Sol–Gel Synthesis of Silica–Poly (Vinylpyrrolidone) Hybrids with Prooxidant Activity and Antibacterial Properties
Abstract
1. Introduction
2. Results and Discussion
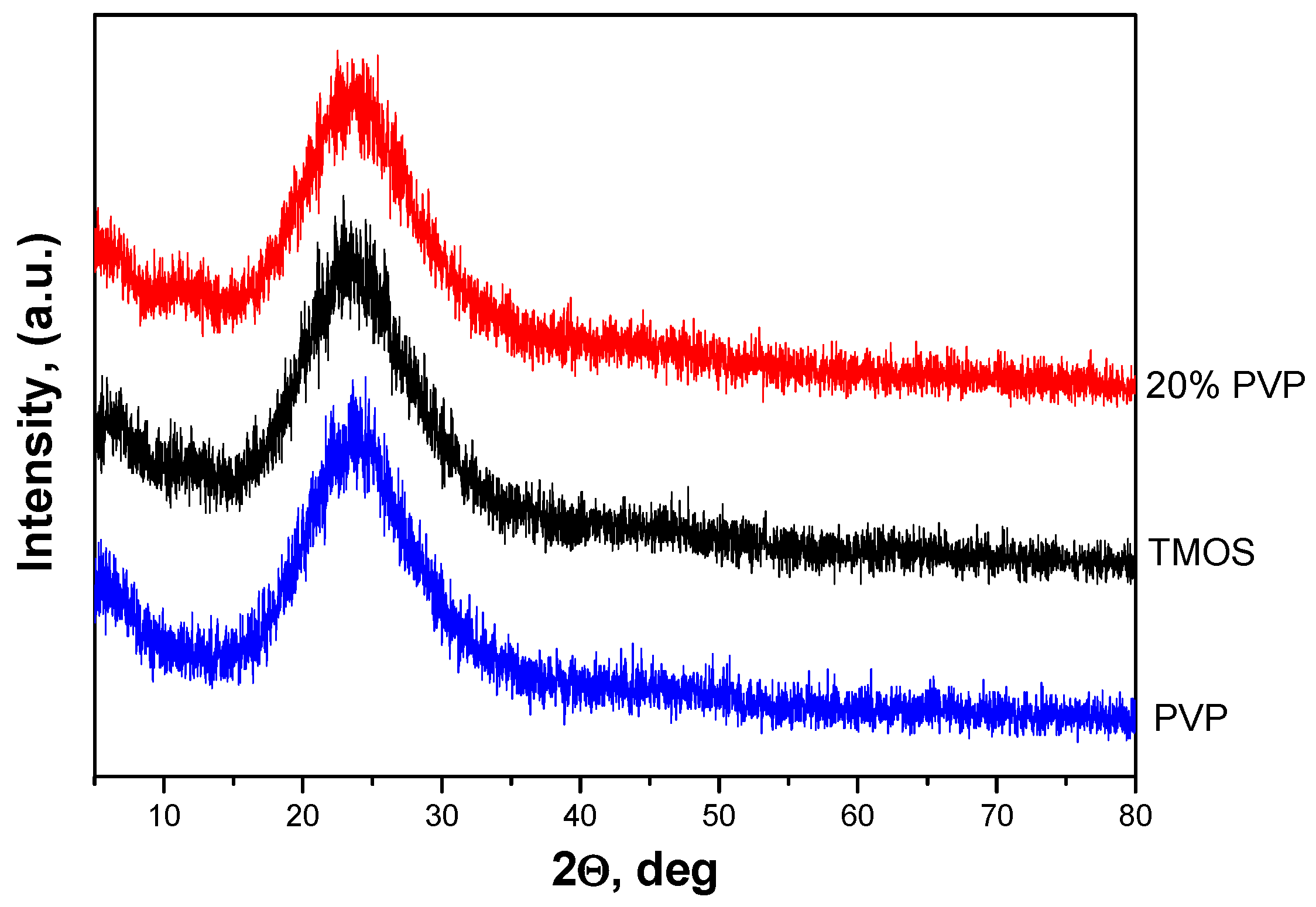
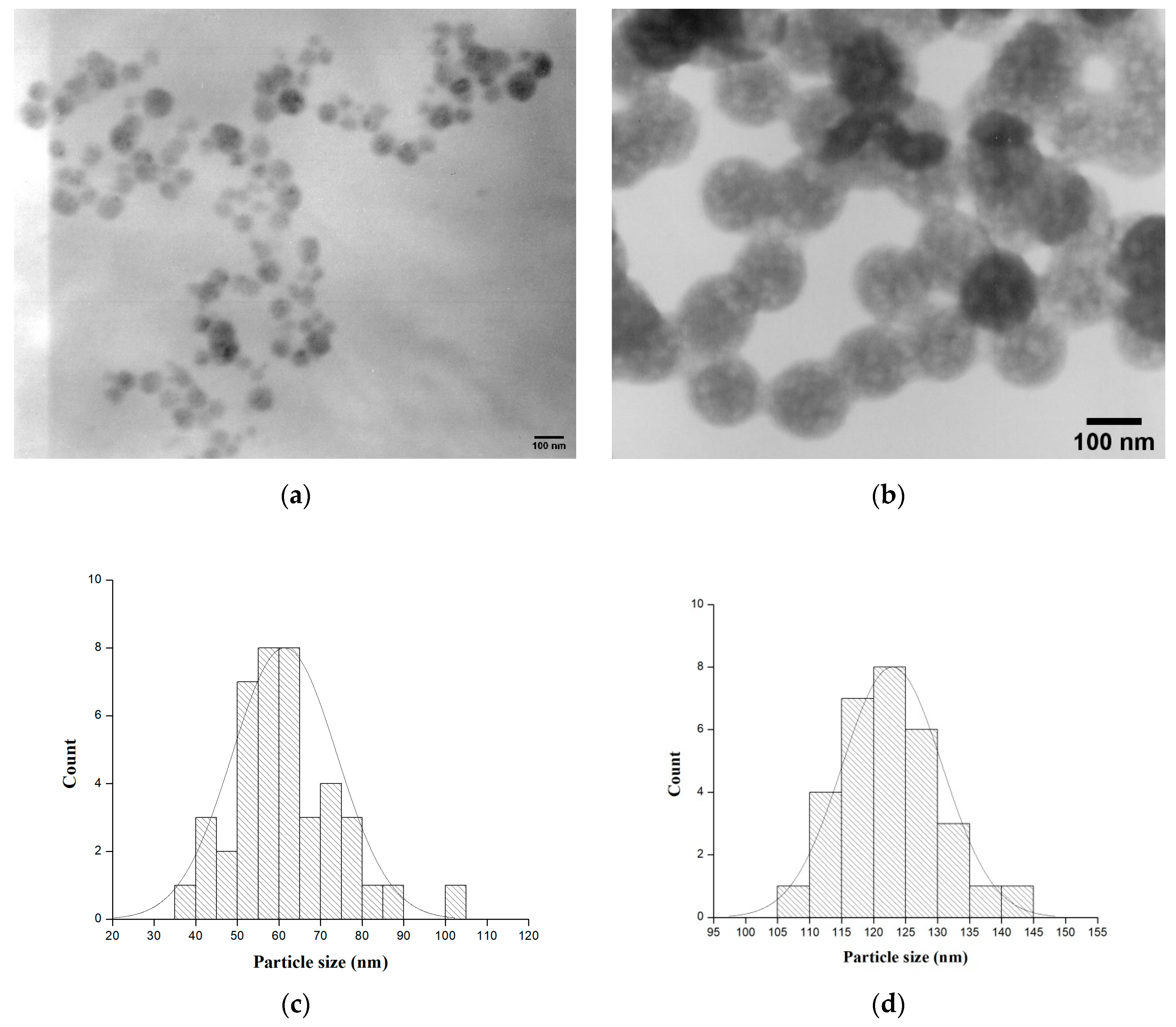
2.1. Phase Transformations, SEM and TEM Observations
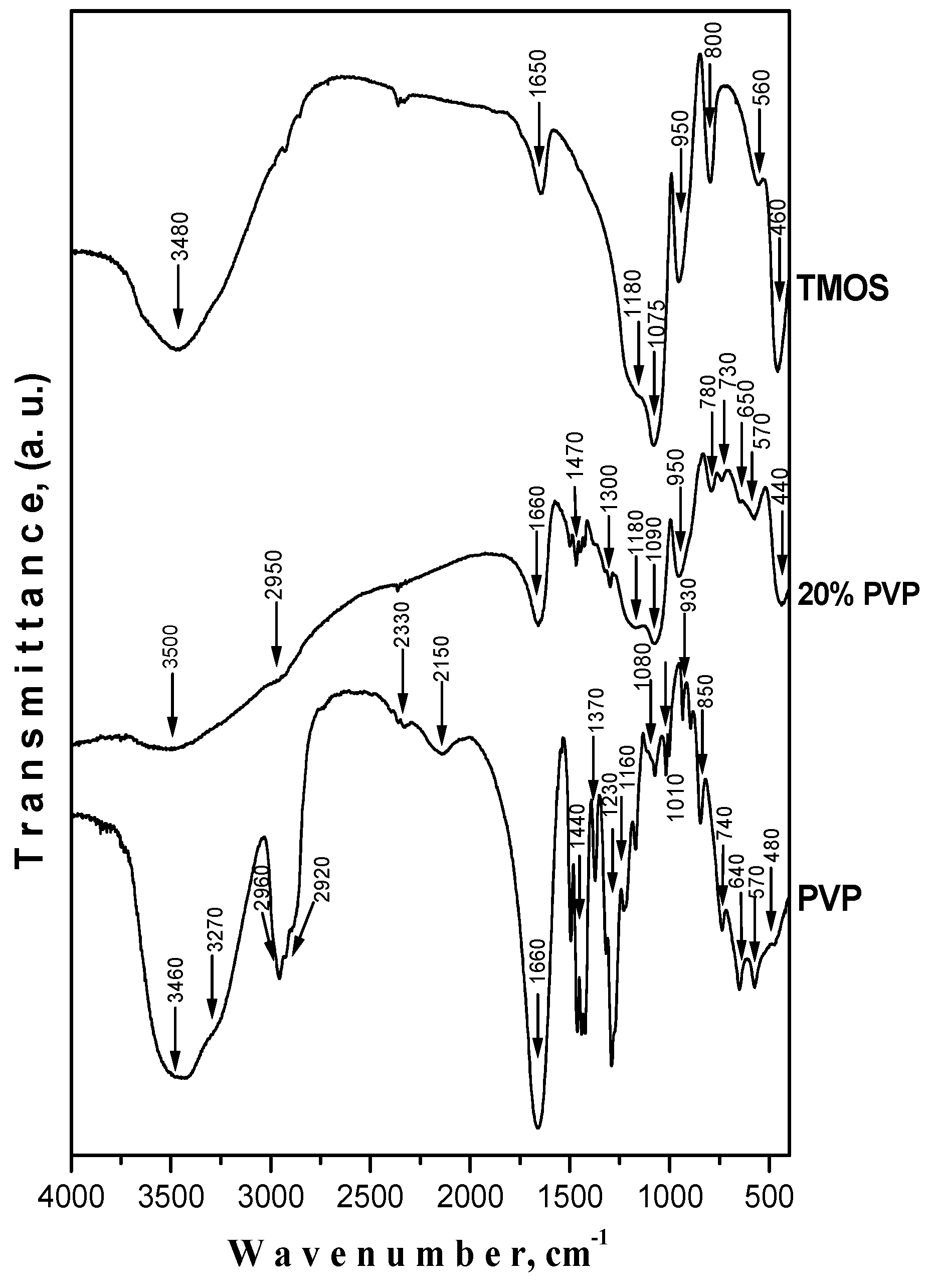
2.2. IR Structural Investigations
2.3. 29Si MAS NMR Spectra
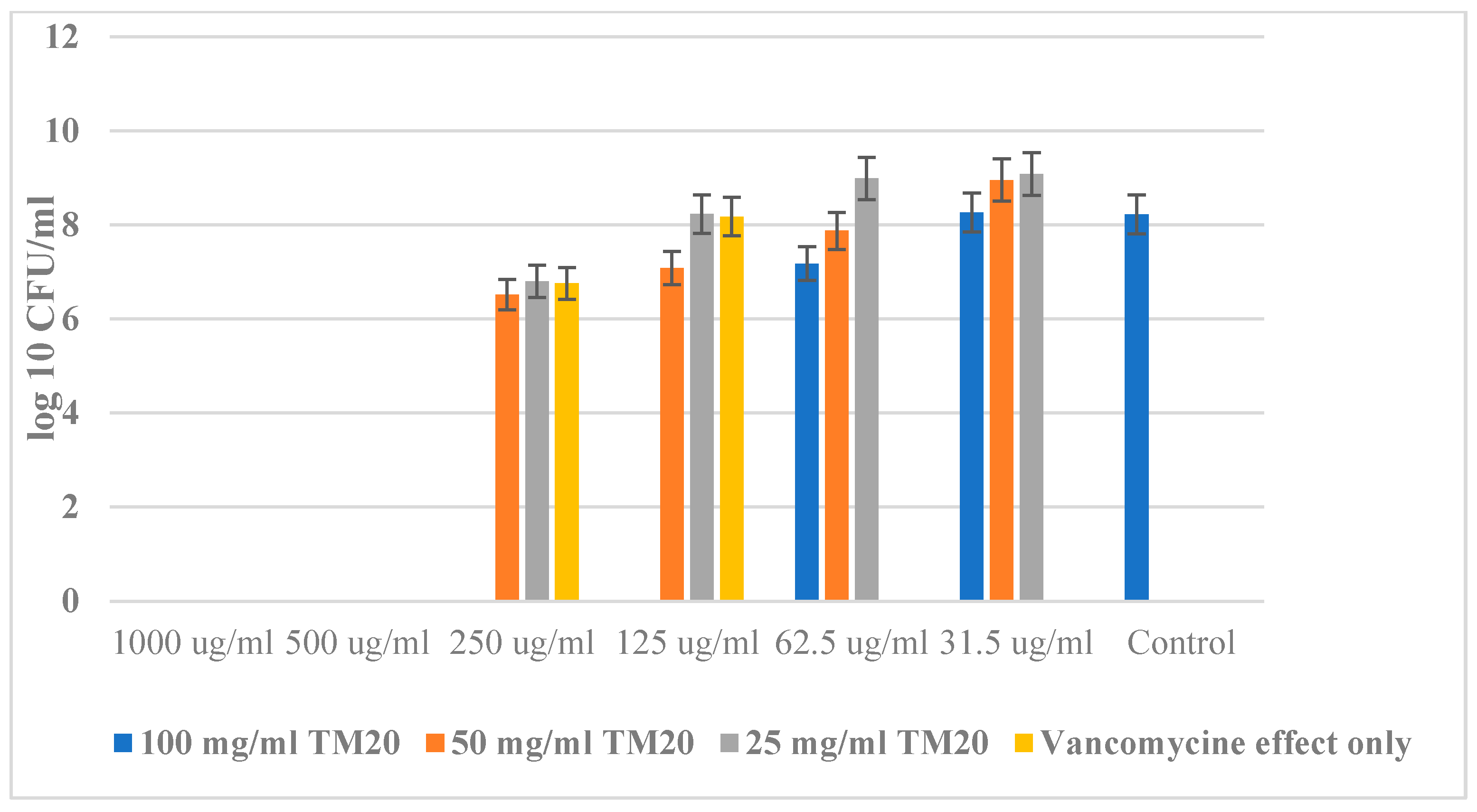
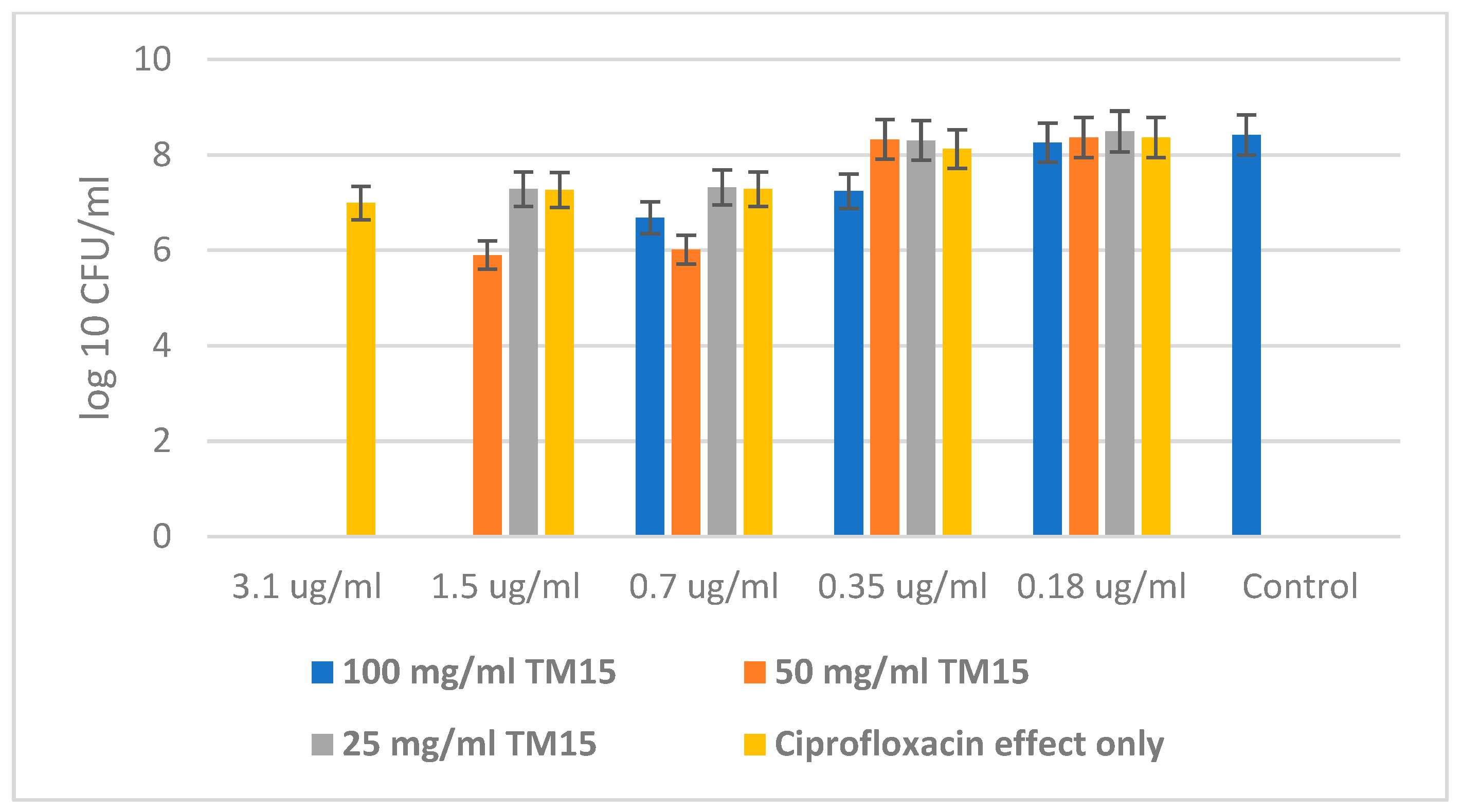
2.4. Antibacterial Properties
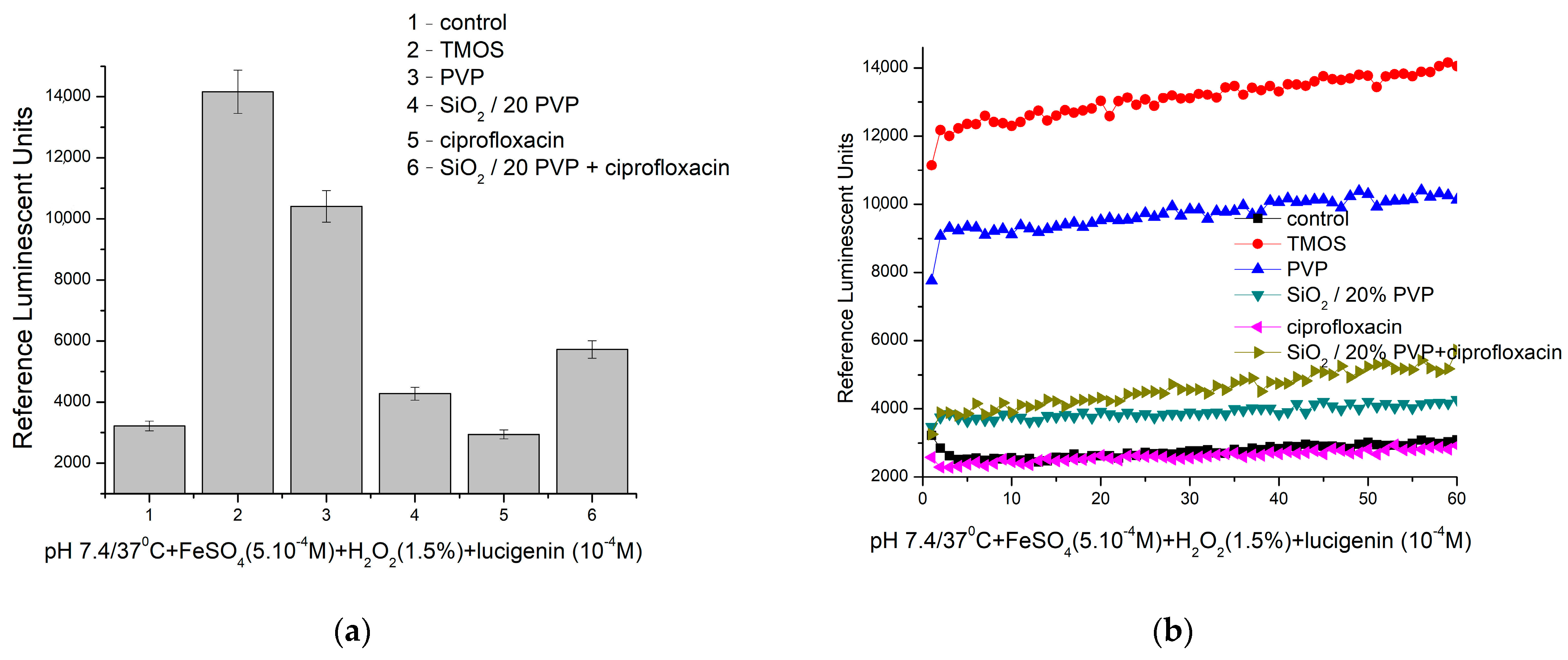
2.5. Chemiluminescence Assay
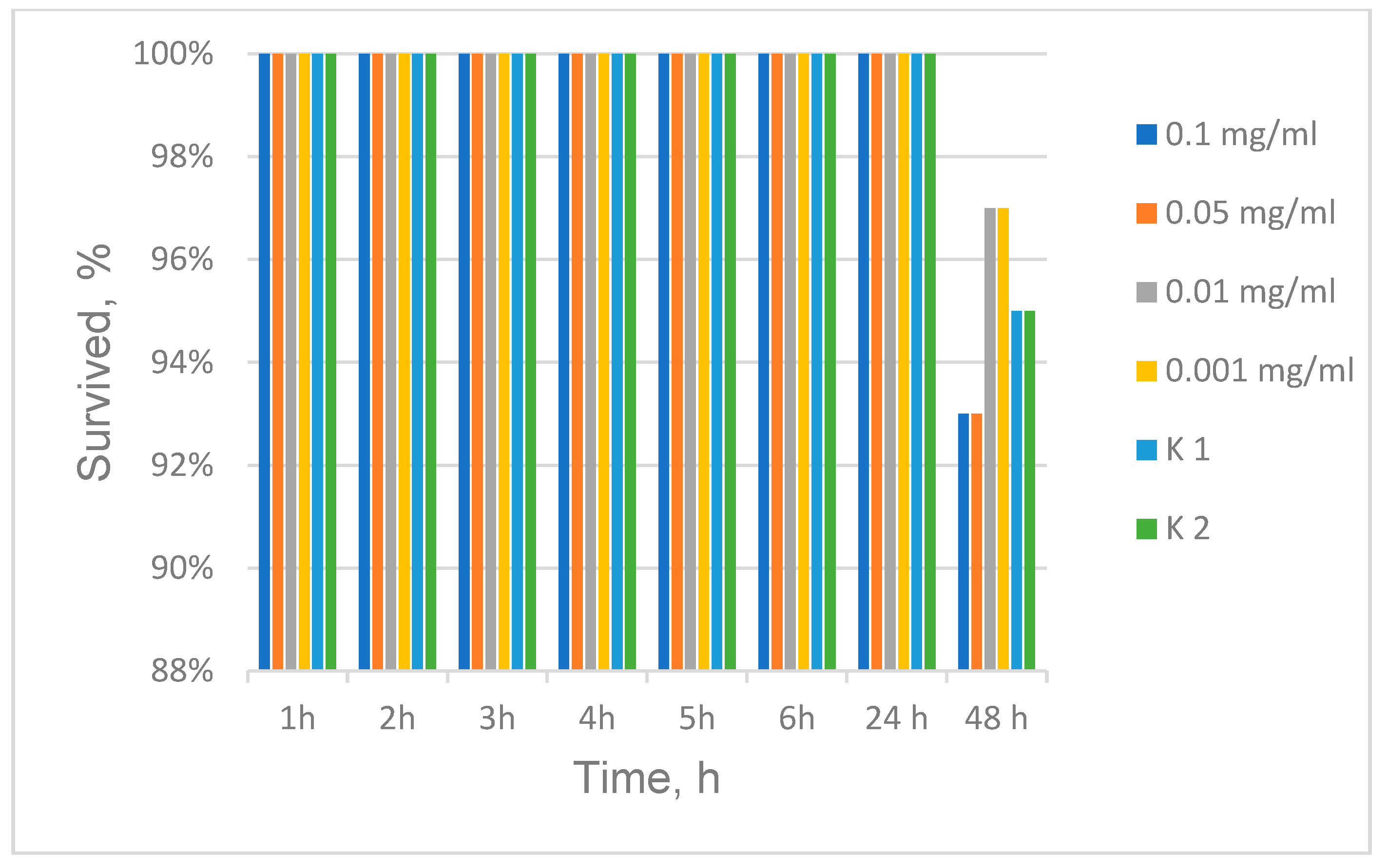
2.6. Daphnia Magna Toxicity
2.7. Analysis of the Results
3. Experimental Procedure
3.1. Materials
3.2. Preparation of Silica–Polyvinylpyrrolidone (SiO2-PVP) Hybrids
3.3. Methods of Characterization
3.4. Antimicrobial Activity Testing
3.5. Methodology for the Evaluation of the Minimum Inhibitory and Minimum Bactericidal Concentration (MBC) and Antibacterial Mode of Inhibition of SiO2/20PVP
3.6. Chemiluminescence Assay
3.6.1. Materials
3.6.2. Method
3.6.3. Statistics
3.7. Daphnia Magna
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babiarczuk, B.; Lewandowski, D.; Szczurek, A.; Kierzek, K.; Meffert, M.; Gerthsen, D.; Jerzy, K.; Krzak, J. Novel approach of silica-PVA hybrid aerogel synthesis by simultaneous sol-gel process and phase separation. J. Supercrit. Fluids 2020, 166, 104997. [Google Scholar] [CrossRef]
- Barrino, F. Hybrid Organic–Inorganic Materials Prepared by Sol–Gel and Sol–Gel-Coating Method for Biomedical Use: Study and Synthetic Review of Synthesis and Properties. Coatings 2024, 14, 425. [Google Scholar] [CrossRef]
- Catauro, M.; Ciprioti, S.V. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef]
