“NO” Time in Fear Response: Possible Implication of Nitric-Oxide-Related Mechanisms in PTSD
Abstract
:1. Introduction
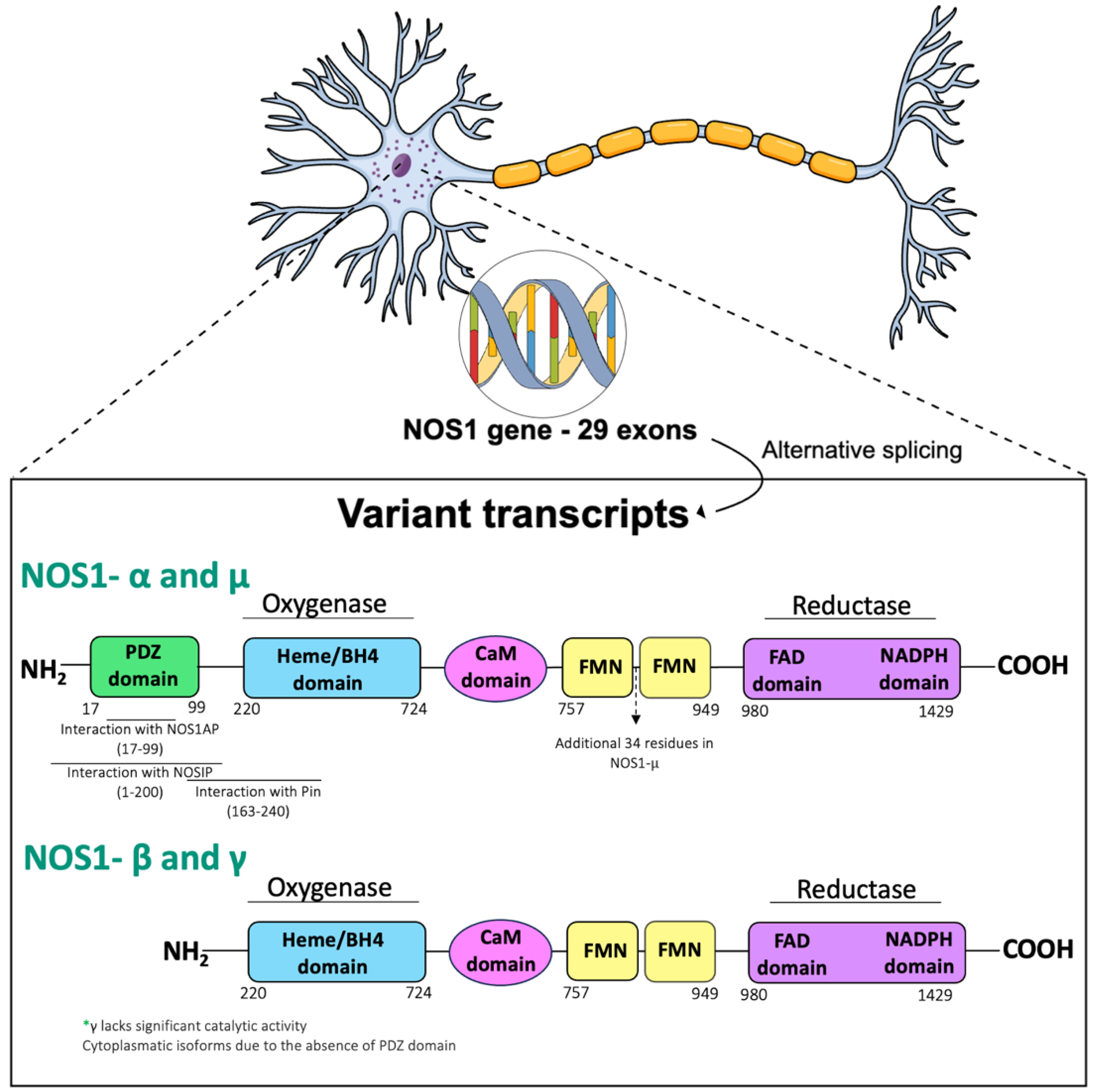
NO Production and Signaling Pathways
2. NO Involvement in Learned Fear
Molecular Pathways Modified by nNOS and Implications for Fear Response
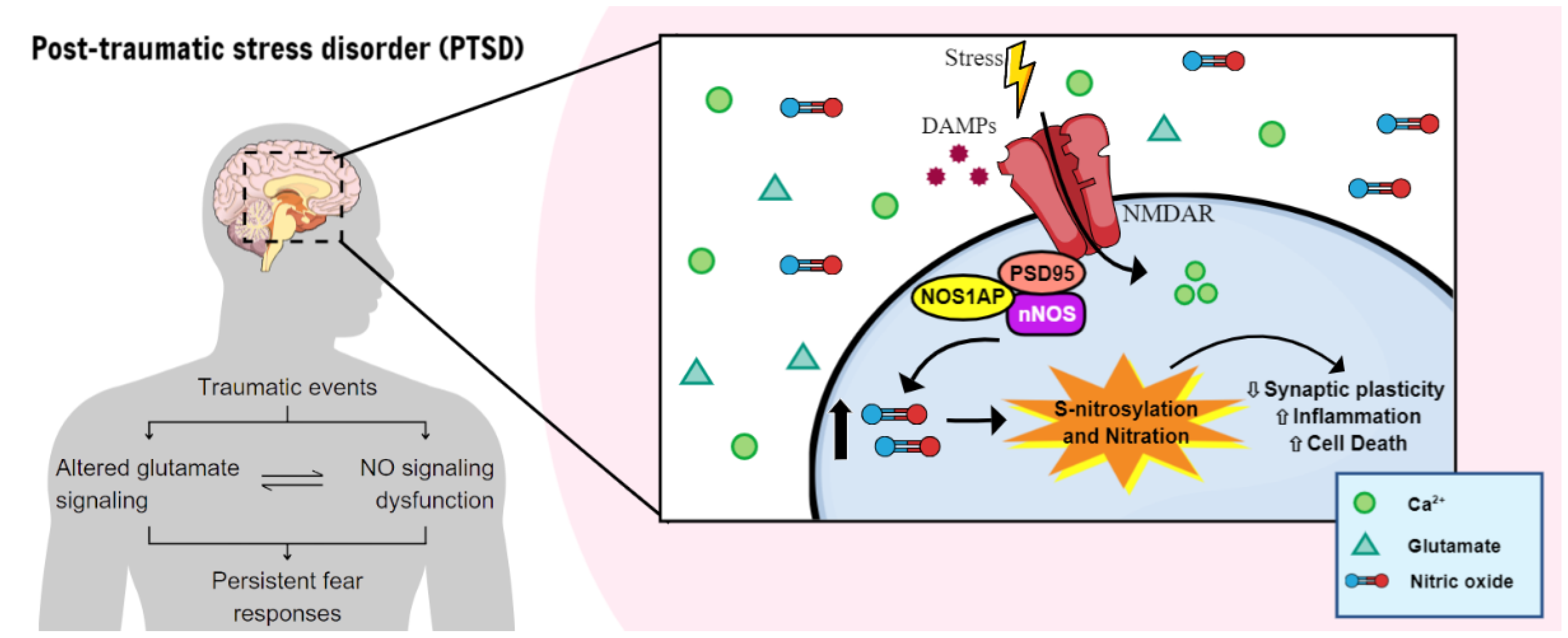
3. nNOS, NO-Related Mechanisms, and Stress Response
3.1. The Interplay between Inflammatory Markers and NO in PTSD
3.2. Cellular Metabolism and Nitrosative Stress
3.3. Role of NO in Cell Death and Excitotoxicity
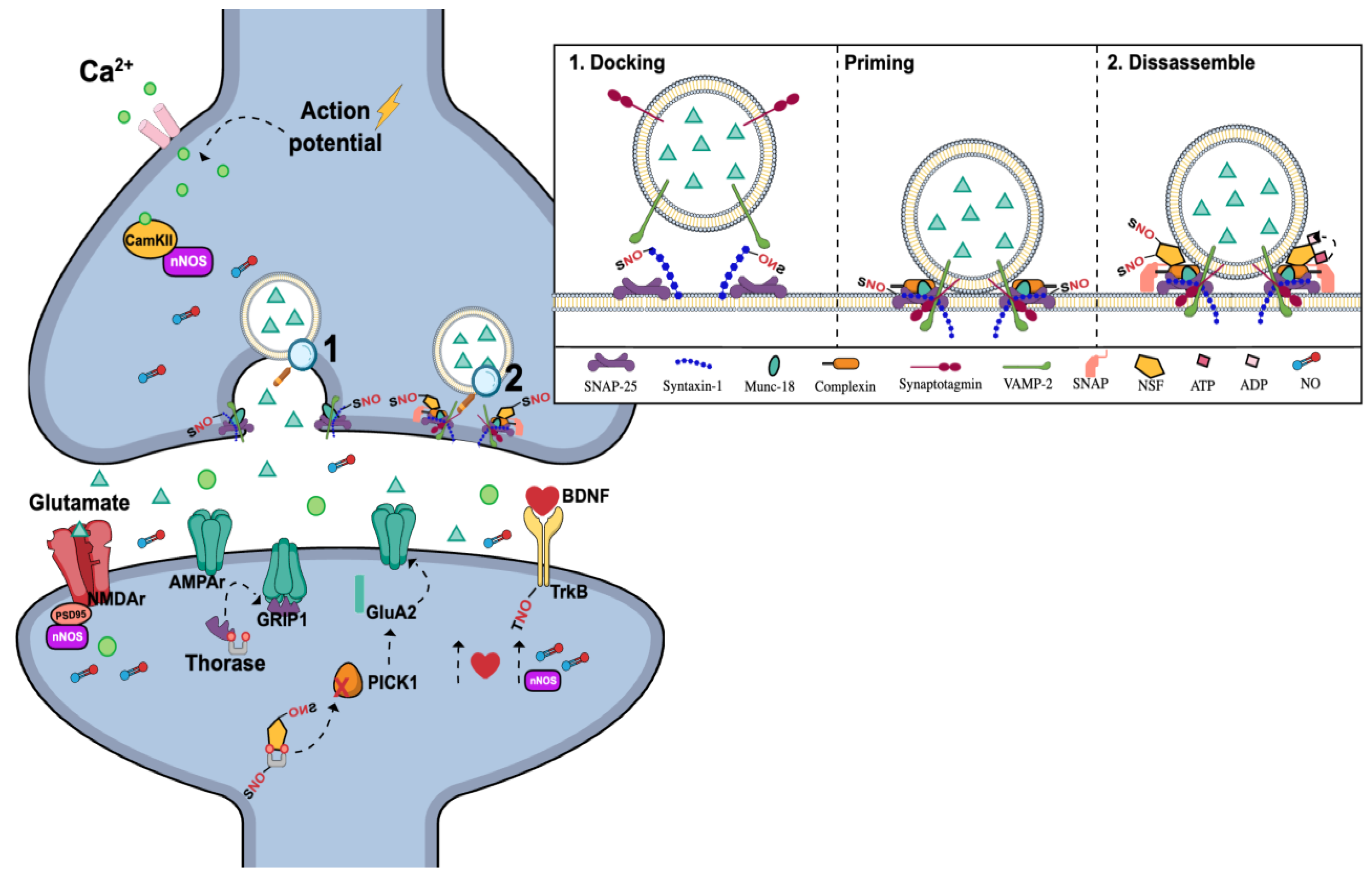
3.4. nNOS and the Regulation of Synaptic Transmission and Plasticity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Schaefke, B.; Wei, P.; Wang, L. Defensive responses: Behaviour, the brain and the body. Nat. Rev. Neurosci. 2023, 24, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.G.; Ressler, K.J. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 2013, 16, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Berretta, S.; Bolshakov, V.Y.; Rosso, I.M.; Meloni, E.G.; Rauch, S.L.; Carlezon, W.A. Post-traumatic stress disorder: Clinical and translational neuroscience from cells to circuits. Nat. Rev. Neurol. 2022, 18, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Staib, L.H.; Kaloupek, D.; Southwick, S.M.; Soufer, R.; Charney, D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol. Psychiatry 1999, 45, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Lanius, R.A.; Williamson, P.C.; Densmore, M.; Boksman, K.; Gupta, M.A.; Neufeld, R.W.; Gati, J.S.; Menon, R.S. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am. J. Psychiatry 2001, 158, 1920–1922. [Google Scholar] [CrossRef]
- Yan, Z.; Rein, B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: Pathophysiological implications. Mol. Psychiatry 2022, 27, 445–465. [Google Scholar] [CrossRef]
- Tovote, P.; Fadok, J.P.; Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015, 16, 317–331. [Google Scholar] [CrossRef]
- Susswein, A.J.; Katzoff, A.; Miller, N.; Hurwitz, I. Nitric oxide and memory. Neuroscientist 2004, 10, 153–162. [Google Scholar] [CrossRef]
- Guimarães, F.S.; Beijamini, V.; Moreira, F.A.; Aguiar, D.C.; de Lucca, A.C. Role of nitric oxide in brain regions related to defensive reactions. Neurosci. Biobehav. Rev. 2005, 29, 1313–1322. [Google Scholar] [CrossRef]
- McNeill, R.V.; Kehrwald, C.; Brum, M.; Knopf, K.; Brunkhorst-Kanaan, N.; Etyemez, S.; Koreny, C.; Bittner, R.A.; Freudenberg, F.; Herterich, S.; et al. Uncovering associations between mental illness diagnosis, nitric oxide synthase gene variation, and peripheral nitric oxide concentration. Brain Behav. Immun. 2022, 101, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.A.; Hemmati, S.; Nassireslami, E.; Yousefi Zoshk, M.; Hosseini, Y.; Abbasian, K.; Chamanara, M. Targeting neuronal nitric oxide synthase and the nitrergic system in post-traumatic stress disorder. Psychopharmacology 2022, 239, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Lawford, B.R.; Morris, C.P.; Swagell, C.D.; Hughes, I.P.; Young, R.M.; Voisey, J. NOS1AP is associated with increased severity of PTSD and depression in untreated combat veterans. J. Affect. Disord. 2013, 147, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Haaker, J.; Glotzbach-Schoon, E.; Schümann, D.; Andreatta, M.; Mechias, M.-L.; Raczka, K.; Gartmann, N.; Büchel, C.; Mühlberger, A.; et al. Converging evidence for an impact of a functional NOS gene variation on anxiety-related processes. Soc. Cogn. Affect. Neurosci. 2016, 11, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Bruenig, D.; Morris, C.P.; Mehta, D.; Harvey, W.; Lawford, B.; Young, R.M.; Voisey, J. Nitric oxide pathway genes (NOS1AP and NOS1) are involved in PTSD severity, depression, anxiety, stress and resilience. Gene 2017, 625, 42–48. [Google Scholar] [CrossRef]
- Harvey, B.H.; Bothma, T.; Nel, A.; Wegener, G.; Stein, D.J. Involvement of the NMDA receptor, NO-cyclic GMP and nuclear factor K-beta in an animal model of repeated trauma. Hum. Psychopharmacol. 2005, 20, 367–373. [Google Scholar] [CrossRef]
- Campos, A.C.; Piorino, E.M.; Ferreira, F.R.; Guimarães, F.S. Increased nitric oxide-mediated neurotransmission in the medial prefrontal cortex is associated with the long lasting anxiogenic-like effect of predator exposure. Behav. Brain Res. 2013, 256, 391–397. [Google Scholar] [CrossRef]
- Chong, P.S.; Poon, C.H.; Fung, M.L.; Guan, L.; Steinbusch, H.W.M.; Chan, Y.S.; Lim, W.L.; Lim, L.W. Distribution of neuronal nitric oxide synthase immunoreactivity in adult male Sprague-Dawley rat brain. Acta Histochem. 2019, 121, 151437. [Google Scholar] [CrossRef]
- Huang, P.L.; Dawson, T.M.; Bredt, D.S.; Snyder, S.H.; Fishman, M.C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 1993, 75, 1273–1286. [Google Scholar] [CrossRef]
- Dinerman, J.L.; Dawson, T.M.; Schell, M.J.; Snowman, A.; Snyder, S.H. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: Implications for synaptic plasticity. Proc. Natl. Acad. Sci. USA 1994, 91, 4214–4218. [Google Scholar] [CrossRef]
- Montezuma, K.; Biojone, C.; Lisboa, S.F.; Cunha, F.Q.; Guimarães, F.S.; Joca, S.R. Inhibition of iNOS induces antidepressant-like effects in mice: Pharmacological and genetic evidence. Neuropharmacology 2012, 62, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Nasyrova, R.F.; Moskaleva, P.V.; Vaiman, E.E.; Shnayder, N.A.; Blatt, N.L.; Rizvanov, A.A. Genetic Factors of Nitric Oxide’s System in Psychoneurologic Disorders. Int. J. Mol. Sci. 2020, 21, 1604. [Google Scholar] [CrossRef] [PubMed]
- Buskila, Y.; Abu-Ghanem, Y.; Levi, Y.; Moran, A.; Grauer, E.; Amitai, Y. Enhanced astrocytic nitric oxide production and neuronal modifications in the neocortex of a NOS2 mutant mouse. PLoS ONE 2007, 2, e843. [Google Scholar] [CrossRef] [PubMed]
- Olivenza, R.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Fernández, A.P.; Rodrigo, J.; Boscá, L.; Leza, J.C. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J. Neurochem. 2000, 74, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Castrillo, A.; Boscá, L.; Leza, J.C. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J. Neurochem. 2001, 76, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Gilhotra, N.; Dhingra, D. Involvement of NO-cGMP pathway in anti-anxiety effect of aminoguanidine in stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.A.; Vila-Verde, C.; Sartim, A.G.; Uliana, D.L.; Braga, L.A.; Guimarães, F.S.; Lisboa, S.F. Inducible Nitric Oxide Synthase Inhibition in the Medial Prefrontal Cortex Attenuates the Anxiogenic-Like Effect of Acute Restraint Stress via CB1 Receptors. Front. Psychiatry 2022, 13, 923177. [Google Scholar] [CrossRef]
- Harvey, B.H.; Oosthuizen, F.; Brand, L.; Wegener, G.; Stein, D.J. Stress–restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology 2004, 175, 494–502. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Andrew, P.J.; Mayer, B. Enzymatic function of nitric oxide synthases. Cardiovasc. Res. 1999, 43, 521–531. [Google Scholar] [CrossRef]
- Feng, C. Mechanism of Nitric Oxide Synthase Regulation: Electron Transfer and Interdomain Interactions. Coord. Chem. Rev. 2012, 256, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Salerno, J.C.; Harris, D.E.; Irizarry, K.; Patel, B.; Morales, A.J.; Smith, S.M.; Martasek, P.; Roman, L.J.; Masters, B.S.; Jones, C.L.; et al. An autoinhibitory control element defines calcium-regulated isoforms of nitric oxide synthase. J. Biol. Chem. 1997, 272, 29769–29777. [Google Scholar] [CrossRef] [PubMed]
- Marletta, M.A. Nitric oxide synthase: Function and mechanism. Adv. Exp. Med. Biol. 1993, 338, 281–284. [Google Scholar] [PubMed]
- Hosseini, N.; Kourosh-Arami, M.; Nadjafi, S.; Ashtari, B. Structure, Distribution, Regulation, and Function of Splice Variant Isoforms of Nitric Oxide Synthase Family in the Nervous System. Curr. Protein Pept. Sci. 2022, 23, 510–534. [Google Scholar] [CrossRef] [PubMed]
