Luminescent Alendronic Acid-Conjugated Micellar Nanostructures for Potential Application in the Bone-Targeted Delivery of Cholecalciferol
Abstract
:1. Introduction
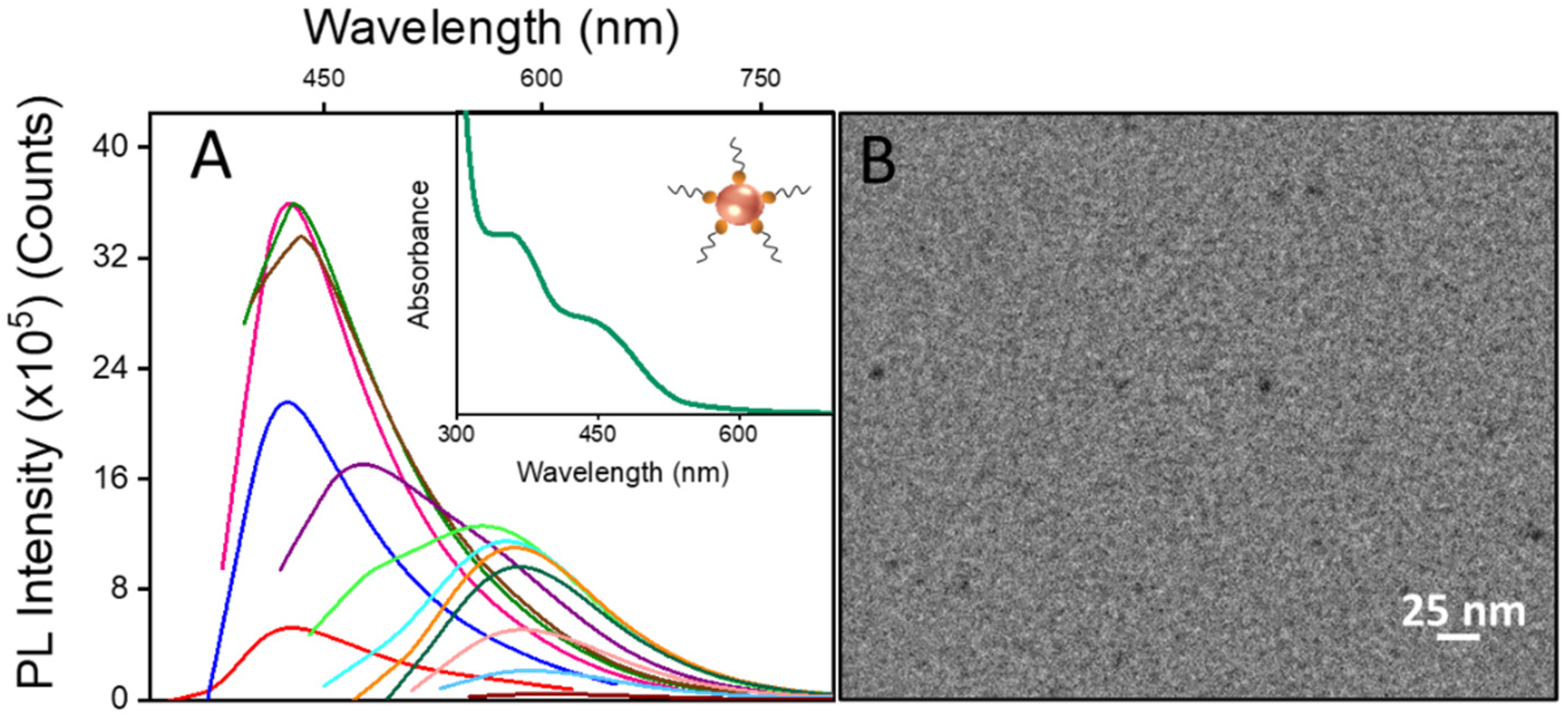
2. Results and Discussion
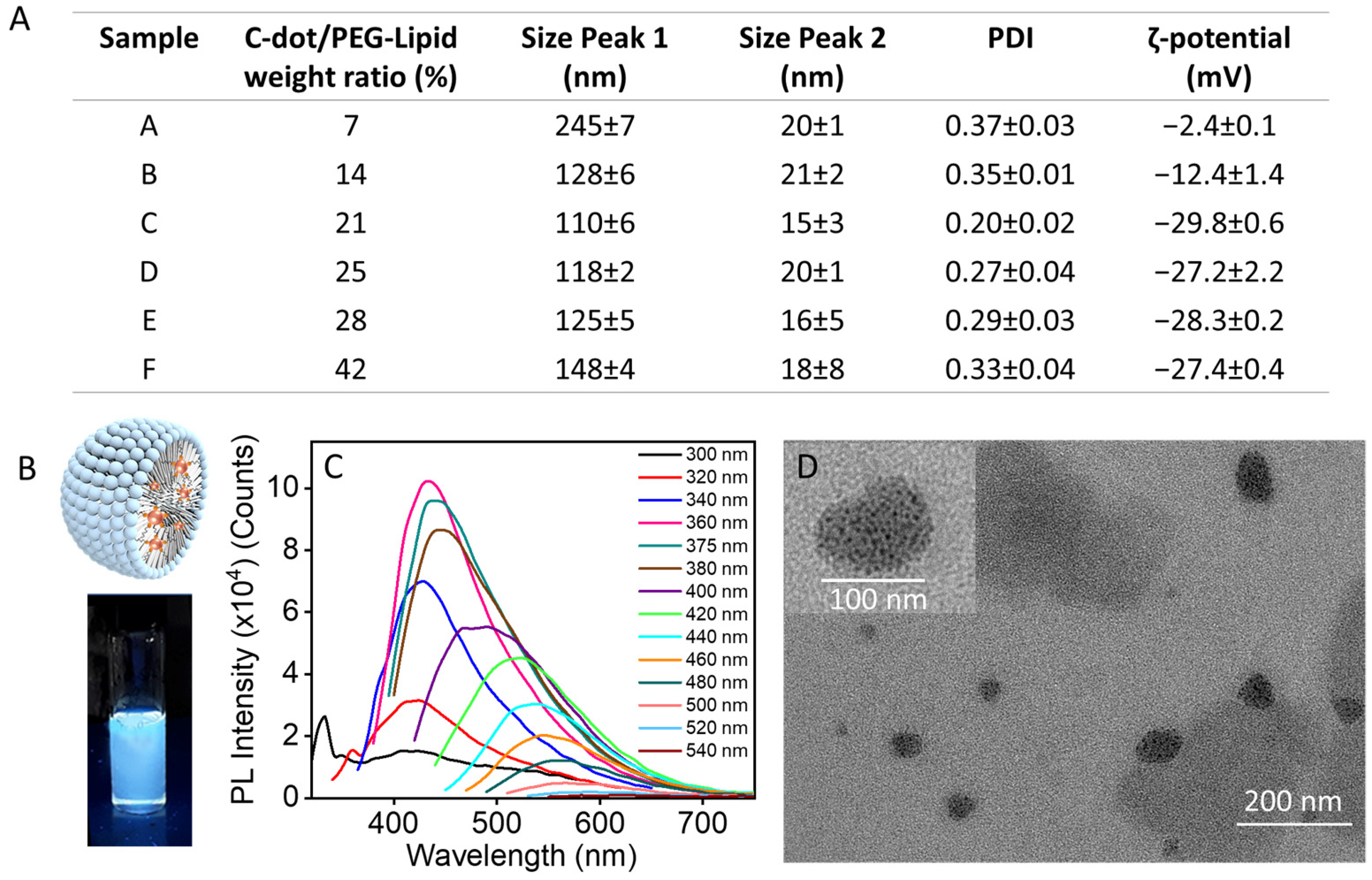
2.1. Preparation of PEGylated Phospholipid-Based Micellar Nanostructures
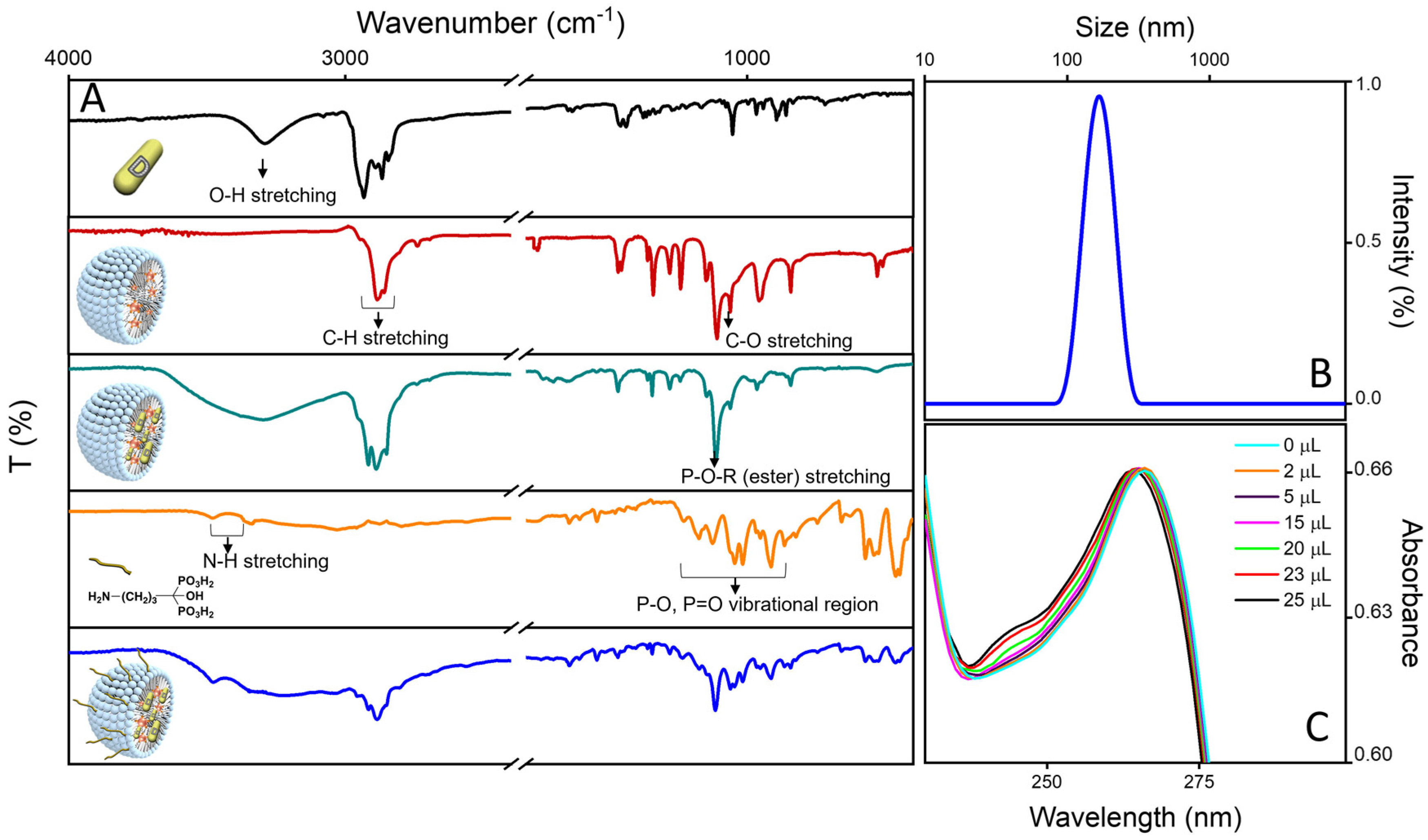
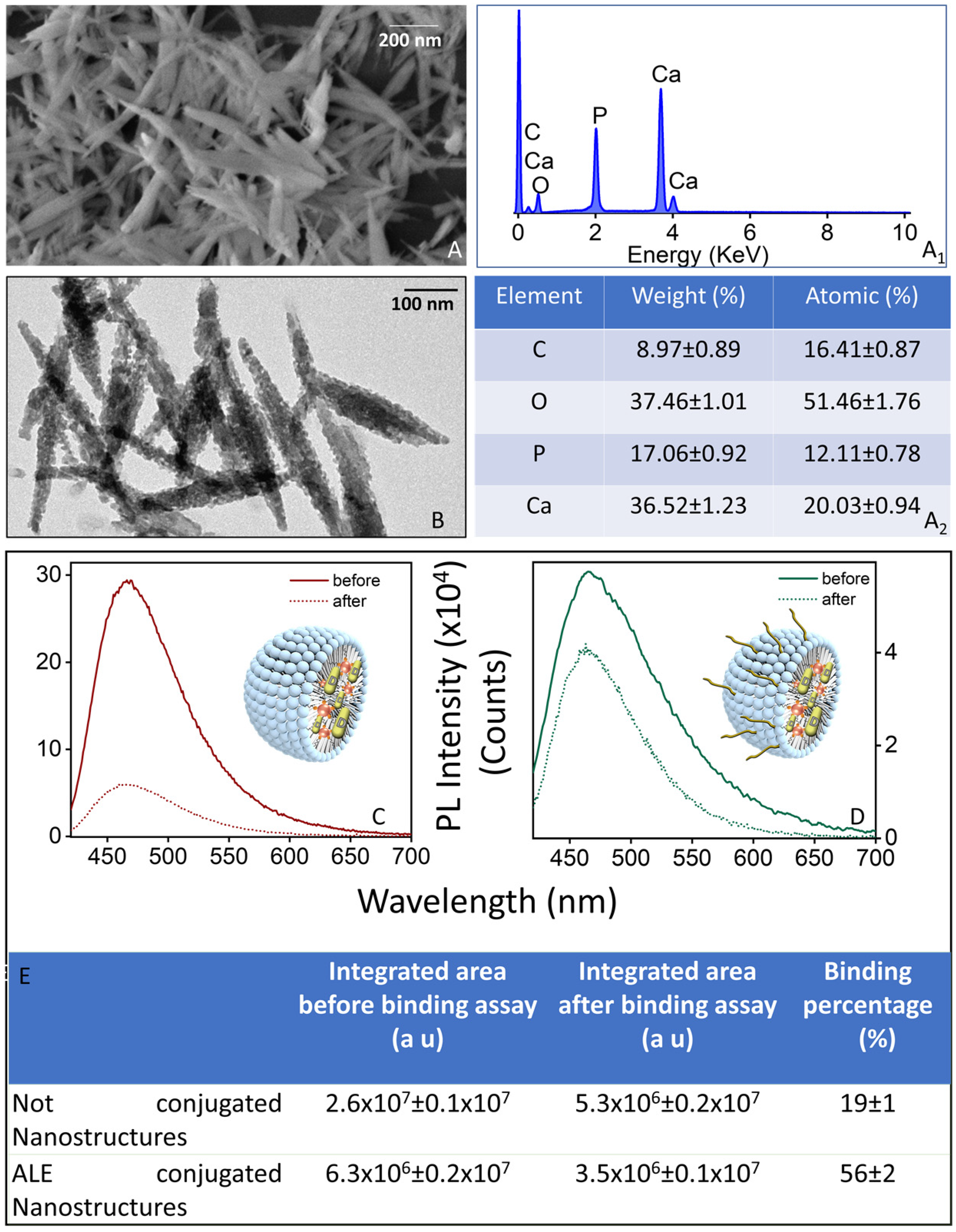
2.2. Conjugation with ALE of the Luminescent Micellar VitD3 Containing Nanostructures
2.3. Binding Affinity Assay
3. Materials and Methods
3.1. Materials
3.2. Preparation of PEG–Micellar Nanostructures Loaded with C-Dots and VitD3
3.3. Determination of Loading and Encapsulation Efficiency Percentages of Vitamin D3 and In Vitro Release Study
3.4. Synthesis of Alendronic Acid
3.5. Alendronate Bioconjugation of the Micellar Nanostructures Loaded with C-Dots and Vitamin D3
3.6. Binding Assay to Synthetic Hydroxyapatite
3.7. DLS Investigation and ζ-Potential Measurements
3.8. TEM and SEM Investigation
3.9. FTIR-ATR Characterizazion
3.10. Photophysical Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines†. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Coleman, R. Bone-Targeted Agents and Metastasis Prevention. Cancers 2022, 14, 3640. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; Misso, G.; Salzano, G.; Caraglia, M. Bisphosphonates and Cancer: What Opportunities from Nanotechnology? J. Drug Deliv. 2013, 2013, 637976. [Google Scholar] [CrossRef] [PubMed]
- Buranrat, B.; Bootha, S. Antiproliferative and antimigratory activities of bisphosphonates in human breast cancer cell line MCF-7. Oncol. Lett. 2019, 18, 1246–1258. [Google Scholar] [CrossRef]
- Altundag, O.; Altundag, K.; Selim Silay, Y.; Gunduz, M.; Demircan, K.; Gullu, I. Calcium and vitamin D supplementation during bisphosphonate administration may increase osteoclastic activity in patients with bone metastasis. Med. Hypotheses 2004, 63, 1010–1013. [Google Scholar] [CrossRef]
