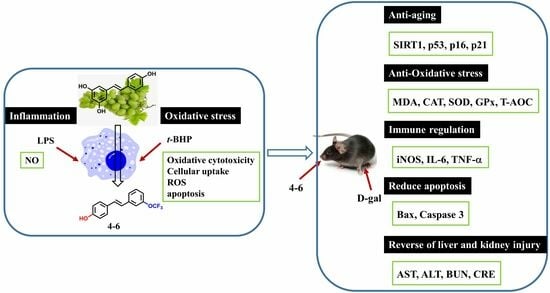
Discovery of a 4-Hydroxy-3′-Trifluoromethoxy-Substituted Resveratrol Derivative as an Anti-Aging Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Resveratrol Derivatives
2.2. In Vitro Study
2.2.1. Initial Screening
Effects on Inflammation in LPS-Stimulated Raw264.7 Cells
Effects on Oxidative Stress in t-BHP-Stimulated Raw264.7 Cells
2.2.2. Cellular Uptake
2.2.3. Effects of Resveratrol 1–1 and Its Active Derivative 4–6 on the Excessive Accumulation of ROS in t-BHP-Stimulated Raw264.7 Cells
2.2.4. Effects of Resveratrol 1–1 and Its Active Derivative 4–6 on Apoptosis in Raw264.7 Cells Induced by t-BHP
2.2.5. Molecular Docking Analysis of 1–1 and Its Derivative 4–6
2.3. In Vivo Study
2.3.1. Biochemical Analyses of Serum for Liver and Kidney Function Test
2.3.2. Effects of 4–6 on D-gal-Stimulated Oxidative Stress in the Serum
2.3.3. Effects of 4-6 on Oxidative Stress Stimulated with D-gal in the Brain and Liver
2.3.4. Effects of 4–6 on D-gal-Induced Inflammation in the Spleen Tissue
2.3.5. Effects on Histopathological Alternations in Aging Mice Caused by D-gal
2.3.6. Effects on AchE and Ach in the Brain of Aging Mice Stimulated with D-gal
2.3.7. Effects on Protein Expression Related to Aging, Oxidative Stress, and Apoptosis in the Brain Homogenate of Aging Mice
2.3.8. Medicinal and Chemical Properties of 4–6
3. Conclusions
4. Materials and Methods
4.1. Synthesis
4.2. Cell Culture
4.2.1. Determination of the Inhibition of NO
4.2.2. t-BHP-Induced Oxidative Cytotoxicity
4.2.3. Cell Cytotoxic Assay
4.2.4. Cellular Uptake
4.2.5. Determination of Intracellular ROS
4.2.6. Determination of Cell Apoptosis
4.3. Molecular Docking Studies of SIRT1 and the Compounds
4.4. Animal
4.4.1. Treatment of Mice
4.4.2. Biochemical Analysis
4.4.3. Histopathological Examination
4.4.4. Western Blot Assays
4.5. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinmura, K. Effects of caloric restriction on cardiac oxidative stress and mitochondrial bioenergetics: Potential role of cardiac sirtuins. Oxid. Med. Cell. Longev. 2013, 2013, 528935. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Orr, W.C. The redox stress hypothesis of aging. Free Radic. Biol. Med. 2012, 52, 539–555. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.T.; Tang, M.L.; Deng, H.M.; Xing, T.R.; Chen, J.T.; Wang, H.L.; Ruan, D.Y. Epigallo-catechin-3-gallate induced primafry cultures of rat hippocampal neurons death linked to calcium overload and oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 551–564. [Google Scholar] [CrossRef]
- Ruan, Q.; Liu, F.; Gao, Z.; Kong, D.; Hu, X.; Shi, D.; Bao, Z.; Yu, Z. The anti-inflamm-aging and hepatoprotective effects of huperzine A in D-galactose-treated rats. Mech. Ageing Dev. 2013, 134, 89–97. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef]
- Candore, G.; Caruso, C.; Colonna-Romano, G. Inflammation, genetic background and longevity. Biogerontology 2010, 11, 565–573. [Google Scholar] [CrossRef]
- Catalina, A.L.; Isabel, V. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharm. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.Y.; Li, Y.J.; Han, L.F.; Yan, X.H. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical application-a comprehensive review. Phytochestry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.H.; Yang, D.; Wang, Y.P.; Yang, R.; Qu, L.B.; Zen, H.J. Effect of resveratrol on the repair of kidney and brain injuries and regulation on klotho gene in D-galactose-induced aging mice. Bioorg. Med. Chem. Lett. 2021, 40, 127913. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Farjana, M.; Moni, A.; Hossain, K.S.; Hannan, M.A.; Ha, H. Prospective pharmacological potential of resveratrol on delaying kidney aging. Int. J. Mol. Sci. 2021, 22, 8258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.F.; Gu, P.L.; Liu, X.M.; Hu, S.H.; Zheng, H.; Liu, T.; Li, C.Y. Gold nanoparticles encapsulated resveratrol as an anti-aging agent of delay cataract development. Pharmaceuticals 2023, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Darrudi, M.; Naseri, K.; Samini, F.; Aschner, M.; Pourbagher-shahri, A.M.; Samarghandian, S. The role of resveratrol in aging and senescence: A focus on molecular mechanisms. Cur. Mol. Med. 2023. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef]
- Kaeberlein, M. Resveratrol and rapamycin: Are they anti-aging drugs? BioEssays 2010, 32, 96–99. [Google Scholar] [CrossRef]
- Kasiotis, K.K.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, A.F.; Noreldin, A.E.; Taha, A.E. The adaptogenic anti-aging potential of resveratrol against heat stress-medicated liver injury in aged rats: Role of HSP70 and NF-κB signaling. J. Therm. Biol. 2019, 83, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Belguendouz, L.; Fremont, L.; Linard, A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997, 53, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Qu, X.Y.; Liu, G.Y.; Liu, R.M. A novel resveratrol derivative induces oxidative stress, G1 cell cycle arrest and premature senescence in A549 cells. Lat. Am. J. Pharm. 2019, 38, 907–917. [Google Scholar]
- Liu, G.Y.; Zhai, Q.; Chen, J.Z.; Zhang, Z.Q.; Yang, J. 2,2′-Fluorine mono-carbonyl curcumin induce reactive oxygen species-Mediated apoptosis in Human lung cancer NCI-H460 cells. Eur. J. Pharmacol. 2016, 786, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Late stage trifluoromethylthiolation strategies for organic compounds. Org. Biomol. Chem. 2016, 14, 7150–7182. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.L.; Cao, Y.X.; Li, Y.R.; Liu, G.Y. 3,5-dihydroxy-4’-fluoro-trans-stilbenzen inhibits the proliferation of A 549 cells via G1 cell cycle arrest and premature senescence. Lat. Am. J. Pharm. 2019, 38, 1838–1845. [Google Scholar]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; Deluca, M.; Ottaviani, E.; Benedictis, G.D. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Abdul, R.P.; Song, J.K. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar]
- Uryga, A.K.; Bennett, M.R. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J. Physiol. 2016, 594, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Y.; Gao, C.; Jia, Y.T.; Huang, Y.Q. Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence. Biochem. Bioph. Res. Commun. 2016, 478, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Zhang, X.Y.; Zheng, S.B.; Khanabdali, R.; Kalionis, B.; Wu, J.Z.; Wan, W.B.; Tai, X.T. An update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Filippo, E.S.D.; Giampietro, L.; Filippis, B.D.; Balaha, M.; Vincenzo, F.; Locatelli, M.; Pietrangelo, T.; Tartaglia, A.; Amoroso, R.; Fulle, S. Synthesis and biological evaluation of halogenated E-stilbenols as promising antiaging agents. Molecules 2020, 25, 5770. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Marco, B.D.; Nocentini, G.; Delfino, D.V. Oxidative stress and apoptosis in immune diseases. Int. J. Immunopathol. Pharmacol. 2002, 15, 157–164. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Hori, Y.S.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. J. Biol. Chem. 2013, 288, 5963–5972. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, Y.; Katayanagi, Y.; Tanaka, K.; Fukutomi, R.; Hiramoto, S.; Imai, S. Alkylresorcinols activate SIRT1 and delay ageing in Drosophila melanogaster. Sci. Rep. 2017, 7, 43679. [Google Scholar] [CrossRef] [PubMed]