- Schuman, E.M.; Madison, D.V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science 1991, 254, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O.; Kiebler, M.; Lee, C.J.; Lev-Ram, V.; Tsien, R.Y.; Kandel, E.R.; Hawkins, R.D. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 1996, 87, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, N.; Dachtler, J.; Fox, K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front. Cell. Neurosci. 2013, 7, 190. [Google Scholar] [CrossRef]
- Ko, G.Y.; Kelly, P.T. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J. Neurosci. 1999, 19, 6784–6794. [Google Scholar] [CrossRef]
- Aso, Y.; Ray, R.P.; Long, X.; Bushey, D.; Cichewicz, K.; Ngo, T.-T.B.; Sharp, B.; Christoforou, C.; Hu, A.; Lemire, A.L.; et al. Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. eLife 2019, 8, e49257. [Google Scholar] [CrossRef]
- Burette, A.; Zabel, U.; Weinberg, R.J.; Schmidt, H.H.H.W.; Valtschanoff, J.G. Synaptic Localization of Nitric Oxide Synthase and Soluble Guanylyl Cyclase in the Hippocampus. J. Neurosci. 2002, 22, 8961–8970. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic. Biol. Med. 2002, 33, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011, 51, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Stamler, J.S. The SNO-proteome: Causation and classifications. Curr. Opin. Chem. Biol. 2011, 15, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef]
- Anand, P.; Stamler, J.S. Enzymatic mechanisms regulating protein S-nitrosylation: Implications in health and disease. J. Mol. Med. 2012, 90, 233–244. [Google Scholar] [CrossRef]
- Hughes, M.N. Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 1999, 1411, 263–272. [Google Scholar] [CrossRef]
- Seth, D.; Hess, D.T.; Hausladen, A.; Wang, L.; Wang, Y.J.; Stamler, J.S. A Multiplex Enzymatic Machinery for Cellular Protein S-nitrosylation. Mol. Cell 2018, 69, 451–464.e6. [Google Scholar] [CrossRef] [PubMed]
- Broniowska, K.A.; Keszler, A.; Basu, S.; Kim-Shapiro, D.B.; Hogg, N. Cytochrome c-mediated formation of S-nitrosothiol in cells. Biochem. J. 2012, 442, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Lipton, S.A. Nitric Oxide-Dependent Protein Post-Translational Modifications Impair Mitochondrial Function and Metabolism to Contribute to Neurodegenerative Diseases. Antioxid. Redox Signal. 2020, 32, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.T.; Stamler, J.S. Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 2012, 287, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.P.; Selvakumar, B.; Mukai, J.; Hester, L.D.; Wang, Y.; Gogos, J.A.; Snyder, S.H. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 2011, 71, 131–141. [Google Scholar] [CrossRef]
- Zareba-Koziol, M.; Bartkowiak-Kaczmarek, A.; Figiel, I.; Krzystyniak, A.; Wojtowicz, T.; Bijata, M.; Wlodarczyk, J. Stress-induced Changes in the S-palmitoylation and S-nitrosylation of Synaptic Proteins. Mol. Cell. Proteom. 2019, 18, 1916–1938. [Google Scholar] [CrossRef]
- Gao, X.; Hannoush, R.N. A Decade of Click Chemistry in Protein Palmitoylation: Impact on Discovery and New Biology. Cell Chem. Biol. 2018, 25, 236–246. [Google Scholar] [CrossRef]
- Gould, N.; Doulias, P.T.; Tenopoulou, M.; Raju, K.; Ischiropoulos, H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J. Biol. Chem. 2013, 288, 26473–26479. [Google Scholar] [CrossRef]
- Kalinina, E.; Novichkova, M. Glutathione in Protein Redox Modulation through S-Glutathionylation and S-Nitrosylation. Molecules 2021, 26, 435. [Google Scholar] [CrossRef]
- Mineka, S.; Oehlberg, K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 2008, 127, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.B.; Balda, M.A.; Anderson, K.L.; Itzhak, Y. Impairments in fear conditioning in mice lacking the nNOS gene. Learn. Mem. 2009, 16, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, H.-J.; Cao, B.; Kong, C.-C.; Yuan, F.; Li, J.; Ni, H.-Y.; Wu, H.-Y.; Chang, L.; Liu, Y.; et al. MGE-derived nNOS+ interneurons promote fear acquisition in nNOS−/− mice. Biochem. Biophys. Res. Commun. 2017, 493, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, S.F.; Gomes, F.V.; Silva, A.L.; Uliana, D.L.; Camargo, L.H.; Guimarães, F.S.; Cunha, F.Q.; Joca, S.R.; Resstel, L.B. Increased Contextual Fear Conditioning in iNOS Knockout Mice: Additional Evidence for the Involvement of Nitric Oxide in Stress-Related Disorders and Contribution of the Endocannabinoid System. Int. J. Neuropsychopharmacol. 2015, 18, pyv005. [Google Scholar] [CrossRef] [PubMed]
- Maren, S. Effects of 7-nitroindazole, a neuronal nitric oxide synthase (nNOS) inhibitor, on locomotor activity and contextual fear conditioning in rats. Brain Res. 1998, 804, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.B.; Anderson, K.L.; Itzhak, Y. Pharmacological modulators of nitric oxide signaling and contextual fear conditioning in mice. Psychopharmacology 2010, 210, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, M.; Wang, Y.; Wang, X.; Yuan, J.; Li, M. Effects of 7-nitroindazole, a selective neural nitric oxide synthase inhibitor, on context-shock associative learning in a two-process contextual fear conditioning paradigm. Neurobiol. Learn. Mem. 2016, 134, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Fabri, D.R.S.; Hott, S.C.; Reis, D.G.; Biojone, C.; Corrêa, F.M.A.; Resstel, L.B.M. The expression of contextual fear conditioning involves activation of a NMDA receptor-nitric oxide-cGMP pathway in the dorsal hippocampus of rats. Eur. Neuropsychopharmacol. 2014, 24, 1676–1686. [Google Scholar] [CrossRef]
- Resstel, L.B.; Corrêa, F.M.; Guimarães, F.S. The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb. Cortex 2008, 18, 2027–2035. [Google Scholar] [CrossRef]
- Cai, C.-Y.; Chen, C.; Zhou, Y.; Han, Z.; Qin, C.; Cao, B.; Tao, Y.; Bian, X.-L.; Lin, Y.-H.; Chang, L.; et al. PSD-95-nNOS Coupling Regulates Contextual Fear Extinction in the Dorsal CA3. Sci. Rep. 2018, 8, 12775. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Cao, B.; Cai, C.Y.; Lin, Y.H.; Li, F.; Wu, H.Y.; Chang, L.; Luo, C.X.; Zhu, D.Y. Disrupting nNOS-PSD-95 coupling in the hippocampal dentate gyrus promotes extinction memory retrieval. Biochem. Biophys. Res. Commun. 2017, 493, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tofigh, A.M.; Amirfakhraei, A.; Chen, X.; Tajik, M.; Xu, D.; Motevalli, S. Modulation of astrocyte activity and improvement of oxidative stress through blockage of NO/NMDAR pathway improve posttraumatic stress disorder (PTSD)-like behavior induced by social isolation stress. Brain Behav. 2022, 12, e2620. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Bian, X.L.; Cai, C.Y.; Chen, C.; Zhou, Y.; Lin, Y.H.; Tao, Y.; Wu, H.Y.; Chang, L.; Luo, C.X.; et al. Uncoupling nNOS-PSD-95 in the ACC can inhibit contextual fear generalization. Biochem. Biophys. Res. Commun. 2019, 513, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vinarskaya, A.K.; Zuzina, A.B.; Balaban, P.M. Nitric Oxide Is Required for Labilization (destabilization) of Contextual Memory in Rats. Neurosci. Behav. Physiol. 2021, 51, 1273–1277. [Google Scholar] [CrossRef]
- Song, S.; Lee, J.; Park, S.; Choi, S. Fear renewal requires nitric oxide signaling in the lateral amygdala. Biochem. Biophys. Res. Commun. 2020, 523, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Fox, M.F.; Vallance, P.; Leiper, J.M. Chromosomal localization, gene structure, and expression pattern of DDAH1: Comparison with DDAH2 and implications for evolutionary origins. Genomics 2000, 68, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Han, L.; Tian, S. Effect of nitric oxide synthase inhibitor l-NAME on fear extinction in rats: A task-dependent effect. Neurosci. Lett. 2014, 572, 13–18. [Google Scholar] [CrossRef]
- Bros, M.; Boissel, J.P.; Gödtel-Armbrust, U.; Förstermann, U. Transcription of human neuronal nitric oxide synthase mRNAs derived from different first exons is partly controlled by exon 1-specific promoter sequences. Genomics 2006, 87, 463–473. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Fang, M.; Snyder, S.H. Nitrosopeptide mapping: A novel methodology reveals s-nitrosylation of dexras1 on a single cysteine residue. Chem. Biol. 2002, 9, 1329–1335. [Google Scholar] [CrossRef]