- Asfour, M.H.; Abd El-Alim, S.H.; Kassem, A.A.; Salama, A.; Gouda, A.S.; Nazim, W.S.; Nashaat, N.H.; Hemimi, M.; Abdel Meguid, N. Vitamin D3-Loaded Nanoemulsions as a Potential Drug Delivery System for Autistic Children: Formulation Development, Safety, and Pharmacokinetic Studies. AAPS PharmSciTech 2023, 24, 58. [Google Scholar] [CrossRef]
- Edlich, R.; Mason, S.S.; Chase, M.E.; Fisher, A.L.; Gubler, K.; Long, W.B., 3rd; Giesy, J.D.; Foley, M.L. Scientific documentation of the relationship of vitamin D deficiency and the development of cancer. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis12345. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A. Vitamin D(3) is more potent than vitamin D(2) in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Gomes, B.; Frasco, M.F.; Coelho, M.A.N.; Pereira, M.C. PLGA nanoparticles as a platform for vitamin D-based cancer therapy. Beilstein J. Nanotechnol. 2015, 6, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Maria, J.R.; Manuel, A.N.C.; Maria, C.P. Nanoparticles for Delivery of Vitamin D: Challenges and Opportunities. In A Critical Evaluation of Vitamin D; Sivakumar, G., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 11. [Google Scholar]
- Agwa, M.M.; Abu-Serie, M.M.; Abdelmonsif, D.A.; Moussa, N.; Elsayed, H.; Khattab, S.N.; Sabra, S. Vitamin D3/phospholipid complex decorated caseinate nanomicelles for targeted delivery of synergistic combination therapy in breast cancer. Int. J. Pharm. 2021, 607, 120965. [Google Scholar] [CrossRef] [PubMed]
- Dałek, P.; Drabik, D.; Wołczańska, H.; Foryś, A.; Jagas, M.; Jędruchniewicz, N.; Przybyło, M.; Witkiewicz, W.; Langner, M. Bioavailability by design—Vitamin D(3) liposomal delivery vehicles. Nanomedicine 2022, 43, 102552. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.A.; Lozano Vigario, F.; Sparrius, R.; van Capel, T.M.M.; van Ree, R.; Tas, S.W.; de Vries, I.J.M.; Geijtenbeek, T.B.H.; Slütter, B.; de Jong, E.C.; et al. Liposomes loaded with vitamin D3 induce regulatory circuits in human dendritic cells. Front. Immunol. 2023, 14, 1137538. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chang, C.; Zhang, X.; Zhang, Y.; Radford, M.J.; Gahler, R.J.; Kuo, Y.C.; Wood, S.; Solnier, J. Designing Vitamin D3 Formulations: An In Vitro Investigation Using a Novel Micellar Delivery System. Nutraceuticals 2023, 3, 290–305. [Google Scholar] [CrossRef]
