A Reliable Multifaceted Solution against Foodborne Viral Infections: The Case of RiLK1 Decapeptide
Abstract
1. Introduction
2. Results and Discussion
2.1. Peptide Design
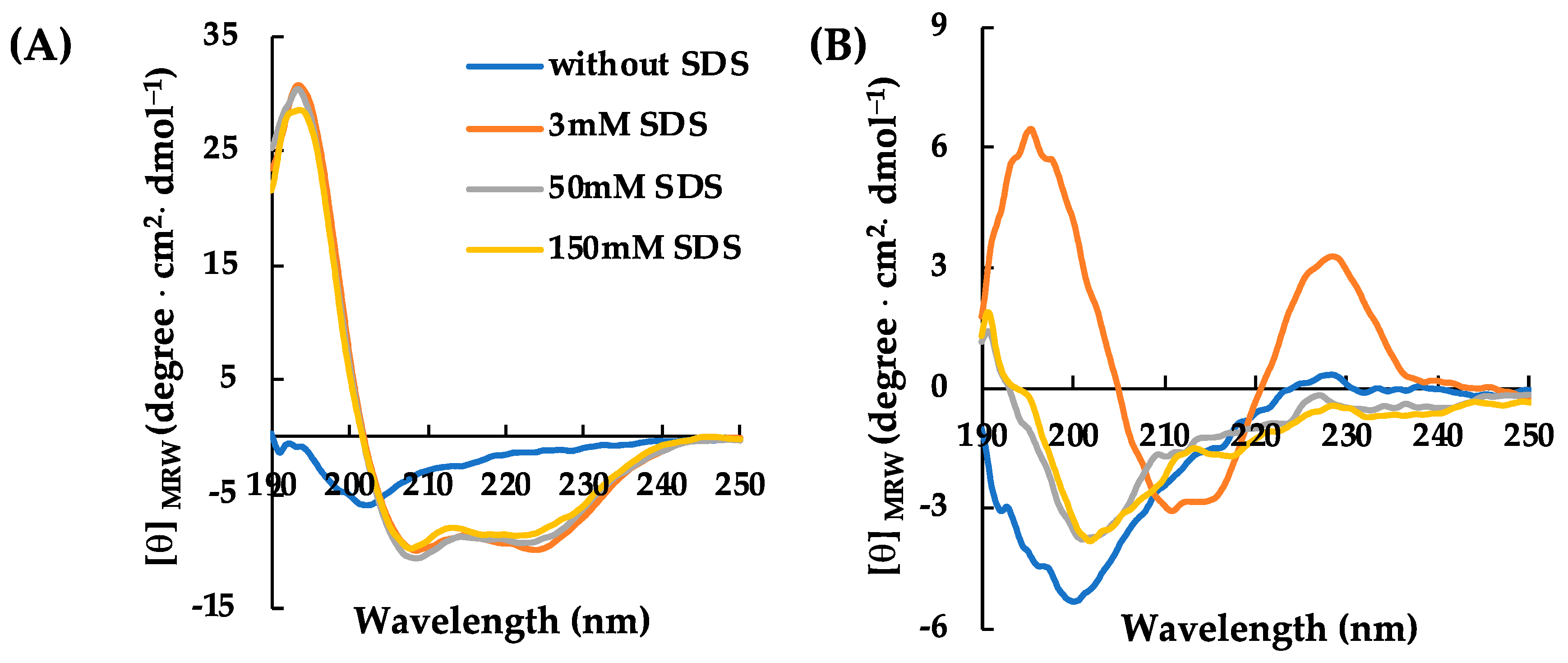
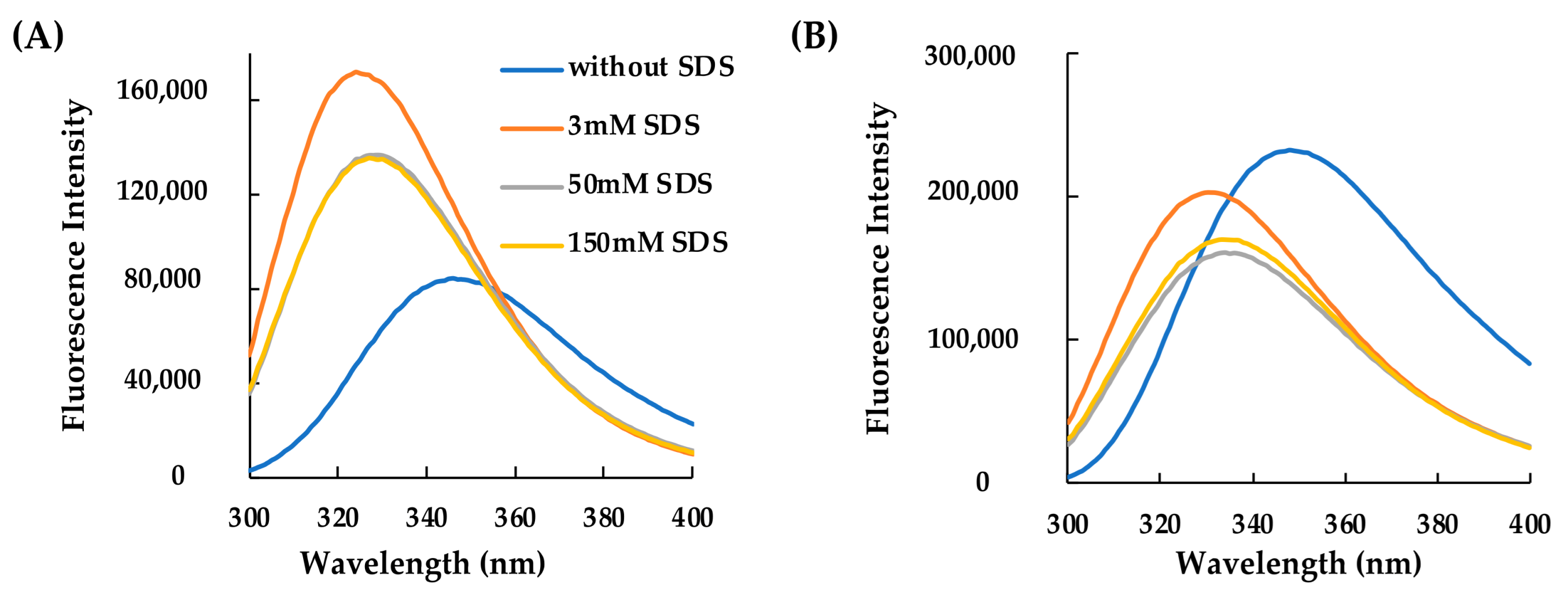
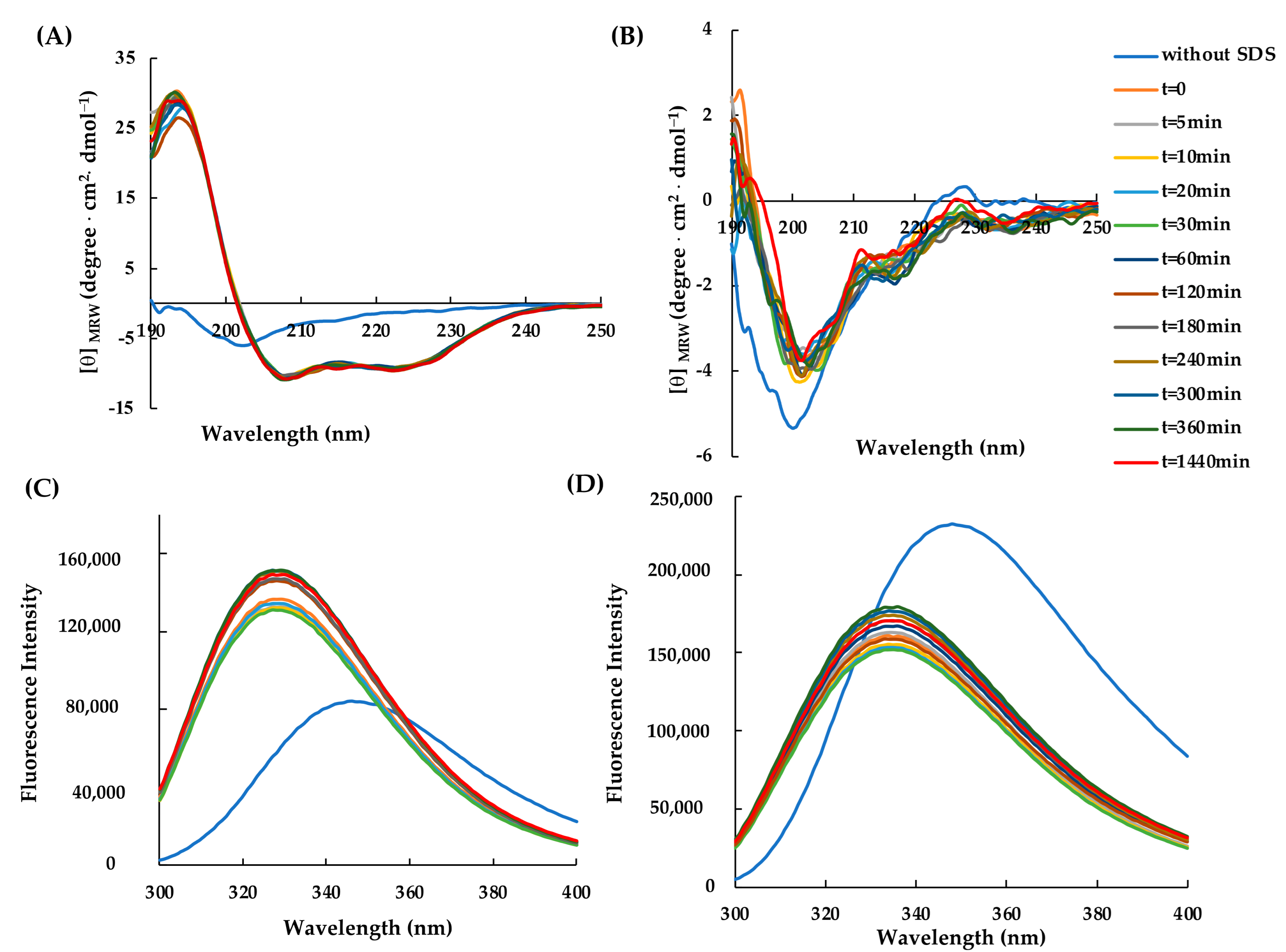
2.2. Structural Characterization of RiLK30 and AVP2
2.3. Antiviral Activity of Peptides
