Preparation of UiO-66 MOF-Bonded Porous-Layer Open-Tubular Columns Using an In Situ Growth Approach for Gas Chromatography
Abstract
:1. Introduction
2. Results and Discussion
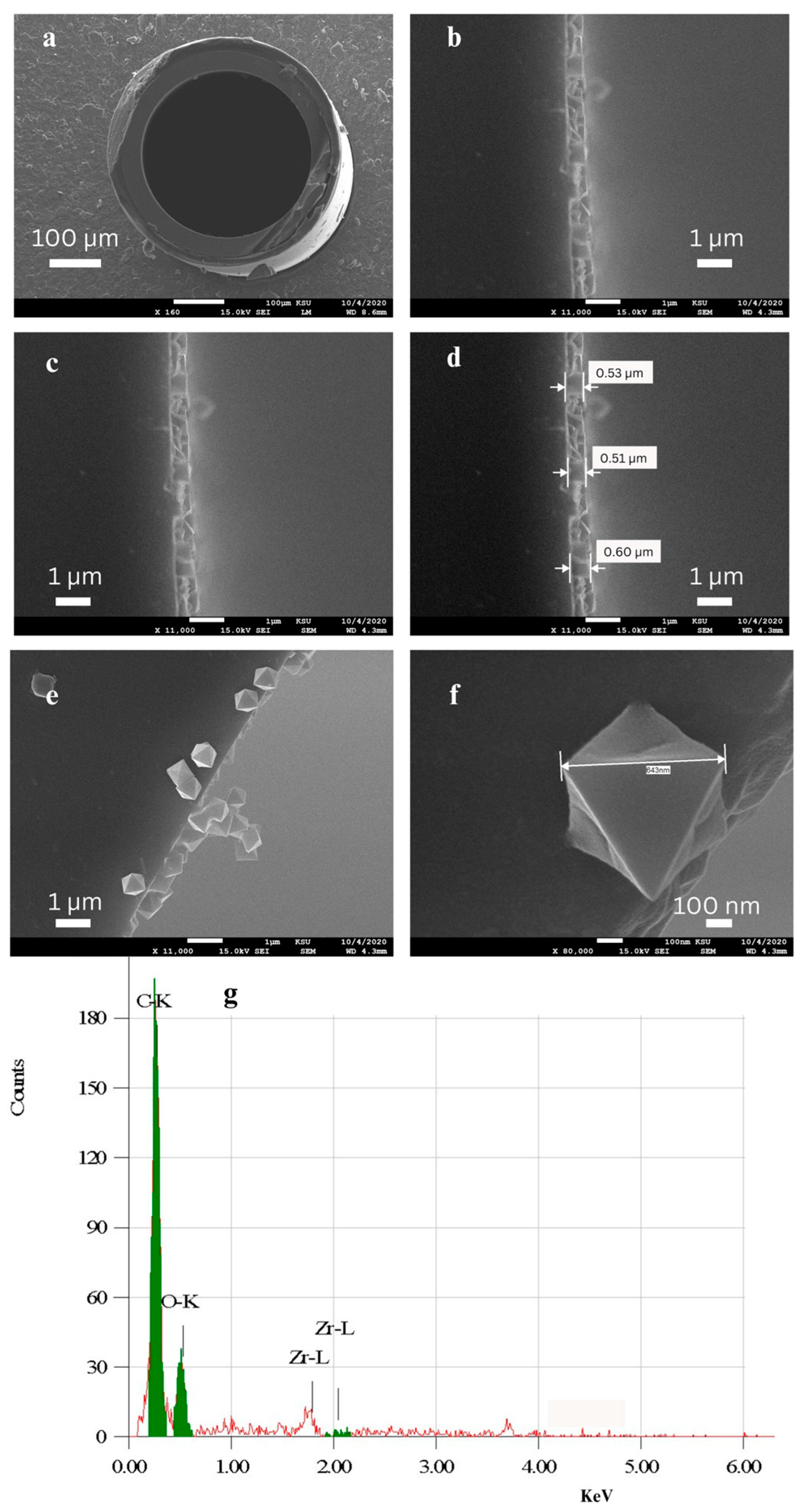
2.1. Characterization of the UiO-66-Bonded PLOT Column
2.2. Polarity of the UiO-66 Stationary Phase
| Column | Retention Index, I | ||||
|---|---|---|---|---|---|
| Benzene | 1-Butanol | 2-Pentanone | Nitropropane | Pyridine | |
| UiO-66 PLOT | 651 | 1408 | 1514 | 1621 | 1533 |
| Squalane | 650 | 600 | 620 | 650 | 709 |
| Column | Stationary Phase | McReynolds Constants *, ΔI | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X’ | Y’ | Z’ | U’ | S’ | Total | ΔIAverage | |||
| Packed MOF-5 | MOF-5 | −101 | −33 | 1 | −28 | −23 | −184 | −37 | [37] |
| Coated MIL-100 (Fe) | MIL-100 (Fe) | −45.8 | −2.6 | 56.4 | 27 | −104.6 | −69.6 | −14 | [32] |
| Coated MIL-100 (Cr) | MIL-100 (Cr) | −32.5 | 97.9 | 44.6 | 43.7 | −42.1 | 111.6 | 22 | [32] |
| SPB-1 | poly(dimethyl siloxane) | 4 | 58 | 43 | 56 | 38 | 199 | 40 | [38] |
| SPB-5 | poly(5% diphenyl-95% dimethyl siloxane) | 19 | 74 | 64 | 93 | 62 | 312 | 63 | [38] |
| Coated MOF-CJ3 | MOF-CJ3 | 61 | 169 | 136 | 242 | 157 | 765 | 153 | [39] |
| SPB-50 | poly(50% diphenyl-50% dimethyl siloxane) | 125 | 175 | 183 | 268 | 220 | 971 | 194 | [38] |
| SP-2340 | poly(bis-cyanopropyl siloxane) | 419 | 654 | 541 | 758 | 637 | 3009 | 602 | [38] |
| UiO-66 PLOT | UiO-66 | 1 | 808 | 894 | 971 | 824 | 3498 | 700 | Present work |