- Dissanayake, R.K.; Perera, K.D.C.; Perera, W.P.T.D.; Wijesinghe, W.P.S.L.; Unagolla, J.M. Enteric Coated Oral Delivery of Hydroxyapatite Nanoparticle for Modified Release Vitamin D3 Formulation. J. Nanomater. 2021, 2021, 9972475. [Google Scholar] [CrossRef]
- Scala, R.; Maqoud, F.; McClenaghan, C.; Harter, T.M.; Perrone, M.G.; Scilimati, A.; Nichols, C.G.; Tricarico, D. Zoledronic Acid Blocks Overactive Kir6.1/SUR2-Dependent K(ATP) Channels in Skeletal Muscle and Osteoblasts in a Murine Model of Cantú Syndrome. Cells 2023, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Scala, R.; Maqoud, F.; Antonacci, M.; Dibenedetto, J.R.; Perrone, M.G.; Scilimati, A.; Castillo, K.; Latorre, R.; Conte, D.; Bendahhou, S.; et al. Bisphosphonates Targeting Ion Channels and Musculoskeletal Effects. Front. Pharmacol. 2022, 13, 837534. [Google Scholar] [CrossRef] [PubMed]
- Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect. Cancers 2019, 11, 206. [Google Scholar] [CrossRef]
- Shao, H.; Varamini, P. Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems. Cells 2022, 11, 388. [Google Scholar] [CrossRef]
- de Miguel, L.; Noiray, M.; Surpateanu, G.; Iorga, B.I.; Ponchel, G. Poly(γ-benzyl-l-glutamate)-PEG-alendronate multivalent nanoparticles for bone targeting. Int. J. Pharm. 2014, 460, 73–82. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, Y.; Huang, Z.; Jiang, Y.; Sun, X.; Shen, Y.; Chu, W.; Zhao, C. Bisphosphonate-functionalized coordination polymer nanoparticles for the treatment of bone metastatic breast cancer. J. Control. Release 2017, 264, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Mehnath, S.; Karthikeyan, K.; Rajan, M.; Jeyaraj, M. Fabrication of bone-targeting hyaluronic acid coupled alendronate-bioactive glass for osteosarcoma therapy. Mater. Chem. Phys. 2021, 273, 125146. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, H.T.; Phung, C.D.; Pathak, S.; Regmi, S.; Ha, D.-H.; Kim, J.O.; Yong, C.S.; Kim, S.K.; Choi, J.-E.; et al. Targeted delivery of doxorubicin for the treatment of bone metastasis from breast cancer using alendronate-functionalized graphene oxide nanosheets. J. Ind. Eng. Chem. 2019, 76, 310–317. [Google Scholar] [CrossRef]