- Naini, R.; Chikati, R.; Vudem, D.R.; Kancha, R.K. Molecular docking analysis of imine stilbene analogs and evalustion of their anti-aging activity using yeast and mammalian cell models. J. Recept. Signal Transd. 2019, 39, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.A.; Shimoga Janakirama, A.R.; Martin, A. SIRT1 activation by Taurine: In vitro evaluation, molecular docking and molecular dynamics simulation studies. J. Nutr. Biochem. 2022, 102, 108948. [Google Scholar]
- ManNa, D.; Bhuyan, R.; Ghosh, R. Probing the mechanism of SIRT1 activation by a 1,4-dihydropyridine. J. Mol. Model. 2018, 24, 340. [Google Scholar] [CrossRef]
- Hou, X.B.; Rooklin, D.; Fang, H.; Zhang, Y.K. Resveratrol serves as a protein-substrate interaction stabilizer in human SIRT1 activation. Sci. Rep. 2016, 6, 38186. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Fan, S.H.; Zheng, Y.L.; Lu, J.; Wu, D.W.; Shan, Q.; Hu, B. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by D-galactose in mouse liver. Food Chem. Toxicol. 2009, 47, 496–501. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.; Dogra, S. Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int. J. Alzheimer’s Dis. 2011, 2011, 347569. [Google Scholar]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef]
- Ho, S.C.; Liu, J.H.; Wu, R.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Tian, Y.; Zhang, Y.; Lu, Y.L.; Li, L.S.; Shi, J.S. Dendrobium alkaloids prevent Aβ25–35-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ 2016, 4, e2739. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Robbins, T.W. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997, 48, 649–684. [Google Scholar] [CrossRef] [PubMed]
- Mohapel, P.; Leanza, G.; Kokaia, M.; Lindvall, O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 2005, 26, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Guarente, L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat. Rev. Mol. Cell Biol. 2005, 6, 298–305. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the regulation of cellular senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef]
- Lockshin, R.A. Programmed cell death: History and future of a concept. J. Soc. Biol. 2005, 199, 169–173. [Google Scholar] [CrossRef]
- Yuan, J.; Yankner, B.A. Apoptosis in the nervous system. Nature 2000, 407, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Higami, Y.; Shimokawa, I. Apoptosis in the aging process. Cell Tissue Res. 2000, 301, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Gokhale, N.; Unni, S.; Deshmukh, P.; Reddyrajula, R.; Bharath, M.M.S.; Dalimba, U.; Padmanabhan, B. Design, synthesis, in-vitro evaluation and molecular docking studies of novel indole derivatives as inhibitors of SIRT1 and SIRT2. Bioorg. Chem. 2019, 92, 103281. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, E.M.; Cermak, S.C.; Wiemer, D.F. Synthesis of schweinfurthin C, a geranylated stilbene from macaranga schweinfurthii. J. Org. Chem. 1999, 64, 8718–8723. [Google Scholar] [CrossRef]
- Yerien, D.E.; Bonesi, S.; Postigo, A. Fluorination methods in drug discovery. Org. Biomol. Chem. 2016, 14, 8398–8427. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Choe, Y.S.; Choi, J.Y.; Lee, K.H.; Kim, B.T. Synthesis and Evaluation of18F-Labeled Styryltriazole and Resveratrol Derivatives forβ-Amyloid Plaque Imaging. J. Med. Chem. 2012, 55, 883–892. [Google Scholar] [CrossRef]