- Brenman, J.E.; Chao, D.S.; Gee, S.H.; McGee, A.W.; Craven, S.E.; Santillano, D.R.; Wu, Z.; Huang, F.; Xia, H.; Peters, M.F.; et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 1996, 84, 757–767. [Google Scholar] [CrossRef]
- Pedersen, S.W.; Albertsen, L.; Moran, G.E.; Levesque, B.; Pedersen, S.B.; Bartels, L.; Wapenaar, H.; Ye, F.; Zhang, M.; Bowen, M.E.; et al. Site-Specific Phosphorylation of PSD-95 PDZ Domains Reveals Fine-Tuned Regulation of Protein-Protein Interactions. ACS Chem. Biol. 2017, 12, 2313–2323. [Google Scholar] [CrossRef]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.F.; Tymianski, M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science 1999, 284, 1845–1848. [Google Scholar] [CrossRef]
- Courtney, M.J.; Li, L.L.; Lai, Y.Y. Mechanisms of NOS1AP action on NMDA receptor-nNOS signaling. Front. Cell. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef]
- Chachlaki, K.; Prevot, V. Nitric oxide signalling in the brain and its control of bodily functions. Br. J. Pharmacol. 2020, 177, 5437–5458. [Google Scholar] [CrossRef]
- Murphy, S.; Coughlan, T. Nitric Oxide Synthases in Brain Function. In Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides; Lajtha, A., Lim, R., Eds.; Springer US: Boston, MA, USA, 2006; pp. 223–247. [Google Scholar]
- Li, L.P.; Dustrude, E.T.; Haulcomb, M.M.; Abreu, A.R.; Fitz, S.D.; Johnson, P.L.; Thakur, G.A.; Molosh, A.I.; Lai, Y.; Shekhar, A. PSD95 and nNOS interaction as a novel molecular target to modulate conditioned fear: Relevance to PTSD. Transl. Psychiatry 2018, 8, 155. [Google Scholar] [CrossRef]
- Gräff, J.; Joseph, N.F.; Horn, M.E.; Samiei, A.; Meng, J.; Seo, J.; Rei, D.; Bero, A.W.; Phan, T.X.; Wagner, F.; et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 2014, 156, 261–276. [Google Scholar] [CrossRef]
- Matsuda, S.; Matsuzawa, D.; Nakazawa, K.; Sutoh, C.; Ohtsuka, H.; Ishii, D.; Tomizawa, H.; Iyo, M.; Shimizu, E. d-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 895–902. [Google Scholar] [CrossRef]
- Zhu, L.J.; Li, T.Y.; Luo, C.X.; Jiang, N.; Chang, L.; Lin, Y.H.; Zhou, H.H.; Chen, C.; Zhang, Y.; Lu, W.; et al. CAPON-nNOS coupling can serve as a target for developing new anxiolytics. Nat. Med. 2014, 20, 1050–1054. [Google Scholar] [CrossRef]
- Qin, C.; Bian, X.-L.; Wu, H.-Y.; Xian, J.-Y.; Lin, Y.-H.; Cai, C.-Y.; Zhou, Y.; Kou, X.-L.; Li, T.-Y.; Chang, L.; et al. Prevention of the return of extinguished fear by disrupting the interaction of neuronal nitric oxide synthase with its carboxy-terminal PDZ ligand. Mol. Psychiatry 2021, 26, 6506–6519. [Google Scholar] [CrossRef]
- Fang, M.; Jaffrey, S.R.; Sawa, A.; Ye, K.; Luo, X.; Snyder, S.H. Dexras1: A G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron 2000, 28, 183–193. [Google Scholar] [CrossRef]
- Richier, L.; Williton, K.; Clattenburg, L.; Colwill, K.; O’Brien, M.; Tsang, C.; Kolar, A.; Zinck, N.; Metalnikov, P.; Trimble, W.S.; et al. NOS1AP associates with Scribble and regulates dendritic spine development. J. Neurosci. 2010, 30, 4796–4805. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Snyder, S.H. PIN: An associated protein inhibitor of neuronal nitric oxide synthase. Science 1996, 274, 774–777. [Google Scholar] [CrossRef]
- Fan, J.S.; Zhang, Q.; Li, M.; Tochio, H.; Yamazaki, T.; Shimizu, M.; Zhang, M. Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme. J. Biol. Chem. 1998, 273, 33472–33481. [Google Scholar] [CrossRef]
- MacAllister, R.J.; Parry, H.; Kimoto, M.; Ogawa, T.; Russell, R.J.; Hodson, H.; Whitley, G.S.; Vallance, P. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br. J. Pharmacol. 1996, 119, 1533–1540. [Google Scholar] [CrossRef]
- Toth, J.; Racz, A.; Kaminski, P.M.; Wolin, M.S.; Bagi, Z.; Koller, A. Asymmetrical dimethylarginine inhibits shear stress-induced nitric oxide release and dilation and elicits superoxide-mediated increase in arteriolar tone. Hypertension 2007, 49, 563–568. [Google Scholar] [CrossRef]
- Rivier, C. Blockade of nitric oxide formation augments adrenocorticotropin released by blood-borne interleukin-1 beta: Role of vasopressin, prostaglandins, and alpha 1-adrenergic receptors. Endocrinology 1995, 136, 3597–3603. [Google Scholar] [CrossRef]
- Lee, M.; Schwab, C.; McGeer, P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011, 59, 152–165. [Google Scholar] [CrossRef]
- Kim, C.K.; Rivier, C.L. Nitric Oxide and Carbon Monoxide Have a Stimulatory Role in the Hypothalamic-Pituitary-Adrenal Response to Physico-Emotional Stressors in Rats. Endocrinology 2000, 141, 2244–2253. [Google Scholar] [CrossRef]
- Vila-Verde, C.; Marinho, A.L.; Lisboa, S.F.; Guimarães, F.S. Nitric oxide in the prelimbic medial prefrontal cortex is involved in the anxiogenic-like effect induced by acute restraint stress in rats. Neuroscience 2016, 320, 30–42. [Google Scholar] [CrossRef]
- Sevgi, S.; Ozek, M.; Eroglu, L. L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 95–99. [Google Scholar] [CrossRef]
- Kaur, R.; Jaggi, A.S.; Bali, A. Investigating the role of nitric oxide in stress adaptive process in electric foot shock stress-subjected mice. Int. J. Neurosci. 2021, 131, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.G.; Hu, Y.; Hua, Y.; Hu, M.; Luo, C.X.; Han, X.; Zhu, X.J.; Wang, B.; Xu, J.S.; Zhu, D.Y. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J. Neurochem. 2007, 103, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Huang, S.Y.; Gao, L.; Lin, Y.H.; Chang, L.; Wu, H.Y.; Zhu, D.Y.; Luo, C.X. Neuronal Nitric Oxide Synthase in Nucleus Accumbens Specifically Mediates Susceptibility to Social Defeat Stress through Cyclin-Dependent Kinase 5. J. Neurosci. 2021, 41, 2523–2539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.G.; Zhu, L.J.; Chen, C.; Wu, H.Y.; Luo, C.X.; Chang, L.; Zhu, D.Y. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J. Neurosci. 2011, 31, 7579–7590. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, M.B.; Guimarães, F.S.; Del Bel, E.A. Acute and delayed restraint stress-induced changes in nitric oxide producing neurons in limbic regions. Neuroscience 2004, 125, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Spiers, J.G.; Sernia, C.; Lavidis, N.A. Acute restraint stress induces specific changes in nitric oxide production and inflammatory markers in the rat hippocampus and striatum. Free Radic. Biol. Med. 2016, 90, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.J.; Lee, J.K.; Sernia, C.; Lavidis, N.A. Neuronal and inducible nitric oxide synthase upregulation in the rat medial prefrontal cortex following acute restraint stress: A dataset. Data Brief 2016, 6, 582–586. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Meijer, O.C.; de Nicola, A.F.; de Rijk, R.H.; Joëls, M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocr. 2018, 49, 124–145. [Google Scholar] [CrossRef]
- de Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat. Rev. Neurosci. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Maples-Keller, J.L.; Yasinski, C.; Manjin, N.; Rothbaum, B.O. Virtual Reality-Enhanced Extinction of Phobias and Post-Traumatic Stress. Neurotherapeutics 2017, 14, 554–563. [Google Scholar] [CrossRef]
- Shahani, N.; Sawa, A. Nitric oxide signaling and nitrosative stress in neurons: Role for S-nitrosylation. Antioxid. Redox Signal. 2011, 14, 1493–1504. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases. Trends Pharmacol. Sci. 2016, 37, 73–84. [Google Scholar] [CrossRef]
- Bradley, S.A.; Steinert, J.R. Nitric Oxide-Mediated Posttranslational Modifications: Impacts at the Synapse. Oxid. Med. Cell. Longev. 2016, 2016, 5681036. [Google Scholar] [CrossRef]
- Marozkina, N.V.; Yemen, S.; Borowitz, M.; Liu, L.; Plapp, M.; Sun, F.; Islam, R.; Erdmann-Gilmore, P.; Townsend, R.R.; Lichti, C.F.; et al. Hsp 70/Hsp 90 organizing protein as a nitrosylation target in cystic fibrosis therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 11393–11398. [Google Scholar] [CrossRef]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef]
- Kemppainen, R.J.; Behrend, E.N. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J. Biol. Chem. 1998, 273, 3129–3131. [Google Scholar] [CrossRef]
