Upregulated Palmitoleate and Oleate Production in Escherichia coli Promotes Gentamicin Resistance
Abstract
:1. Introduction
2. Results
2.1. Changes in the Metabolic Profiling of Gentamicin-Resistant E. coli
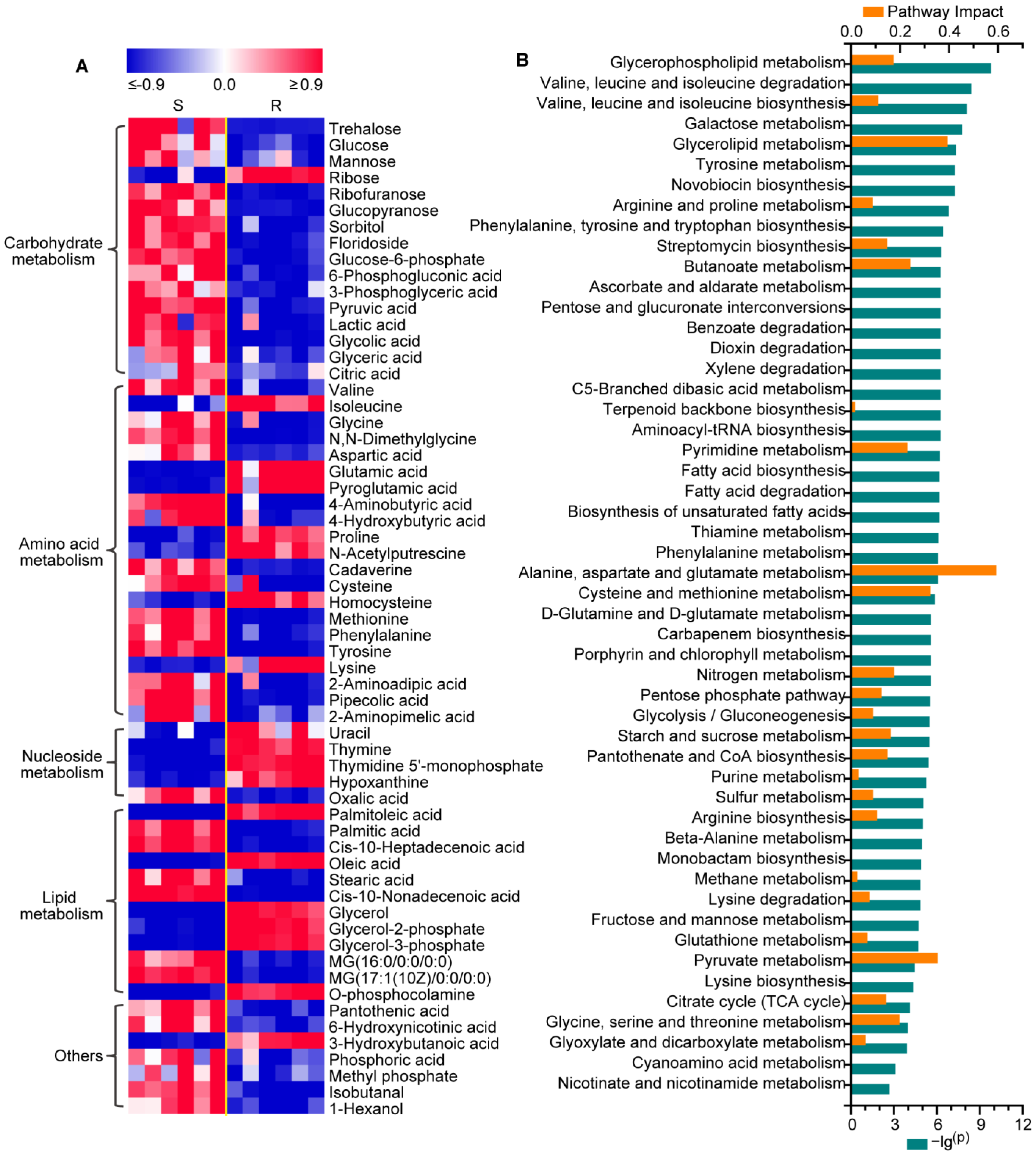
2.2. Metabolic Reprogramming in Gentamicin-Resistant E. coli
2.3. Reprogramming of Carbohydrate and Nucleotide Metabolism in Gentamicin-Resistant E. coli
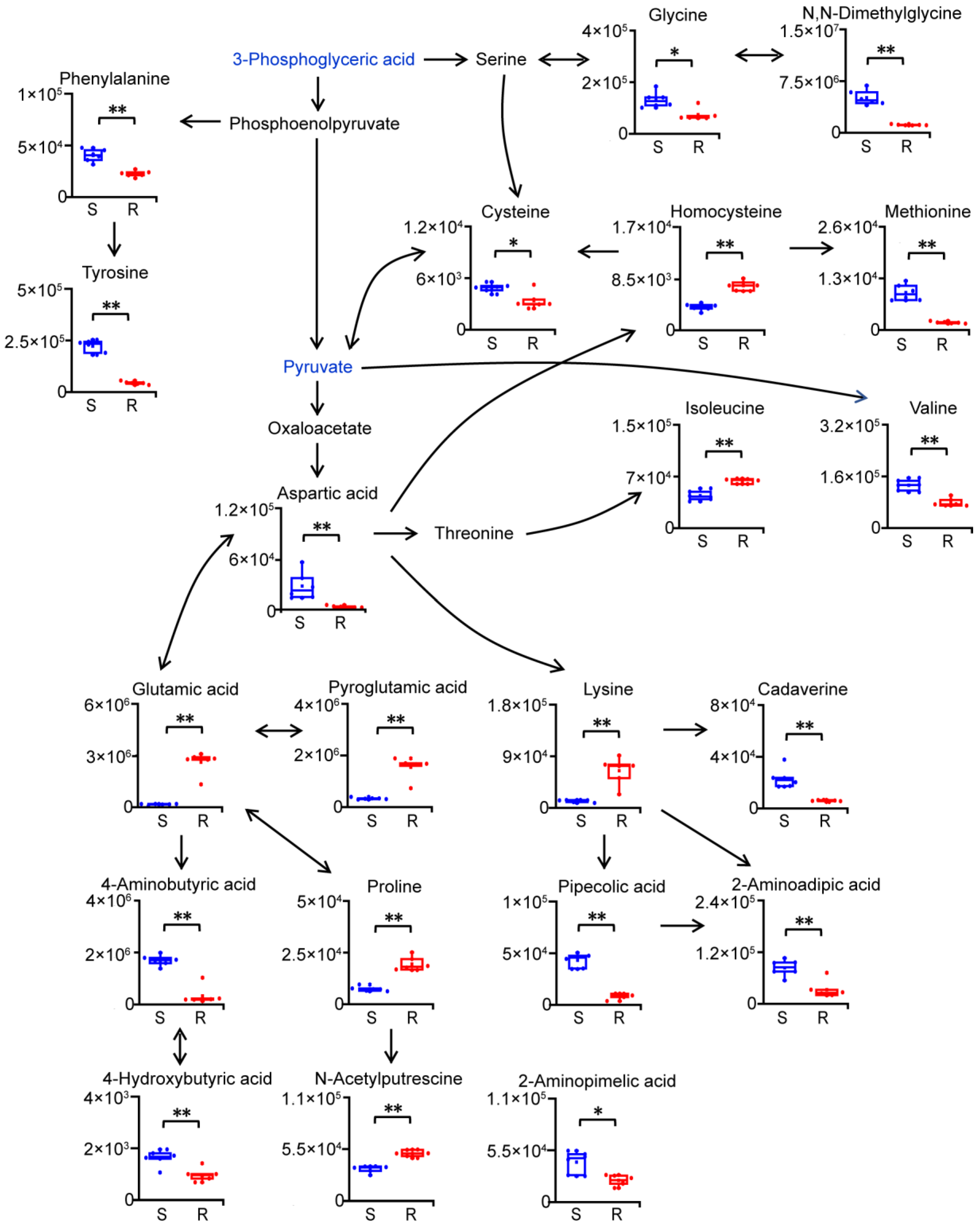
2.4. Reprogramming of Amino Acid Metabolism in Gentamicin-Resistant E. coli
2.5. Reprogramming of Lipid Metabolism in Gentamicin-Resistant E. coli
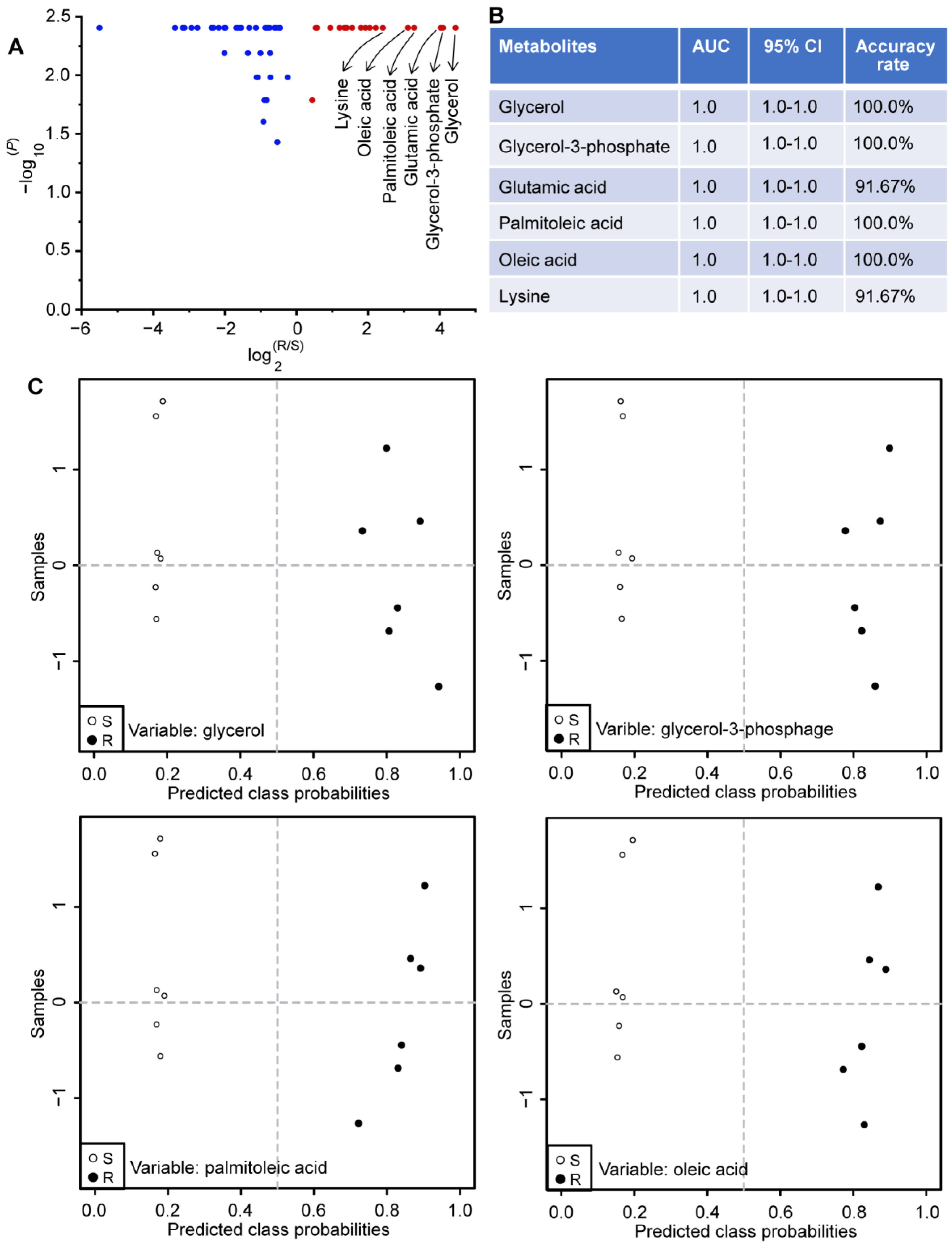
2.6. Potential Biomarkers Related to Gentamicin Resistance in E. coli
2.7. Palmitoleate and Oleate Promote Gentamicin Resistance in E. coli
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strain and Growth Condition
4.3. Growth Curve Measurement
4.4. Determination of MIC
4.5. Screening of Gentamicin-Resistant E. coli
4.6. Sample Collection for the Metabolomic Approach
4.7. Sample Preparation for the Metabolomic Approach
4.8. Instrumental Analysis for the Metabolomic Approach
4.9. Data Preprocessing for the Metabolomic Approach
4.10. Antibiotic Bactericidal Assay
4.11. Determination of Intracellular Gentamicin
4.12. Determination of ROS
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Hershberg, R. Antibiotic-independent adaptive effects of antibiotic resistance mutations. Trends Genet. 2017, 33, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.B.; Peng, B.; Li, H.; Cheng, Z.X.; Zhang, T.T.; Zhu, J.X.; Li, D.; Li, M.Y.; Ye, J.Z.; Du, C.C.; et al. Pyruvate cycle increases aminoglycoside efficacy and provides respiratory energy in bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E1578–E1587. