMR (CDCl3, 600 MHz) δ/ppm: 8.34 (d, J = 9.1 Hz, 1H), 8.11 (d, J = 9.1 Hz, 1H), 7.99 (d, J = 5.7 Hz, 1H), 7.86 (d, J = 5.7 Hz, 1H), 7.49 (dd, J = 8.9, 4.5 Hz, 2H), 7.13 (t, J = 8.6 Hz, 2H), 6.42 (s, 2H), 4.83 (s, 3H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 135.1, 133.0, 131.2, 130.1, 130.0, 127.2, 122.9, 120.5, 116.9, 116.8, 109.6, 108.6, 56.7, 29.7 (the characteristic CF coupling is not detected); MS (ESI) m/z (%, fragment): 298 (100); HRMS (m/z) for C16H13FN3S: [M + H]+calcd = 297.0736, and [M + H]+measured = 297.0742.

- 3-methyl-1-(4-(trifluoromethyl)benzyl)-4,5-dihydro-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 50: 9 mg, 98% isolated yield; yellow powder; Rf (DCM (100%)) = 0.19; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.71 (d, J = 8.4 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 7.29 (d, J = 5.2 Hz, 1H), 7.03 (d, J = 5.7 Hz, 1H), 5.92 (s, 2H), 4.49 (s, 3H), 3.35 (s, 4H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 145.4, 144.4, 139.7, 136.6, 134.6, 127.9, 126.8, 126.7, 126.7, 120.9, 55.0, 39.8, 23.4, 20.9 (the characteristic CF3 coupling is not detected due to an insufficient number of scans); MS (ESI) m/z (%, fragment): 350 (100); HRMS (m/z) for C17H15F3N3S: [M + H]+calcd = 349.0860, and [M + H]+measured = 349.0864.
- 3-methyl-1-(2-morpholinoethyl)-4,5-dihydro-1H-thieno[3′,2′:3,4]benzo[1,2-d][1,2,3]triazol-3-ium iodide 51: 4 mg, 30% isolated yield; yellow powder; Rf (DCM (100%)) = 0.10; 1H NMR (CDCl3, 600 MHz) δ/ppm: 7.39 (d, J = 5.8 Hz, 1H), 7.34 (d, J = 5.5 Hz, 1H), 4.76 (t, J = 6.6 Hz, 2H), 4.45 (s, 3H), 3.61 (t, J = 4.5 Hz, 4H), 3.56 (d, J = 7.3 Hz, 2H), 3.52 (d, J = 7.7 Hz, 2H), 3.01 (t, J = 6.6 Hz, 2H), 2.54 (t, J = 4.1 Hz, 4H); 13C NMR (CDCl3, 150 MHz) δ/ppm: 143.9, 138.9, 136.3, 126.5, 121.2, 120.9, 66.7, 56.5, 53.7, 50.1, 39.8, 23.5, 20.9; MS (ESI) m/z (%, fragment): 305 (100), 214 (45); HRMS (m/z) for C15H21N4OS: [M + H]+calcd = 304.1358, and [M + H]+measured = 304.1364.