- Castrillo, P.; Olmos, D.; Amador, D.; González-Benito, J. Real dispersion of isolated fumed silica nanoparticles in highly filled PMMA prepared by high energy ball milling. J. Colloid Interface Sci. 2007, 308, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Chujo, Y. Organic–inorganic polymer hybrids prepared by the sol-gel method. Compos. Interfaces 2005, 11, 539–566. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Rejab, M.R.; Hamdan, M.H.; Quanjin, M.; Siregar, J.P.; Bachtiar, D.; Muchlis, Y. Historical Development of Hybrid Materials. Mat. Sci. Mat. Eng. 2020, 4, 445–455. [Google Scholar]
- Vichery, C.; Nedelec, J.-M. Bioactive glass nanoparticles: From synthesis to materials design for biomedical applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non Cryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Innocenzi, P. The Sol to Gel Transition; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Schmidt, M.; Schwertfeger, F. Applications for silica aerogel products. J. Non Cryst. Solids 1998, 225, 364–368. [Google Scholar] [CrossRef]
- Kuttor, A.; Szalóki, M.; Rente, T.; Kerényi, F.; Bakó, J.; Fábián, I.; Lázár, I.; Jenei, A.; Hegedü, C. Preparation and Application of Highly Porous Aerogel-based Bioactive Materials in Dentistry. Front. Mater. Sci. 2014, 8, 46–52. [Google Scholar] [CrossRef]
- Pajonk, G.M. Some applications of silica aerogels. Colloid Polym. Sci. 2003, 281, 637–651. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Babooram, K.; Francis, B.; Bissessur, R.; Narain, R. Synthesis and characterization of novel (amide–imide)-silica composites by the sol–gel process. Compos. Sci. Technol. 2008, 68, 617–624. [Google Scholar] [CrossRef]