- Sun, W.; Han, Y.; Li, Z.; Ge, K.; Zhang, J. Bone-Targeted Mesoporous Silica Nanocarrier Anchored by Zoledronate for Cancer Bone Metastasis. Langmuir 2016, 32, 9237–9244. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Balas, F.; Colilla, M.; Manzano, M.; Vallet-Regí, M. Functionalization degree of SBA-15 as key factor to modulate sodium alendronate dosage. Microporous Mesoporous Mater. 2008, 116, 4–13. [Google Scholar] [CrossRef]
- Cong, Y.; Quan, C.; Liu, M.; Liu, J.; Huang, G.; Tong, G.; Yin, Y.; Zhang, C.; Jiang, Q. Alendronate-decorated biodegradable polymeric micelles for potential bone-targeted delivery of vancomycin. J. Biomater. Sci. Polym. Ed. 2015, 26, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Kadu, K.; Hemmadi, V.; Biswas, M.; Kowshik, M.; Ramanan, S.R. Novel hydroxyapatite nanoparticle-based antibiotic alternative to combat methicillin-resistant S. aureus: A mechanism by targeting the structural and functional stability of MRSA membrane protein. J. Mater. Res. 2023, 38, 1609–1619. [Google Scholar] [CrossRef]
- Farrell, K.B.; Karpeisky, A.; Thamm, D.H.; Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018, 9, 47–60. [Google Scholar] [CrossRef]
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef]
- Pascaud, P.; Gras, P.; Coppel, Y.; Rey, C.; Sarda, S. Interaction between a Bisphosphonate, Tiludronate, and Biomimetic Nanocrystalline Apatites. Langmuir 2013, 29, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Rubini, K.; Bigi, A. Composite Nanocrystals Provide New Insight on Alendronate Interaction with Hydroxyapatite Structure. Adv. Mater. 2007, 19, 2499–2502. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Depalo, N.; Iacobazzi, R.M.; Valente, G.; Arduino, I.; Villa, S.; Canepa, F.; Laquintana, V.; Fanizza, E.; Striccoli, M.; Cutrignelli, A.; et al. Sorafenib delivery nanoplatform based on superparamagnetic iron oxide nanoparticles magnetically targets hepatocellular carcinoma. Nano Res. 2017, 10, 2431–2448. [Google Scholar] [CrossRef]