3.6. ChEs Inhibition
3.7. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radić Stojković, M.; Škugor, M.; Tomić, S.; Grabar, M.; Smrečki, V.; Dudek, Ł.; Grolik, J.; Eilmes, J.; Piantanida, I. A short, rigid linker between pyrene and guanidiniocarbonyl-pyrrole induced a new set of spectroscopic responses to the ds-DNA secondary structure. Org. Biomol. Chem. 2013, 11, 4077–4085. [Google Scholar] [CrossRef]
- Kashyap, A.; Silakari, O. Key Heterocycle Cores for Designing Multitargeting Molecules, Triazoles: Multidimensional 5-Membered Nucleus for Designing Multitargeting Agents; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–342. [Google Scholar]
- Guan, L.P.; Jin, Q.H.; Tian, G.R.; Chai, K.Y.; Quan, Z.S. Facile and convenient synthesis of pyrimidine, 4H-3,1-benzoxazin-4-one, pyrazolo[5,1-b]quinazoline, pyrido[1,2-a]quinazoline, and chromeno[3′,4′:4,5]pyrido[1,2-a]quinazoline derivatives. J. Pharm. Pharm. Sci. 2007, 10, 254–262. [Google Scholar]
- Gujjar, R.; Marwaha, A.; Mazouni, F.E.; White, J.; White, K.L.; Creason, S.; Shackleford, D.M.; Baldwin, J.; Charman, W.N.; Buckner, F.S.; et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J. Med. Chem. 2009, 52, 1864–1872. [Google Scholar] [CrossRef]
- Chen, M.; Lu, S.; Yuan, G.; Yang, S.; Du, X. Synthesis and antibacterial activity of some heterocyclic β-enamino ester derivatives with 1,2,3-triazole. Heterocycl. Commun. 2000, 6, 421–426. [Google Scholar] [CrossRef]
- Al-Soud, Y.A.; Al-Dweri, M.N.; Al-Masoudi, N.A. Synthesis, antitumor and antiviral properties of some 1,2,4-triazole derivatives. Il Farmaco 2004, 59, 775–783. [Google Scholar] [CrossRef]
- Mohammad, Y.; Fazili, K.M.; Bhat, K.A.; Ara, T. Synthesis and biological evaluation of novel 3-O-tethered triazoles of diosgenin as potent antiproliferative agents. Steroids 2017, 118, 1–8. [Google Scholar]
- Huang, M.; Deng, Z.; Tian, J.; Liu, T. Synthesis and biological evaluation of salinomycin triazole analogues as anticancer agents. Eur. J. Med. Chem. 2017, 127, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Chemlal, L.; Taoufik, J.; Cherrah, Y.; Radi, S.; El Abbes Faouzi, M.; Ansar, M. Synthesis, antioxidant and analgesic activities of Schiff bases of 4-amino-1,2,4-triazole derivatives containing a pyrazole moiety. Ann. Pharm. Fr. 2016, 74, 431–438. [Google Scholar] [CrossRef]
- Lass-Flörl, C. Triazole antifungal agents in invasive fungal infections: A comparative review. Drugs 2011, 71, 2405–2419. [Google Scholar] [CrossRef]
- Khaligh, P.; Salehi, P.; Bararjanian, M.; Aliahmadi, A.; Khavasi, H.R.; Ebrahimi, S.N. Synthesis and in vitro antibacterial evaluation of novel 4-substituted 1-menthyl-1,2,3-triazoles. Chem. Pharm. Bull. 2016, 64, 1589–1596. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Z.; Wang, J.; Li, Y.; Li, J. Synthesis, in vitro evaluation and molecular docking studies of novel triazine-triazole derivatives as potential α-glucosidase inhibitors. Eur. J. Med. Chem. 2017, 125, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Choe, Y.S.; Choi, J.Y.; Lee, K.H.; Kim, B.T. Synthesis and evaluation of 18F-labeled styryltriazole and resveratrol derivatives for β-amyloid plaque imaging. J. Med. Chem. 2012, 55, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lv, J.; Jin, J.; Jin, Z.; Li, T.; Wu, J. Research advances on the bioactivity of 1,2,3-triazolium salts. Int. J. Mol. Sci. 2023, 24, 10694. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.T.; Sobczyk, J.M.; Gwazdacz, S.C.; Blanck, A.J. Antimicrobial 1,3,4-trisubstituted-1,2,3-triazolium salts. Bioorg. Med. Chem. Lett. 2018, 28, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Godard, J.; Gibbons, D.; Leroy-Lhez, S.; Williams, R.M.; Villandier, N.; Ouk, T.S.; Brégier, F.; Sol, V. Development of phenalenonetriazolium salt derivatives for aPDT: Synthesis and antibacterial screening. Antibiotics 2021, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef]
- Steiner, I.; Stojanovic, N.; Bolje, A.; Brozovic, A.; Polancec, D.; Ambriovic-Ristov, A.; Stojkovic, M.R.; Piantanida, I.; Eljuga, D.; Kosmrlj, J.; et al. Discovery of ‘click’ 1,2,3-triazolium salts as potential anticancer drugs. Radiol. Oncol. 2016, 50, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lucio, H.; Revuelto, A.; Carriles, A.A.; de Castro, S.; Garcia-Gonzalez, S.; Garcia-Soriano, J.C.; Alcon-Calderon, M.; Sanchez-Murcia, P.A.; Hermoso, J.A.; Gago, F.; et al. Identification of 1,2,3-triazolium salt-based inhibitors of Leishmania infantum trypanothione disulfide reductase with enhanced antileishmanial potency in cellulo and increased selectivity. Eur. J. Med. Chem. 2022, 244, 114878. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, J.Z.; Lazar, C.; Crandall, I.E.; Szarek, W.A. Anti-Plasmodium activity of imidazolium and triazolium salts. Bioorg. Med. Chem. 2010, 18, 6184–6196. [Google Scholar] [CrossRef]
- Porcheron, A.; Sadek, O.; Ba Sowid, S.; Bridonneau, N.; Hippolyte, L.; Mercier, D.; Marcus, P.; Mutalliev, L.; Chauvier, C.; Chanéac, C.; et al. Direct synthesis of mesoionic carbene (MIC)-stabilized gold nanoparticles from 1,2,3-triazolium salts. Chem. Mater. 2023, 35, 6865–6876. [Google Scholar] [CrossRef]
- Lau, G.P.S.; Tsao, H.N.; Zakeeruddin, S.M.; Grätzel, M.; Dyson, P.J. Highly stable dye-sensitized solar cells based on novel 1,2,3-triazolium ionic liquids. ACS Appl. Mater. Interfaces 2014, 6, 13571–13577. [Google Scholar] [CrossRef] [PubMed]
- Horsten, T.; de Jong, F.; Theunissen, D.; Van der Auweraer, M.; Dehaen, W. Synthesis and spectroscopic properties of 1,2,3-triazole BOPAHY dyes and their water-soluble triazolium salts. J. Org. Chem. 2021, 86, 13774–13782. [Google Scholar] [CrossRef] [PubMed]
- Yacob, Z.; Liebscher, J. Chemistry of 1,2,3-triazolium salts. In Chemistry of 1,2,3-Triazoles; Dehaen, W., Bakulev, V.A., Eds.; Springer International Publishing: Cham, Germany, 2015; Volume 40, pp. 167–210. [Google Scholar]
- Soreq, H.; Seidman, S. Acetylcholinesterase-new roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Faraho, I.; Odak, I.; Kovačević, B.; Raspudić, A.; Šagud, I.; Bosnar, M.; Škorić, I.; Barić, D. Cholinesterase inhibitory and anti-inflammatory activity of the naphtho- and thienobenzo-triazole photoproducts: Experimental and computational study. Int. J. Mol. Sci. 2023, 24, 14676. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Odak, I.; Faraho, I.; Talić, S.; Bosnar, M.; Lasić, K.; Barić, D.; Škorić, I. New naphtho/thienobenzo-triazoles with interconnected anti-inflammatory and cholinesterase inhibitory activity. Eur. J. Med. Chem. 2022, 24, 114616. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Selec, I.; Ćaleta, I.; Odak, I.; Barić, D.; Ratković, A.; Molčanov, K.; Škorić, I. New thienobenzo/naphtho-triazoles as butyrylcholinesterase inhibitors: Design, synthesis and computational study. Int. J. Mol. Sci. 2023, 24, 5879. [Google Scholar] [CrossRef] [PubMed]
