Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications
Abstract
1. Introduction
2. Hydrogen Storage Properties and Mechanisms of Magnesium-Based Alloys
2.1. Thermodynamic and Kinetic Properties
2.2. Hydrogen Storage Mechanisms
3. Synthesis Methods for Magnesium-Based Hydrogen Storage Alloys
3.1. Mechanical Alloying and Reactive Ball Milling
3.2. Solvothermal Synthesis
3.3. Vapor Deposition and Electrochemical Methods
4. Modification Strategies for Enhancing Hydrogen Storage Performance
4.1. Multiple Alloying
4.2. Nanostructuring
4.3. Surface Modification
5. Hydrogen Storage Performance of Magnesium-Based Alloys
5.1. Hydrogen Absorption/Desorption Properties
5.2. Comparison of Different Magnesium-Based Alloy Systems
5.3. Effect of Modification Strategies on Hydrogen Storage Performance
6. Applications and Future Perspectives
6.1. Potential Applications of Magnesium-Based Hydrogen Storage Alloys
6.2. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Z.; Ma, T.; Lu, C.T.; Ma, W.; Shaw, L. Predicting the hydrogen release ability of LiBH4-based mixtures by ensemble machine learning. Energy Storage Mater. 2020, 27, 466–477. [Google Scholar] [CrossRef]
- Jain, I.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4 + Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New insights into the solid-state hydrogen storage of nanostructured LiBH4-MgH2 system. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloys Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Yang, H.; Ding, Z.; Li, Y.-T.; Li, S.-Y.; Wu, P.-K.; Hou, Q.-H.; Zheng, Y.; Gao, B.; Huo, K.-F.; Du, W.-J.; et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met. 2023, 42, 2906–2927. [Google Scholar] [CrossRef]
- Guo, S.; Yu, Z.; Li, Y.; Fu, Y.; Zhang, Z.; Han, S. Preparation of Mg-Mg2Ni/C composite and its excellent hydrogen storage properties. J. Alloys Compd. 2024, 976, 173035. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, H.; Zong, Y.; Xu, H.; Luo, J.; Liu, X.; Xu, J. Studies of Ni-Mg catalyst for stable high efficiency hydrogen storage. J. Alloys Compd. 2022, 905, 164279. [Google Scholar] [CrossRef]
- de Rango, P.; Wen, J.; Skryabina, N.; Laversenne, L.; Fruchart, D.; Borges, M. Hydrogen Storage Properties of Mg-Ni Alloys Processed by Fast Forging. Energies 2020, 13, 3509. [Google Scholar] [CrossRef]
- Baum, L.; Meyer, M.; Mendoza-Zélis, L. Hydrogen storage properties of the Mg/Fe system. Phys. B Condens. Matter 2007, 389, 189–192. [Google Scholar] [CrossRef]
- Andreani, G.F.D.L.; Triques, M.R.M.; Leiva, D.R.; Roche, V.; Cardoso, K.R.; Ishikawa, T.T.; Botta, W.J.; Jorge, A.M. Hydrogen storage properties of 2 Mg–Fe mixtures processed by hot extrusion: Effect of ram speeds. Int. J. Hydrogen Energy 2019, 44, 20203–20212. [Google Scholar] [CrossRef]
- Andreani, G.D.L.; Miglioli, M.; Triques, M.; Roche, V.; Kiminami, C.; Botta, W.; Jorge, A. Hydrogen storage properties of 2Mg-Fe mixtures processed by hot extrusion at different temperatures. Int. J. Hydrogen Energy 2017, 42, 11493–11500. [Google Scholar] [CrossRef]
- Dai, Z.; Xiao, L.; Zhang, B.; Kimura, H.; Xie, X.; Ni, C.; Sun, X.; Du, W. Recent progress of the effect of Co/Ni/Fe-based containing catalysts addition on hydrogen storage of Mg. J. Mater. Sci. 2022, 58, 46–62. [Google Scholar] [CrossRef]
- Anik, M.; Akay, I.; Özdemir, G.; Baksan, B. Electrochemical hydrogen storage performance of Mg–Ti–Zr–Ni alloys. Int. J. Hydrogen Energy 2009, 34, 9765–9772. [Google Scholar] [CrossRef]
- Ölmez, R.; Çakmak, G.; Öztürk, T. Combinatorial search for hydrogen storage alloys: Mg–Ni and Mg–Ni–Ti. Int. J. Hydrogen Energy 2010, 35, 11957–11965. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Fu, J.; Guo, Q.; Pan, F.; Deng, S.; Li, J.; Zhao, G. The synthesis and hydrogen storage properties of Mg2Ni substituted with Cu, Co. J. Mater. Res. 2009, 24, 1311–1316. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y. Improved hydrogen storage properties of a V decorated Mg nanoblade array. Phys. Chem. Chem. Phys. 2009, 11, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, J.; Ju, S.; Yang, Y.; Li, Z.; Fang, F.; Sun, D.; Xia, G.; Pan, H.; Yu, X. Understanding and unlocking the role of V in boosting the reversible hydrogen storage performance of MgH2. J. Mater. Chem. A 2023, 11, 9762–9771. [Google Scholar] [CrossRef]