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Z.; Lin, X.M.; Cheng, Z.X.; Su, Y.B.; Li, W.X.; Ali, F.M.; Zheng, J.; Peng, B. Identification and efficacy of glycine, serine and threonine metabolism in potentiating kanamycin-mediated killing of Edwardsiella piscicida. J. Proteom. 2018, 183, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Liu, S.R.; Peng, B.; Li, D.; Cheng, Z.X.; Zhu, J.X.; Zhang, S.; Peng, Y.M.; Li, H.; Zhang, T.T.; et al. Exogenous l-valine promotes phagocytosis to kill multidrug-resistant bacterial pathogens. Front. Immunol. 2017, 8, 207. [Google Scholar] [CrossRef]
- Baek, S.H.; Li, A.H.; Sassetti, C.M. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 2011, 9, e1001065. [Google Scholar] [CrossRef]
- Belenky, P.; Ye, J.D.; Porter, C.B.; Cohen, N.R.; Lobritz, M.A.; Ferrante, T.; Jain, S.; Korry, B.J.; Schwarz, E.G.; Walker, G.C.; et al. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 2015, 13, 968–980. [Google Scholar] [CrossRef]
- Chain, C.; Sheehan, J.P.; Xu, X.; Ghaffari, S.; Godbole, A.; Kim, H.; Freundlich, J.S.; Rabinowitz, J.D.; Gitai, Z. A folate inhibitor exploits metabolic differences in Pseudomonas aeruginosa for narrow-spectrum targeting. Nat. Microbiol. 2024, 9, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, F.; Qin, Q.; Qiao, L. Molecular responses during bacterial filamentation reveal inhibition methods of drug-resistant bacteria. Proc. Natl. Acad. Sci. USA 2023, 120, e2301170120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Su, Y.B.; Ye, J.Z.; Li, H.; Kuang, S.F.; Wu, J.H.; Li, S.H.; Peng, X.X.; Peng, B. Ampicillin-controlled glucose metabolism manipulates the transition from tolerance to resistance in bacteria. Sci. Adv. 2023, 9, eade8582. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Su, Y.-B.; Li, H.; Han, Y.; Guo, C.; Tian, Y.-M.; Peng, X.-X. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015, 21, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Sirakova, T.D.; Dubey, V.S.; Deb, C.; Daniel, J.; Korotkova, T.A.; Abomoelak, B.; Kolattukudy, P.E. Identification of a diacylglycerol acyltransferase gene involved in accumulation of triacylglycerol in Mycobacterium tuberculosis under stress. Microbiology 2006, 152, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Mercier, R.; Mickiewicz, K.; Serafini, A.; Sório de Carvalho, L.P.; Errington, J. Crucial role for central carbon metabolism in the bacterial L-form switch and killing by β-lactam antibiotics. Nat. Microbiol. 2019, 4, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Kuang, S.F.; Lai, S.S.; Zhang, S.; Yang, J.; Peng, B.; Peng, X.X.; Chen, Z.G.; Li, H. Na(+)-NQR confers aminoglycoside resistance via the regulation of l-alanine metabolism. mBio 2020, 11, e02086-20. [Google Scholar] [CrossRef] [PubMed]
- Händel, N.; Schuurmans, J.M.; Brul, S.; ter Kuile, B.H. Compensation of the metabolic costs of antibiotic resistance by physiological adaptation in Escherichia coli. Antimicrob. Agents Chemother. 2013, 57, 3752–3762. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef]
- Schulz zur Wiesch, P.; Engelstädter, J.; Bonhoeffer, S. Compensation of fitness costs and reversibility of antibiotic resistance mutations. Antimicrob. Agents Chemother. 2010, 54, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhu, B.; Yao, Z.; Yin, P.; Lu, X.; Kong, H.; Fan, F.; Jiao, B.; Xu, G. Analysis of urinary metabolic signatures of early hepatocellular carcinoma recurrence after surgical removal using gas chromatography–mass spectrometry. J. Proteome Res. 2012, 11, 4361–4372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Hu, C.; Xie, Z.; Li, H.; Wei, S.; Wang, D.; Wen, C.; Xu, G. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J. Pharm. Biomed. 