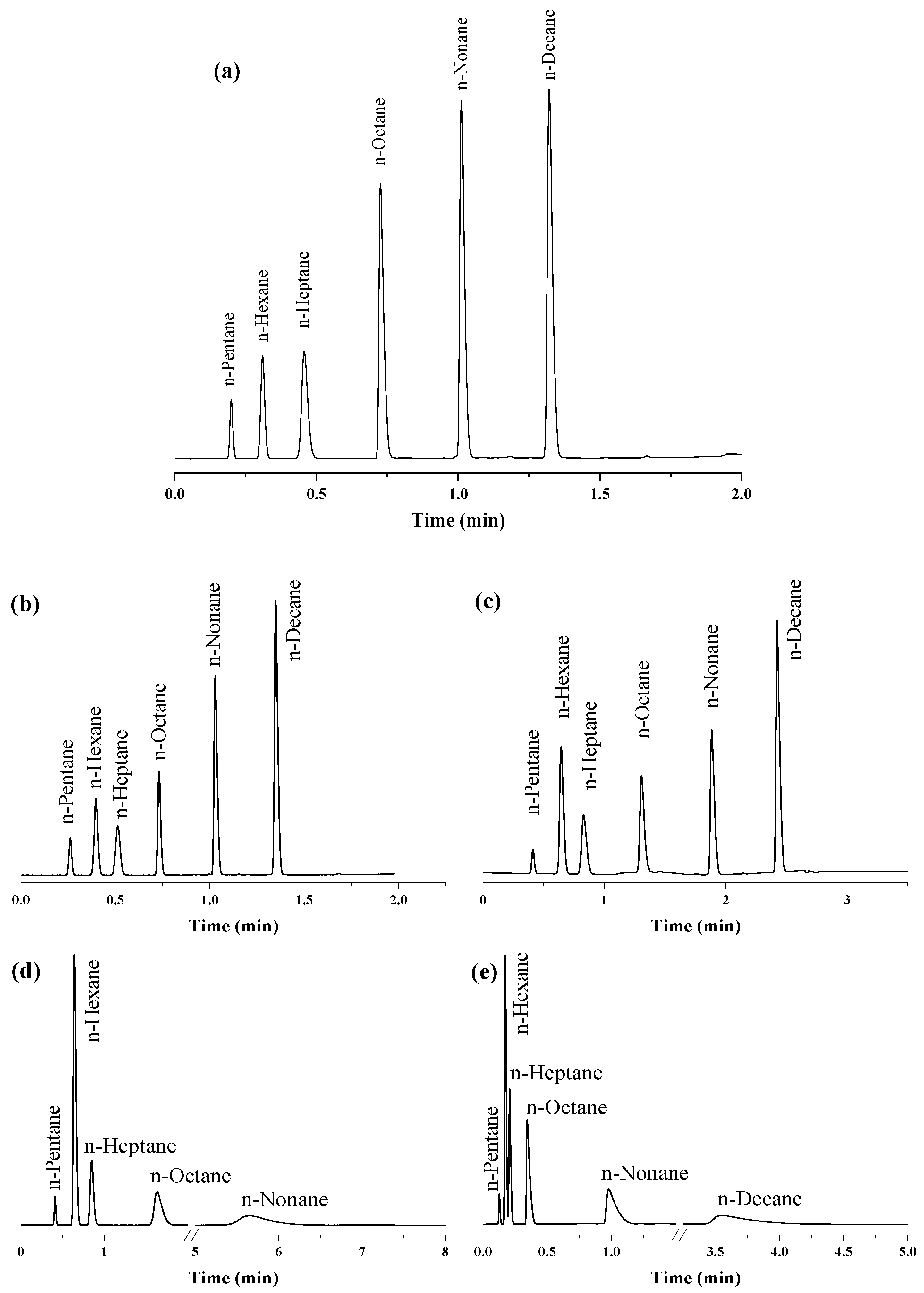
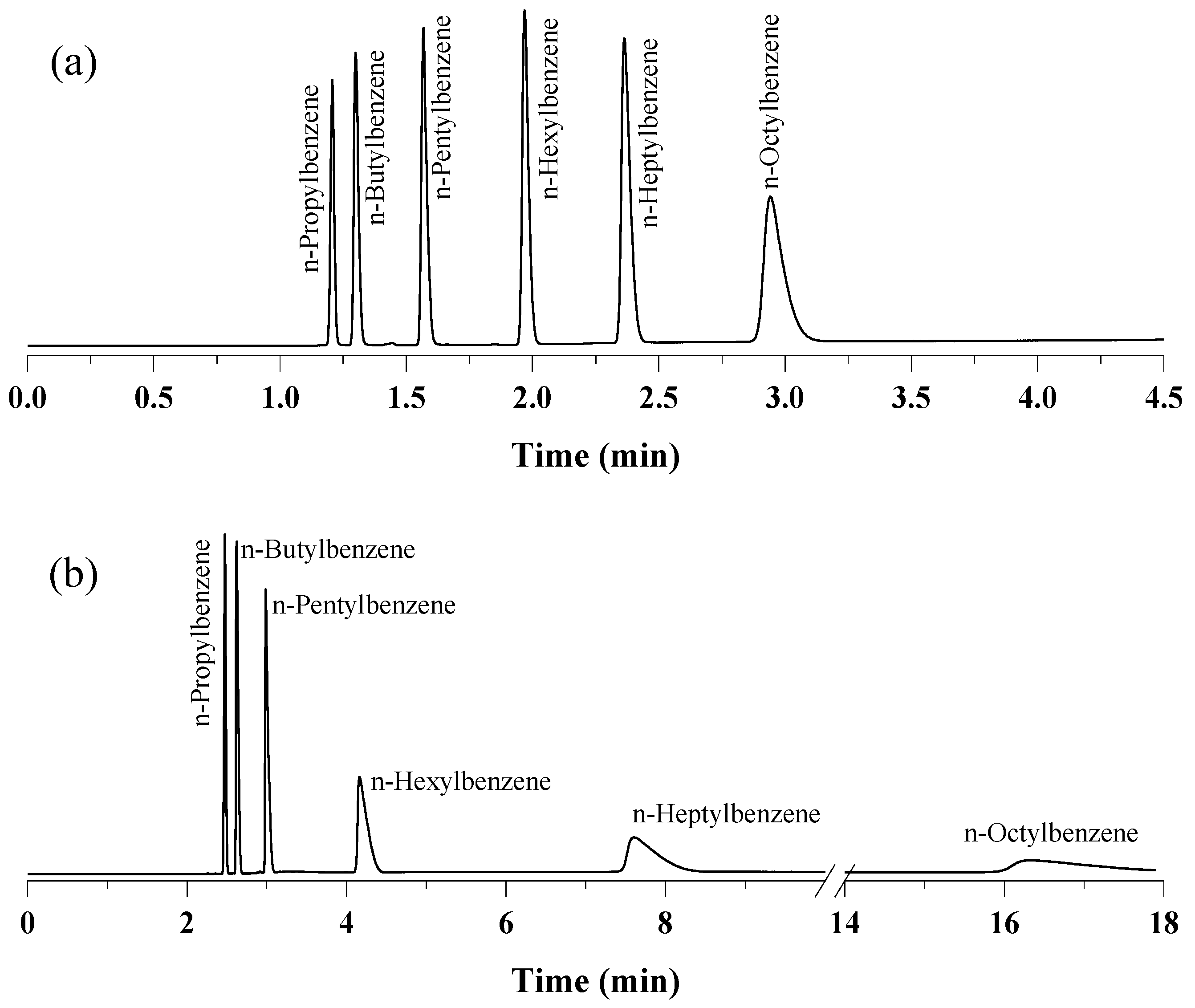
2.3. Separation Performance of the UiO-66 PLOT Column
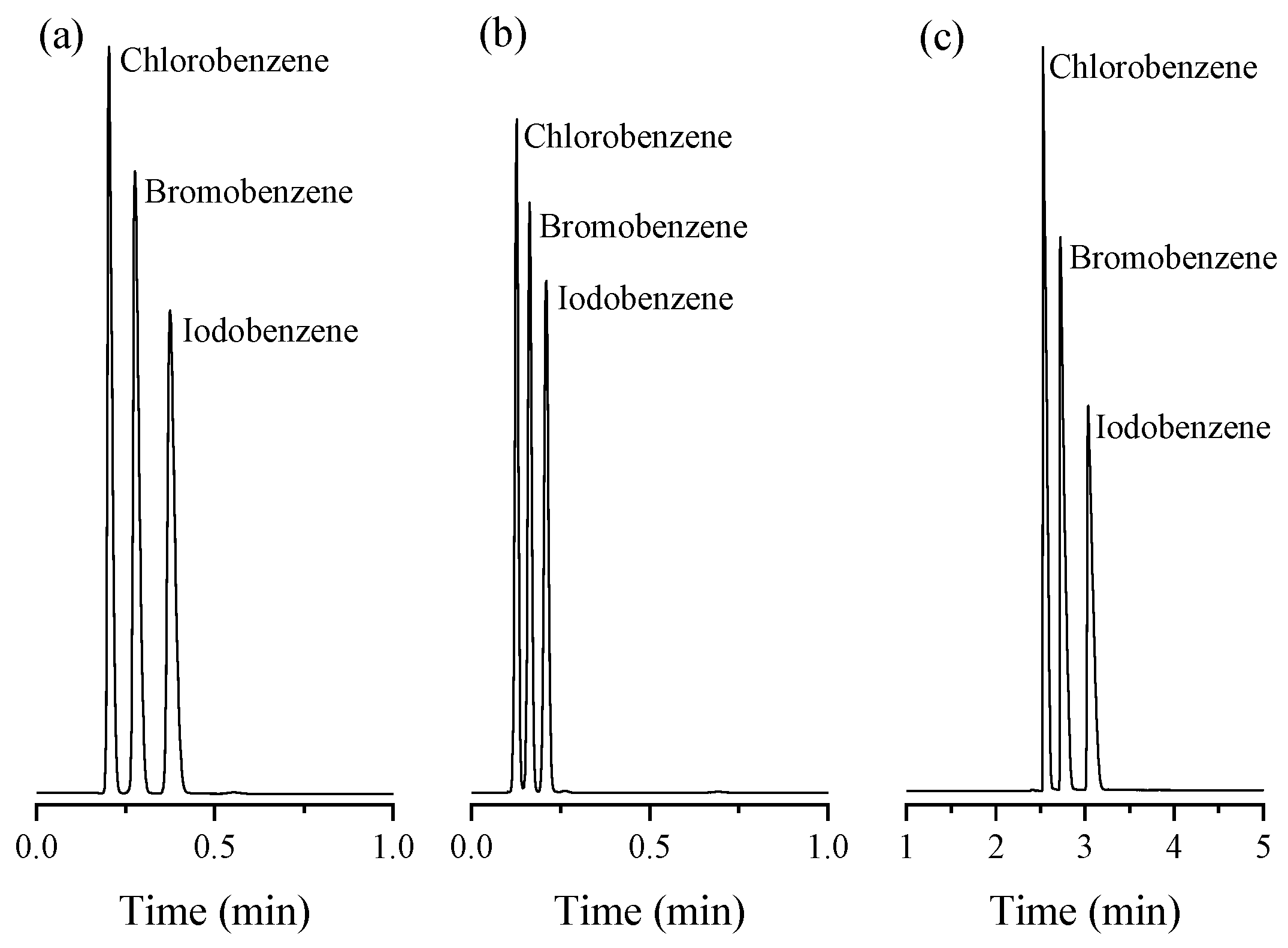
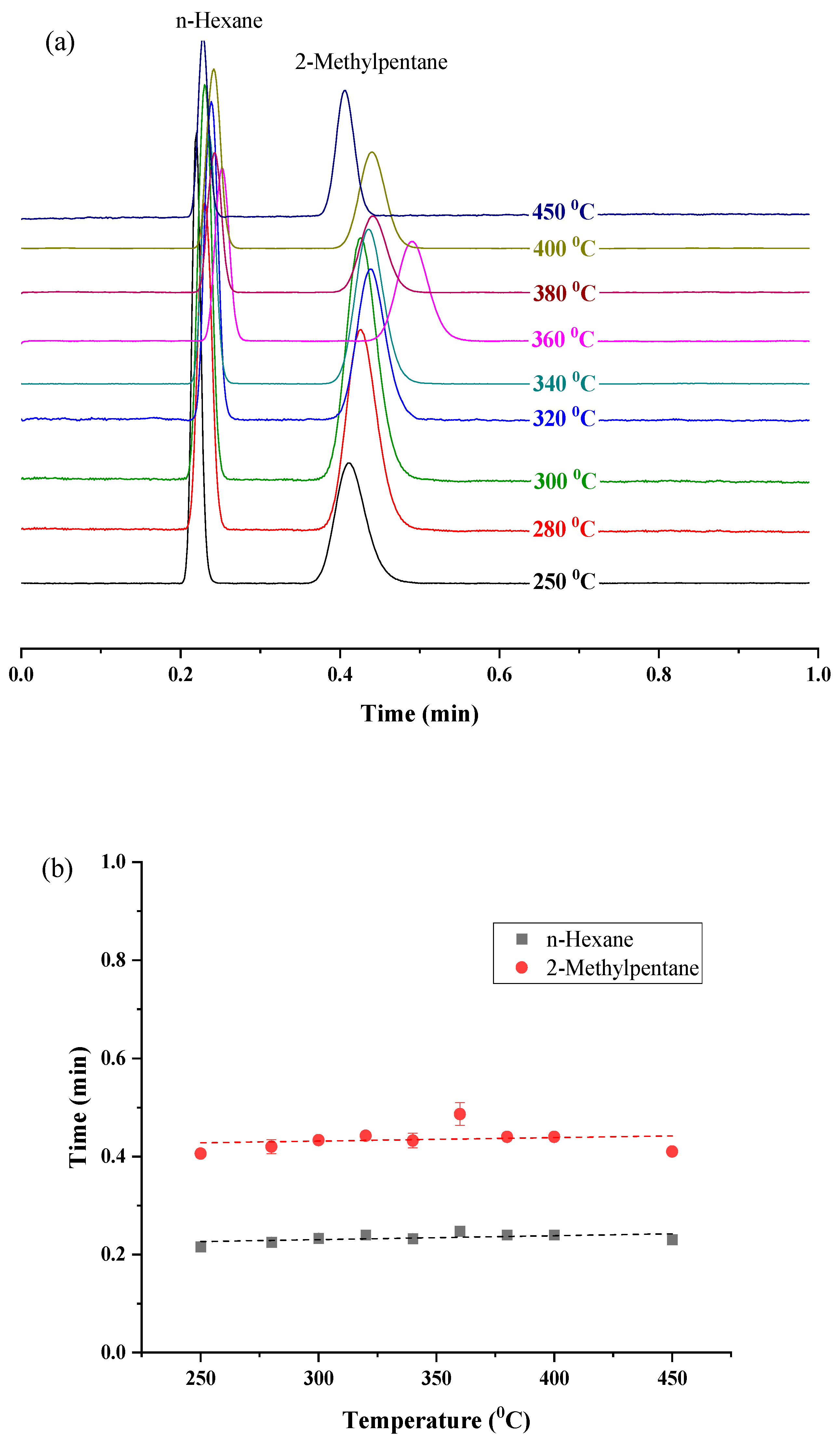
2.4. Exceptional Thermal Stability of UiO-66 PLOT Columns
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Preparation of the Carboxyl-Terminated Capillary (SAM)
3.4. Preparation of the UiO-66-Bonded PLOT Column
3.5. Determination of the McReynolds Constants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barry, E.F.; Grob, R.L. Columns for Gas Chromatography; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Dorman, F.L.; Dawes, P. Gas Chromatography; Poole, C.F., Ed.; Elsevier Inc.: Waltham, MA, USA, 2012; pp. 79–96. [Google Scholar]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Hoffmann, F.; Fröba, M. The Chemistry of Metal-Organic Frameworks; Kaskel, S., Ed.; Wiley-VCH: Weinheim, Germany, 2016; pp. 5–40. [Google Scholar]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal–organic frameworks for separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Almáši, M. A review on state of art and perspectives of Metal-Organic frameworks (MOFs) in the fight against coronavirus SARS-CoV-2. J. Coord. Chem. 2021, 74, 2111–2127. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-Organic frameworks (MOFs): Synthesis, structure and function. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 3–10. