Enhanced Efficiency and Stability of Sky Blue Perovskite Light-Emitting Diodes via Introducing Lead Acetate
Abstract
1. Introduction
2. Results
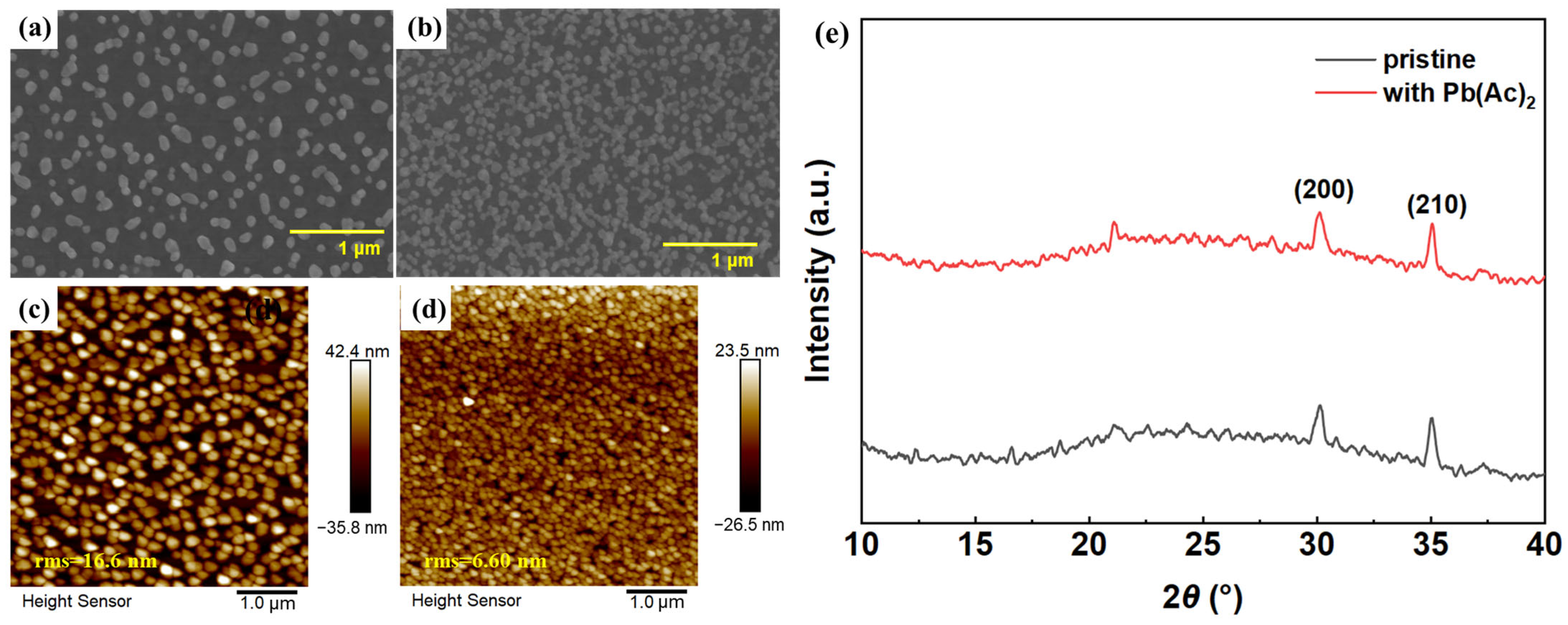
2.1. The Morphology and Film Quality of Perovskite Film
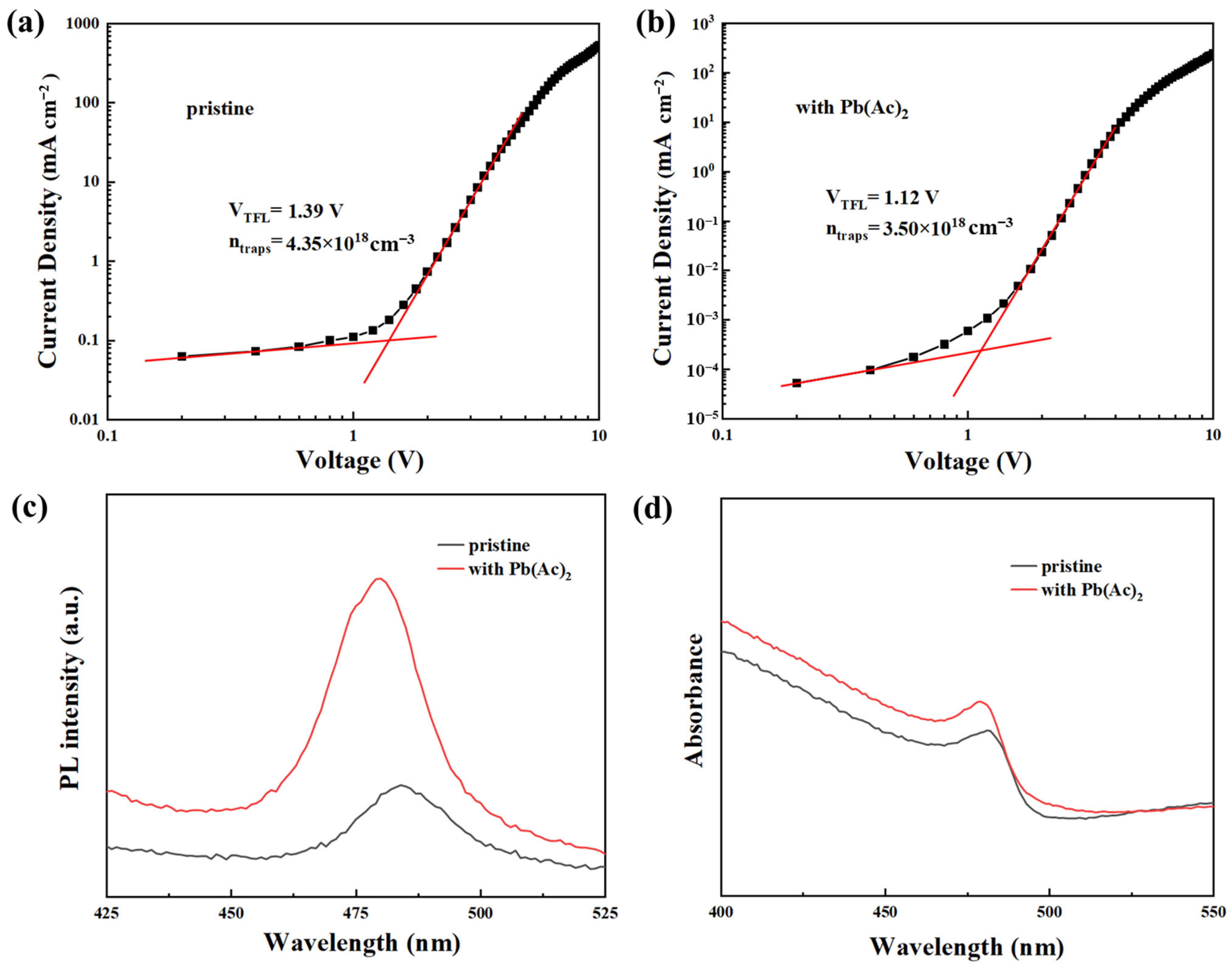
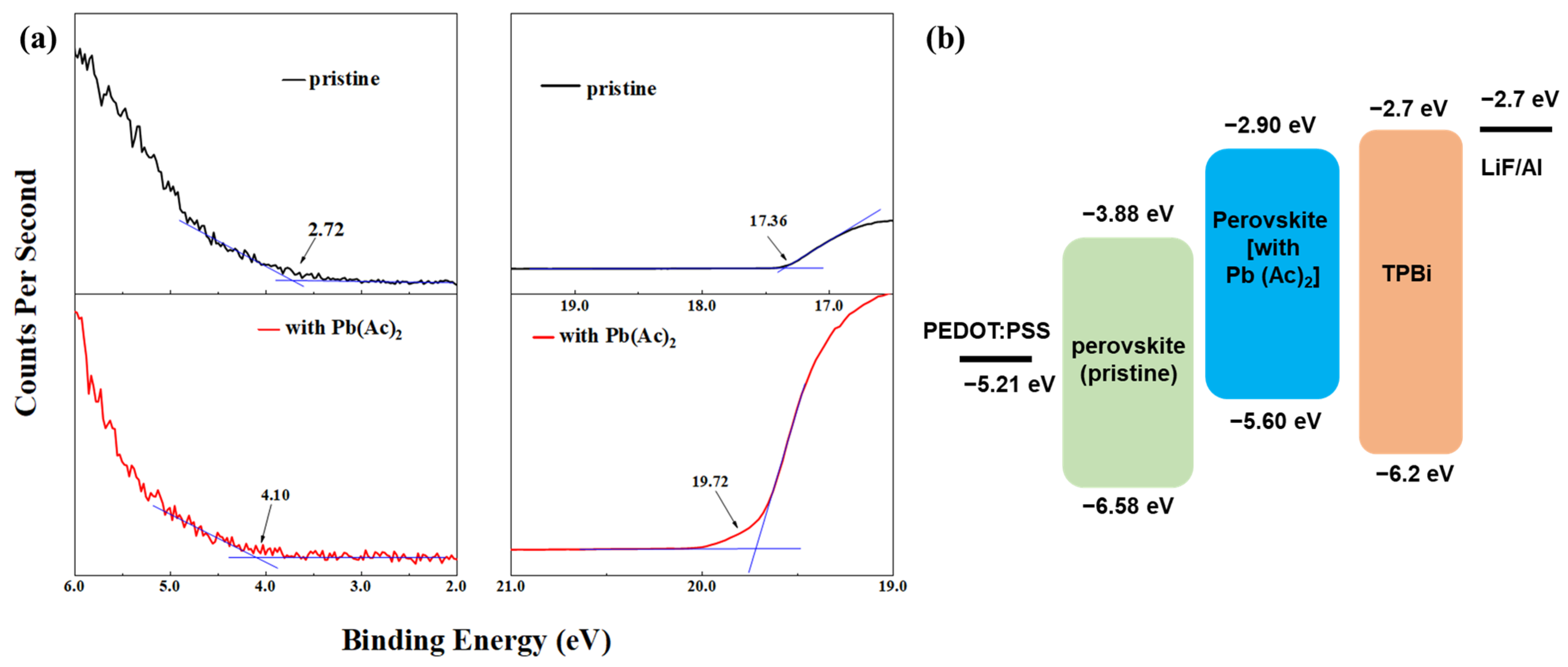
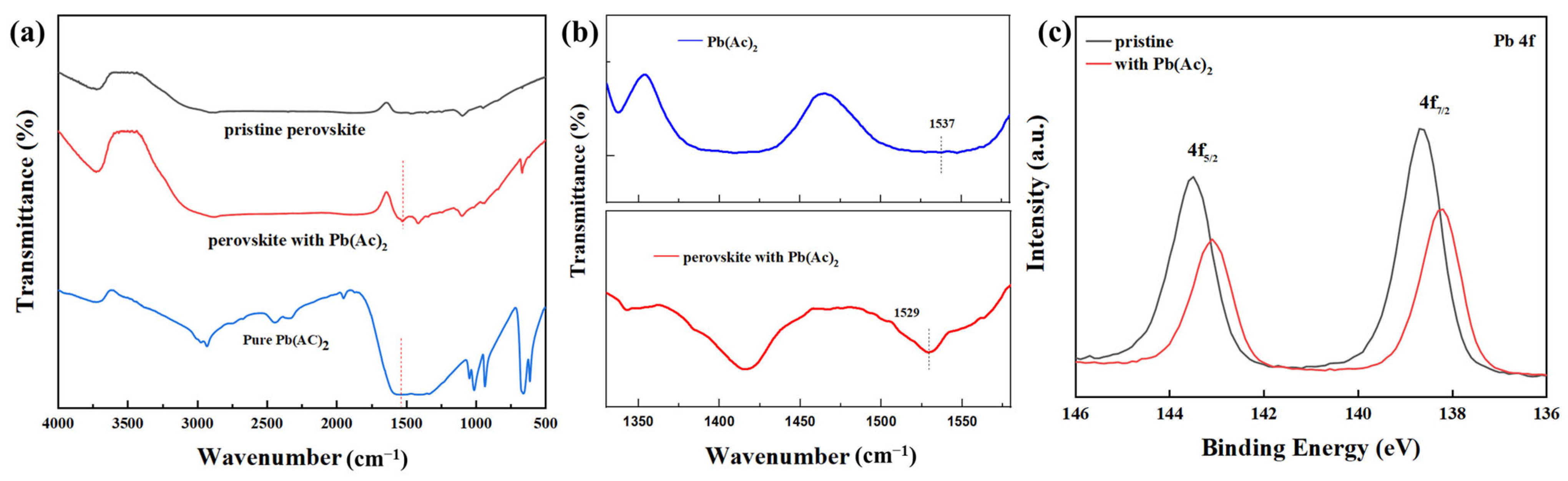
2.2. The Interaction between Pb(Ac)2 and Perovskite
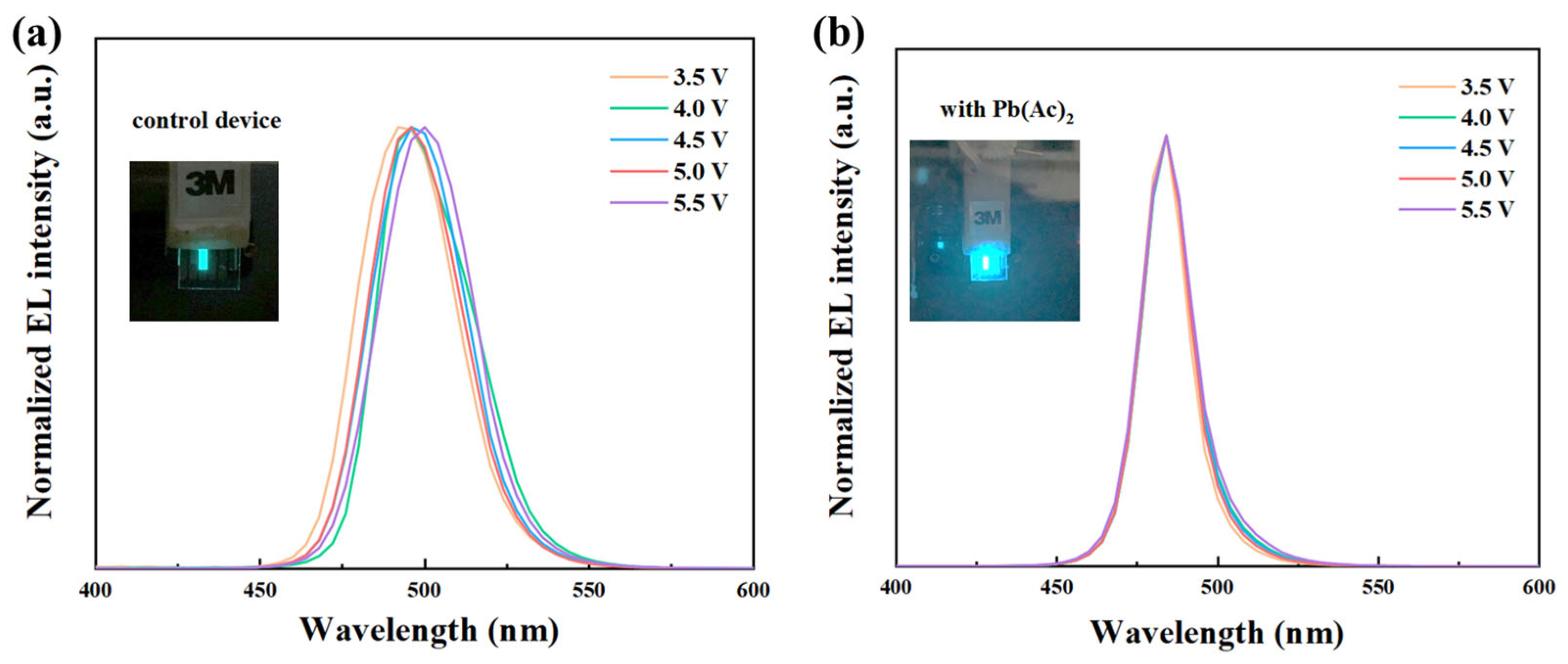
2.3. Device Performance
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Solution Preparation
4.3. Device Fabrication
4.4. Measurement and Characterization
4.5. Preparation for Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deschler, F.; Price, M.; Pathak, S.; Klintberg, L.E.; Jarausch, D.D.; Higler, R.; Huttner, S.; Leijtens, T.; Stranks, S.D.; Snaith, H.J.; et al. High Photoluminescence Efficiency and Optically Pumped Lasing in Solution-Processed Mixed Halide Perovskite Semiconductors. J. Phys. Chem. Lett. 2014, 5, 1421–1426. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Q.; Gu, W.; Tang, Z.; Zhang, B.; Wu, C.; Zhang, X.; Chen, H.; Zhang, X.; Cao, R.; et al. Phase dimensions resolving of efficient and stable perovskite light-emitting diodes at high brightness. Nat. Electron. 2024, 18, 363–370. [Google Scholar] [CrossRef]