- Seo, S.-J.; Kim, J.-J.; Kim, J.-H.; Lee, J.-Y.; Shin, U.S.; Lee, E.-J.; Kim, H.-W. Enhanced mechanical properties and bone bioactivity of chitosan/silica membrane by functionalized-carbon nanotube incorporation. Compos. Sci. Technol. 2014, 96, 31–37. [Google Scholar] [CrossRef]
- Gadalla, A.M.; Yun, S.J. Characterization of gels prepared from silicon ethoxide in presence of HCl and HF. J. Non-Cryst. Solids 1992, 143, 121–132. [Google Scholar] [CrossRef]
- Haraguchi, K.; Usami, Y.; Ono, Y. The preparation and characterization of hybrid materials composed of phenolic resin and silica. J. Mater. Sci. 1998, 33, 3337–3344. [Google Scholar] [CrossRef]
- Morikawa, A.; Lyoku, Y.; Kakimoto, M.; Mai, Y. Preparation of Silica-Containing Polyvinylpyrrolidone Films by Sol-Gel Process Polymer. J. Mater. Sci. 1992, 24, 689–692. [Google Scholar]
- Hsiao, C.N.; Huang, K.S. Synthesis, Characterization, and Applications of Polyvinylpyrrolidone/SiO2. Hybrid Materials. J. Appl. Polym. Sci. 2005, 96, 1936–1942. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Singh, S.; Satnalika, N.; Khandelwal, A.; Jeon, S.H. Nanotechnology, big things from a tiny world: A review. Int. J. U E Serv. Sci. Technol. 2009, 2, 29–38. [Google Scholar]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.-T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnology 2022, 375, 1477–3155. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.-I. Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance-Unraveling Mechanisms and Enhancing Medication Efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.M.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. Int. J. Nanomed. 2018, 13, 6779–6790. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.; Kumari, M. Emergence of microbial resistance against nanoparticles: Mechanisms and strategies. Front. Microbiol. 