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Siemensmeyer, K.; Peeples, C.A.; Tholen, P.; Schmitt, F.-J.; Çoşut, B.; Hanna, G.; Yücesan, G. Phosphonate Metal–Organic Frameworks: A Novel Family of Semiconductors. Adv. Mater. 2020, 32, 2000474. [Google Scholar] [CrossRef]
- Leelasree, T.; Goel, S.; Aggarwal, H. MOF-Based Dual Sensor for the Electrochemical and Fluorescence Detection of Nicotine. ACS Appl. Nano Mater. 2022, 5, 16753–16759. [Google Scholar] [CrossRef]
- Király, N.; Capková, D.; Gyepes, R.; Vargová, N.; Kazda, T.; Bednarčík, J.; Yudina, D.; Zelenka, T.; Čudek, P.; Zeleňák, V.; et al. Sr(II) and Ba(II) Alkaline Earth Metal–Organic Frameworks (AE-MOFs) for Selective Gas Adsorption, Energy Storage, and Environmental Application. Nanomaterials 2023, 13, 234. [Google Scholar] [CrossRef]
- Ye, Y.; Xian, S.; Cui, H.; Tan, K.; Gong, L.; Liang, B.; Pham, T.; Pandey, H.; Krishna, R.; Lan, P.C.; et al. Metal–Organic Framework Based Hydrogen-Bonding Nanotrap for Efficient Acetylene Storage and Separation. J. Am. Chem. Soc. 2022, 144, 1681–1689. [Google Scholar] [CrossRef]
- Kuzharov, A.A.; Gritsai, M.A.; Butova, V.V.; Soldatov, M.A.; Polyakov, V.A.; Rud, P.A.; Rusalev, Y.V.; Kubrin, S.P.; Roldugin, V.A.; Trigub, A.L.; et al. One-step electrochemical synthesis of γ-Fe2O3@MIL-88a magnetic composite for heterogeneous Fenton-like catalysis. Ceram. Int. 2022, 48, 34864–34876. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Wu, Y.; Wang, C.; Guo, T.; Zhang, J.; Sun, L. Cross-linked γ-cyclodextrin metal-organic framework—A new stationary phase for the separations of benzene series and polycyclic aromatic hydrocarbons. Microchim Acta 2021, 188, 245. [Google Scholar] [CrossRef]