- Cheah, J.H.; Kim, S.F.; Hester, L.D.; Clancy, K.W.; Patterson, S.E., 3rd; Papadopoulos, V.; Snyder, S.H. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 2006, 51, 431–440. [Google Scholar] [CrossRef]
- Chen, Y.; Khan, R.S.; Cwanger, A.; Song, Y.; Steenstra, C.; Bang, S.; Cheah, J.H.; Dunaief, J.; Shindler, K.S.; Snyder, S.H.; et al. Dexras1, a small GTPase, is required for glutamate-NMDA neurotoxicity. J. Neurosci. 2013, 33, 3582–3587. [Google Scholar] [CrossRef]
- White, R.S.; Bhattacharya, A.K.; Chen, Y.; Byrd, M.; McMullen, M.F.; Siegel, S.J.; Carlson, G.C.; Kim, S.F. Lysosomal iron modulates NMDA receptor-mediated excitation via small GTPase, Dexras1. Mol. Brain 2016, 9, 38. [Google Scholar] [CrossRef]
- Huang, Y.; Man, H.Y.; Sekine-Aizawa, Y.; Han, Y.; Juluri, K.; Luo, H.; Cheah, J.; Lowenstein, C.; Huganir, R.L.; Snyder, S.H. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron 2005, 46, 533–540. [Google Scholar] [CrossRef]
- Yu, X.M.; Askalan, R.; Keil, G.J., 2nd; Salter, M.W. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 1997, 275, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Pitcher, G.M.; Pelkey, K.A.; Salter, M.W. PSD-95 is a negative regulator of the tyrosine kinase Src in the NMDA receptor complex. Embo J. 2006, 25, 4971–4982. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010, 90, 419–463. [Google Scholar] [CrossRef] [PubMed]
- Averill, L.A.; Purohit, P.; Averill, C.L.; Boesl, M.A.; Krystal, J.H.; Abdallah, C.G. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci. Lett. 2017, 649, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Schafe, G.E.; Atkins, C.M.; Swank, M.W.; Bauer, E.P.; Sweatt, J.D.; LeDoux, J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 2000, 20, 8177–8187. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, J.S.; Barea-Rodriguez, E.J. ERK phosphorylation is required for retention of trace fear memory. Neurobiol. Learn. Mem. 2006, 85, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Chen, Z.G.; Liu, W.T.; Chi, Z.Q.; He, L.; Liu, J.G. Dorsal hippocampal NMDA receptor blockade impairs extinction of naloxone-precipitated conditioned place aversion in acute morphine-treated rats by suppressing ERK and CREB phosphorylation in the basolateral amygdala. Br. J. Pharmacol. 2015, 172, 482–491. [Google Scholar] [CrossRef]
- Li, J.; Tong, L.; Schock, B.C.; Ji, L.-L. Post-traumatic Stress Disorder: Focus on Neuroinflammation. Mol. Neurobiol. 2023, 60, 3963–3978. [Google Scholar] [CrossRef]
- Shanazz, K.; Nalloor, R.; Lucas, R.; Vazdarjanova, A. Neuroinflammation is a susceptibility factor in developing a PTSD-like phenotype. Front. Behav. Neurosci. 2023, 17, 1112837. [Google Scholar] [CrossRef]
- Tursich, M.; Neufeld, R.W.; Frewen, P.A.; Harricharan, S.; Kibler, J.L.; Rhind, S.G.; Lanius, R.A. Association of trauma exposure with proinflammatory activity: A transdiagnostic meta-analysis. Transl. Psychiatry 2014, 4, e413. [Google Scholar] [CrossRef]
- Baker, D.G.; Ekhator, N.N.; Kasckow, J.W.; Hill, K.K.; Zoumakis, E.; Dashevsky, B.A.; Chrousos, G.P.; Geracioti, T.D., Jr. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 2001, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav. Immun. 2013, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maslanik, T.; Mahaffey, L.; Tannura, K.; Beninson, L.; Greenwood, B.N.; Fleshner, M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav. Immun. 2013, 28, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Meir, T.; Klein, E.; Volpin, G.; Assaf, M.; Pollack, S. Cytokine Levels as Potential Biomarkers for Predicting the Development of Posttraumatic Stress Symptoms in Casualties of Accidents. Int. J. Psychiatry Med. 2011, 42, 117–131. [Google Scholar] [CrossRef]
- Eraly, S.A.; Nievergelt, C.M.; Maihofer, A.X.; Barkauskas, D.A.; Biswas, N.; Agorastos, A.; O’Connor, D.T.; Baker, D.G.; Marine Resiliency Study Team. Assessment of Plasma C-Reactive Protein as a Biomarker of Posttraumatic Stress Disorder Risk. JAMA Psychiatry 2014, 71, 423–431. [Google Scholar] [CrossRef]
- Katrinli, S.; Oliveira, N.C.S.; Felger, J.C.; Michopoulos, V.; Smith, A.K. The role of the immune system in posttraumatic stress disorder. Transl. Psychiatry 2022, 12, 313. [Google Scholar] [CrossRef]
- Enomoto, S.; Kato, T.A. Involvement of microglia in disturbed fear memory regulation: Possible microglial contribution to the pathophysiology of posttraumatic stress disorder. Neurochem. Int. 2021, 142, 104921. [Google Scholar] [CrossRef]
- Li, S.; Liao, Y.; Dong, Y.; Li, X.; Li, J.; Cheng, Y.; Cheng, J.; Yuan, Z. Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice. J. Neuroinflammation 2021, 18, 7. [Google Scholar] [CrossRef]
- Carrillo-de Sauvage, M.; Maatouk, L.; Arnoux, I.; Pasco, M.; Sanz Diez, A.; Delahaye, M.; Herrero, M.T.; Newman, T.A.; Calvo, C.F.; Audinat, E.; et al. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 2013, 20, 1546–1557. [Google Scholar] [CrossRef]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav. Immun. 2015, 48, 1–7. [Google Scholar] [CrossRef]
- Frank, M.G.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress induction of the alarmin HMGB1 and reduction of the microglia checkpoint receptor CD200R1 in limbic brain structures. Brain Behav. Immun. 2019, 80, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, B.; Yang, J.; Lian, Y.-J.; Yu, H.-Z.; Wang, Y.-X. HMGB1 in depression: An overview of microglial HMBG1 in the pathogenesis of depression. Brain Behav. Immun.—Health 2023, 30, 100641. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.Y.; Pribis, J.P.; Lotze, M.; Mollen, K.P.; Shapiro, R.; Loughran, P.; Scott, M.J.; Billiar, T.R. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol. Med. 2013, 19, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lian, Y.J.; Su, W.J.; Peng, W.; Dong, X.; Liu, L.L.; Gong, H.; Zhang, T.; Jiang, C.L.; Wang, Y.X. HMGB1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav. Immun. 2018, 72, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, S.; Tomohiro, A.; Ukeshima, S.; Liu, K.; Wake, H.; Kimura, S.H.; Yamamoto, Y.; Nishibori, M.; Furuyashiki, T. Repeated Social Defeat Stress Induces HMGB1 Nuclear Export in Prefrontal Neurons, Leading to Social Avoidance in Mice. Cells 2023, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Karki, A.; Du, D.Y.; Zhao, X.J.; Xiang, X.Y.; Lu, Z.Q. Plasma levels of high mobility group box 1 increase in patients with posttraumatic stress disorder after severe blunt chest trauma: A prospective cohort study. J. Surg. Res. 2015, 193, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, Y.; Li, H.; Huang, W.; Tu, D.; Yang, M.; Liu, X.; Hong, J.S.; Gao, H.M. Posttranslational S-nitrosylation modification regulates HMGB1 secretion and promotes its proinflammatory and neurodegenerative effects. Cell Rep. 2022, 40, 111330. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Kim, J.; Won, J.S.; Singh, A.K.; Sharma, A.K.; Singh, I. STAT3 regulation by S-nitrosylation: Implication for inflammatory disease. Antioxid. Redox Signal. 2014, 20, 2514–2527. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Toki, Y.; Takenouchi, T.; Harada, H.; Tanuma, S.-i.; Kitani, H.; Kojima, S.; Tsukimoto, M. Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1β, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochem. Biophys. Res. Commun. 2015, 458, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.E.; Roncalho, A.L.; Glaser, T.; Ulrich, H.; Wegener, G.; Joca, S. P2X7 Receptor Signaling in Stress and Depression. Int. J. Mol. Sci. 2019, 20, 2778. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, O.; Rivera-Escobales, Y.; Castillo-Ocampo, Y.; Velazquez, B.; Colón, M.; Porter, J.T. Purinergic P2X7 receptor-mediated inflammation precedes PTSD-related behaviors in rats. Brain Behav. Immun. 2023, 110, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.T.; Cox, A.J.; Voigt, M.M. Molecular Structure of P2X Receptors. Curr. Top. Med. Chem. 2004, 4, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Jindrichova, M.; Kuzyk, P.; Li, S.; Stojilkovic, S.S.; Zemkova, H. Conserved ectodomain cysteines are essential for rat P2X7 receptor trafficking. Purinergic Signal. 2012, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Saunders, B.M.; Petrou, S.; Wiley, J.S. P2X7 Is a Scavenger Receptor for Apoptotic Cells in the Absence of Its Ligand, Extracellular ATP. J. Immunol. 2011, 187, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Kim, J.-E. Protein disulfide isomerase-mediated S-nitrosylation facilitates surface expression of P2X7 receptor following status epilepticus. J. Neuroinflammation 2021, 18, 14. [Google Scholar] [CrossRef]