2023, 14, 1102615. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Moradi, F.; Ghaedi, A.; Fooladfar, Z.; Bazrgar, A. Recent advance on nanoparticles or nanomaterials with anti-multidrug resistant bacteria and anti-bacterial biofilm properties: A systematic review. Heliyon 2023, 9, e22105. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Pant, G.; Hossain, K.; Ahmad, A.; Alshammari, M.B. Perspectives on Usage of Functional Nanomaterials in Antimicrobial Therapy for Antibiotic-Resistant Bacterial Infections. ACS Omega 2023, 8, 13492–13508. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale 2020, 15, 190. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Toshkovska, R.D.; Doncheva, T.E.; Ivanova, I.A. Prooxidant and antimicrobic effects of iron and titanium oxide nanoparticles and thalicarpine. Arch. Microbiol. 2020, 202, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.L.; Savov, V.M. Chemiluminescent in vitro estimation of the inhibitory constants of antioxidants ascorbic and uric acids in Fenton’s reaction in urine. Biochemistry 2006, 71, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, K.; Fridovich, I. Luminol and lucigenin as detectors for O2ṡ−. Free. Radic. Biol. Med. 1993, 15, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. Sci. Total Environ. 2021, 763, 143038. [Google Scholar] [CrossRef] [PubMed]
- Hubai, K. Daphnia magna Acute Immobilization Test: An Opportunity to Test the Ecotoxicity of Alternative Fuels. Hung. J. Ind. Chem. 2021, 49, 77–82. [Google Scholar] [CrossRef]
- Briffa, S.M.; Nasser, F.; Valsami, E.; Jones, I.L. Uptake and impacts of polyvinylpyrrolidone (PVP) capped metal oxide nanoparticles on Daphnia magna: Role of core composition and acquired corona. Environ. Sci. Nano 2018, 7, 1745–1756. [Google Scholar] [CrossRef]