2016, 127, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Maifiah, M.H.M.; Velkov, T.; Schreiber, F.; Li, J. Metabolic responses to polymyxin treatment in Acinetobacter baumannii ATCC 19606: Integrating transcriptomics and metabolomics with genome-scale metabolic modeling. mSystems 2019, 4, e00157-18. [Google Scholar] [CrossRef] [PubMed]
- Bryła, J.; Lietz, T.; Jarzyna, R.; Michalik, M.; Pietkiewicz, J. Differential in vivo and in vitro effect of gentamicin on glutamate synthesis and glutamate deamination in rabbit kidney-cortex tubules and mitochondria. Pharmacol. Res. 1992, 26, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Arab, H.H.; Maghrabi, I.A. Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct. 2018, 9, 6632–6642. [Google Scholar] [CrossRef] [PubMed]
- Beshay, O.N.; Ewees, M.G.; Abdel-Bakky, M.S.; Hafez, S.; Abdelrehim, A.B.; Bayoumi, A.M.A. Resveratrol reduces gentamicin-induced EMT in the kidney via inhibition of reactive oxygen species and involving TGF-β/Smad pathway. Life Sci. 2020, 258, 118178. [Google Scholar] [CrossRef]
- Devbhuti, P.; Saha, A.; Sengupta, C. Gentamicin induced lipid peroxidation and its control with ascorbic acid. Acta Pol. Pharm. 2009, 66, 363–369. [Google Scholar] [PubMed]
- Kuhad, A.; Tirkey, N.; Pilkhwal, S.; Chopra, K. Effect of Spirulina, a blue green algae, on gentamicin-induced oxidative stress and renal dysfunction in rats. Fundam. Clin. Pharmacol. 2006, 20, 121–128. [Google Scholar] [CrossRef]
- Ruzheinikov, S.N.; Taal, M.A.; Sedelnikova, S.E.; Baker, P.J.; Rice, D.W. Substrate-induced conformational changes in Bacillus subtilis glutamate racemase and their implications for drug discovery. Structure 2005, 13, 1707–1713. [Google Scholar] [CrossRef]