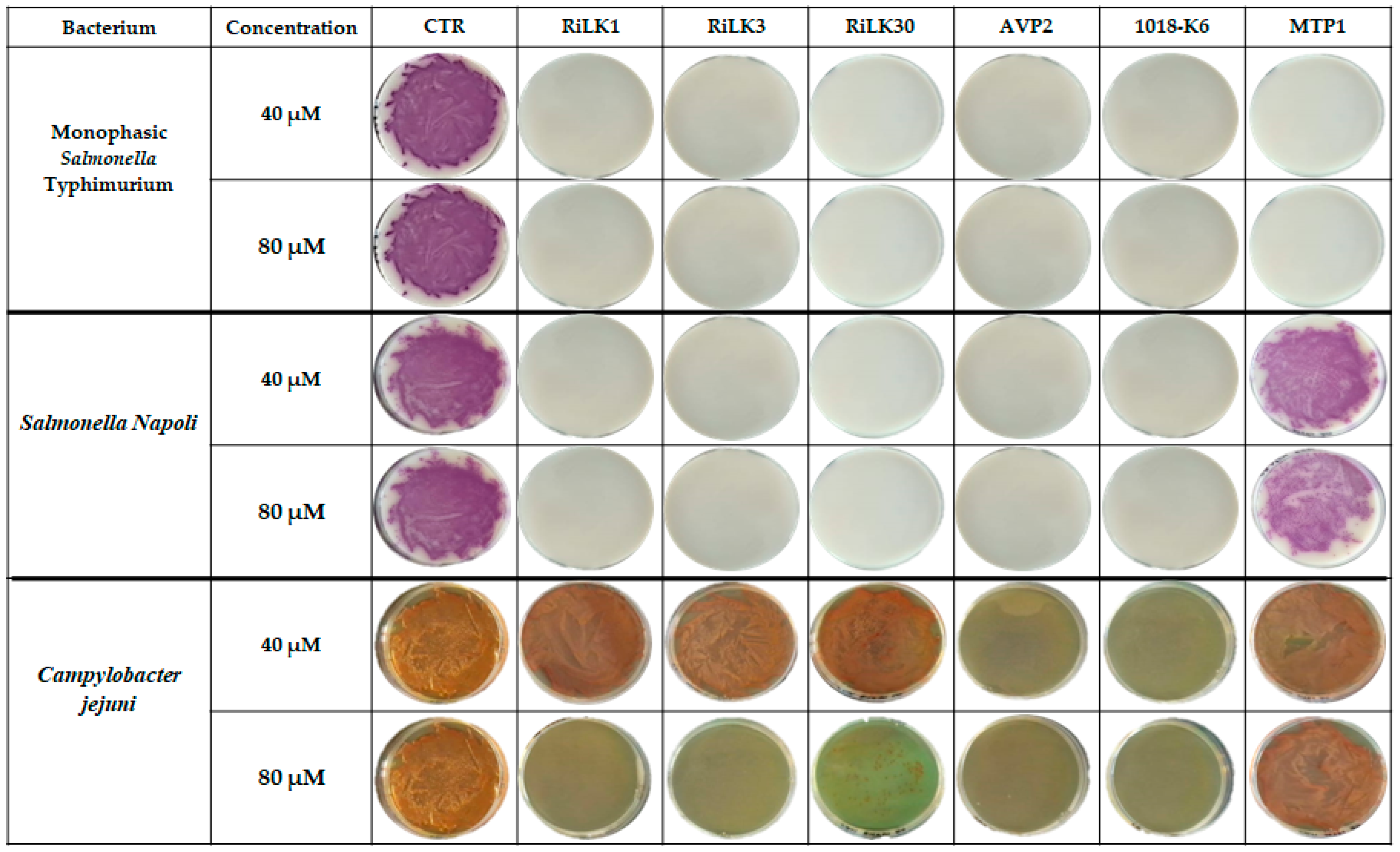
2.4. Antibacterial Activity of Peptides
3. Materials and Methods
3.1. Virus Strains and Cell Lines
3.2. Antimicrobial Peptides
3.3. Circular Dichroism (CD) Spectroscopy
3.4. Fluorescence Spectroscopy
3.5. Cytotoxicity Determination of Peptides on Cells
3.6. Virucidal Effect of Peptides
3.7. Antibacterial Assays
3.8. Mechanism of Action of RiLK1 and RiLK3 Peptides
- i)
- A virus pre-treatment assay to assess the direct interaction between the virus and peptide: the incubation of the peptide with viral suspension for 1 h at room temperature was carried out, followed by its incubation on cells for 1 h at 37 °C. Then, cell monolayers were washed twice with PBS and incubated with 500 µL DMEM/MEM supplemented with 2% of FBS to determine the TCID50/mL.