- Tarring, E.C.; Robison-Smith, C.; Cable, J.; Durance, I.; Harbottle, M.; Ward, B.D. Detection of Polyvinylpyrrolidone in Daphnia Magna: Development of a Refractive Index Quantification Method for Water-Soluble Polymers in Aquatic Organisms. Sci. Total Environ. 2024, 935, 173428. [Google Scholar] [CrossRef] [PubMed]
- Mondellini, S.; Schott, M.; Löder, M.G.J.; Agarwal, S.; Greiner, A.; Laforsch, C. Beyond microplastics: Water soluble synthetic polymers exert sublethal adverse effects in the fresh water cladoceran Daphnia magna. Sci. Total Environ. 2022, 847, 157608. [Google Scholar] [CrossRef] [PubMed]
- Shahmiri, M.; Bayat, S.; Kharrazi, S. Catalytic performance of PVP-coated CuO nanosheets under environmentally friendly conditions. RSC Adv. 2023, 13, 13213–13223. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, C.; Cao, Y. PVP modified Fe3O4@SiO2 nanoparticles as a new adsorbent for hydrophobic substances. J. Phys. Chem. Solids 2019, 133, 28–34. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Synthesis of poly (vinylpyrrolidone)/Fe3O4@SiO2 nanoporous catalyst by γ-rays and evaluation their sono-photo-Fenton degradation of toluidine blue under magnetic field. Appl. Organomet. Chem. 2021, 35, e6388. [Google Scholar] [CrossRef]
- Ding, D.; Yu, J.; Guo, Q.; Guo, X.; Xiao, X.; Mao, D.; Lu, G. The effects of PVP-modified SiO2 on the catalytic performance of CO hydrogenation over Rh–Mn–Li/SiO2 catalyst. RSC Adv. 2017, 7, 48420. [Google Scholar] [CrossRef]
- Velikova, N.; Vueva, Y.; Ivanova, Y.; Georgieva, R.; Detcheva, A. Synthesis and characterization of sol-gel mesoporous organosilicas functionalized with amine groups. J. Non-Cryst. Solids 2013, 378, 89–95. [Google Scholar] [CrossRef]