- Shi, G.; Huang, Z.; Qiao, R.; Chen, W.; Li, Z.; Li, Y.; Mu, K.; Si, T.; Xiao, Z. Manipulating solvent fluidic dynamics for large-area perovskite film-formation and white light-emitting diodes. Nat. Commun. 2024, 15, 1066. [Google Scholar] [CrossRef]
- Yuan, F.; Folpini, G.; Liu, T.; Singh, U.; Treglia, A.; Lim, J.W.M.; Klarbring, J.; Simak, S.I.; Abrikosov, I.A.; Sum, T.C.; et al. Bright and stable near-infrared lead-free perovskite light-emitting diodes. Nat. Photonics 2024, 18, 170–176. [Google Scholar] [CrossRef]
- Jiang, J.; Chu, Z.; Yin, Z.; Li, J.; Yang, Y.; Chen, J.; Wu, J.; You, J.; Zhang, X. Red Perovskite Light-Emitting Diodes with Efficiency Exceeding 25% Realized by Co-Spacer Cations. Adv. Mater. 2022, 34, 2204460. [Google Scholar] [CrossRef]
- Bai, W.; Xuan, T.; Zhao, H.; Dong, H.; Cheng, X.; Wang, L.; Xie, R.J. Perovskite Light-Emitting Diodes with an External Quantum Efficiency Exceeding 30. Adv. Mater. 2023, 35, 2302283. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, Y.; Wang, D.; Lu, P.; Wang, X.; Lu, M.; Wu, Y.; Chen, P.; Hu, J.; Bai, X.; et al. Phase distribution management for high-efficiency and bright blue perovskite light-emitting diodes. Nano Energy 2024, 120, 109144. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhang, D.; Zhang, Q.; Qiu, X.; Zhou, Y.; Poddar, S.; Fu, Y.; Zhu, Y.; Liao, J.-F.; Shu, L.; et al. High-efficiency, flexible and large-area red/green/blue all-inorganic metal halide perovskite quantum wires-based light-emitting diodes. Nat. Commun. 2023, 14, 4611. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX(3), X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhao, H.; Wang, Y.; Li, Z.; Wang, X.; Cao, W. Research status of all-inorganic perovskite solar cells: A review. J. Solid State Chem. 2023, 328, 124299. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72 % Efficiency. Angew. Chem. Int. Ed. 2018, 130, 3849. [Google Scholar] [CrossRef]
- Taylor, A.D.; Sun, Q.; Goetz, K.P.; An, Q.; Schramm, T.; Hofstetter, Y.; Litterst, M.; Paulus, F.; Vaynzof, Y. A general approach to high-efficiency perovskite solar cells by any antisolvent. Nat. Commun. 2021, 12, 1878. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Zhou, Y.; Liu, H.; Xue, Q.; Li, X.; Jen, A.K. Highly efficient all-inorganic perovskite solar cells with suppressed non-radiative recombination by a Lewis base. Nat. Commun. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Xie, Y.; Fung, M. All-Inorganic Perovskite Solar Cells: Modification Strategies and Challenges. Adv. Energy Sustain. Res. 2024, 2300263. [Google Scholar] [CrossRef]
- Wu, T.; Li, X.; Qi, Y.; Zhang, Y.; Han, L. Defect Passivation for Perovskite Solar Cells: From Molecule Design to Device Performance. ChemSusChem 2021, 14, 4354–4376. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xie, P.; Pan, Z.; Rao, H.; Zhong, X. One-step solution deposition of CsPbBr3 based on precursor engineering for efficient all-inorganic perovskite solar cells. J. Mater. Chem. A 2019, 7, 22420. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, X.; Zheng, G.; Wang, Y.; Li, Y.; Li, B.; Yang, Y.; Liang, J.; Chen, D.; Hou, L.; et al. High-Quality Lead Acetate–Based Ruddlesden–Popper Perovskite Films for Efficient Solar Cells. Solar RRL 2023, 7, 2300111. [Google Scholar] [CrossRef]
- An, H.J.; Kim, Y.C.; Kim, D.H.; Myoung, J.M. High-Performance Green Light-Emitting Diodes Based on MAPbBr3 with pi-Conjugated Ligand. ACS Appl. Mater. Interfaces 2020, 12, 16726–16735. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Yu, Y.; Zhang, W.; Chen, Y.; Zhang, J.; Yang, L.; Feng, J.; Cheng, G.; Liu, L.; Chen, S. Efficient Pure Blue Light-Emitting Diodes Based on CsPbBr3 Quantum-Confined Nanoplates. ACS Appl. Mater. Interfaces 2022, 14, 5682–5691. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Piao, J.; Wang, Y.; Liu, C.; Chen, J.; Cao, K.; Chen, S. Trifunctional Trichloroacetic Acid Incorporated Mixed-Halide Perovskites for Spectrally Stable Blue Light-Emitting Diodes. J. Phys. Chem. Lett. 2023, 14, 4734–4741. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Pan, M.; Liu, G.; Jiang, X.; Wen, X.; Li, W.; Chen, S.; Zhou, W.; Wang, H.; Lu, Y.; et al. Electron-withdrawing organic ligand for high-efficiency all-perovskite tandem solar cells. Nat. Energy 2024, 9, 298–307. [Google Scholar] [CrossRef]
- Yan, N.; Ren, X.; Fang, Z.; Jiang, X.; Xu, Z.; Zhang, L.; Ren, S.; Jia, L.; Zhang, J.; Du, Y.; et al. Ligand-Anchoring-Induced Oriented Crystal Growth for High-Efficiency Lead-Tin Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2201384. [Google Scholar] [CrossRef]