- Pereira, V.S.; Casarotto, P.C.; Hiroaki-Sato, V.A.; Sartim, A.G.; Guimarães, F.S.; Joca, S.R.L. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: Involvement of nitric oxide. Eur. Neuropsychopharmacol. 2013, 23, 1769–1778. [Google Scholar] [CrossRef]
- Tian, J.; Kim, S.F.; Hester, L.; Snyder, S.H. S-nitrosylation/activation of COX-2 mediates NMDA neurotoxicity. Proc. Natl. Acad. Sci. USA 2008, 105, 10537–10540. [Google Scholar] [CrossRef]
- Chen, C. COX-2’s new role in inflammation. Nat. Chem. Biol. 2010, 6, 401–402. [Google Scholar] [CrossRef]
- Kim, S.F.; Huri, D.A.; Snyder, S.H. Inducible Nitric Oxide Synthase Binds, S-Nitrosylates, and Activates Cyclooxygenase-2. Science 2005, 310, 1966–1970. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Duan, F.; Wu, J.; Min, Q.; Huang, Q.; Luo, M.; He, Z. Effect of cyclooxygenase-2 inhibition on the development of post-traumatic stress disorder in rats. Mol. Med. Rep. 2018, 17, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Gamble-George, J.C.; Baldi, R.; Halladay, L.; Kocharian, A.; Hartley, N.; Silva, C.G.; Roberts, H.; Haymer, A.; Marnett, L.J.; Holmes, A.; et al. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. Elife 2016, 5, e14137. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.E.; Hess, D.T.; Stamler, J.S. S-nitrosylation: Physiological regulation of NF-kappaB. Proc. Natl. Acad. Sci. USA 2004, 101, 8841–8842. [Google Scholar] [CrossRef] [PubMed]
- Lander, H.M.; Ogiste, J.S.; Pearce, S.F.A.; Levi, R.; Novogrodsky, A. Nitric Oxide-stimulated Guanine Nucleotide Exchange on p21ras. J. Biol. Chem. 1995, 270, 7017–7020. [Google Scholar] [CrossRef] [PubMed]
- Lander, H.M.; Hajjar, D.P.; Hempstead, B.L.; Mirza, U.A.; Chait, B.T.; Campbell, S.; Quilliam, L.A. A Molecular Redox Switch on p21ras: STRUCTURAL BASIS FOR THE NITRIC OXIDE-p21ras INTERACTION. J. Biol. Chem. 1997, 272, 4323–4326. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci. Signal. 2014, 7, ra106. [Google Scholar] [CrossRef]
- Kelleher, Z.T.; Matsumoto, A.; Stamler, J.S.; Marshall, H.E. NOS2 Regulation of NF-κB by S-Nitrosylation of p65. J. Biol. Chem. 2007, 282, 30667–30672. [Google Scholar] [CrossRef]
- Reynaert, N.L.; Ckless, K.; Korn, S.H.; Vos, N.; Guala, A.S.; Wouters, E.F.; van der Vliet, A.; Janssen-Heininger, Y.M. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc. Natl. Acad. Sci. USA 2004, 101, 8945–8950. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Ni, H.-Y.; Chen, R.; Chang, L.; Shi, H.-J.; Qiu, D.; Zhang, Z.; Wu, D.-L.; Jiang, Z.-C.; Xin, H.-L.; et al. Hippocampal nuclear factor kappa B accounts for stress-induced anxiety behaviors via enhancing neuronal nitric oxide synthase (nNOS)-carboxy-terminal PDZ ligand of nNOS-Dexras1 coupling. J. Neurochem. 2018, 146, 598–612. [Google Scholar] [CrossRef]
- Si, J.; Yang, J.; Xue, L.; Yang, C.; Luo, Y.; Shi, H.; Lu, L. Activation of NF-κB in basolateral amygdala is required for memory reconsolidation in auditory fear conditioning. PLoS ONE 2012, 7, e43973. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mohammed, Z.; Singh, I. Bruton’s tyrosine kinase drives neuroinflammation and anxiogenic behavior in mouse models of stress. J. Neuroinflammation 2021, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Fang, M.; Ye, S.; Fang, Z.; Amin, N.; Chen, Y. Fluoxetine protects against inflammation and promotes autophagy in mice model of post-traumatic stress disorder. Behav. Brain Res. 2022, 433, 114004. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, H.; Zhang, R.; Chen, Y.; Xue, F.; Nie, H.; Chen, Y.; Wu, D.; Wang, Y.; Wang, H.; et al. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol. Res. 2013, 62, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Smith, D.E.; Ibáñez-Sandoval, O.; Sims, J.E.; Friedman, W.J. Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein. J. Neurosci. 2011, 31, 18048–18059. [Google Scholar] [CrossRef]
- Navarrete, M.; Cuartero, M.I.; Palenzuela, R.; Draffin, J.E.; Konomi, A.; Serra, I.; Colié, S.; Castaño-Castaño, S.; Hasan, M.T.; Nebreda, Á.R.; et al. Astrocytic p38α MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nat. Commun. 2019, 10, 2968. [Google Scholar] [CrossRef]
- Young, M.B.; Howell, L.L.; Hopkins, L.; Moshfegh, C.; Yu, Z.; Clubb, L.; Seidenberg, J.; Park, J.; Swiercz, A.P.; Marvar, P.J. A peripheral immune response to remembering trauma contributes to the maintenance of fear memory in mice. Psychoneuroendocrinology 2018, 94, 143–151. [Google Scholar] [CrossRef]
- Bersani, F.S.; Wolkowitz, O.M.; Lindqvist, D.; Yehuda, R.; Flory, J.; Bierer, L.M.; Makotine, I.; Abu-Amara, D.; Coy, M.; Reus, V.I.; et al. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav. Immun. 2016, 52, 153–160. [Google Scholar] [CrossRef]
- Monsour, M.; Croci, D.M.; Agazzi, S. The role of IL-6 in TBI and PTSD, a potential therapeutic target? Clin. Neurol. Neurosurg. 2022, 218, 107280. [Google Scholar] [CrossRef]
- Rossato, J.I.; Bevilaqua, L.R.M.; Lima, R.H.; Medina, J.H.; Izquierdo, I.; Cammarota, M. On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience 2006, 143, 15–23. [Google Scholar] [CrossRef]
- Alonso, M.; Bevilaqua, L.R.M.; Izquierdo, I.; Medina, J.H.; Cammarota, M. Memory formation requires p38MAPK activity in the rat hippocampus. NeuroReport 2003, 14, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.C.; Xu, C.; Duman, R.S. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav. Immun. 2018, 72, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, E.; Daskalakis, N.P.; Agorastos, A. Oxidative Dysregulation in Early Life Stress and Posttraumatic Stress Disorder: A Comprehensive Review. Brain Sci. 2021, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Galkina, O.V. The specific features of free-radical processes and the antioxidant defense in the adult brain. Neurochem. J. 2013, 7, 89–97. [Google Scholar] [CrossRef]
- Reed, E.C.; Case, A.J. Defining the nuanced nature of redox biology in post-traumatic stress disorder. Front. Physiol. 2023, 14, 1130861. [Google Scholar] [CrossRef] [PubMed]
- Peruzzolo, T.L.; Pinto, J.V.; Roza, T.H.; Shintani, A.O.; Anzolin, A.P.; Gnielka, V.; Kohmann, A.M.; Marin, A.S.; Lorenzon, V.R.; Brunoni, A.R.; et al. Inflammatory and oxidative stress markers in post-traumatic stress disorder: A systematic review and meta-analysis. Mol. Psychiatry 2022, 27, 3150–3163. [Google Scholar] [CrossRef]
- Nathan, C. The Moving Frontier in Nitric Oxide–Dependent Signaling. Sci. STKE 2004, 2004, pe52. [Google Scholar] [CrossRef]
- Corpas, F.J. Reactive Nitrogen Species (RNS) in Plants Under Physiological and Adverse Environmental Conditions: Current View. In Progress in Botany Vol. 78; Cánovas, F.M., Lüttge, U., Matyssek, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 97–119. [Google Scholar]