- Aengjun, N.; Vibulyaseak, K.; Ogawa, M. Heterostructural transformation of mesoporous silica–titania hybrids. Sci. Rep. 2021, 11, 3210. [Google Scholar] [CrossRef]
- Yang, S.M.; Coombs, N.; Ozin, G.A. Micromolding in inverted polymer opals (MIPO): Synthesis of hexagonal mesoporous silica opals. Adv. Mater. 2000, 12, 1940–1944. [Google Scholar] [CrossRef]
- Diaz, J.F.; Balkus, J.K.; Bedioui, F.; Kurshev, V.; Kevan, L. Synthesis and Characterization of Cobalt-Complex Functionalized MCM-41. Chem. Mater. 1997, 9, 61–67. [Google Scholar] [CrossRef]
- Linsenbuhler, M.; Werth, J.H.; Dammer, S.M.; Knudsen, H.A.; Hinrichsen, H.; Wirth, K.-E.; Wolf, D.E. Cluster size distribution of charged nanopowders in suspensions. Powder Technol. 2006, 167, 124–133. [Google Scholar] [CrossRef]
- Al-Harbi, L.M.; Kosa, S.A.; Baloch, M.K.; Bhatti, Q.A.; El-Mossalamy, E.S. Adsorption of Polyvinylpyrrolidone over the Silica Surface: As Affected by Pretreatment of Adsorbent and Molar Mass of Polymer Adsorbate. Int. J. Polym. Sci. 2016, 2016, 2417292. [Google Scholar] [CrossRef]
- Wahab, M.A.; Kim, I.I.; Ha, C.S. Hybrid periodic mesoporous organosilica materials prepared from 1,2-bis(triethoxysilyl)ethane and (3-cyanopropyl)triethoxysilane. Microporous Mesoporous Mater. 2004, 69, 19–27. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Progress. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Shalaby, A.; Angelova, T.; Bachvarova-Nedelcheva, A.; Georgieva, N.; Iordanova, R.; Staneva, A.; Dimitriev, Y. Sol-gel synthesis of materials in the system SiO2/ZnO/TiO2/RGO and their antimicrobial efficiency against E. coli K12. Comptes Rendus L’academie Bulg. Des. Sci. 2016, 69, 25–30. [Google Scholar]