- Depalo, N.; Carrieri, P.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Bertinetti, L.; Innocenti, C.; Sangregorio, C.; Curri, M.L. Biofunctionalization of Anisotropic Nanocrystalline Semiconductor–Magnetic Heterostructures. Langmuir 2011, 27, 6962–6970. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.; Depalo, N.; de Paola, I.; Iacobazzi, R.M.; Denora, N.; Laquintana, V.; Comparelli, R.; Altamura, E.; Latronico, T.; Altomare, M.; et al. Integrin-targeting with peptide-bioconjugated semiconductor-magnetic nanocrystalline heterostructures. Nano Res. 2016, 9, 644–662. [Google Scholar] [CrossRef]
- Depalo, N.; Mallardi, A.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L. Luminescent nanocrystals in phospholipid micelles for bioconjugation: An optical and structural investigation. J. Colloid Interface Sci. 2008, 325, 558–566. [Google Scholar] [CrossRef]
- Depalo, N.; De Leo, V.; Corricelli, M.; Gristina, R.; Valente, G.; Casamassima, E.; Comparelli, R.; Laquintana, V.; Denora, N.; Fanizza, E.; et al. Lipid-based systems loaded with PbS nanocrystals: Near infrared emitting trackable nanovectors. J. Mater. Chem. B 2017, 5, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Depalo, N.; Valente, G.; Fanizza, E.; Laquintana, V.; Denora, N.; Fasano, A.; Striccoli, M.; Colella, M.; Agostiano, A.; et al. Cytotoxicity Study on Luminescent Nanocrystals Containing Phospholipid Micelles in Primary Cultures of Rat Astrocytes. PLoS ONE 2016, 11, e0153451. [Google Scholar] [CrossRef]
- Panniello, A.; Di Mauro, A.E.; Fanizza, E.; Depalo, N.; Agostiano, A.; Curri, M.L.; Striccoli, M. Luminescent Oil-Soluble Carbon Dots toward White Light Emission: A Spectroscopic Study. J. Phys. Chem. C 2018, 122, 839–849. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Liang, W. Pegylated Phospholipid Micelles Induce Endoplasmic Reticulum-Dependent Apoptosis of Cancer Cells but not Normal Cells. ACS Nano 2012, 6, 5018–5030. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, J.; Ge, Q.; Xing, D.; Zhou, X.; Qian, Y.; Jiang, G. The optimized drug delivery systems of treating cancer bone metastatic osteolysis with nanomaterials. Drug Deliv. 2021, 28, 37–53. [Google Scholar] [CrossRef]