- Ratković, A.; Mlakić, M.; Dehaen, W.; Opsomer, T.; Barić, D.; Škorić, I. Synthesis and photochemistry of novel 1,2,3-triazole di-heterostilbenes. An experimental and computational study. Spectr. Acta A Mol. Biomol. Spectr. 2021, 261, 120056. [Google Scholar] [CrossRef]
- Ali, R.; Lang, T.; Saleh, S.M.; Meier, R.J.; Wolfbeis, O.S. Optical sensing scheme for carbon dioxide using a solvatochromic probe. Anal. Chem. 2011, 83, 2846–2851. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef]
- Niko, Y.; Klymchenko, A.S. Emerging solvatochromic push–pull dyes for monitoring the lipid order of biomembranes in live cells. J. Biochem. 2021, 170, 163–174. [Google Scholar] [CrossRef]
- Demeunynck, M.; Bailly, C.; Wilson, W.D. Small Molecule DNA and RNA Binders: From Synthesis to Nucleic Acid Complexes; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 2004. [Google Scholar]
- Lee, P.; Wu, X. Modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtnex, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Hasselgren, C.; Bercu, J.; Cayley, A.; Cross, K.; Glowienke, S.; Kruhlak, N.; Muster, W.; Nicolette, J.; Vijayaraj Reddy, M.; Saiakhov, R.; et al. Management of pharmaceutical ICH M7 (Q)SAR predictions—The impact of model updates. Regul. Toxicol. Pharmacol. 2020, 118, 104807. [Google Scholar] [CrossRef]
- Schulte, L.N.; Heinrich, B.; Janga, H.; Schmeck, B.T.; Vázquez, O. A far-red fluorescent DNA binder for interaction studies of live multidrug-resistant pathogens and host Cells. Angew. Chem. Int. Ed. 2018, 57, 11564–11568. [Google Scholar] [CrossRef]
- Mergny, J.L.; Lacroix, L. Analysis of thermal melting curves. Oligonucleotides 2003, 13, 515–537. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDock-823 Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Carletti, E.; Li, H.; Li, B.; Ekstrom, F.; Nicolet, Y.; Loiodice, M.; Gillon, E.; Froment, M.T.; Lockridge, O.; Schopfer, L.M.; et al. Aging of cholinesterases phosphylated by tabun proceeds through O-dealkylation. J. Am. Chem. Soc. 2008, 130, 16011–16020. [Google Scholar] [CrossRef] [PubMed]











| Compound | λmax/nm | ε/mol dm−3 cm−1 | 1 λem/nm | Apparent Stokes Shift/nm |
|---|---|---|---|---|
| 45 | 310 | 12,851.5 | 480 | 170 |
| 50 | 296 265 | 12,529.3 10,693.2 | 456 | 160 |
| Compound | AChE | BChE | ||
|---|---|---|---|---|
| % Inhibition * | IC50/μM | % Inhibition * | IC50/μM | |
| 23 | 30.1 ± 0.3 (250) | - | 75.4 ± 3.0 (250) | 70.0 |
| 24 | 70.8 ± 4.2 (200) | 113.0 | 68.9 ± 2.7 (100) | 31.0 |
| 25 | 32.6 ± 6.9 (100) | - | 70.0 ± 2.5 (75) | 29.3 |
| 26 | 26.5 ± 1.0 (100) | - | 69.7 ± 1.7 (75) | 20.6 |
| 27 | 24.3 ± 1.2 (100) | - | 64.0 ± 3.4 (100) | 31.1 |
| 28 | 44.7 ± 1.6 (500) | - | 71.9 ± 4.4 (150) | 79.4 |