- Martínez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Cotgreave, I.A.; Weis, M.; Berggren, M.; Sandy, M.S.; Moldéus, P.W. Determination of the intracellular protein thiol distribution of hepatocytes using monobromobimane derivatisation of intact cells and isolated subcellular fractions. J. Biochem. Biophys. Methods 1988, 16, 247–254. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls: The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Rosso, I.M.; Crowley, D.J.; Silveri, M.M.; Rauch, S.L.; Jensen, J.E. Hippocampus Glutamate and N-Acetyl Aspartate Markers of Excitotoxic Neuronal Compromise in Posttraumatic Stress Disorder. Neuropsychopharmacology 2017, 42, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Maulik, D.; Ashraf, Q.M.; Delivoria-Papadopoulos, M. Nitration of N-methyl-D-aspartate receptor subunits following in vitro dephosphorylation of cerebral cortical membranes of newborn piglets. Neurosci. Lett. 2002, 317, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, S.A.; Ashraf, Q.M.; Delivoria-Papadopoulos, M.; Mishra, O.P. Peroxynitrite-induced modification of the N-methyl-d-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci. Lett. 2000, 296, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Broniowska, K.A.; Hogg, N. The chemical biology of S-nitrosothiols. Antioxid. Redox Signal. 2012, 17, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Dahm, C.C.; Moore, K.; Murphy, M.P. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: Implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 2006, 281, 10056–10065. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, S.; Sugita, H.; Takamori, H.; Horino, K.; Nakahara, O.; Okabe, H.; Miyake, K.; Tanaka, H.; Beppu, T.; Baba, H. NO donor and MEK inhibitor synergistically inhibit proliferation and invasion of cancer cells. Int. J. Oncol. 2012, 40, 807–815. [Google Scholar] [CrossRef]
- Michels, L.; Schulte-Vels, T.; Schick, M.; O’Gorman, R.L.; Zeffiro, T.; Hasler, G.; Mueller-Pfeiffer, C. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Res. 2014, 224, 288–295. [Google Scholar] [CrossRef]
- Duffy, S.L.; Lagopoulos, J.; Cockayne, N.; Hermens, D.F.; Hickie, I.B.; Naismith, S.L. Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. J. Affect. Disord. 2015, 180, 29–35. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ogłodek, E.A. Changes in the concentrations of inflammatory and oxidative status biomediators (MIP-1 α, PMN elastase, MDA, and IL-12) in depressed patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2018, 70, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; de Castro Gomes, V.; Pinton, S.; Batista Teixeira da Rocha, J.; Landeira-Fernandez, J. Association between oxidative stress and contextual fear conditioning in Carioca high- and low-conditioned freezing rats. Brain Res. 2013, 1512, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yermilov, V.; Rubio, J.; Ohshima, H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995, 376, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Alhasawi, A.; Legendre, F.; Jagadeesan, S.; Appanna, V.; Appanna, V. Chapter 10—Biochemical Strategies to Counter Nitrosative Stress: Nanofactories for Value-Added Products. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 153–169. [Google Scholar]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front. Physiol. 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Kasamatsu, S.; Yanai, S.; Endo, S.; Akaike, T.; Ihara, H. 8-Nitro-cGMP attenuates context-dependent fear memory in mice. Biochem. Biophys. Res. Commun. 2019, 511, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Bombeck, C.A.; Billiar, T.R. Nitric Oxide as a Bifunctional Regulator of Apoptosis. Circ. Res. 1999, 84, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Y.; Wang, J.; Liu, Y.; Xiao, M.; Song, C.; Bai, Y.; Yinuo Han, N.; Han, F. Synapse impairment associated with enhanced apoptosis in post-traumatic stress disorder. Synapse 2020, 74, e22134. [Google Scholar] [CrossRef]
- Li-Li, L.; Vanessa, G.; Xiaonan, L.; Olga, V.; Minna, T.; Marc, M.; Christophe, B.; Julien, P.; Anita, C.T.; Michael, J.C. The nNOS-p38MAPK Pathway Is Mediated by NOS1AP during Neuronal Death. J. Neurosci. 2013, 33, 8185. [Google Scholar] [CrossRef]
- Brüne, B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003, 10, 864–869. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ju, J.-W.; Oh, C.-D.; Yoon, Y.-M.; Song, W.K.; Kim, J.-H.; Yoo, Y.J.; Bang, O.-S.; Kang, S.-S.; Chun, J.-S. ERK-1/2 and p38 Kinase Oppositely Regulate Nitric Oxide-induced Apoptosis of Chondrocytes in Association with p53, Caspase-3, and Differentiation Status. J. Biol. Chem. 2002, 277, 1332–1339. [Google Scholar] [CrossRef]
- Wang, X.; Zalcenstein, A.; Oren, M. Nitric oxide promotes p53 nuclear retention and sensitizes neuroblastoma cells to apoptosis by ionizing radiation. Cell Death Differ. 2003, 10, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X. Cytochrome C-Mediated Apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.S.; Cadenas, E. Nitric Oxide and Cell Signaling Pathways in Mitochondrial-Dependent Apoptosis. Biol. Chem. 2002, 383, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Han, F.; Liu, D.J.; Shi, Y.X. Single-prolonged stress induced mitochondrial-dependent apoptosis in hippocampus in the rat model of post-traumatic stress disorder. J. Chem. Neuroanat. 2010, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Fenster, R.J.; Lebois, L.A.M.; Ressler, K.J.; Suh, J. Brain circuit dysfunction in post-traumatic stress disorder: From mouse to man. Nat. Rev. Neurosci. 2018, 19, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Hausladen, A.; Liu, L.; Hess, D.T.; Zeng, M.; Miao, Q.X.; Kane, L.S.; Gow, A.J.; Stamler, J.S. Fas-Induced Caspase Denitrosylation. Science 1999, 284, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Wang, L.; Wong, C.C.L.; Scott, F.L.; Eckelman, B.P.; Han, X.; Tzitzilonis, C.; Meng, F.; Gu, Z.; Holland, E.A.; et al. Transnitrosylation of XIAP Regulates Caspase-Dependent Neuronal Cell Death. Mol. Cell 2010, 39, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, N.; Ma, B.; Wang, Y.; Zhang, G.; Yan, X.; Hu, S.; Xu, T. Procaspase-9 induces its cleavage by transnitrosylating XIAP via the Thioredoxin system during cerebral ischemia-reperfusion in rats. Sci. Rep. 2016, 6, 24203. [Google Scholar] [CrossRef]
- Espey, M.G.; Miranda, K.M.; Feelisch, M.; Fukuto, J.O.N.; Grisham, M.B.; Vitek, M.P.; Wink, D.A. Mechanisms of Cell Death Governed by the Balance between Nitrosative and Oxidative Stress. Ann. N. Y. Acad. Sci. 2000, 899, 209–221. [Google Scholar] [CrossRef]
- Mohr, S.; Stamler, J.S.; Brüne, B. Posttranslational Modification of Glyceraldehyde-3-phosphate Dehydrogenase by S-Nitrosylation and Subsequent NADH Attachment. J. Biol. Chem. 1996, 271, 4209–4214. [Google Scholar] [CrossRef]
- Hara, M.R.; Snyder, S.H. Nitric Oxide–GAPDH–Siah: A Novel Cell Death Cascade. Cell. Mol. Neurobiol. 2006, 26, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Yamashita, H.; Takahashi, T.; Kishida, S.; Nakamura, T.; Iseki, E.; Hattori, N.; Mizuno, Y.; Kikuchi, A.; Matsumoto, M. Siah-1 Facilitates Ubiquitination and Degradation of Synphilin-1. J. Biol. Chem. 2003, 278, 51504–51514. [Google Scholar] [CrossRef] [PubMed]
- Moriyoshi, K.; Iijima, K.; Fujii, H.; Ito, H.; Cho, Y.; Nakanishi, S. Seven in absentia homolog 1A mediates ubiquitination and degradation of group 1 metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 8614–8619. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Hara, M.R.; Ahmad, A.S.; Cascio, M.B.; Kamiya, A.; Ehmsen, J.T.; Agrawal, N.; Hester, L.; Doré, S.; Snyder, S.H.; et al. GOSPEL: A neuroprotective protein that binds to GAPDH upon S-nitrosylation. Neuron 2009, 63, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, E.J. Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway. J. Biol. Chem. 2001, 276, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Dastoor, Z.; Dreyer, J.L. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J. Cell Sci. 2001, 114, 1643–1653. [Google Scholar] [CrossRef]
- Meyer-Siegler, K.; Mauro, D.J.; Seal, G.; Wurzer, J.; deRiel, J.K.; Sirover, M.A. A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. USA 1991, 88, 8460–8464. [Google Scholar] [CrossRef]
- Patterson, R.L.; van Rossum, D.B.; Kaplin, A.I.; Barrow, R.K.; Snyder, S.H. Inositol 1,4,5-trisphosphate receptor/GAPDH complex augments Ca2+ release via locally derived NADH. Proc. Natl. Acad. Sci. USA 2005, 102, 1357–1359. [Google Scholar] [CrossRef]