- ii)
- A cell pre-treatment assay to assess the interaction between the peptide and cells before the viral infection: cell monolayers were preincubated with 100 µL of peptide solution for 1 h at 37 °C, followed by the removal of the peptide, addition to cells of viral suspensions for 1 h at 37 °C, washing step, and culture with 2% FBS DMEM/MEM.
- iii)
- An attachment assay to assess the possible action of the peptide at the stage of virus attachment to cells: cell monolayers were incubated with 100 µL of a suspension containing both the peptide and virus and were incubated for 1 h at 4 °C. Subsequently, the cell monolayers were washed with PBS and incubated for 1 h at 37 °C with a culture medium followed by a washing step and the addition of culture with 2% FBS DMEM/MEM.
- iv)
- An entry assay to assess the possible action of the peptide at the stage of virus internalization into cells: cell monolayers were incubated with 100 µL of the viral suspension for 1 h at 4 °C, followed by the addition of the peptide and incubation for 1 h at 37 °C. Cells were then washed with PBS, and 2% FBS DMEM/MEM was finally added.
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Tohma, K.; Saito, M.; Pajuelo, M.J.; Mayta, H.; Zimic, M.; Lepore, C.J.; Ford-Siltz, L.A.; Gilman, R.H.; Parra, G.I. Viral intra-host evolution in immunocompetent children contributes to human norovirus diversification at the global scale. Emerg. Microbes Infect. 2021, 10, 1717–1730. [Google Scholar] [CrossRef]
- Refaat, A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- Pagán, R.; García-Gonzalo, D. Influence of environmental factors on bacterial biofilm formation in the food industry: A review. Postdoc J. 2015, 3, 3–13. [Google Scholar]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Rowe, G.; Bolger, F. Final report on “the identification of food safety priorities using the Delphi technique”. EFSA Support. Publ. 2016, 13, 1007E. [Google Scholar] [CrossRef]
- Wang, X.; Ren, J.; Gao, Q.; Hu, Z.; Sun, Y.; Li, X.; Rowlands, D.J.; Yin, W.; Wang, J.; Stuart, D.I.; et al. Hepatitis A virus and the origins of picornaviruses. Nature 2015, 517, 85–88. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses-The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef]
- Payne, D.C.; Vinje, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and medically attended gastroenteritis in US children. N. Engl. J. Med. 2013, 368, 1121–1130. [Google Scholar] [CrossRef]
- Yu, Z.; Shao, Q.; Xu, Z.; Chen, C.; Li, M.; Jiang, Y.; Cheng, D. Immunogenicity and Blocking Efficacy of Norovirus GII. Recombinant P Protein Vaccine. Vaccines 2023, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; van Beek, J.; Koopmans, M.P. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 2016, 14, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, C.; Faber, M.; Werber, D.; Stark, K.; Bitzegeio, J.; Michaelis, K.; Sagebiel, D.; Wenzel, J.J.; Enkelmann, J. Resurgence of an international hepatitis A outbreak linked to imported frozen strawberries, Germany, 2018 to 2020. Eurosurveillance 2020, 25, 1900670–1900679. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.P.; Weng, M.K.; Hofmeister, M.G.; Moore, K.L.; Doshani, M.; Kamili, S.; Koneru, A.; Haber, P.; Hagan, L.; Romero, J.R.; et al. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm. Rep. 2020, 69, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Mishra, N.; Saxena, R. Advances in antiviral drug discovery and development: Part II: Advancements in antiviral drug development. Future Virol. 2009, 4, 209–215. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Casciaro, B.; Genovese, A.; Brancaccio, D.; Marcocci, M.E.; Novellino, E.; Carotenuto, A.; Palamara, A.T.; Mangoni, M.L.; Nencioni, L. Temporin G, an amphibian antimicrobial peptide against influenza and parainfluenza respiratory viruses: Insights into biological activity and mechanism of action. FASEB J. 2021, 35, e21358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.C.; Feng, H.; Lin, Y.C.; Guo, X.R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The Amphibian Antimicrobial Peptide Temporin B Inhibits In Vitro Herpes Simplex Virus 1 Infection. Antimicrob. Agents Chemother. 2018, 62, e02367-17. [Google Scholar] [CrossRef]