- Mackie, J.; Kumar, H.; Bearne, S.L. Changes in quaternary structure cause a kinetic asymmetry of glutamate racemase-catalyzed homocysteic acid racemization. FEBS Lett. 2018, 592, 3399–3413. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Jin, Z.; Li, L.; Zhang, J.; Kong, X. GC-MS-based metabolomics analysis reveals L-aspartate enhances the antibiotic sensitivity of neomycin sulfate-resistant Aeromonas hydrophila. J. Fish Dis. 2018, 41, 1831–1841. [Google Scholar] [CrossRef]
- Zaprasis, A.; Bleisteiner, M.; Kerres, A.; Hoffmann, T.; Bremer, E. Uptake of amino acids and their metabolic conversion into the compatible solute proline confers osmoprotection to Bacillus subtilis. Appl. Environ. Microbiol. 2015, 81, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Wang, Z. Cysteine potentiates bactericidal antibiotics activity against Gram-negative bacterial persisters. Infect. Drug Resist. 2020, 13, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Su, Y.; Qian, C.; Yuan, D.; Miao, K.; Lee, D.; Ng, A.H.C.; Wijker, R.S.; Ribas, A.; Levine, R.D.; et al. Raman-guided subcellular pharmaco-metabolomics for metastatic melanoma cells. Nat. Commun. 2020, 11, 4830. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mi, T.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Metabolomics analysis of salt tolerance of Zygosaccharomyces rouxii and guided exogenous fatty acid addition for improved salt tolerance. J. Sci. Food Agric. 2022, 102, 6263–6272. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lin, X.; Ren, H.; Zhang, Y.; Wang, S.; Peng, X. Analysis of outer membrane proteome of Escherichia coli related to resistance to ampicillin and tetracycline. Proteomics 2006, 6, 462–473. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, M.; Xu, D.; Peng, B.; Peng, X.X.; Li, H. Reduced redox-dependent mechanism and glucose-mediated reversal in gentamicin-resistant Vibrio alginolyticus. Environ. Microbiol. 2019, 21, 4724–4739. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, X.; Liu, X.; Liao, X.; Zhang, H.; Yan, C.; Lin, Y.; Huang, Q. Polystyrene microplastics induce metabolic disturbances in marine medaka (Oryzias melastigmas) liver. Sci. Total Environ. 2021, 782, 146885. [Google Scholar] [CrossRef]
- Ye, G.; Gao, H.; Zhang, X.; Liu, X.; Chen, J.; Liao, X.; Zhang, H.; Huang, Q. Aryl hydrocarbon receptor mediates benzo[a]pyrene-induced metabolic reprogramming in human lung epithelial BEAS-2B cells. Sci. Total Environ. 2021, 756, 144130. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 2011, 473, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.B.; Peng, B.; Han, Y.; Li, H.; Peng, X.X. Fructose restores susceptibility of multidrug-resistant Edwardsiella tarda to kanamycin. J. Proteome Res. 2015, 14, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.I.; Bhagabati, N.K.; Braisted, J.C.; Liang, W.; Sharov, V.; Howe, E.A.; Li, J.; Thiagarajan, M.; White, J.A.; Quackenbush, J. TM4 microarray software suite. Methods Enzym. 2006, 411, 134–193. [Google Scholar]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, G.; Fan, L.; Zheng, Y.; Liao, X.; Huang, Q.; Su, Y. Upregulated Palmitoleate and Oleate Production in Escherichia coli Promotes Gentamicin Resistance. Molecules 2024, 29, 2504. https://doi.org/10.3390/molecules29112504
Ye G, Fan L, Zheng Y, Liao X, Huang Q, Su Y. Upregulated Palmitoleate and Oleate Production in Escherichia coli Promotes Gentamicin Resistance. Molecules. 2024; 29(11):2504. https://doi.org/10.3390/molecules29112504
Chicago/Turabian StyleYe, Guozhu, Lvyuan Fan, Yuhong Zheng, Xu Liao, Qiansheng Huang, and Yubin Su. 2024. "Upregulated Palmitoleate and Oleate Production in Escherichia coli Promotes Gentamicin Resistance" Molecules 29, no. 11: 2504. https://doi.org/10.3390/molecules29112504