- Gao, B.; Huang, M.; Zhang, Z.; Yang, Q.; Su, B.; Yang, Y.; Ren, Q.; Bao, Z. Hybridization of metal–organic framework and monodisperse spherical silica for chromatographic separation of xylene isomers. Chin. J. Chem. Eng. 2019, 27, 818–826. [Google Scholar] [CrossRef]
- Yang, S.; Xie, H.; Zhu, H.; Zhang, L.; Zhou, Y.; Zhang, H.; Zhao, Z. Highly effective hydrogen isotope separation by cryogenic gas chromatography in a new stationary phase material MnCl2@CPL-1@γ-Al2O3. Int. J. Hydrogen Energy 2018, 43, 7973–7981. [Google Scholar] [CrossRef]
- Li, Z.; Mao, Z.; Zhou, W.; Chen, Z. Incorporation of homochiral metal-organic cage into ionic liquid based monolithic column for capillary electrochromatography. Anal. Chim. Acta 2020, 1094, 160–167. [Google Scholar] [CrossRef]
- Yusuf, K.; Badjah-Hadj-Ahmed, A.Y.; Aqel, A.; Alothman, Z.A. Monolithic metal-organic framework MIL-53(Al)-polymethacrylate composite column for the reversed-phase capillary liquid chromatography separation of small aromatics. J. Sep. Sci. 2016, 39, 880–888. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Yang, C.-X.; Yan, X.-P. An in situ growth approach to the fabrication of zeolite imidazolate framework-90 bonded capillary column for gas chromatography separation. Analyst 2015, 140, 3107–3112. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Jiang, J.Q.; Yan, X.P. Fabrication of isoreticular metal-organic framework coated capillary columns for high-resolution gas chromatographic separation of persistent organic pollutants. Anal. Chem. 2011, 83, 5093–5100. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Münch, A.S.; Seidel, J.; Obst, A.; Weber, E.; Mertens, F.O.R.L. High-separation performance of chromatographic capillaries coated with MOF-5 by the controlled SBU approach. Chem. A Eur. J. 2011, 17, 10958–10964. [Google Scholar] [CrossRef]
- Gu, Z.G.; Zhang, J. Epitaxial growth and applications of oriented metal–organic framework thin films. Coord. Chem. Rev. 2019, 378, 513–532. [Google Scholar] [CrossRef]
- Tang, P.; Bao, T.; Chen, Z. Novel Zn-based MOFs stationary phase with large pores for capillary electrochromatography. Electrophoresis 2016, 37, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Zhang, J.; Zhang, W.; Chen, Z. Growth of metal–organic framework HKUST-1 in capillary using liquid-phase epitaxy for open-tubular capillary electrochromatography and capillary liquid chromatography. J. Chromatogr. A 2015, 1381, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, W.; Chen, X. In situ rapid preparation of homochiral metal-organic framework coated column for open tubular capillary electrochromatography. J. Chromatogr. A 2016, 1427, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Lv, W.J.; Ren, C.L.; Niu, X.Y.; Chen, H.L.; Chen, X.G. In situ preparation of multilayer