| 29 | 53.2 ± 0.8 (100) | - | 57.3 ± 0.8 (200) | 92.2 |
| 30 | 63.3 ± 0.8 (250) | 171.0 | 33.2 ± 1.7 (250) | - |
| 31 | 50.1 ± 2.4 (241) | - | 53.4 ± 1.99 (241) | 232.5 |
| 32 | 58.1 ± 2.0 (250) | 195.4 | 71.6 ± 2.7 (250) | 71.0 |
| 33 | 33.9 ± 1.8(250) | - | 52.1 ± 1.7 (250) | 195.4 |
| 34 | 50.3 ± 1.9 (200) | - | 39.7 ± 0.8 (150) | - |
| 44 | 78.7 ± 2.6 (25) | 1.8 | 71.3 ± 2.0 (1) | 0.3 |
| 45 | 80.6 ± 1.8 (50) | 4.3 | 74.0 ± 7.0 (50) | 9.2 |
| 46 | 82.6 ± 0.6 (250) | 6.9 | 79.4 ± 2.2 (25) | 1.8 |
| 47 | 74.6 ± 0.6 (250) | 15.3 | 86.5 ± 5.1 (150) | 9.8 |
| 48 | 57.2 ± 0.7 (250) | 167.6 | 78.6 ± 2.0 (250) | 37.2 |
| 49 | 82.7 ± 4.2 (250) | 6.7 | 82.7 ± 4.2 (250) | 9.4 |
| 50 | 76.3 ± 2.1 (250) | 29.3 | 81.0 ± 0.3 (250) | 29.7 |
| 51 | 74.1 ± 5.0 (250) | 20.3 | 74.1 ± 5.0 (250) | 17.5 |
| Galantamine | 90.0 ± 1.5 (60) | 0.15 | 90.1 ± 3.4 (4.5) | 7.9 |
| Structure | ICH M7 Class | Derek Prediction | Sarah Prediction | Similarity to API | Overall In Silico |
|---|---|---|---|---|---|
| 23 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 24 | Class 3 |  |  | No Derek Alerts found | Positive |
| 25 | Class 3 |  |  | No Derek Alerts found | Positive |
| 26 | Class 3 |  |  | No Derek Alerts found | Positive |
| 27 | Class 3 |  |  | No Derek Alerts found | Positive |
| 28 | Class 5 |  |  | No Derek Alerts found | Negative |
| 29 | Class 3 |  |  | No Derek Alerts found | Positive |
| 30 | Class 3 |  |  | No Derek Alerts found | Positive |
| 31 | Class 3 |  |  | No Derek Alerts found | Positive |
| 32 | Class 5 |  |  | No Derek Alerts found | Negative |
| 33 | Class 3 |  |  | No Derek Alerts found | Positive |
| 34 | Class 3 |  |  | No Derek Alerts found | Positive |
| 44 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 45 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 46 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 47 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 48 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 49 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 50 | Inconclusive |  |  | No Derek Alerts found | Negative |
| 51 | Inconclusive |  |  | No Derek Alerts found | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlakić, M.; Barić, D.; Ratković, A.; Šagud, I.; Čipor, I.; Piantanida, I.; Odak, I.; Škorić, I. New Charged Cholinesterase Inhibitors: Design, Synthesis, and Characterization. Molecules 2024, 29, 1622. https://doi.org/10.3390/molecules29071622
Mlakić M, Barić D, Ratković A, Šagud I, Čipor I, Piantanida I, Odak I, Škorić I. New Charged Cholinesterase Inhibitors: Design, Synthesis, and Characterization. Molecules. 2024; 29(7):1622. https://doi.org/10.3390/molecules29071622
Chicago/Turabian StyleMlakić, Milena, Danijela Barić, Ana Ratković, Ivana Šagud, Ivona Čipor, Ivo Piantanida, Ilijana Odak, and Irena Škorić. 2024. "New Charged Cholinesterase Inhibitors: Design, Synthesis, and Characterization" Molecules 29, no. 7: 1622. https://doi.org/10.3390/molecules29071622
APA StyleMlakić, M., Barić, D., Ratković, A., Šagud, I., Čipor, I., Piantanida, I., Odak, I., & Škorić, I. (2024). New Charged Cholinesterase Inhibitors: Design, Synthesis, and Characterization. Molecules, 29(7), 1622. https://doi.org/10.3390/molecules29071622