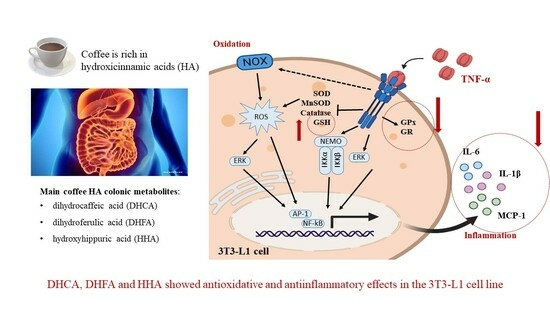
Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells
Abstract
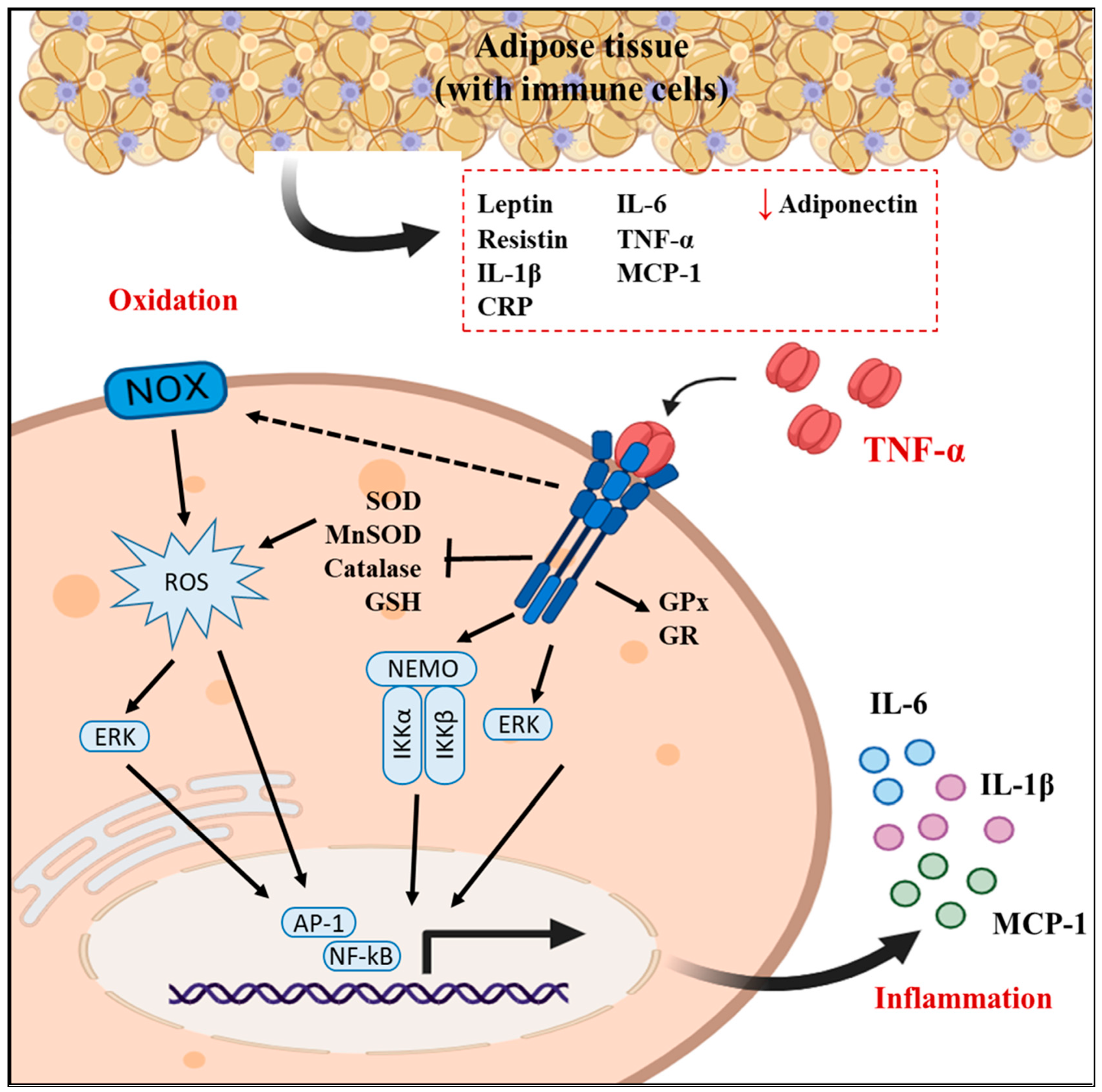
1. Introduction
2. Results
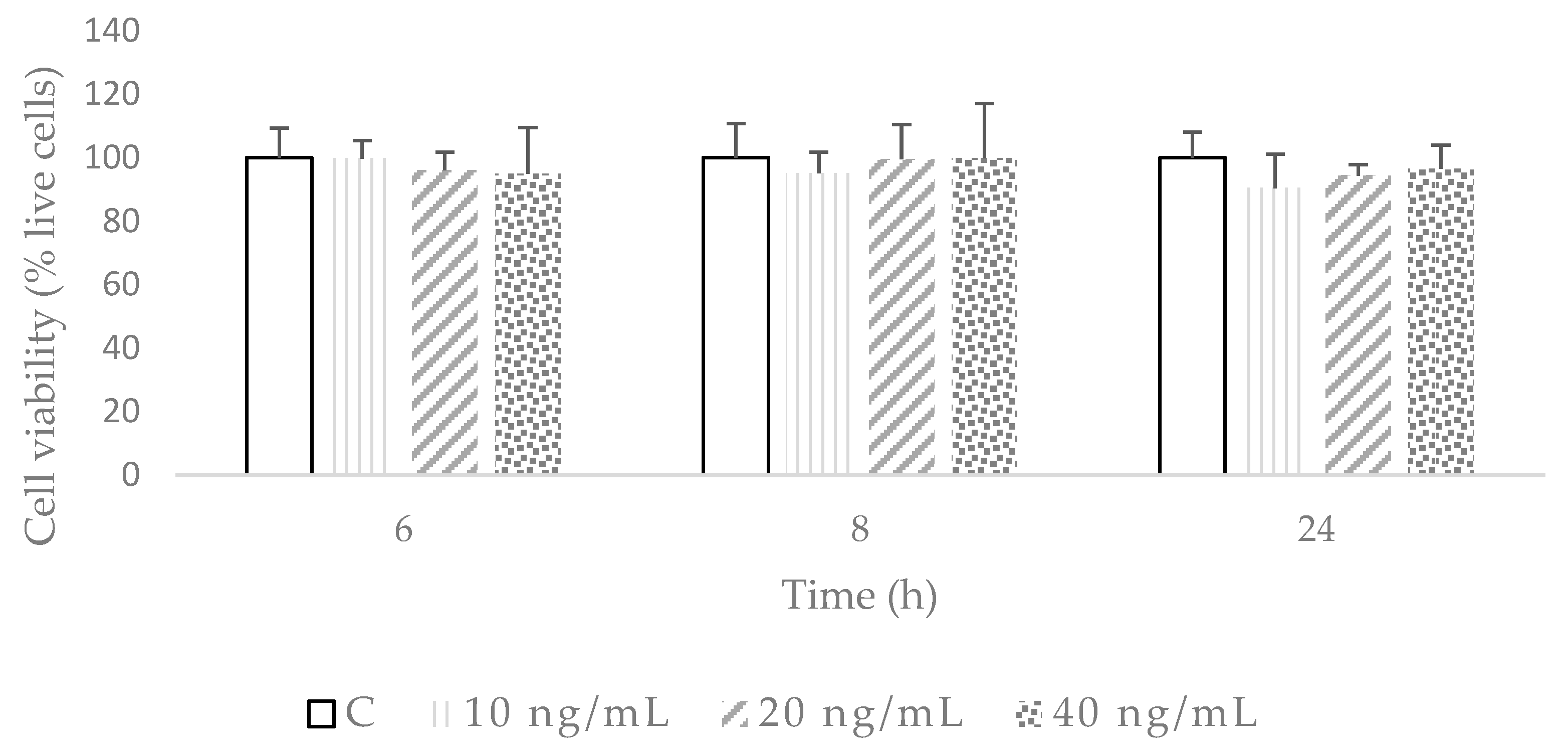
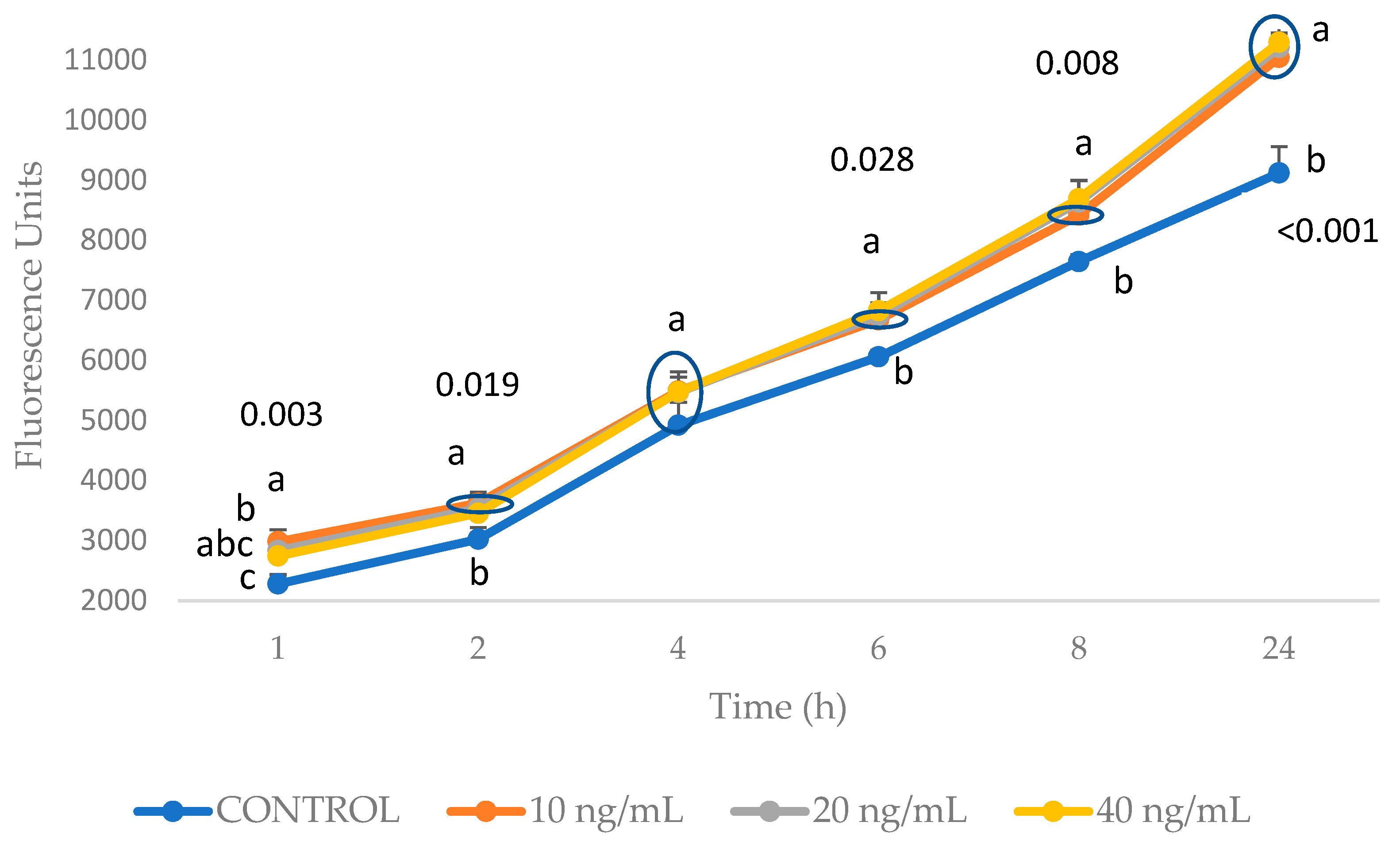
2.1. Establishment of the Inflammation and Oxidation Model in 3T3-L1 Fibroblasts
2.2. Direct Effects of DHCA, DHFA, and HHA on 3T3-L1 Cell Line Viability and ROS Production
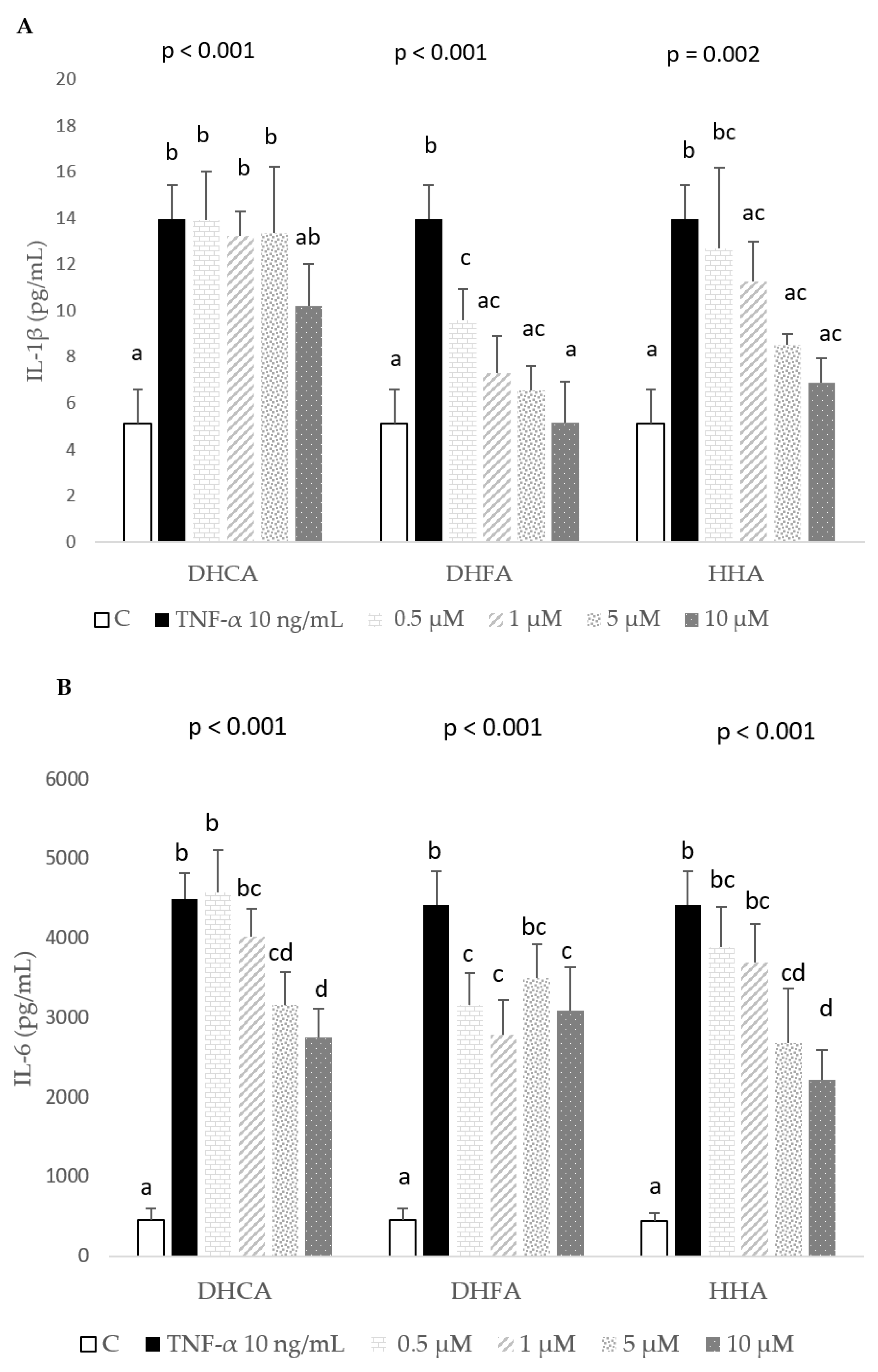
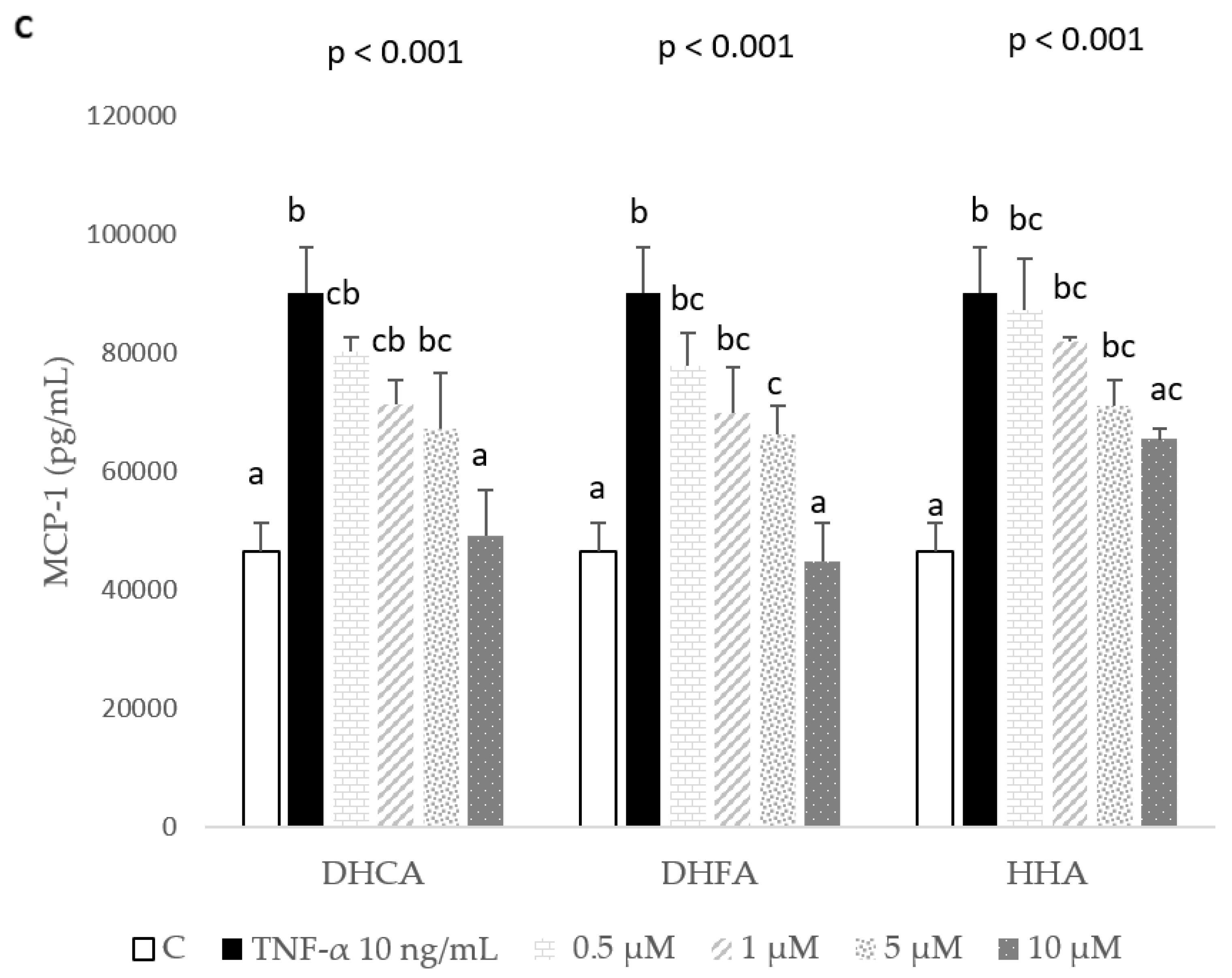
2.3. Protective Effects of DHCA, DHFA, and HHA on 3T3-L1 Cell Line on IL-1β, IL-6, and MCP-1 Inflammation Markers
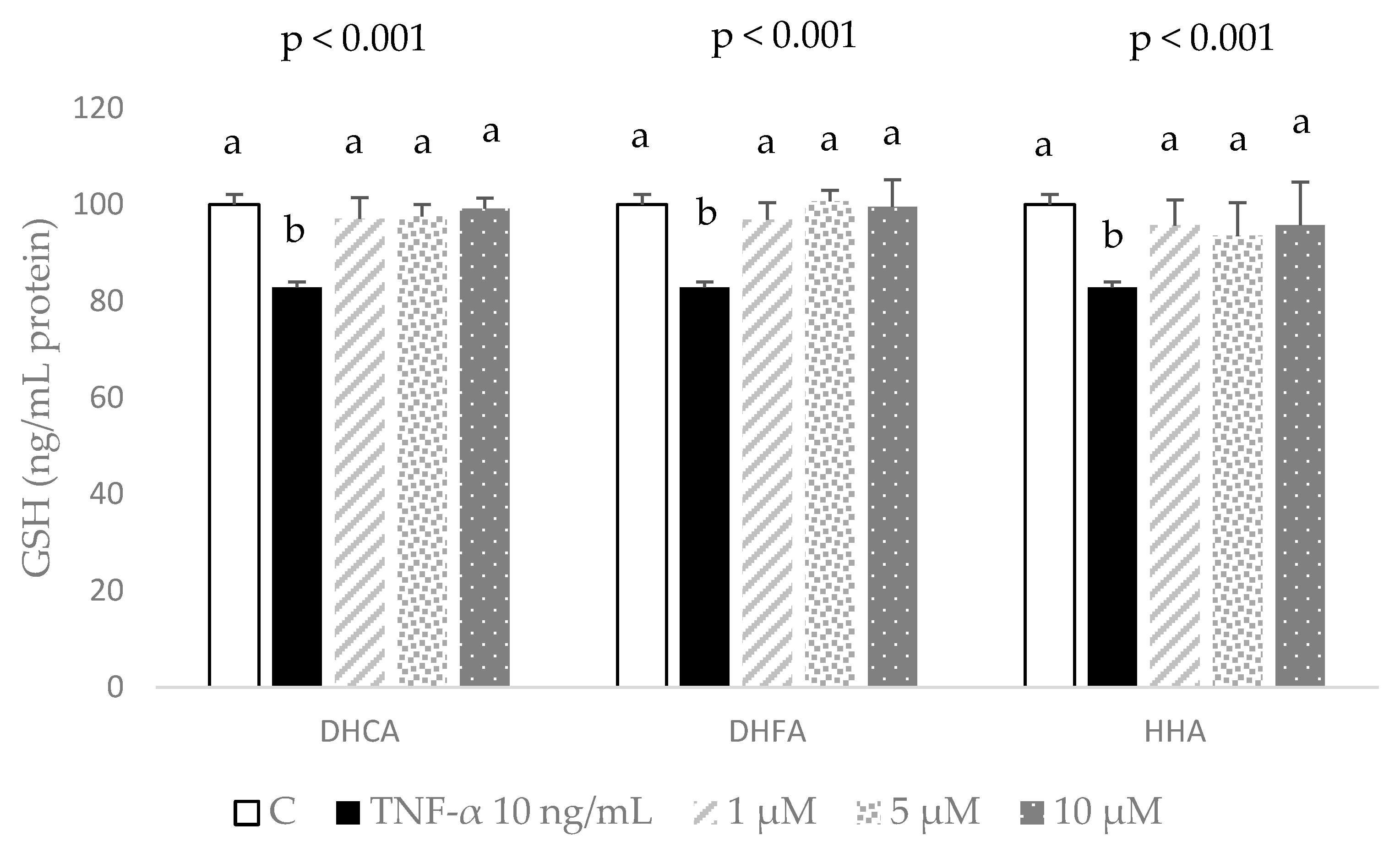
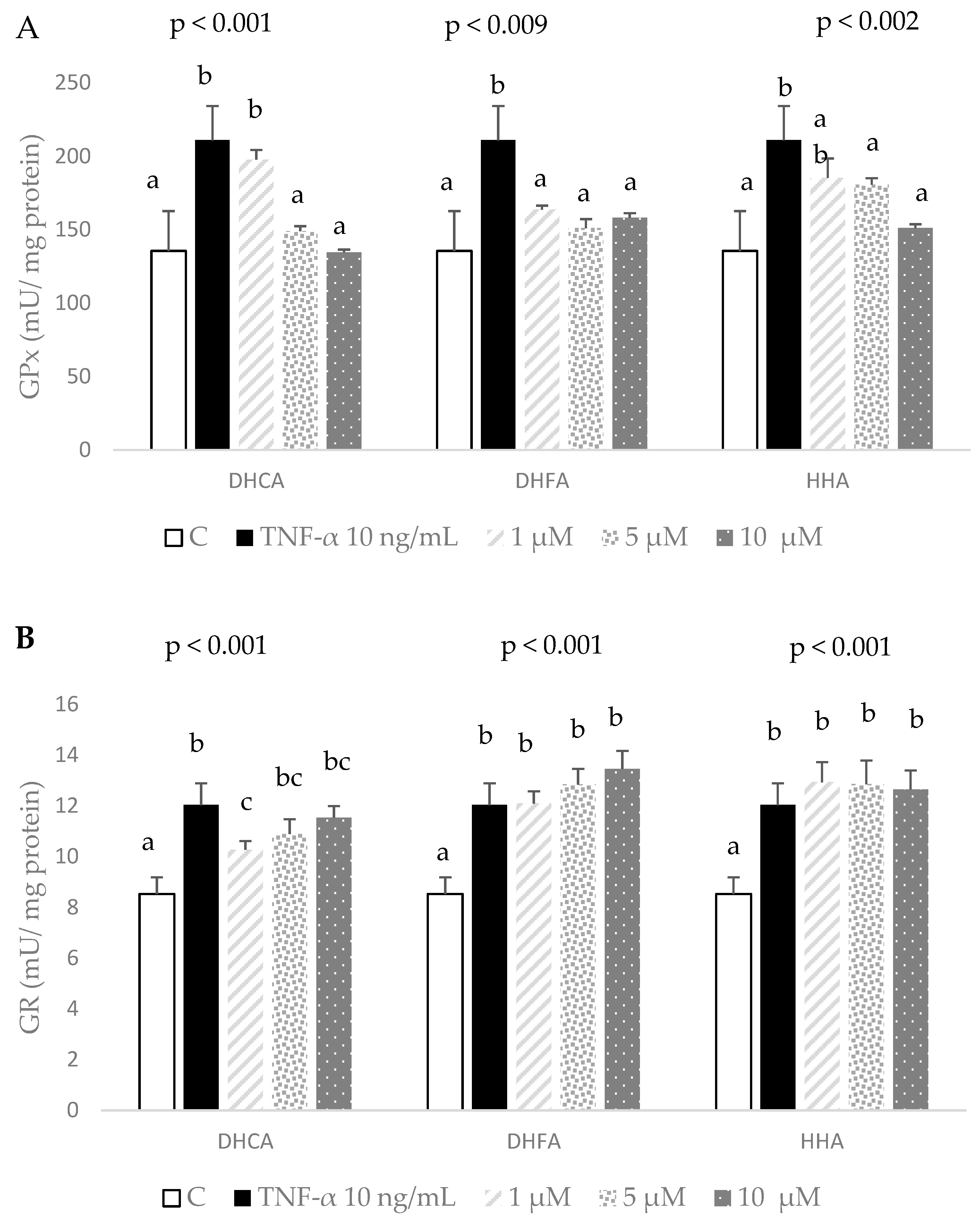
2.4. Protective Effects of DHCA, DHFA, and HHA on Oxidative Stress Markers in 3T3-L1 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Induced Inflammation/Oxidation Model in 3T3-L1 Cells with TNF-α
4.3.1. Pro-Inflammatory Treatment
4.3.2. Evaluation of Cell Viability
4.3.3. ROS Production
4.3.4. Measurement of IL-6 Production in Cells Supernatant
4.4. Direct Effects and Protective Effects against Inflammation/Oxidation of the Three Coffee Colonic Metabolites, DHCA, DHFA, and HHA, in 3T3-L1 Cells