- Polavarapu, L.; Nickel, B.; Feldmann, J.; Urban, A.S. Advances in Quantum-Confined Perovskite Nanocrystals for Optoelectronics. Adv. Energy Mater. 2017, 7, 1700267. [Google Scholar] [CrossRef]
- Dai, L.; Deng, Z.; Auras, F.; Goodwin, H.; Zhang, Z.; Walmsley, J.C.; Bristowe, P.D.; Deschler, F.; Greenham, N.C. Slow carrier relaxation in tin-based perovskite nanocrystals. Nat. Photon. 2021, 15, 696–702. [Google Scholar] [CrossRef]
- Wang, S.; Yao, H.; Zhu, W.; Wu, C.; Tang, Z.; Liu, J.; Ding, L.; Hao, F. Stabilization of Perovskite Lattice and Suppression of Sn2+/Sn4+ Oxidation via Formamidine Acetate for High Efficiency Tin Perovskite Solar Cells. Adv. Funct. Mater. 2023, 33, 2215041. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Gao, P. Lessons learned: How to report XPS data incorrectly about lead-halide perovskites. Mater. Chem. Front. 2023, 7, 3797–3802. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Guo, X.; Lu, C.; Wei, J.; Fang, J. Constructing heterojunctions by surface sulfidation for efficient inverted perovskite solar cells. Science 2022, 375, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, Q.; Wang, Z.; Bai, Y.; Liu, L.; Wang, F.; Zhou, E.; Hayat, T.; Alsaedi, A.; Tan, Z. Efficient Planar Structured Perovskite Solar Cells with Enhanced Open-Circuit Voltage and Suppressed Charge Recombination Based on a Slow Grown Perovskite Layer from Lead Acetate Precursor. ACS Appl. Mater. Interfaces 2017, 9, 41937–41944. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, N.K.; Dey, A.; Kumar, A.; Gopinathan, S.P.; Narasimhan, K.L.; Kabra, D. Band Gap Tuning of CH3NH3PbBr1-xClx3 Hybrid Perovskite for Blue Electroluminescence. ACS Appl. Mater. Interfaces 2015, 7, 13119–13124. [Google Scholar] [CrossRef]
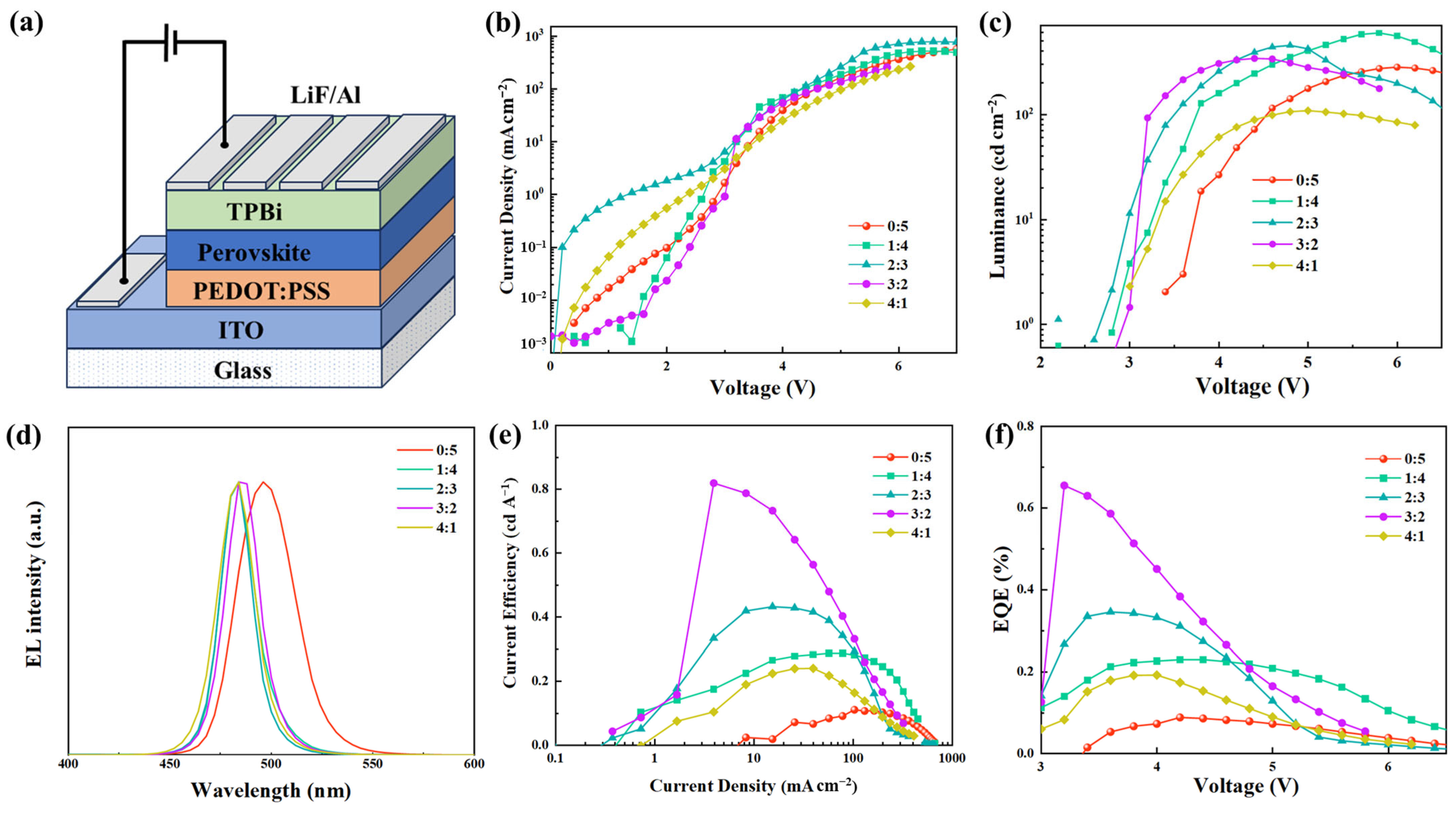
| Pb(AC)2:PbBr2 (Mole Ratio) | Vth (V) | Max. L a (cd m−2) | Max. LE b (cd A−1) | Max. EQE c (%) | EL Peak (nm) | CIE |
|---|---|---|---|---|---|---|
| 0:5 | 3.4 | 281.92 | 0.11 | 0.09 | 496 | (0.065, 0.447) |
| 1:4 | 2.8 | 597.17 | 0.29 | 0.23 | 484 | (0.086, 0.209) |
| 2:3 | 2.6 | 454.55 | 0.43 | 0.35 | 484 | (0.090, 0.181) |
| 3:2 | 2.8 | 341.79 | 0.82 | 0.66 | 484 | (0.080, 0.224) |