- Brie, A.; Boudaud, N.; Mssihid, A.; Loutreul, J.; Bertrand, I.; Gantzer, C. Inactivation of murine norovirus and hepatitis a virus on fresh raspberries by gaseous ozone treatment. Food Microbiol. 2018, 70, 1–6. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Pérez-Pérez, R.E.; Niemira, B.A.; Fan, X. Fan Evaluation of gaseous chlorine dioxide for the inactivation of Tulane virus on blueberries. Int. J. Food Microbiol. 2018, 273, 28–32. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Pourmand, M.R. Venom-derived peptide Mastoparan-1 eradicates planktonic and biofilm embedded methicillin-resistant Staphylococcus aureus isolates. Microb. Pathog. 2018, 119, 72–80. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Carvalho, A.; Gomes, V.M. Plant defensins—Prospects for the biological functions and biotechnological properties. Peptides 2009, 30, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Urmi, U.L.; Vijay, A.K.; Kuppusamy, R.; Islam, S.; Willcox, M.D.P. A review of the antiviral activity of cationic antimicrobial peptides. Peptides 2023, 166, 171024. [Google Scholar] [CrossRef] [PubMed]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The broad-spectrum antiviral potential of the amphibian peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef]
- Bastian, A.; Schafer, H. Human alpha-defensin 1 (hnp-1) inhibits adenoviral infection in vitro. Regul. Pept. 2001, 101, 157–161. [Google Scholar] [CrossRef]
- Horne, W.S.; Wiethoff, C.M.; Cui, C.; Wilcoxen, K.M.; Amorin, M.; Ghadiri, M.R.; Nemerow, G.R. Antiviral cyclic D,L-α-peptides: Targeting a general biochemical pathway in virus infections. Bioorg. Med. Chem. 2005, 13, 5145–5153. [Google Scholar] [CrossRef]
- Robinson, W.E., Jr.; McDougall, B.; Tran, D.; Selsted, M.E. Anti-hiv-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 1998, 63, 94–100. [Google Scholar] [CrossRef]
- Sitaram, N.; Nagaraj, R. Interaction of antimicrobial peptides with biological and model membranes: Structural and charge requirements for activity. Biochim. Biophys. Acta 1999, 1462, 29–54. [Google Scholar] [CrossRef]
- Yasin, B.; Wang, W.; Pang, M.; Cheshenko, N.; Hong, T.; Waring, A.J.; Herold, B.C.; Wagar, E.A.; Lehrer, R.I. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004, 78, 5147–5156. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, H.; Ishihara, T.; Otaka, A.; Murakami, T.; Ibuka, T.; Waki, M.; Matsumoto, A.; Yamamoto, N.; Fujii, N. Analysis of the interaction of an anti-hiv peptide, t22 ([tyr5, 12, lys7] polyphemusin ii), with gp120 and cd4 by surface plasmon resonance. Biochim. Biophys. Acta 1996, 1298, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Song, B.H.; Lee, G.C.; Moon, M.S.; Cho, Y.H.; Lee, C.H. Human cytomegalovirus binding to heparan sulfate proteoglycans on the cell surface and/or entry stimulates the expression of human leukocyte antigen class I. J. Gen. Virol. 2001, 82, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B. Lunatusin, a trypsin-stable antimicrobial peptide from lima beans (Phaseolus lunatus L.). Peptides 2005, 26, 2086–2092. [Google Scholar] [CrossRef]