- Fernandes, H.S.; Popik, B.; de Oliveira Alvares, L. Effects of hippocampal IP3R inhibition on contextual fear memory consolidation, retrieval, reconsolidation and extinction. Neurobiol. Learn. Mem. 2022, 188, 107587. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Volbracht, C.; Kühnle, S.; Fava, E.; Ferrando-May, E.; Nicotera, P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol. Med. 1997, 3, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Dawson, V.L.; Dawson, T.M.; London, E.D.; Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 1991, 88, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Cascio, M.B.; Sawa, A. GAPDH as a sensor of NO stress. Biochim. Biophys. Acta 2006, 1762, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Averill, L.A.; Jiang, L.; Purohit, P.; Coppoli, A.; Averill, C.L.; Roscoe, J.; Kelmendi, B.; De Feyter, H.M.; de Graaf, R.A.; Gueorguieva, R.; et al. Prefrontal Glutamate Neurotransmission in PTSD: A Novel Approach to Estimate Synaptic Strength in Vivo in Humans. Chronic Stress 2022, 6, 24705470221092734. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Girgenti, M.J.; Davis, M.T.; Pietrzak, R.H.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Southwick, S.; Duman, R.S.; Carson, R.E.; et al. Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc. Natl. Acad. Sci. USA 2017, 114, 8390–8395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sheerin, C.; Mandel, H.; Banducci, A.N.; Myrick, H.; Acierno, R.; Amstadter, A.B.; Wang, Z. Variation in SLC1A1 is related to combat-related posttraumatic stress disorder. J. Anxiety Disord. 2014, 28, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Meffert, M.K.; Premack, B.A.; Schulman, H. Nitric oxide stimulates Ca(2+)-independent synaptic vesicle release. Neuron 1994, 12, 1235–1244. [Google Scholar] [CrossRef]
- Palmer, Z.J.; Duncan, R.R.; Johnson, J.R.; Lian, L.Y.; Mello, L.V.; Booth, D.; Barclay, J.W.; Graham, M.E.; Burgoyne, R.D.; Prior, I.A.; et al. S-nitrosylation of syntaxin 1 at Cys145 is a regulatory switch controlling Munc18-1 binding. Biochem. J. 2008, 413, 479–491. [Google Scholar] [CrossRef]
- Jahn, R.; Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature 2012, 490, 201–207. [Google Scholar] [CrossRef]
- Kaeser, P.S.; Regehr, W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014, 76, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; Burgoyne, R.D. Membrane Traffic: Controlling Membrane Fusion by Modifying NSF. Curr. Biol. 2004, 14, R968–R970. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Park, K.; Lee, S.; Yi, J.H.; Woo, C.; Kang, S.J.; Shin, K.S. Auditory fear conditioning facilitates neurotransmitter release at lateral amygdala to basal amygdala synapses. Biochem. Biophys. Res. Commun. 2021, 584, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Suvrathan, A.; Bennur, S.; Ghosh, S.; Tomar, A.; Anilkumar, S.; Chattarji, S. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130151. [Google Scholar] [CrossRef] [PubMed]
- Umanah, G.K.E.; Ghasemi, M.; Yin, X.; Chang, M.; Kim, J.W.; Zhang, J.; Ma, E.; Scarffe, L.A.; Lee, Y.I.; Chen, R.; et al. AMPA Receptor Surface Expression Is Regulated by S-Nitrosylation of Thorase and Transnitrosylation of NSF. Cell Rep. 2020, 33, 108329. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, W.; Nicoll, R.A.; Bredt, D.S. Stargazin is an AMPA receptor auxiliary subunit. Proc. Natl. Acad. Sci. USA 2005, 102, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, B.; Jenkins, M.A.; Hussain, N.K.; Huganir, R.L.; Traynelis, S.F.; Snyder, S.H. S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc. Natl. Acad. Sci. USA 2013, 110, 1077–1082. [Google Scholar] [CrossRef]
- Schnell, E.; Sizemore, M.; Karimzadegan, S.; Chen, L.; Bredt, D.S.; Nicoll, R.A. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. USA 2002, 99, 13902–13907. [Google Scholar] [CrossRef]
- El-Husseini Ael, D.; Schnell, E.; Dakoji, S.; Sweeney, N.; Zhou, Q.; Prange, O.; Gauthier-Campbell, C.; Aguilera-Moreno, A.; Nicoll, R.A.; Bredt, D.S. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 2002, 108, 849–863. [Google Scholar] [CrossRef]
- Patel, J.; Haulcomb, M.; Li, L.; Jiang, G.; Dustrude, E.; Liu, Y.; Lai, Y.; Molosh, A.; Shekhar, A. 77822 PSD95-nNOS interaction alters the basolateral amygdala transcriptome following fear conditioning: Implications for molecular mechanisms underlying PTSD. J. Clin. Transl. Sci. 2021, 5, 23. [Google Scholar] [CrossRef]
- Humeau, Y.; Reisel, D.; Johnson, A.W.; Borchardt, T.; Jensen, V.; Gebhardt, C.; Bosch, V.; Gass, P.; Bannerman, D.M.; Good, M.A.; et al. A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J. Neurosci. 2007, 27, 10947–10956. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, S.; LeDoux, J.; Zador, A.; Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science 2005, 308, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Park, K.; Hong, I.; Song, B.; Son, G.; Park, H.; Kim, W.R.; Park, E.; Choe, H.K.; et al. Amygdala depotentiation and fear extinction. Proc. Natl. Acad. Sci. USA 2007, 104, 20955–20960. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.L.; Huganir, R.L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 2010, 330, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.L.; Wang, Y.T.; Floresco, S.B.; Phillips, A.G. Disruption of AMPA Receptor Endocytosis Impairs the Extinction, but not Acquisition of Learned Fear. Neuropsychopharmacology 2008, 33, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Trent, S.; Barnes, P.; Hall, J.; Thomas, K.L. AMPA receptors control fear extinction through an Arc-dependent mechanism. Learn. Mem. 2017, 24, 375–380. [Google Scholar] [CrossRef]
- Zushida, K.; Sakurai, M.; Wada, K.; Sekiguchi, M. Facilitation of extinction learning for contextual fear memory by PEPA: A potentiator of AMPA receptors. J. Neurosci. 2007, 27, 158–166. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Thoenen, H. Neurotrophins and neuronal plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef]
- Rattiner, L.M.; Davis, M.; French, C.T.; Ressler, K.J. Brain-Derived Neurotrophic Factor and Tyrosine Kinase Receptor B Involvement in Amygdala-Dependent Fear Conditioning. J. Neurosci. 2004, 24, 4796–4806. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Morinobu, S.; Yamamoto, S.; Fuchikami, M.; Matsumoto, T.; Yamawaki, S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J. Psychiatr. Res. 2011, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Biojone, C.; Casarotto, P.C.; Joca, S.R.; Castrén, E. Interplay between Nitric Oxide and Brain-Derived Neurotrophic Factor in Neuronal Plasticity. CNS Neurol. Disord. Drug Targets 2015, 14, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Biojone, C.; Casarotto, P.C.; Cannarozzo, C.; Fred, S.M.; Herrera-Rodríguez, R.; Lesnikova, A.; Voipio, M.; Castrén, E. nNOS-induced tyrosine nitration of TRKB impairs BDNF signaling and restrains neuronal plasticity. Prog. Neurobiol. 2023, 222, 102413. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Sciarretta, C.; Rodríguez-Moreno, A.; Al Banchaabouchi, M.; Negrete-Díaz, V.; Costanzi, M.; Berno, V.; Egorov, A.V.; von Bohlen Und Halbach, O.; Cestari, V.; et al. TrkB modulates fear learning and amygdalar synaptic plasticity by specific docking sites. J. Neurosci. 2009, 29, 10131–10143. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Vidal, L.E.; Do-Monte, F.H.; Sotres-Bayon, F.; Quirk, G.J. Hippocampal–Prefrontal BDNF and Memory for Fear Extinction. Neuropsychopharmacology 2014, 39, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Nott, A.; Watson, P.M.; Robinson, J.D.; Crepaldi, L.; Riccio, A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 2008, 455, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, R.; González, A.; Caviedes, A.; Pancetti, F.; Smalla, K.H.; Kaehne, T.; Michea, L.; Gundelfinger, E.D.; Wyneken, U. Homeostatic NMDA receptor down-regulation via brain derived neurotrophic factor and nitric oxide-dependent signalling in cortical but not in hippocampal neurons. J. Neurochem. 2011, 118, 760–772. [Google Scholar] [CrossRef]
- Sasaki, M.; Gonzalez-Zulueta, M.; Huang, H.; Herring, W.J.; Ahn, S.; Ginty, D.D.; Dawson, V.L.; Dawson, T.M. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc. Natl. Acad. Sci. USA 2000, 97, 8617–8622. [Google Scholar] [CrossRef]