- Kang, S.S.; Cuendet, M.; Endringer, D.C.; Croy, V.L.; Pezzuto, J.M. Synthesis and biological evaluation of a library of resveratrol analogues as inhibitors of COX-1, COX-2 and NF-κB. Bioorgan. Med. Chem. 2009, 17, 1044–1054. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.Y.; Chen, J.Z.; Guo, S.J. Resveratrol derivative containing fluorine group, and preparation method and application. CN Patent 2017,107011127A, 4 August 2017. [Google Scholar]
- Shang, Y.J.; Qian, Y.P.; Liu, X.D.; Dai, F.; Shang, X.L.; Jia, W.Q.; Liu, Q.; Fang, J.G.; Zhou, B. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical, and substitution. J. Org. Chem. 2009, 74, 5025–5031. [Google Scholar] [CrossRef]
- Lion, C.J.; Matthews, C.S.; Stevens, M.F.G.; Westwell, A.D. Synthesis, Antitumor Evaluation, and Apoptosis-Inducing Activity of Hydroxylated (E)-Stilbenes. J. Med. Chem. 2005, 48, 1292–1295. [Google Scholar] [CrossRef]
- Chan, S.Y.; Loh, Y.C.; Oo, C.W.; Yam, M.F. In vitro study and structure-activity relationship analysis of stilbenoid derivatives as powerful vasorelaxants: Discovery of new lead compound. Bioorg. Chem. 2020, 104, 104239. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.K.; Chen, J.; Zhang, W.; Song, J.G.; Balzo, U.D.; Brown, L.; Boddupalli, S.; Bobzin, S.; Gilat, S.; et al. Cytoprotective compounds, pharmaceutical and cosmetic formulations, and methods. U.S. Patent 2003,73712, 17 April 2003. [Google Scholar]
- Zhang, Y.; Wang, Q.; Li, L.L.; Le, Y.; Liu, L.; Yang, J.; Li, Y.L.; Bao, G.C.; Yan, L.J. Synthesis and preliminary structure-activity relationship study of 3-methylquinazolinone derivatives as EGFR inhibitors with enhanced antiproliferative activities against tumour cells. J. Enzym. Inhib. Med. Chem. 2021, 36, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Bavo, F.; Pallavicini, M.; Pucci, S.; Appiani, R.; Giraudo, A.; Oh, H.; Kneisley, D.L.; Eaton, B.; Lucero, L.; Gotti, C.; et al. Subnanomolar affinity and selective antagonism at α7 nicotinic receptor by combined modifications of 2-triethylammonium ethyl ether of 4-Stilbenol (MG624). J. Med. Chem. 2023, 66, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Yang, L.; Wu, S.; Liu, S.S.; Cushman, M.; Tian, J.; Li, N.M.; Yang, Q.H.; Zhang, H.A.; Qiu, Y.J.; et al. Discovery of efficient stimulators for adult hippocampal neurogenesis based on scaffolds in dragon’s blood. Eur. J. Med. Chem. 2017, 136, 382–392. [Google Scholar] [CrossRef]















| Num | IC50 (μM) | Num | IC50 (μM) | Num | IC50 (μM) |
|---|---|---|---|---|---|
| 1–2 | 45.8 ± 2.26 | 1–3 | 82.5 ± 1.70 | 1–4 | 30.5 ± 1.55 |
| 2–2 | 16.5 ± 1.45 | 2–3 | 22.7 ± 0.99 | 2–4 | 23.6 ± 2.10 |
| 3–2 | 22.9 ± 1.38 | 3–3 | 21.9 ± 2.69 | 3–4 | 13.1 ± 0.20 |
| 4–1 | 23.4 ± 0.13 | 4–2 | 23.2 ± 1.76 | 4–3 | 26.0 ± 1.36 |
| 4–4 | 14.9 ± 1.12 | 4–5 | 13.8 ± 1.06 | 4–6 | 11.1 ± 1.05 |
| 1–1 | 33.5 ± 3.03 | 2–1 | 2.2 ± 0.01 | 3–1 | 3.02 ± 0.03 |
| 2–5 | 18.8 ± 1.75 | 3–5 | 7.96 ± 0.23 |
| Num | cLogP a | Molecular Weight MW (g/mol) | Polar Surface Area PSA (Å2) a |
|---|---|---|---|
| 4-6 | 4.96 | 280.25 | 29.46 |
| 1-1 | 2.99 | 228.25 | 60.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Chen, X.; Teng, Z.; Wang, X.; Yang, J.; Liu, G. Discovery of a 4-Hydroxy-3′-Trifluoromethoxy-Substituted Resveratrol Derivative as an Anti-Aging Agent. Molecules 2024, 29, 86. https://doi.org/10.3390/molecules29010086
Liang Y, Chen X, Teng Z, Wang X, Yang J, Liu G. Discovery of a 4-Hydroxy-3′-Trifluoromethoxy-Substituted Resveratrol Derivative as an Anti-Aging Agent. Molecules. 2024; 29(1):86. https://doi.org/10.3390/molecules29010086
Chicago/Turabian StyleLiang, Yinhu, Xi Chen, Zhifeng Teng, Xuekun Wang, Jie Yang, and Guoyun Liu. 2024. "Discovery of a 4-Hydroxy-3′-Trifluoromethoxy-Substituted Resveratrol Derivative as an Anti-Aging Agent" Molecules 29, no. 1: 86. https://doi.org/10.3390/molecules29010086