- Yu, J.; Dai, Y.; Fu, Y.; Wang, K.; Yang, Y.; Li, M.; Xu, W.; Wei, L. Cathelicidin antimicrobial peptides suppress EV71 infection via regulating antiviral response and inhibiting viral binding. Antivir. Res. 2021, 187, 105021. [Google Scholar] [CrossRef]
- Stelitano, D.; Franci, G.; Chianese, A.; Galdiero, S.; Morelli, G.; Galdiero, M. HSV membrane glycoproteins, their function in viral entry and their use in vaccine studies. In Amino Acids, Peptides and Proteins; Royal Society of Chemistry: London, UK, 2019; pp. 14–43. [Google Scholar]
- Roy, M.; Lebeau, L.; Chessa, C.; Damour, A.; Ladram, A.; Oury, B.; Boutolleau, D.; Bodet, C.; Leveque, N. Comparison of Anti-Viral Activity of Frog Skin Anti-Microbial Peptides Temporin-Sha and [K(3)]SHa to LL-37 and Temporin-Tb against Herpes Simplex Virus Type 1. Viruses 2019, 11, 77. [Google Scholar] [CrossRef]
- Palmieri, G.; Balestrieri, M.; Proroga, Y.T.; Falcigno, L.; Facchiano, A.; Riccio, A.; Capuano, F.; Marrone, R.; Neglia, G.; Anastasio, A. New antimicrobial peptides against foodborne pathogens: From in silico design to experimental evidence. Food Chem. 2016, 211, 546–554. [Google Scholar] [CrossRef]
- Palmieri, G.; Balestrieri, M.; Capuano, F.; Proroga, Y.T.R.; Pomilio, F.; Centorame, P.; Riccio, A.; Marrone, R.; Anastasio, A. Bactericidal and antibiofilm activity of bactenecin-derivative peptides against the food-pathogen Listeria monocytogenes: New perspectives for food processing industry. Int. J. Food Microbiol. 2018, 279, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, B.; Proroga, Y.T.R.; Gogliettino, M.; Balestrieri, M.; Tatè, R.; Nicolais, L.; Palmieri, G. A Safe and Multitasking Antimicrobial Decapeptide: The Road from De Novo Design to Structural and Functional Characterization. Int. J. Mol. Sci. 2020, 21, 6952. [Google Scholar] [CrossRef] [PubMed]
- Falcigno, L.; D’Auria, G.; Palmieri, G.; Gogliettino, M.; Agrillo, B.; Tatè, R.; Dardano, P.; Nicolais, L.; Balestrieri, M. Key Physicochemical Determinants in the Antimicrobial Peptide RiLK1 Promote Amphipathic Structures. Int. J. Mol. Sci. 2021, 22, 10011. [Google Scholar] [CrossRef] [PubMed]
- Agrillo, B.; Porritiello, A.; Gratino, L.; Balestrieri, M.; Proroga, Y.T.; Mancusi, A.; Cozzi, L.; Vicenza, T.; Dardano, P.; Miranda, B.; et al. Antimicrobial activity, membrane interaction and structural features of short arginine-rich antimicrobial peptides. Front. Microbiol. 2023, 14, 1244325. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Watson, K.M.; Peterkofsky, A.; Buckheit, R.W., Jr. Identification of novel human immunodeficiency virus type 1-inhibitory peptides based on the antimicrobial peptide database. Antimicrob. Agents Chemother. 2010, 54, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Smetanska, I. Special issue “Bioactive compounds from natural sources (2020, 2021)”. Molecules 2022, 27, 1929. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Findlay, E.G.; Currie, S.M.; Davidson, D.J. Antiviral potential of cathelicidins. Future Microbiol. 2014, 9, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, J.; Qin, Z.; Dong, C.; Yang, H.; Sun, J.; Xu, W.; Wei, L. Endogenous cathelicidin is required for protection against ZIKV-caused testis damage via inactivating virions. Antivir. Res. 2022, 198, 105248. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, M.; Palmieri, G.; Neglia, G.; Anastasio, A.; Capuano, F.; De Stefano, L.; Nicolais, L. Antimicrobial Peptides against Listeria Monocytogenes (Brevetto Internazionale n. WO2019012158A1); European Patent Office: Munich, Germany, 2022. [Google Scholar]
- Palmieri, G.; Balestrieri, M.; Nicolais, L. Antimicrobial Peptides (Brevetto Internazionale n. WO2021191851A1); European Patent Office: Munich, Germany, 2021. [Google Scholar]