- Xie, L.; Xu, M.; Zhang, C.; Wu, T. Composition dependent hydrogen storage performance and desorption factors of Mg–Ce based alloys. Int. J. Hydrogen Energy 2020, 45, 9865–9876. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Zhang, W.; Yuan, Z.; Wei, X.; Gao, J.; Ren, H. Improvement of substituting La with Ce on hydrogen storage thermodynamics and kinetics of Mg-based alloys. Int. J. Hydrogen Energy 2021, 46, 28719–28733. [Google Scholar] [CrossRef]
- Wagemans, R.W.P.; Van Lenthe, J.H.; De Jongh, P.E.; Van Dillen, A.J.; De Jong, K.P. Hydrogen storage in magnesium clusters: Quantum chemical study. J. Am. Chem. Soc. 2005, 127, 16675–16680. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Huot, J.; Swainson, I.; Schulz, R. Phase transformation in magnesium hydride induced by ball milling. Eur. J. Control. 2006, 31, 135–144. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Manickam, K.; Hirscher, M.; Besenbacher, F.; Jensen, T.R. Confinement of MgH2 nanoclusters within nanoporous aerogel scaffold materials. ACS Nano 2009, 3, 3521–3528. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Ma, H.; Chen, J. Magnesium nanowires: Enhanced kinetics for hydrogen absorption and desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Towata, S.; Seno, Y.; Hirose, Y.; Nishibori, E.; Takata, M.; Sakata, M. Chemical bonding of hydrogen in MgH2. Appl. Phys. Lett. 2002, 81, 2008–2010. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic Tuning of Mg-Based Hydrogen Storage Alloys: A Review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; Ding, Z.; Jiang, H.; Yang, H.; Du, W.; Zheng, Y.; Huo, K.; Shaw, L.L. MOFs-Based Materials for Solid-State Hydrogen Storage: Strategies and Perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of mag-nesium hydride nanoparticles. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Rather, S.U.; Taimoor, A.A.; Muhammad, A.; Alhamed, Y.A.; Zaman, S.F.; Ali, A.M. Kinetics of hydrogen adsorption on MgH2/CNT composite. Mater. Res. Bull. 2016, 77, 23–28. [Google Scholar] [CrossRef]
- Révész, D.; Fátay, T. Spassov, Hydriding kinetics of ball-milled nanocrystalline MgH2 powders. J. Mater. Res. 2007, 22, 3144–3151. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Zhang, X.; Qu, J.; Wang, Y.; Li, X. Catalytic effect of Ni nanoparticles on the improvement of hydrogen storage kinetics of MgH2 powders. J. Alloys Compd. 2009, 482, 388–392. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials. J. Magnes. Alloys 2021, 9, 1922–1941. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, Z.-G.; Wang, X.; Fu, X.-P.; Hong, Y.; Shi, Y.-T.; Zhang, P. First-principles prediction of Mg decoration on monolayer g-C6N7 as a promising a hydrogen storage media. Int. J. Hydrogen Energy 2024, 50, 136–147. [Google Scholar] [CrossRef]
- Čermák, J.; Král, L. Hydrogen diffusion in Mg–H and Mg–Ni–H alloys. Acta Mater. 2008, 56, 2677–2686. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, C.; Li, Y.; Zhang, Q. Crystallite growth characteristics of Mg during hydrogen desorption of MgH2. Prog. Nat. Sci. Mater. Int. 2020, 30, 246–250. [Google Scholar] [CrossRef]
- Lyu, J.; Kudiiarov, V.; Lider, A. Experimentally Observed Nucleation and Growth Behavior of Mg/MgH2 during De/Hydrogenation of MgH2/Mg: A Review. Materials 2022, 15, 8004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Hosokai, S.; Akiyama, T. Growth Mechanism for the Controlled Synthesis of MgH2/Mg Crystals via a Vapor–Solid Process. Cryst. Growth Des. 2011, 11, 4166–4174. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Metallic glassy Ti2Ni grain-growth inhibitor powder for enhancing the hydrogenation/dehydrogenation kinetics of MgH2. RSC Adv. 2019, 9, 1036–1046. [Google Scholar] [CrossRef]