coated capillary column with HKUST-1 for separation of neutral small organic molecules by open tubular capillary electrochromatography. J. Chromatogr. A 2018, 1532, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chemie Int. Ed. 2006, 45, 1390–1393. [Google Scholar] [CrossRef]
- Chang, N.; Gu, Z.Y.; Yan, X.P. Zeolitic imidazolate framework-8 nanocrystal coated capillary for molecular sieving of branched alkanes from linear alkanes along with high-resolution chromatographic separation of linear alkanes. J. Am. Chem. Soc. 2010, 132, 13645–13647. [Google Scholar] [CrossRef] [PubMed]
- Münch, A.S.; Mertens, F.O.R.L. HKUST-1 as an open metal site gas chromatographic stationary phase—Capillary preparation, separation of small hydrocarbons and electron donating compounds, determination of thermodynamic data. J. Mater. Chem. 2012, 22, 10228–10234. [Google Scholar] [CrossRef]
- Fan, L.; Yan, X.P. Evaluation of isostructural metal-organic frameworks coated capillary columns for the gas chromatographic separation of alkane isomers. Talanta 2012, 99, 944–950. [Google Scholar] [CrossRef]
- Xie, S.M.; Zhang, M.; Fei, Z.X.; Yuan, L.M. Experimental comparison of chiral metal-organic framework used as stationary phase in chromatography. J. Chromatogr. A 2014, 1363, 137–143. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Yan, X.P. Exploring reverse shape selectivity and molecular sieving effect of metal-organic framework UIO-66 coated capillary column for gas chromatographic separation. J. Chromatogr. A 2012, 1257, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sladekova, K.; Campbell, C.; Grant, C.; Fletcher, A.J.; Gomes, J.R.B.; Jorge, M. The effect of atomic point charges on adsorption isotherms of CO2 and water in metal organic frameworks. Adsorption 2019, 26, 663–685. [Google Scholar] [CrossRef]
- Gu, Z.; Jiang, D.; Wang, H.; Cui, X.; Yan, X. Adsorption and separation of xylene isomers and ethylbenzene on two Zn-terephthalate metal-organic frameworks. J. Phys. Chem. C 2010, 114, 311–316. [Google Scholar] [CrossRef]
- Supelco-Inc. The retention index system in gas chromatography: McReynolds constants. Supelco GC Bull. 1997, 880, 8. [Google Scholar]
- Fang, Z.L.; Zheng, S.R.; Tan, J.B.; Cai, S.L.; Fan, J.; Yan, X.; Zhang, W.G. Tubular metal-organic framework-based capillary gas chromatography column for separation of alkanes and aromatic positional isomers. J. Chromatogr. A 2013, 1285, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Sheldon, L.S.; Croghan, C.W.; Chuang, J.C.; Lordo, R.A.; Wilson, N.K.; Lyu, C.; Brinkman, M.; Morse, N.; Chou, Y.L.; et al. A Pilot Study of Children’s Total Exposure to Persistent Pesticides and Other Persistent Organic Pollutants (CTEPP); U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Agilent, Performance and Durability for High-Temperature GC. 2020. Available online: https://www.agilent.com/cs/library/brochures/brochure-high-temp-gc-columns-5994-1384en-agilent.pdf (accessed on 31 March 2024).