- Giordano, F.; Lenna, S.; Rampado, R.; Brozovich, A.; Hirase, T.; Tognon, M.G.; Martini, F.; Agostini, M.; Yustein, J.T.; Taraballi, F. Nanodelivery Systems Face Challenges and Limitations in Bone Diseases Management. Adv. Ther. 2021, 4, 2100152. [Google Scholar] [CrossRef]
- Di Nunno, L.; Di Nunno, L.; Scilimati, A. Synthesis of 3-aryl-4, 5-dihydro-5-hydroxy-1,2-oxazoles by reaction of substituted benzonitrile oxides with the enolate ion of acetaldehyde. Tetrahedron 1987, 43, 2181–2189. [Google Scholar] [CrossRef]
- Kuljanin, J.; Janković, I.; Nedeljković, J.; Prstojević, D.; Marinković, V. Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe(III) ions. J. Pharm. Biomed. Anal. 2002, 28, 1215–1220. [Google Scholar] [CrossRef]
- Savino, S.; Toscano, A.; Purgatorio, R.; Profilo, E.; Laghezza, A.; Tortorella, P.; Angelelli, M.; Cellamare, S.; Scala, R.; Tricarico, D.; et al. Novel bisphosphonates with antiresorptive effect in bone mineralization and osteoclastogenesis. Eur. J. Med. Chem. 2018, 158, 184–200. [Google Scholar] [CrossRef]
- Wang, S.; Mamedova, N.; Kotov, N.A.; Chen, W.; Studer, J. Antigen/Antibody Immunocomplex from CdTe Nanoparticle Bioconjugates. Nano Lett. 2002, 2, 817–822. [Google Scholar] [CrossRef]
- Reissig, F.; Hübner, R.; Steinbach, J.; Pietzsch, H.-J.; Mamat, C. Facile preparation of radium-doped, functionalized nanoparticles as carriers for targeted alpha therapy. Inorg. Chem. Front. 2019, 6, 1341–1349. [Google Scholar] [CrossRef]
- Liu, X.; Qu, S.; Lu, X.; Ge, X.; Leng, Y. Time-of-flight secondary ion mass spectrometry study on the distribution of alendronate sodium in drug-loaded ultra-high molecular weight polyethylene. Biomed. Mater. 2009, 4, 065008. [Google Scholar] [CrossRef]