4.4.1. Direct Effects of DHCA, DHFA, and HHA in 3T3-L1 Cells
4.4.2. Protective Effects of DHCA, DHFA, and HHA in 3T3-L1 Cells Treated with TNF-α
4.4.3. Anti-Inflammatory Effects of DHCA, DHFA, and HHA in 3T3-L1 Cells Treated with TNF-α
4.5. Antioxidant Effects of DHCA, DHFA, and HHA in 3T3-L1 Cells Treated with TNF-α
4.5.1. Reduced Glutathione
4.5.2. Antioxidant Enzymes
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, E.; Choi, J.M.; Kim, W.J.; Rhee, E.J.; Oh, K.W.; Lee, W.Y.; Park, S.E.; Park, S.W.; Park, C.Y. Restoration of adiponectin expression via the ERK pathway in TNFα-treated 3T3-L1 adipocytes. Mol. Med. Rep. 2014, 10, 905–910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yen, G.-C.; Chen, Y.-C.; Chang, W.-T.; Hsu, C.-L. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011, 59, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Weinberg, S.; Chandel, N.S. Mitochondrial control of immunity: Beyond ATP. Nat. Rev. Immunol. 2017, 17, 608–620. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2013, 395, 203–230. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress and oxidative distress. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 2020; pp. 3–12. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Silva, S.D.; Jara, Z.P.; Peres, R.; Lima, L.S.; Scavone, C.; Montezano, A.C.; Touyz, R.M.; Casarini, D.; Michelini, L.C. Temporal changes in cardiac oxidative stress, inflammation and remodeling induced by exercise in hypertension: Role for local angiotensin II reduction. PLoS ONE 2017, 12, 0189535. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Liu, J.-F.; Chen, P.-C.; Chang, T.-M.; Hou, C.-H. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J. Exp. Clin. Cancer Res. 2020, 39, 254. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Bilal Hussain, M.; Hassan, S.; Waheed, M.; Javed, A.; Adil Farooq, M.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Intech Open: London, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Martín, M.A.; Sarriá, B.; Ramos, S.; Mateos, R.; Bravo, L. Effect of cocoa and its flavonoids on biomarkers of inflammation: Studies in cell culture, animals and humans. Nutrients 2016, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean dietary polyphenol intake and obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef]
- Surma, S.; Sahebkar, A.; Banach, M. Coffee or tea: Anti-inflammatory properties in the context of atherosclerotic cardiovascular disease prevention. Pharmacol. Res. 2022, 187, 106596. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Chen, C.H.; Lee, M.F.; Chang, T.; Yu, Y.M. Chlorogenic acid attenuates adhesion molecules up-regulation in IL-1-treated endothelial cells. Eur. J. Nutr. 2010, 49, 267–275. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial phenolic metabolites: Which molecules actually have an effect on human health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef]
- Márquez-Campos, E.; Jakobs, L.; Simon, M.-C. Antidiabetic effects of flavan-3-ols and their microbial metabolites. Nutrients 2020, 12, 1592. [Google Scholar] [CrossRef]
- Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo-Clemente, L.; Goya, L.; Sarriá, B. Colonic coffee phenols metabolites, dihydrocaffeic, dihydroferulic, and hydroxyhippuric acids protect hepatic cells from TNF-induced inflammation and oxidative stress. Int. J. Mol. Sci. 2023, 24, 1440. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Seguido, M.Á.; Tarradas, R.M.; González-Rámila, S.; García-Cordero, J.; Sarriá, B.; Bravo-Clemente, L.; Mateos, R. Sustained consumption of a decaffeinated green coffee nutraceutical has limited effects on phenolic metabolism and bioavailability in overweight/obese subjects. Nutrients 2022, 14, 2445. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Sarriá, B.; Mateos, R.; Goya, L.; Bravo, L. TNF-α induced inflammation in human EA.hy926 endothelial cells is prevented by yerba mate and green coffee extracts, their main hydroxycinnamic acids, and microbial metabolites. Int. J. Food Sci. Nutr. 2019, 70, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Cave, E.; Crowther, N.J. The use of 3T3-L1 murine preadipocytes as a model of adipogenesis. Methods Mol. Biol. 2019, 1916, 263–272. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, B.L.; Liu, K.; Tang, N.; Huang, J.; An, Y.; Li, L. Establishment of the insulin resistance induced by inflammatory response in 3T3-L1 preadipocytes cell line. Inflammation 2008, 31, 355–364. [Google Scholar] [CrossRef]
- Jiao, W.; Mi, S.; Sang, Y.; Jin, Q.; Chitrakar, B.; Wang, X.; Wang, S. Integrated network pharmacology and cellular assay for the investigation of an anti-obesity effect of 6-shogaol. Food Chem. 2022, 374, 131755. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Mateos, R.; Bravo, L. Dihydrocaffeic acid, a mayor microbial metabolite of chlorogenic acids, shows similar protective effect than a yerba mate phenolic extract against oxidative stress in HepG2 cells. Food Res. Int. 2016, 87, 25–33. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Landolt, L.; Spagnoli, G.C.; Hertig, A.; Brocheriou, I.; Marti, H.-P. Fibrosis and cancer: Shared features and mechanisms suggest common targeted therapeutic approaches. Nephrol. Dial. Transpl. 2022, 37, 1024–1032. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of phytochemicals in skin photoprotection via regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef]
- Araki, S.; Dobashi, K.; Kubo, K.; Yamamoto, Y.; Asayama, K.; Shirahata, A. N-acetylcysteine attenuates TNF-alpha induced changes in secretion of interleukin-6, plasminogen activator inhibitor-1 and adiponectin from 3T3-L1 adipocytes. Life Sci. 2006, 79, 2405–2412. [Google Scholar] [CrossRef]
- Chen, X.; Xun, K.; Chen, L.; Wang, Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem. Funct. 2009, 27, 407–416. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Lopez-Oliva, M.E.; Ramos, S.; Martín, M.A. Preventive effect of cocoa flavanols against glucotoxicity-induced vascular inflammation in the arteria of diabetic rats and on the inflammatory process in TNF-α-stimulated endothelial cells. Food Chem. Toxicol. 2020, 146, 111824. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lee, W.H. Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J. Nutr. Biochem. 2008, 19, 819–825. [Google Scholar] [CrossRef]