- Miyamoto, M.; Kohmura, S.; Iwatsuka, H.; Oumi, Y.; Uemiya, S. In situ solvothermal growth of highly oriented Zr-based metal organic framework UiO-66 film with monocrystalline layer. CrystEngComm 2015, 17, 3422–3425. [Google Scholar] [CrossRef]
- Barry, E.F. Modern Practice of Gas Chromatography; Grob, R.L., Barry, E.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 65–191. [Google Scholar]
- Sun, T.; Tian, L.; Li, J.; Qi, M.; Fu, R.; Huang, X. Dithienyl benzothiadiazole derivatives: A new type of stationary phases for capillary gas chromatography. J. Chromatogr. A 2013, 1321, 109–118. [Google Scholar] [CrossRef]




| Analyte a | tR, min b Before Heating at 450 °C | tR, min b After Heating at 450 °C | %RPD c |
|---|---|---|---|
| n-Hexane | 0.28 | 0.24 | 13.59 |
| 2-methylpntane | 0.64 | 0.47 | 30.63 |
| n-pentane | 0.22 | 0.20 | 12.05 |
| n-Hexane | 0.32 | 0.30 | 6.45 |
| n-Heptane | 0.41 | 0.43 | 3.59 |
| n-Octane | 0.65 | 0.69 | 5.97 |
| n-Nonane | 0.95 | 0.99 | 3.62 |
| n-Decane | 1.27 | 1.29 | 1.56 |
| n-Probylbenzene | 1.21 | 1.23 | 1.64 |
| n-butylbenzene | 1.30 | 1.39 | 6.69 |
| n-Pentylbenzene | 1.57 | 1.67 | 6.17 |
| n-Hexylbenzene | 1.97 | 2.05 | 3.99 |
| n-Heptylbenzene | 2.36 | 2.45 | 3.74 |
| n-Octylbenzene | 2.94 | 3.20 | 8.31 |
| iso-butylbenzene | 0.31 | 0.36 | 14.93 |
| n-Butylbenzene | 0.37 | 0.41 | 11.13 |
| tert-Butylbenzene | 0.70 | 0.70 | 0.72 |
| Chlorobenzene | 0.12 | 0.12 | 0.00 |
| Bromobenzene | 0.16 | 0.16 | 0.00 |
| Iodobenzene | 0.2 | 0.21 | 4.80 |
| p-Chlorotoluene | 0.23 | 0.23 | 2.20 |
| m-Chlorotoluene | 0.28 | 0.29 | 0.00 |
| o-Chlorotoluene | 0.51 | 0.49 | 4.00 |
| p-Xylene | 0.80 | 0.88 | 9.52 |
| m-Xylene | 1.08 | 1.10 | 1.83 |
| Ethylbenzene | 1.54 | 1.50 | 2.63 |
| o-Xylene | 2.86 | 2.59 | 9.91 |
| Stationary Phase | Polarity | Common Name | Maximum Temperature, °C |
|---|---|---|---|
| 100% Dimethylpolysiloxane | Non-polar | DB-1ht | 400 |
| (5%-Phenyl)-methylpolysiloxane | Non-polar | DB-5ht | 400 |
| (50%-Phenyl)-methylpolysiloxane | Mid-polarity | DB-17ht | 365 |
| UiO-66 | High polar | UiO-66-bonded PLOT | 450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaif, K.D.; Badjah-Hadj-Ahmed, A.-Y.; ALOthman, Z.A. Preparation of UiO-66 MOF-Bonded Porous-Layer Open-Tubular Columns Using an In Situ Growth Approach for Gas Chromatography. Molecules 2024, 29, 2505. https://doi.org/10.3390/molecules29112505
Otaif KD, Badjah-Hadj-Ahmed A-Y, ALOthman ZA. Preparation of UiO-66 MOF-Bonded Porous-Layer Open-Tubular Columns Using an In Situ Growth Approach for Gas Chromatography. Molecules. 2024; 29(11):2505. https://doi.org/10.3390/molecules29112505
Chicago/Turabian StyleOtaif, Khadejah D., Ahmed-Yacine Badjah-Hadj-Ahmed, and Zeid Abdullah ALOthman. 2024. "Preparation of UiO-66 MOF-Bonded Porous-Layer Open-Tubular Columns Using an In Situ Growth Approach for Gas Chromatography" Molecules 29, no. 11: 2505. https://doi.org/10.3390/molecules29112505