| 4:1 | 3.0 | 108.62 | 0.24 | 0.19 | 484 | (0.093, 0.178) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Niu, Q.; Chai, B.; Xiong, J.; Chen, Y.; Zeng, W.; Peng, X.; Iwuoha, E.I.; Xia, R. Enhanced Efficiency and Stability of Sky Blue Perovskite Light-Emitting Diodes via Introducing Lead Acetate. Molecules 2024, 29, 2425. https://doi.org/10.3390/molecules29112425
Zhang Z, Niu Q, Chai B, Xiong J, Chen Y, Zeng W, Peng X, Iwuoha EI, Xia R. Enhanced Efficiency and Stability of Sky Blue Perovskite Light-Emitting Diodes via Introducing Lead Acetate. Molecules. 2024; 29(11):2425. https://doi.org/10.3390/molecules29112425
Chicago/Turabian StyleZhang, Zequan, Qiaoli Niu, Baoxiang Chai, Junhao Xiong, Yuqing Chen, Wenjin Zeng, Xinwen Peng, Emmanuel Iheanyichukwu Iwuoha, and Ruidong Xia. 2024. "Enhanced Efficiency and Stability of Sky Blue Perovskite Light-Emitting Diodes via Introducing Lead Acetate" Molecules 29, no. 11: 2425. https://doi.org/10.3390/molecules29112425
APA StyleZhang, Z., Niu, Q., Chai, B., Xiong, J., Chen, Y., Zeng, W., Peng, X., Iwuoha, E. I., & Xia, R. (2024). Enhanced Efficiency and Stability of Sky Blue Perovskite Light-Emitting Diodes via Introducing Lead Acetate. Molecules, 29(11), 2425. https://doi.org/10.3390/molecules29112425