- Turhanen, P.A.; Vepsäläinen, J.J.; Peräniemi, S. Advanced material and approach for metal ions removal from aqueous solutions. Sci. Rep. 2015, 5, 8992. [Google Scholar] [CrossRef]
- Ke, J.; Dou, H.; Zhang, X.; Uhagaze, D.S.; Ding, X.; Dong, Y. Determination of pKa values of alendronate sodium in aqueous solution by piecewise linear regression based on acid-base potentiometric titration. J. Pharm. Anal. 2016, 6, 404–409. [Google Scholar] [CrossRef]
- Hengst, V.; Oussoren, C.; Kissel, T.; Storm, G. Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int. J. Pharm. 2007, 331, 224–227. [Google Scholar] [CrossRef]
- Miller, K.; Eldar-Boock, A.; Polyak, D.; Segal, E.; Benayoun, L.; Shaked, Y.; Satchi-Fainaro, R. Antiangiogenic Antitumor Activity of HPMA Copolymer–Paclitaxel–Alendronate Conjugate on Breast Cancer Bone Metastasis Mouse Model. Mol. Pharm. 2011, 8, 1052–1062. [Google Scholar] [CrossRef]
- Ozcan, I.; Bouchemal, K.; Segura-Sánchez, F.; Ozer, O.; Güneri, T.; Ponchel, G. Synthesis and characterization of surface-modified PBLG nanoparticles for bone targeting: In vitro and in vivo evaluations. J. Pharm. Sci. 2011, 100, 4877–4887. [Google Scholar] [CrossRef]
- Torres Martin de Rosales, R.; Finucane, C.; Mather, S.J.; Blower, P.J. Bifunctional bisphosphonate complexes for the diagnosis and therapy of bone metastases. Chem. Commun. 2009, 32, 4847–4849. [Google Scholar] [CrossRef]
- Uludag, H.; Kousinioris, N.; Gao, T.; Kantoci, D. Bisphosphonate Conjugation to Proteins as a Means to Impart Bone Affinity. Biotechnol. Prog. 2000, 16, 258–267. [Google Scholar] [CrossRef]
- Chen, H.; Li, G.; Chi, H.; Wang, D.; Tu, C.; Pan, L.; Zhu, L.; Qiu, F.; Guo, F.; Zhu, X. Alendronate-conjugated amphiphilic hyperbranched polymer based on Boltorn H40 and poly(ethylene glycol) for bone-targeted drug delivery. Bioconjug. Chem. 2012, 23, 1915–1924. [Google Scholar] [CrossRef]
- Habibah, T.U.; Salisbury, H.G. Hydroxyapatite Dental Material. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Beaufils, S.; Rouillon, T.; Millet, P.; Le Bideau, J.; Weiss, P.; Chopart, J.-P.; Daltin, A.-L. Synthesis of calcium-deficient hydroxyapatite nanowires and nanotubes performed by template-assisted electrodeposition. Mater. Sci. Eng. C 2019, 98, 333–346. [Google Scholar] [CrossRef]