- Yang, H.L.; Chang, H.C.; Lin, S.W.; Senthil Kumar, K.J.; Liao, C.H.; Wang, H.M.; Lin, K.Y.; Hseu, Y.C. Antrodia salmonea inhibits TNF-a-induced angiogenesis and atherogenesis in human endothelial cells through the down-regulation of NF-κB and up-regulation of Nrf2 signaling pathways. J. Ethnopharmacol. 2014, 151, 394–406. [Google Scholar] [CrossRef]
- Coimbra, S.; Catarino, C.; Santos-Silva, A. The role of adipocytes in the modulation of iron metabolism in obesity. Obes. Rev. 2013, 14, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007, 364, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, W.; Wu, X.; Yu, Y.; Lv, J.; Liu, C.; Mao, X.; Zhu, Y.; Xu, K.; Han, X.; et al. Anti-inflammatory effect of resveratrol on TNF-R-induced MCP-1 expression in adipocytes. Biochem. Biophys. Res. Commun. 2008, 369, 471–477. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2016, 60, 2602–2610. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Carlsen, H.; Nordstrom, O.; Blomhoff, R.; Moskaug, J.O.C. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
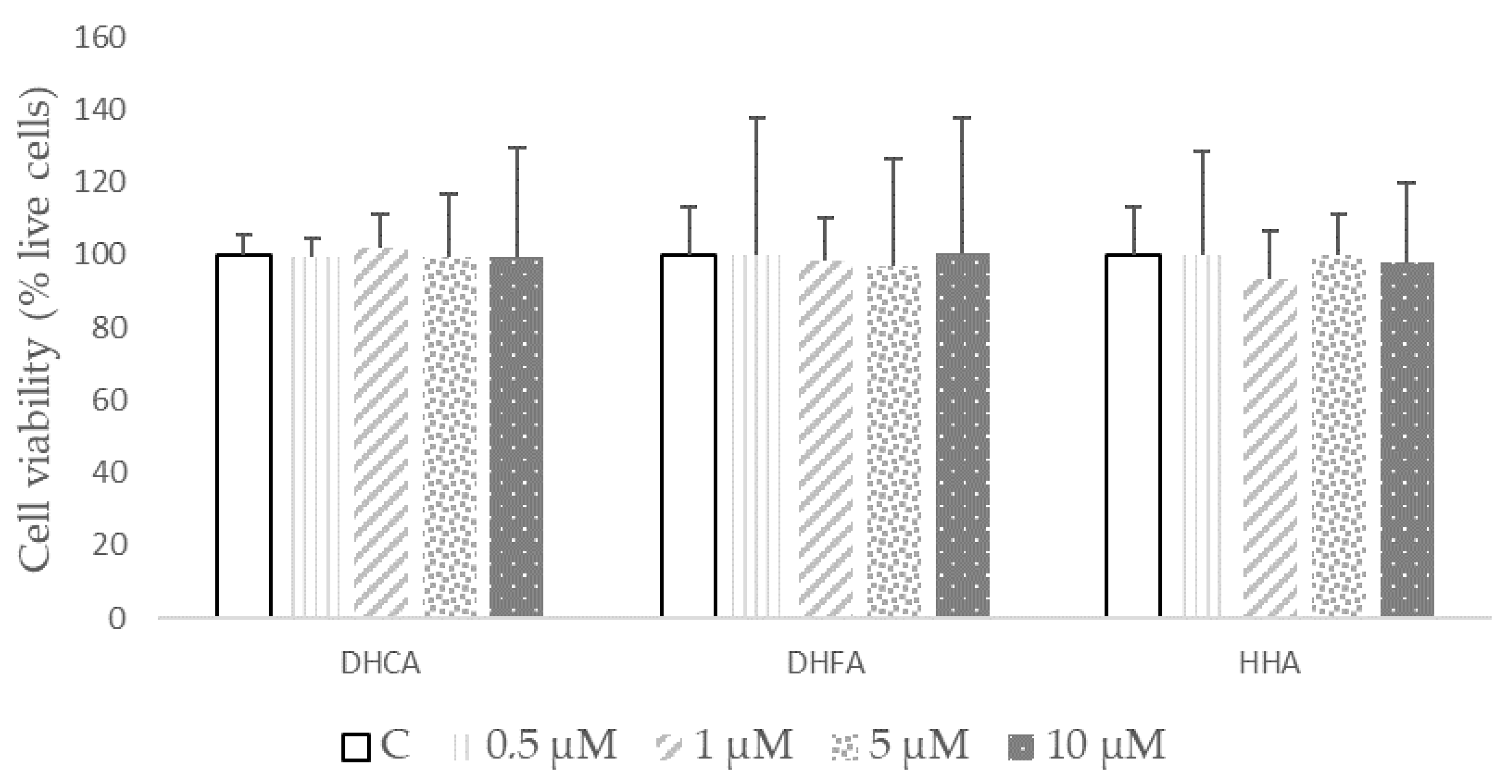

| µM | DHCA | DHFA | HHA |
|---|---|---|---|
| 0 (C) | 100.01 ± 2.31 | 100.12 ± 2.03 | 100.6 ± 3.6 |
| 0.5 | 99.7 ± 0.2 | 97.4 ± 3.4 | 97.1 ± 2.2 |
| 1 | 101.4 ± 1.3 | 97.7 ± 1.6 | 99.7 ± 2.2 |
| 5 | 101.01 ± 0.63 | 96.12± 1.03 | 96.7 ± 1.5 |
| 10 | 100.1 ± 1.8 | 95.8 ± 0.4 | 96.8 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goya, L.; Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo, L.; Sarriá, B. Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules 2024, 29, 88. https://doi.org/10.3390/molecules29010088
Goya L, Sánchez-Medina A, Redondo-Puente M, Dupak R, Bravo L, Sarriá B. Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules. 2024; 29(1):88. https://doi.org/10.3390/molecules29010088
Chicago/Turabian StyleGoya, Luis, Andrea Sánchez-Medina, Mónica Redondo-Puente, Rudolf Dupak, Laura Bravo, and Beatriz Sarriá. 2024. "Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells" Molecules 29, no. 1: 88. https://doi.org/10.3390/molecules29010088
APA StyleGoya, L., Sánchez-Medina, A., Redondo-Puente, M., Dupak, R., Bravo, L., & Sarriá, B. (2024). Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules, 29(1), 88. https://doi.org/10.3390/molecules29010088