Eliciting Polyphenols in Strawberry Leaves: Preliminary Experiments in Fragaria × ananassa cv. Festival
Abstract
:1. Introduction
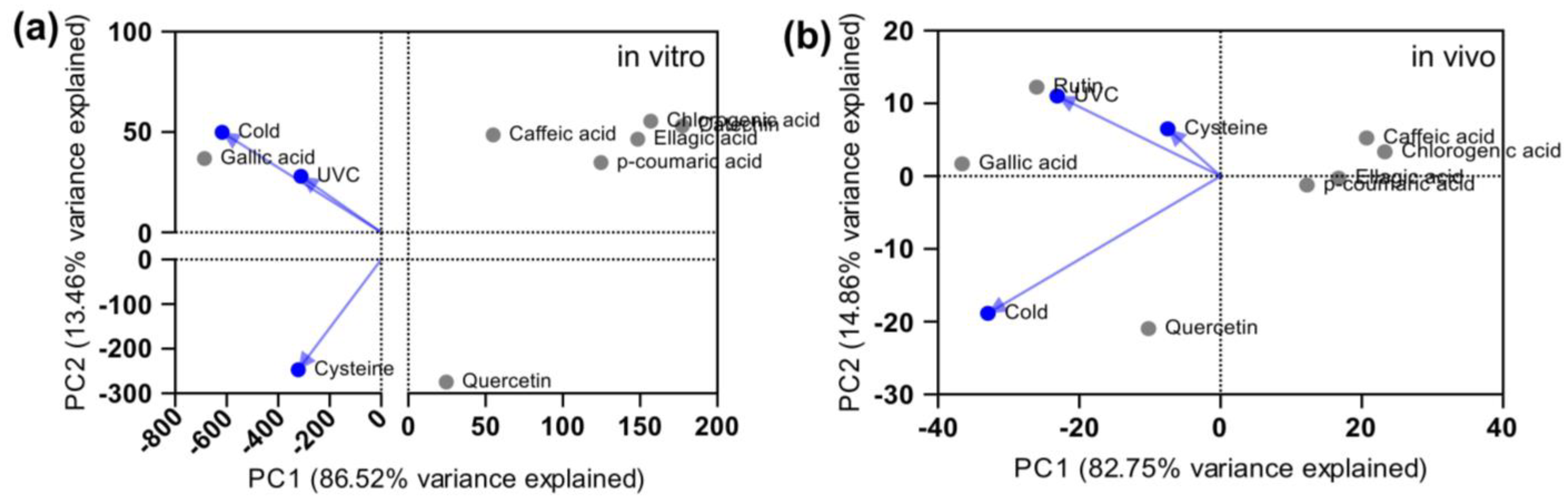
2. Results and Discussion
3. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ. Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, H.M.; Ma, Y.X.; Wang, X.D. Developments in Extraction, Purification, and Structural Elucidation of Proanthocyanidins (2000–2019). Stud. Nat. Prod. Chem. 2021, 68, 347–391. [Google Scholar]
- Gündüz, K. Chapter 30 - Strawberry: Phytochemical Composition of Strawberry (Fragaria × ananassa). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 733–752. [Google Scholar]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria genus: Chemical composition and biological activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Extracts from strawberry by-products rich in phenolic compounds reduce the activity of apple polyphenol oxidase. LWT Food Sci. Technol. 2020, 133, 110097. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Strawberry agro-industrial by-products as a source of bioactive compounds: Effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour. Bioprocess. 2021, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria × ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules 2023, 28, 1865. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Lobo-Vázquez, M.; Gómez-González, J.C.; Arnáez-Serrano, E.; Zamora-Fallas, G.; Sánchez-Zúñiga, K.; Centeno-Cerdas, C. Bioactive potential of tropical highland apple (Malus domestica cv. Anna) crude extract: Opportunities for food waste revalorization. Future J. Pharm. Sci. 2022, 8, 57. [Google Scholar] [CrossRef]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.M.; Al-Khayri, J.M. Impact of Abiotic Elicitors on In vitro Production of Plant Secondary Metabolites: A Review. J. Adv. Res. Biotechnol. 2016, 1, 1–7. [Google Scholar]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Cabrera-De la Fuente, M.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry Biostimulation: From Mechanisms of Action to Plant Growth and Fruit Quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Kousar, S.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Anjum, S. Nano-elicitation as an effective and emerging strategy for in vitro production of industrially important flavonoids. Appl. Sci. 2021, 11, 1694. [Google Scholar] [CrossRef]