- Mikut, R.; Ruden, S.; Reischl, M.; Breitling, F.; Volkmer, R.; Hilpert, K. Improving short antimicrobial peptides despite elusive rules for activity. BBA Biomembr. 2016, 1858, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. Meta-iAVP: A Sequence-Based Meta-Predictor for Improving the Prediction of Antiviral Peptides Using Effective Feature Representation. Int. J. Mol. Sci. 2019, 20, 5743. [Google Scholar] [CrossRef]
- Mathur, D.; Singh, S.; Mehta, A.; Agrawal, P.; Raghava, G.P.S. In silico approaches for predicting the half-life of natural and modified peptides in blood. PLoS ONE 2018, 13, e0196829. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Walse, B.; Svensson, B.; Malmsten, M.; Schmidtchen, A. Rational design of antimicrobial C3a analogues with enhanced effects against Staphylococci using an integrated structure and function-based approach. Biochemistry 2008, 47, 9057–9070. [Google Scholar] [CrossRef] [PubMed]
- Galanth, C.; Abbassi, F.; Lequin, O.; Ayala-Sanmartin, J.; Ladram, A.; Nicolas, P.; Amiche, M. Mechanism of antibacterial action of dermaseptin B2: Interplay between helix-hinge-helix structure and membrane curvature strain. Biochemistry 2009, 48, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Joeng, J.H.; Kim, Y. Design, Characterization, and Antimicrobial Activity of a Novel Antimicrobial Peptide Derived from Bovine Lactophoricin. J. Microbiol. Biotechnol. 2017, 27, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kamarasu, P.; Hsu, H.Y.; Moore, M.D. Research progress in viral inactivation utilizing human norovirus surrogates. Front. Sustain. Food Syst. 2018, 2, 89. [Google Scholar] [CrossRef]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W., 4th. Murine norovirus: A model system to study norovirus biology and pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Schelhaas, M. Concepts of papillomavirus entry into host cells. Curr. Opin. Virol. 2014, 4, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoint. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Wekell, M.M. Tetrazolium-based cell bioassay for neurotoxins active on voltage-sensitive sodium channels: Semiautomated assay for saxitoxins, brevetoxins, and ciguatoxins. Anal. Biochem. 1993, 214, 190–194. [Google Scholar] [CrossRef]
- Findlay, E.G.; Currie, S.M.; Davidson, D.J. Cationic host defense peptides: Potential as antiviral therapeutics. BioDrugs 2013, 27, 479–493. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Sequence | MW (Da) | Net Charge | BI (kcal/mol) | Hydrophobicity | AVP § |
|---|---|---|---|---|---|---|
| RiLK1 a | RLKWVRIWRR-NH2 (10 aa) | 1467.8 | +6 | 4.70 | −0.56 | 1 |
| RiLK3 b | RLRWVRIWRR-NH2 (10 aa) | 1495.8 | +6 | 5.6 | −0.63 | 0.932 |
| RiLK30 | KLRWVKIWKK-NH2 (10 aa) | 1383.8 | +6 | 1.85 | −0.36 | 0.994 |
| 1018-K6 c | VRLIVKVRIWRR-NH2 (12 aa) | 1594.0 | +6 | 3.00 | −0.35 | 0.558 |
| MTP1 d | KVSGVLFGTGLWVAL-NH2 (15 aa) | 1545.8 | +2 | −1.68 | 0.23 | 0.744 |
| AVP2 e | GWFDVVKHIAKRF-NH2 (13 aa) | 1601.9 | +4 | 1.18 | −0.10 | 0.926 |
| Viral Titre after Treatment (logTCID50/mL ± SD) | LRV (logTCID50/mL ± SD) | |||
|---|---|---|---|---|
| Treatment | 4 °C | RT | 4 °C | RT |
| Untreated HAV | 4.7 ± 0.2 | 4.7 ± 0.2 a | n.r. | n.r. a |
| RiLK1 [80 µM] | 3.6 ± 0.1 * | 3.3 ± 0.2 *,a | 1.1 ± 0.3 * | 1.4 ± 0.4 *,a |
| RiLK1 [40 µM] | 3.9 ± 0.2 | 3.6 ± 0.1 *,a | 0.8 ± 0.4 | 1.1 ± 0.3 *,a |
| RiLK3 [80 µM] | 4.6 ± 0.1 | 4.4 ± 0.1 a | 0.1 ± 0.3 | 0.3 ± 0.3 a |
| RiLK3 [40 µM] | 4.6 ± 0.3 | 4.4 ± 0.1 a | 0.1 ± 0.2 | 0.3 ± 0.3 a |
| RiLK30 [80 µM] | 4.3 ± 0.2 | 4.1 ± 0.2 | 0.4 ± 0.4 | 0.6 ± 0.4 |
| RiLK30 [40 µM] | 4.3 ± 0.2 | 4.1 ± 0.2 | 0.4 ± 0.4 | 0.6 ± 0.4 |
| 1018-K6 [80 µM] | 3.3 ± 0.2 * | 3.3 ± 0.2 * | 1.4 ± 0.4 * | 1.4 ± 0.4 * |
| 1018-K6 [40 µM] | 3.4 ± 0.1 * | 3.3 ± 0.2 * | 1.3 ± 0.3 * | 1.4 ± 0.4 * |