- Latronico, T.; Rizzi, F.; Panniello, A.; Laquintana, V.; Arduino, I.; Denora, N.; Fanizza, E.; Milella, S.; Mastroianni, C.M.; Striccoli, M.; et al. Luminescent PLGA Nanoparticles for Delivery of Darunavir to the Brain and Inhibition of Matrix Metalloproteinase-9, a Relevant Therapeutic Target of HIV-Associated Neurological Disorders. ACS Chem. Neurosci. 2021, 12, 4286–4301. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, F.; Panniello, A.; Comparelli, R.; Arduino, I.; Fanizza, E.; Iacobazzi, R.M.; Perrone, M.G.; Striccoli, M.; Curri, M.L.; Scilimati, A.; et al. Luminescent Alendronic Acid-Conjugated Micellar Nanostructures for Potential Application in the Bone-Targeted Delivery of Cholecalciferol. Molecules 2024, 29, 2367. https://doi.org/10.3390/molecules29102367
Rizzi F, Panniello A, Comparelli R, Arduino I, Fanizza E, Iacobazzi RM, Perrone MG, Striccoli M, Curri ML, Scilimati A, et al. Luminescent Alendronic Acid-Conjugated Micellar Nanostructures for Potential Application in the Bone-Targeted Delivery of Cholecalciferol. Molecules. 2024; 29(10):2367. https://doi.org/10.3390/molecules29102367
Chicago/Turabian StyleRizzi, Federica, Annamaria Panniello, Roberto Comparelli, Ilaria Arduino, Elisabetta Fanizza, Rosa Maria Iacobazzi, Maria Grazia Perrone, Marinella Striccoli, Maria Lucia Curri, Antonio Scilimati, and et al. 2024. "Luminescent Alendronic Acid-Conjugated Micellar Nanostructures for Potential Application in the Bone-Targeted Delivery of Cholecalciferol" Molecules 29, no. 10: 2367. https://doi.org/10.3390/molecules29102367
APA StyleRizzi, F., Panniello, A., Comparelli, R., Arduino, I., Fanizza, E., Iacobazzi, R. M., Perrone, M. G., Striccoli, M., Curri, M. L., Scilimati, A., Denora, N., & Depalo, N. (2024). Luminescent Alendronic Acid-Conjugated Micellar Nanostructures for Potential Application in the Bone-Targeted Delivery of Cholecalciferol. Molecules, 29(10), 2367. https://doi.org/10.3390/molecules29102367