- Guru, A.; Dwivedi, P.; Kaur, P.; Pandey, D.K. Exploring the role of elicitors in enhancing medicinal values of plants under in vitro condition. S. Afr. J. Bot. 2022, 149, 1029–1043. [Google Scholar] [CrossRef]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Kotsupiy, O.; Karpova, E.; Trofimova, E.; Novikova, T.; Ambros, E. Transformation of Strawberry Plants’ Phenolic Profile after Treatment with a Mechanocomposite Based on Silicon Chelates in the Course of Development under In Vitro, Ex Vitro, and In Vivo Conditions. Horticulturae 2023, 9, 157. [Google Scholar] [CrossRef]
- Koehler, G.; Rohloff, J.; Wilson, R.C.; Kopka, J.; Erban, A.; Winge, P.; Bones, A.M.; Davik, J.; Alsheikh, M.K.; Randall, S.K. Integrative “omic” analysis reveals distinctive cold responses in leaves and roots of strawberry, Fragaria × ananassa ‘Korona’. Front. Plant Sci. 2015, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Ishibashi, M.; Fukuda, I.; Okino, A.; Osawa, R.; Uno, Y. Pre- and Post-Harvest Conditions Affect Polyphenol Content in Strawberry (Fragaria × ananassa). Plants 2022, 11, 2220. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Bonilla, V.; Abdelnour-Esquivel, A. Protocolo de micropropagación de arándano nativo de Costa Rica (Vaccinium consanguinium). Rev. Tecnol. Marcha 2018, 31, 144–159. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant 1962, 15, 474–497. [Google Scholar] [CrossRef]
- Santiago, R.; Reid, L.M.; Arnason, J.T.; Zhu, X.Y.; Martinez, N.; Malvar, R.A. Phenolics in maize genotypes differing in susceptibility to Gibberella stalk rot (Fusarium graminearum Schwabe). J. Agric. Food Chem. 2007, 55, 5186–5193. [Google Scholar] [CrossRef]
- Lux, P.E.; Freiling, M.; Stuetz, W.; Von Tucher, S.; Carle, R.; Steingass, C.B.; Frank, J. (Poly)phenols, Carotenoids, and Tocochromanols in Corn (Zea mays L.) Kernels As Affected by Phosphate Fertilization and Sowing Time. J. Agric. Food Chem. 2020, 68, 612–622. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Pérez, A.M.; Vaillant, F.; Pineda-Castro, M.L. Physicochemical and Antioxidant Composition of Fresh Peach Palm (Bactris gasipaes Kunth) Fruits in Costa Rica. Braz. J. Food Technol. 2016, 19, e2015097. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
| Treatment | Total Polyphenols (mg GAE g−1 DW) | |
|---|---|---|
| In Vitro | In Vivo | |
| Untreated control | 212.27 * | 116.18 ± 17.06 |
| UVC (254 nm) | 250.45 ± 119.94 | 117.54 ± 13.94 |
| Cold (4 °C) | 203.33 ± 35.09 | 112.77 ± 19.12 |
| Cysteine (200 mg L−1) | 215.90 ± 81.30 | 110.54 ± 16.75 |
| Peak | Compound | Retention Time (min) | UV/Vis Wavelength (nm) | [M–H]:Ion Products (m/z) | Polyphenol Concentration in µg mg−1 DW | |||
|---|---|---|---|---|---|---|---|---|
| Control | UVC (254 nm) | Cold (4 °C) | Cysteine (200 mg L−1) | |||||