- Marchetti, A.; Yin, J.; Su, Y.; Kong, X. Solid-state NMR in the field of drug delivery: State of the art and new perspectives. Magn. Reson. Lett. 2021, 1, 28–70. [Google Scholar] [CrossRef]
- Popova, M.; Mihaylova, R.; Momekov, G.; Momekova, D.; Lazarova, H.; Trendafilova, I.; Mitova, V.; Koseva, N.; Mihályi, J.; Shestakova, P.; et al. Verapamil delivery systems on the basis of mesoporous ZSM-5/KIT-6 and ZSM-5/SBA-15 polymer nanocomposites as a potential tool to overcome MDR in cancer cells. Eur. J. Pharm. Biopharm. 2019, 142, 460–472. [Google Scholar] [CrossRef]
- Szegedi, Á.; Shestakova, P.; Trendafilova, I.; Mihályi, J.; Tsacheva, I.; Mitova, V.; Kyulavska, M.; Koseva, N.; Momekova, D.; Konstantinov, S.; et al. Modified mesoporous silica nanoparticles coated by polymer complex as novel curcumin delivery carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 700–712. [Google Scholar] [CrossRef]
- Trendafilova, I.; Szegedi, Á.; Yoncheva, K.; Shestakova, P.; Mihály, J.; Ristic, A.; Konstantinov, S.; Popova, M. A pH dependent delivery of mesalazine from polymer coated and drug-loaded SBA-16 systems. Eur. J. Pharm. Sci. 2016, 81, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, e1900669. [Google Scholar] [CrossRef]
- Llopis-Lorente, A.; Lozano-Torres, B.; Bernardos, A.; Martínez-Máñez, R.; Sancenón, F.J. Mesoporous silica materials for controlled delivery based on enzymes. Mater. Chem. B 2017, 5, 3069–3083. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Tang, F. Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems. Int. Mater. Rev. 2017, 62, 57–77. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez, R.C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.L.; Ivanova, I.A.; Staneva, A.D.; Kostadinova, A.S.; Kichukova, D.G.; Yocheva, L.D. Prooxidant, antioxidant and biological activity of nanocomposites of reduced graphene oxide, silver, copper and their combinations. Chem. Pap. 2022, 76, 6789–6800. [Google Scholar] [CrossRef]
- Poirier, D.G.; Westlake, G.F.; Abernethy, S.G. Daphnia Magna Acute Lethality Toxicity Protocol; Ontario Ministry of the Environment: Toronto, ON, Canada, 1988. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachvarova-Nedelcheva, A.; Kostova, Y.; Yordanova, L.; Nenova, E.; Shestakova, P.; Ivanova, I.; Pavlova, E. Sol–Gel Synthesis of Silica–Poly (Vinylpyrrolidone) Hybrids with Prooxidant Activity and Antibacterial Properties. Molecules 2024, 29, 2675. https://doi.org/10.3390/molecules29112675
Bachvarova-Nedelcheva A, Kostova Y, Yordanova L, Nenova E, Shestakova P, Ivanova I, Pavlova E. Sol–Gel Synthesis of Silica–Poly (Vinylpyrrolidone) Hybrids with Prooxidant Activity and Antibacterial Properties. Molecules. 2024; 29(11):2675. https://doi.org/10.3390/molecules29112675
Chicago/Turabian StyleBachvarova-Nedelcheva, Albena, Yoanna Kostova, Lilia Yordanova, Elena Nenova, Pavletta Shestakova, Iliana Ivanova, and Elitsa Pavlova. 2024. "Sol–Gel Synthesis of Silica–Poly (Vinylpyrrolidone) Hybrids with Prooxidant Activity and Antibacterial Properties" Molecules 29, no. 11: 2675. https://doi.org/10.3390/molecules29112675
APA StyleBachvarova-Nedelcheva, A., Kostova, Y., Yordanova, L., Nenova, E., Shestakova, P., Ivanova, I., & Pavlova, E. (2024). Sol–Gel Synthesis of Silica–Poly (Vinylpyrrolidone) Hybrids with Prooxidant Activity and Antibacterial Properties. Molecules, 29(11), 2675. https://doi.org/10.3390/molecules29112675