- Moreno-López, B.; Romero-Grimaldi, C.; Noval, J.A.; Murillo-Carretero, M.; Matarredona, E.R.; Estrada, C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J. Neurosci. 2004, 24, 85–95. [Google Scholar] [CrossRef]
- Packer, M.A.; Stasiv, Y.; Benraiss, A.; Chmielnicki, E.; Grinberg, A.; Westphal, H.; Goldman, S.A.; Enikolopov, G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 9566–9571. [Google Scholar] [CrossRef] [PubMed]
- Jatzko, A.; Rothenhöfer, S.; Schmitt, A.; Gaser, C.; Demirakca, T.; Weber-Fahr, W.; Wessa, M.; Magnotta, V.; Braus, D.F. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J. Affect. Disord. 2006, 94, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Alvania, R.S.; Lonze, B.E.; Ramanan, N.; Kim, T.; Huang, Y.; Dawson, T.M.; Snyder, S.H.; Ginty, D.D. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell 2006, 21, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.I.; Martínez-Ruiz, A.; Araújo, I.M. S-nitrosation and neuronal plasticity. Br. J. Pharmacol. 2015, 172, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Lipton, S.A. S-Nitrosylation in neurogenesis and neuronal development. Biochim. Biophys. Acta 2015, 1850, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Rei, D.; Mason, X.; Seo, J.; Gräff, J.; Rudenko, A.; Wang, J.; Rueda, R.; Siegert, S.; Cho, S.; Canter, R.G.; et al. Basolateral amygdala bidirectionally modulates stress-induced hippocampal learning and memory deficits through a p25/Cdk5-dependent pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 7291–7296. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Nakamura, T.; Holland, E.A.; McKercher, S.R.; Lipton, S.A. S-nitrosylation of Cdk5: Potential implications in amyloid-β-related neurotoxicity in Alzheimer disease. Prion 2012, 6, 364–370. [Google Scholar] [CrossRef]
- Morabito, M.A.; Sheng, M.; Tsai, L.H. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J. Neurosci. 2004, 24, 865–876. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, W.-Y.; Fu, A.K.Y.; Ip, N.Y. S-nitrosylation-dependent proteasomal degradation restrains Cdk5 activity to regulate hippocampal synaptic strength. Nat. Commun. 2015, 6, 8665. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakabayashi, T.; Mita, N.; Jin, X.; Aikawa, Y.; Sasamoto, K.; Miyoshi, G.; Miyata, M.; Inoue, T.; Ohshima, T. Involvement of Cdk5 activating subunit p35 in synaptic plasticity in excitatory and inhibitory neurons. Mol. Brain 2022, 15, 37. [Google Scholar] [CrossRef]
- Dao, V.T.-V.; Elbatreek, M.H.; Fuchß, T.; Grädler, U.; Schmidt, H.H.H.W.; Shah, A.M.; Wallace, A.; Knowles, R. Nitric Oxide Synthase Inhibitors into the Clinic at Last. In Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 169–204. [Google Scholar]

| Paradigm | Animals | Experimental Condition | Treatment | Main Results | Refs. |
|---|---|---|---|---|---|
| NO modu-lation | Male Sprague Dawley rats | TDS stress | 7-NI (20 mg/kg; i.p.) | Reversal of ↑ NOx levels in HIP (7 days post-stress) | [16] |
| Male iNOS KO mice | - | - | ↑ NOS activity in neocortex | [23] | |
| L-NAME (50 mg/kg; i.p.) | Reversal of anxiety-like behavior in the EPM | ||||
| Male Wistar rats | 7-, 14- or 21-days RS (360 min each) | - | ↑ NOS activity and NOx after 7 days in cortex; ↑ iNOS expression after 14 days in cortex | [24] | |
| AG (400 mg/kg; i.p.) from day 7 to 21 | Reversal of ↑ NOx levels in cortex | ||||
| Male and female Swiss mice | Acute RS (360 min) | AG (12.5, 25, and 50 mg/kg; i.p.) | Reversal of anxiety-like behavior in the EPM and LDT (50 mg/kg); ↓ plasma nitrite levels | [26] | |
| SIL (1 mg/kg; i.p.) | Anxiety-like behavior in the EPM and LDT; ↑ plasma nitrite levels | ||||
| Male Wistar rats | Acute RS (120 min) | 1400 W (10−4, 10−3, and 10−2 nmol; intra-PL PFC) | Reversal of anxiety-like behavior in the EPM (10−3 nmol) | [27] | |
| Male Sprague Dawley rats | TDS stress | - | ↑ NOS activity after the initial stressors and on day 42 in HIP; ↓ GABA levels and NMDA receptor density on day 42 in HIP | [28] | |
| AG (50 mg/kg; i.p.) from day 1 to 21 | Reversal of ↑ NOS activity on day 42 in HIP | ||||
| Male iNOS KO mice | - | - | ↑ CFC; ↑ NOx and nNOS expression 24 h after conditioning in medial PFC | [64] | |
| 7-NI (30 mg/kg; i.p.) | Reversal of ↑ CFC response | ||||
| Male Sprague Dawley rats | Acute FSS (1 mA) | L-NAME (50 µL; i.c.v. or 50 mg/kg; s.c.) | Reversal of ↑ plasma ACTH levels (50 μL); ↓ Anterior pituitary NOS activity (50 mg/kg); ↓ Hypothalamic NOS activity | [99] | |
| Male Wistar rats | Acute RS (180 min) | NPLA (0.04 nmol; intra-PL PFC) | Anxiety-like behavior in the EPM; ↑ nNOS expression after 24 h or 7 days | [100] | |
| Male Wistar rats | Acute RS (120 min) | L-NAME (10 mg/kg; i.p.) | Reversal of anxiety-like behavior in the EPM; Reversal of depression-like behavior in the FST | [101] | |
| 15 days RS (120 min each) | |||||
| Swiss mice (unk. sex) | Acute or 5-days FSS (0.5 mA or 1.5 mA) | L-NAME (10 and 30 mg/kg; i.p.) | Reversal of ↑ serum CORT levels (1.5 mA; 30 mg/kg) | [102] | |
| L-arginine (100 and 300 mg/kg; i.p.) | Augment the ↑ serum CORT levels (0.5 mA; 300 mg/kg) | ||||
| L-NAME (30 mg/kg) + L-arginine (300 mg/kg); i.p. | Inhibited L-NAME effects (1.5 mA) | ||||
| Male WT mice | CMS | - | ↑ nNOS expression after 4, 21, and 56 days in HIP; ↑ NOx and nNOS activity after 21 days in HIP; Depression-like behavior in the TST | [103] | |
| Male WT mice | 7-NI (30 mg/kg; i.p.) for 7 days | Reversal of depression-like behavior in the TST; ↑ BrdU+ cells in the DG | |||
| Male nNOS KO mice | - | Reversal of depression-like behavior in the TST; ↑ BrdU+ cells in the DG | |||
| Male C57BL/6 mice | CSDS | - | Depression-like behavior in the TST and SPT; ↑ Density of neurons expressing nNOS, nNOS expression and activity in NAc shell | [104] | |
| L-VNIO (1.5 mM; intra-NAc shell) | |||||
| Carboxy-PTIO (10 µM; intra-NAc shell) | Reversal of depression-like behavior in the TST and SPT | ||||
| CMS | - | Depression-like behavior in the TST and SPT; ↑ nNOS expression in HIP | |||
| Male WT mice | CMS | - | ↑ nNOS expression and density of neurons expressing nNOS in HIP | [105] | |
| 7-NI (10 µM; intra-HIP) | |||||
| CORT-induced chronic stress model | 7-NI (10 µM; intra-HIP) | Reversal of depression-like behavior in the TST, FST, and SPT; Reversal of ↑ GR expression in HIP; ↓ CORT plasma levels | |||
| Stress Expos-ure on NO signal-ing | Male Wistar rats | Predator exposure | - | Anxiety-like behavior in the EPM; ↑ NOx and density of neurons expressing nNOS in PFC | [17] |
| Anxiety-like behavior in the EPM; ↑ Density of neurons expressing nNOS in BLA | |||||
| Male Wistar rats | Acute RS (360 min) | - | ↑ NOS activity after 6 h in cortex; ↑ NF-kB translocation to the nucleus; ↑ iNOS expression in cortex | [25] | |
| Male Wistar rats | Acute RS (120 min) | - | ↑ Density of neurons expressing nNOS in CeA | [106] | |
| 5-days RS (120 min each) | - | ↑ Density of neurons expressing nNOS in HIP and entorhinal cortex | |||
| Male Wistar rats | Acute RS (60, 120, or 240 min) | - | ↑ CORT plasma levels, NOx and constitutive NOS activity after 60 min in HIP; ↑ iNOS gene expression and activity after 240 min in HIP | [107] | |
| ↑ iNOS gene expression and activity after 120 min in striatum | |||||
| Male Wistar rats | Acute RS (60, 120, or 240 min) | - | ↑ nNOS and iNOS gene expression after 240 min in PFC | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronza, M.G.; Ferreira, B.F.; Pavan-Silva, I.; Guimarães, F.S.; Lisboa, S.F. “NO” Time in Fear Response: Possible Implication of Nitric-Oxide-Related Mechanisms in PTSD. Molecules 2024, 29, 89. https://doi.org/10.3390/molecules29010089
Fronza MG, Ferreira BF, Pavan-Silva I, Guimarães FS, Lisboa SF. “NO” Time in Fear Response: Possible Implication of Nitric-Oxide-Related Mechanisms in PTSD. Molecules. 2024; 29(1):89. https://doi.org/10.3390/molecules29010089
Chicago/Turabian StyleFronza, Mariana G., Bruna F. Ferreira, Isabela Pavan-Silva, Francisco S. Guimarães, and Sabrina F. Lisboa. 2024. "“NO” Time in Fear Response: Possible Implication of Nitric-Oxide-Related Mechanisms in PTSD" Molecules 29, no. 1: 89. https://doi.org/10.3390/molecules29010089