| 1 | Gallic acid | 2.06 | 201/220/270 | 169:79, 81, 125 | 132.48 | 355.34 ± 69.43 | 690.03 ± 250.47 | 379.62 ± 38.53 |
| 2 | (+)-Catechin | 7.13 | 201/278 | 289:203, 205, 245 | 0.0019 | 0.003 ± 0.0006 | 0.002 ± 0.0003 | 0.002 ± 0.0001 |
| 3 | Caffeic acid | 8.51 | 216/240/322 | 179:107, 117, 135 | 9.99 | 49.95 ± 9.77 | 96.93 ± 31.47 | 55.81 ± 12.20 |
| 4 | Chlorogenic acid | 8.69 | 215/320 | 353:135, 179, 191 | n.d. | 15.05 ± 1.36 | 14.18 ± 0.81 | 6.66 ± 1.39 |
| 5 | p-coumaric acid | 10.51 | 209/310 | 163:93, 117, 119 | 11.30 | 26.13 ± 4.39 | 31.53 ± 14.87 | 39.00 ± 14.02 |
| 7 | Ellagic acid | 11.15 | 196/254/366 | 301:185, 229, 257 | 11.39 | 22.58 ± 5.56 | 14.66 ± 9.25 | 18.45 ± 2.84 |
| 8 | Quercetin | 13.91 | 201/255/370 | 301:121, 151 | 23.82 | n.d. | n.d. | 361.79 ± 54.98 |
| Peak | Compound | Retention Time (min) | UV/Vis Wavelength (nm) | [M–H]:Ion Products (m/z) | Polyphenol Concentration in µg mg−1 DW Extract | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Insoluble Fraction | Soluble Fraction | |||||||||
| UVC | Cold | Cysteine | UVC | Cold | Cysteine | |||||
| 1 | Gallic acid | 2.06 | 201/220/270 | 169:79, 81, 125 | 35.01 ± 6.74 | 51.17 ± 11.49 | 5.47 ± 0.72 | 0.58 ± 0.26 | 0.35 ± 0.12 | 0.26 ± 0.03 |
| 3 | Caffeic acid | 8.51 | 216/240/322 | 179:107, 117, 135 | 2.41 ± 0.58 | 3.66 ± 0.56 | 2.03 ± 0.56 | 0.36 ± 0.27 | 0.37 ± 0.08 | 3.66 ± 0.56 |
| 4 | Chlorogenic acid | 8.69 | 215/320 | 353:135, 179, 191 | n.d. | 2.61 ± 0.75 | n.d. | 1.30 ± 0.66 | 0.51 ± 0.15 | 0.82 ± 0.22 |
| 5 | p-coumaric acid | 10.51 | 209/310 | 163:93, 117, 119 | 2.31 ± 0.23 | 14.32 ± 6.33 | 1.61 ± 0.08 | 0.66 ± 0.01 | 0.86 ± 0.36 | 0.35 ± 0.17 |
| 6 | Rutin | 10.63 | 201/256/355 | 609:151, 255, 271, 300, 301 | 30.52 ± 3.02 | 37.92 ± 14.13 | 19.87 ± 1.30 | 4.36 ± 0.67 | 1.89 ± 0.65 | 3.52 ± 0.43 |
| 7 | Ellagic acid | 11.15 | 196/254/366 | 301:185, 229, 257 | 0.65 ± 0.29 | 10.12 ± 4.50 | 0.76 ± 0.40 | 0.01 ± 0.01 | 0.19 ± 0.19 | 0.001 ± 0.001 |
| 8 | Quercetin | 13.91 | 201/255/370 | 301:121, 151 | n.d. | 44.19 ± 9.37 | n.d. | 0.13 ± 0.01 | 0.16 ± 0.01 | 0.12 ± 0.01 |
| Parameter | Control | UVC (254 nm) | Cold (4 °C) | Cysteine (200 mg L−1) |
|---|---|---|---|---|
| Length of the longest root (cm) | 0.89 ± 0.93 | 1.49 ± 0.90 | 1.39 ± 0.48 | 1.33 ± 0.81 |
| Length of the longest stem (cm) | 2.14 ± 0.66 | 2.93 ± 0.99 | 2.66 ± 1.01 | 2.49 ± 0.89 |
| Length of the longest leaf (cm) | 0.94 ± 0.25 | 1.23 ± 0.26 * | 1.21 ± 0.30 * | 1.10 ± 0.32 |
| Width of the longest leaf (cm) | 0.99 ± 0.23 | 1.18 ± 0.29 | 1.06 ± 0.31 | 1.15 ± 0.33 |
| Total amount of leaves | 19.50 ± 5.10 | 38.53 ± 24.9 * | 25.93 ± 9.22 | 21.30 ± 4.49 |