| MTP1 [80 µM] | 3.8 ± 0.2 * | 3.5 ± 0.1 * | 0.9 ± 0.4 * | 1.2 ± 0.3 * |
| MTP1 [40 µM] | 3.6 ± 0.3 * | 3.2 ± 0.2 * | 1.1 ± 0.2 * | 1.5 ± 0.4 * |
| AVP2 [80 µM] | 4.3 ± 0.2 | 4.0 ± 0.3 | 0.4 ± 0.4 | 0.7 ± 0.5 |
| AVP2 [40 µM] | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.4 ± 0.4 | 0.4 ± 0.4 |
| Viral Titre after Treatment (logTCID50/mL ± SD) | LRV (logTCID50/mL ± SD) | |||
|---|---|---|---|---|
| Treatment | 4 °C | RT | 4 °C | RT |
| Untreated MNV-1 | 4.5 ± 0.2 | 4.5 ± 0.2 | n.r. | n.r. |
| RiLK1 [80 µM] | 4.3 ± 0.2 | 3.3 ± 0.2 * | 0.2 ± 0.4 | 1.2 ± 0.4 * |
| RiLK1 [40 µM] | 4.3 ± 0.2 | 3.5 ± 0.2 * | 0.2 ± 0.4 | 1.0 ± 0.4 * |
| RiLK3 [80 µM] | 4.5 ± 0.2 | 4.5 ± 0.1 | 0 ± 0.4 | 0 ± 0.3 |
| RiLK3 [40 µM] | 4.5 ± 0.1 | 4.5 ± 0.2 | 0 ± 0.3 | 0 ± 0.4 |
| RiLK30 [80 µM] | 3.9 ± 0.2 | 4.5 ± 0.1 | 0.6 ± 0.4 | 0 ± 0.3 |
| RiLK30 [40 µM] | 4.3 ± 0.3 | 4.5 ± 0.2 | 0.2 ± 0.5 | 0 ± 0.4 |
| 1018-K6 [80 µM] | 4.3 ± 0.2 | 4.3 ± 0.2 | 0.2 ± 0.4 | 0.2 ± 0.4 |
| 1018-K6 [40 µM] | 4.5 ± 0.1 | 4.5 ± 0.2 | 0 ± 0.3 | 0 ± 0.4 |
| MTP1 [80 µM] | 4.5 ± 0.1 | 4.5 ± 0.2 | 0 ± 0.3 | 0 ± 0.4 |
| MTP1 [40 µM] | 4.4 ± 0.1 | 4.5 ± 0.2 | 0.1 ± 0.3 | 0 ± 0.4 |
| AVP2 [80 µM] | 3.6 ± 0.1 * | 3.5 ± 0.1 * | 0.9 ± 0.3 * | 1.0 ± 0.3 * |
| AVP2 [40 µM] | 3.6 ± 0.1 * | 3.6 ± 0.1 * | 0.9 ± 0.3 * | 0.9 ± 0.3 * |
| RiLK1 | RiLK3 | |||
|---|---|---|---|---|
| Viral Titre (logTCID50/mL ± SD) | LRV (logTCID50/mL ± SD) | Viral Titre (logTCID50/mL ± SD) | LRV (logTCID50/mL ± SD) | |
| untreated virus | 4.7 ± 0.2 | - | 4.7 ± 0.2 | - |
| virus pre-treatment | 3.3 ± 0.2 * | 1.4 ± 0.4 * | 4.4 ± 0.1 | 0.3 ± 0.3 |
| cell pre-treatment | 4.6 ± 0.1 | 0.1 ± 0.3 | 3.8 ± 0.2 * | 0.9 ± 0.4 * |
| attachment | 3.3 ± 0.2 * | 1.4 ± 0.4 * | 3.7 ± 0.2 * | 1.0 ± 0.4 * |
| entry | 4.6 ± 0.1 | 0.1 ± 0.3 | 4.2 ± 0.2 | 0.5 ± 0.4 |
| RiLK1 | RiLK3 | |||
|---|---|---|---|---|
| Viral Titre (logTCID50/mL ± SD) | LRV (logTCID50/mL ± SD) | Viral Titre (logTCID50/mL ± SD) | LRV (logTCID50/mL ± SD) | |
| untreated virus | 4.5 ± 0.2 | - | 4.5 ± 0.2 | - |
| virus pre-treatment | 3.3 ± 0.2 * | 1.2 ± 0.4 * | 4.5 ± 0.1 | 0 ± 0.3 |
| cell pre-treatment | 4.2 ± 0.2 | 0.3 ± 0.4 | 3.5 ± 0.1 * | 1.0 ± 0.3 * |
| attachment | 3.1 ± 0.2 * | 1.4 ± 0.4 * | 3.5 ± 0.1 * | 1.0 ± 0.3 * |
| entry | 3.5 ± 0.2 * | 1.0 ± 0.4 * | 3.5 ± 0.1 * | 1.0 ± 0.3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galatola, E.; Agrillo, B.; Gogliettino, M.; Palmieri, G.; Maccaroni, S.; Vicenza, T.; Proroga, Y.T.R.; Mancusi, A.; Di Pasquale, S.; Suffredini, E.; et al. A Reliable Multifaceted Solution against Foodborne Viral Infections: The Case of RiLK1 Decapeptide. Molecules 2024, 29, 2305. https://doi.org/10.3390/molecules29102305
Galatola E, Agrillo B, Gogliettino M, Palmieri G, Maccaroni S, Vicenza T, Proroga YTR, Mancusi A, Di Pasquale S, Suffredini E, et al. A Reliable Multifaceted Solution against Foodborne Viral Infections: The Case of RiLK1 Decapeptide. Molecules. 2024; 29(10):2305. https://doi.org/10.3390/molecules29102305
Chicago/Turabian StyleGalatola, Emanuela, Bruna Agrillo, Marta Gogliettino, Gianna Palmieri, Serena Maccaroni, Teresa Vicenza, Yolande T. R. Proroga, Andrea Mancusi, Simona Di Pasquale, Elisabetta Suffredini, and et al. 2024. "A Reliable Multifaceted Solution against Foodborne Viral Infections: The Case of RiLK1 Decapeptide" Molecules 29, no. 10: 2305. https://doi.org/10.3390/molecules29102305
APA StyleGalatola, E., Agrillo, B., Gogliettino, M., Palmieri, G., Maccaroni, S., Vicenza, T., Proroga, Y. T. R., Mancusi, A., Di Pasquale, S., Suffredini, E., & Cozzi, L. (2024). A Reliable Multifaceted Solution against Foodborne Viral Infections: The Case of RiLK1 Decapeptide. Molecules, 29(10), 2305. https://doi.org/10.3390/molecules29102305