| Total amount of buds | 3.00 ± 0.82 | 5.47 ± 2.74 * | 3.87 ± 1.76 | 4.47 ± 1.41 |
| Fresh weight of leaves (g) | 0.55 ± 0.28 | 0.59 ± 0.42 | 0.39 ± 0.37 | 0.36 ± 0.24 |
| Parameter | Control | UVC (254 nm) | Cold (4 °C) | Cysteine (200 mg L−1) |
|---|---|---|---|---|
| Length of the longest root (cm) | 11.30 ± 1.47 | 10.78 ± 1.63 | 14.08 ± 2.66 * | 13.68 ± 0.85 |
| Length of the longest stem (cm) | 7.28 ± 0.54 | 8.96 ± 1.35 | 9.52 ± 0.39 * | 9.07 ± 1.15 * |
| Length of the longest leaf (cm) | 3.47 ± 0.37 | 3.20 ± 0.49 | 3.63 ± 0.16 | 3.50 ± 0.62 |
| Width of the longest leaf (cm) | 3.03 ± 0.20 | 2.78 ± 0.37 | 3.20 ± 0.20 | 2.95 ± 0.36 |
| Total amount of leaves | 12.67 ± 3.14 | 13.50 ± 1.64 | 14.33 ± 3.50 | 16.00 ± 4.10 |
| Total amount of buds | 4.33 ± 1.21 | 5.17 ± 0.75 | 5.33 ± 1.21 | 5.83 ± 2.04 |
| Fresh weight of leaves (g) | 1.08 ± 0.21 | 0.98 ± 0.23 | 1.37 ± 0.41 | 1.35 ± 0.59 |
| Treatment | Disinfectant Steps | Working Concentration | Incubation Time in Agitation (min) |
|---|---|---|---|
| A | Surgical soap | - | 10 |
| Water | - | 5 | |
| Agri-mycin® + Afungil® | 5 g L−1, 4 g L−1 | 15 | |
| NaClO | 3% | 15 | |
| B | Surgical soap | - | 10 |
| Water | - | 5 | |
| Agri-mycin® + Afungil® | 0.8 g L−1, 0.8 g L−1 | 15 | |
| Gamba Oxi | 40% | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Arias, K.; Irías-Mata, A.; Sánchez-Calvo, L.; Brenes-Zárate, M.F.; Abdelnour-Esquivel, A.; Villalta-Romero, F.; Calvo-Castro, L.A. Eliciting Polyphenols in Strawberry Leaves: Preliminary Experiments in Fragaria × ananassa cv. Festival. Molecules 2024, 29, 2467. https://doi.org/10.3390/molecules29112467
Salas-Arias K, Irías-Mata A, Sánchez-Calvo L, Brenes-Zárate MF, Abdelnour-Esquivel A, Villalta-Romero F, Calvo-Castro LA. Eliciting Polyphenols in Strawberry Leaves: Preliminary Experiments in Fragaria × ananassa cv. Festival. Molecules. 2024; 29(11):2467. https://doi.org/10.3390/molecules29112467
Chicago/Turabian StyleSalas-Arias, Karla, Andrea Irías-Mata, Laura Sánchez-Calvo, María Fernanda Brenes-Zárate, Ana Abdelnour-Esquivel, Fabián Villalta-Romero, and Laura A. Calvo-Castro. 2024. "Eliciting Polyphenols in Strawberry Leaves: Preliminary Experiments in Fragaria × ananassa cv. Festival" Molecules 29, no. 11: 2467. https://doi.org/10.3390/molecules29112467
APA StyleSalas-Arias, K., Irías-Mata, A., Sánchez-Calvo, L., Brenes-Zárate, M. F., Abdelnour-Esquivel, A., Villalta-Romero, F., & Calvo-Castro, L. A. (2024). Eliciting Polyphenols in Strawberry Leaves: Preliminary Experiments in Fragaria × ananassa cv. Festival. Molecules, 29(11), 2467. https://doi.org/10.3390/molecules29112467