- Bobet, J.L.; Akiba, E.; Nakamura, Y.; Darriet, B. Study of Mg-M (M = Co, Ni and Fe) mixture elaborated by reactive mechanical alloying-hydrogen sorption properties. Int. J. Hydrogen Energy 2000, 25, 987–996. [Google Scholar] [CrossRef]
- Giraldo, C.; Ferraro, F.; Hadad, C.Z.; García-Beltrán, O.; Osorio, E. Structural, thermodynamic and kinetic factors in the desorption/absorption of a hydrogen molecule in the M3AlH10−xNa (M = Be or Mg; x = 0 or 2) hydrides. New J. Chem. 2019, 43, 18041–18048. [Google Scholar] [CrossRef]
- Friedrichs, O.; Kolodziejczyk, L.; Sánchez-López, J.; López-Cartés, C.; Fernández, A. Synthesis of nanocrystalline MgH2 powder by gas-phase condensation and in situ hydridation: TEM, XPS and XRD study. J. Alloys Compd. 2007, 434, 721–724. [Google Scholar] [CrossRef]
- Bortz, M.; Bertheville, B.; Böttger, G.; Yvon, K. Structure of the high pressure phase γ-MgH2 by neutron powder diffraction. J. Alloys Compd. 1999, 287, L4–L6. [Google Scholar] [CrossRef]
- Kurko, S.; Mamula, B.P.; Rmuš, J.; Novaković, J.G.; Novaković, N. DFT study of boron doped MgH2: Bonding mechanism, hydrogen diffusion and desorption. Int. J. Hydrogen Energy 2020, 45, 7947–7957. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, S.; Chen, X.; Liu, L.; Deng, Y.; Zhou, L.; Cai, X. Mechanism of hydrogenation and dehydrogenation in Mg/Cu9Al4 @Mg and MgH2/Cu9Al4 @MgH2: A DFT and experimental investigation. J. Alloys Compd. 2024, 978, 173542. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogen storage properties of nanosized MgH2−0.1TiH2 prepared by ultrahigh-energy-high-pressure milling. J. Am. Chem. Soc. 2010, 131, 15843–15852. [Google Scholar] [CrossRef]
- Zhang, J.; Cuevas, F.; Zaïdi, W.; Bonnet, J.-P.; Aymard, L.; Bobet, J.-L.; Latroche, M. Highlighting of a Single Reaction Path during Reactive Ball Milling of Mg and TM by Quantitative H2 Gas Sorption Analysis To Form Ternary Complex Hydrides (TM = Fe, Co, Ni). J. Phys. Chem. C 2011, 115, 4971–4979. [Google Scholar] [CrossRef]
- Zolriasatein, A.; Shokuhfar, A.; Safari, F.; Abdi, N. Comparative study of SPEX and planetary milling methods for the fabrication of complex metallic alloy nanoparticles. Micro Nano Lett. 2018, 13, 448–451. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Z.; Cheng, H.; Lu, G. Hydrogen diffusion and effect of grain size on hydrogenation kinetics in magnesium hydrides. J. Mater. Res. 2008, 23, 336–340. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Banyan, M.; Al-Ajmi, F. Discovering a new MgH2 metastable phase. RSC Adv. 2018, 8, 32003–32008. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Floriano, R.; Leiva, D.R.; Deledda, S.; Hauback, B.C.; Botta, W.J. Nanostructured MgH2 obtained by cold rolling combined with short-time high-energy ball milling. Mater. Res. 2013, 16, 158–163. [Google Scholar] [CrossRef]
- Czujko, T.; Oleszek, E.E.; Szot, M. New Aspects of MgH2 Morphological and Structural Changes during High-Energy Ball Milling. Materials 2020, 13, 4550. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Han, X.; Zang, S.; Liu, W.; Yang, G.; Lv, L.; Wang, X.; Duan, J.; Wang, S. Effect of LiH on the fast hydrolysis and hydrogen generation of MgH2 by ball milling. New J. Chem. 2022, 46, 19900–19908. [Google Scholar] [CrossRef]
- Imamura, H.; Tanaka, K.; Kitazawa, I.; Sumi, T.; Sakata, Y.; Nakayama, N.; Ooshima, S. Hydrogen storage properties of nanocrystalline MgH2 and MgH2/Sn nanocomposite synthesized by ball milling. J. Alloys Compd. 2009, 484, 939–942. [Google Scholar] [CrossRef]
- Yamasaki, N.; Miyazawa, H.; Ohyanagi, M.; Munir, Z.A. Accelerated hydrogen desorption from MgH2 by high-energy ball-milling with Al2O3. J. Mater. Sci. 2012, 47, 3577–3584. [Google Scholar] [CrossRef]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef]
- Pinto, A.H.; Shin, S.W.; Isaac, E.; Knutson, T.R.; Aydil, E.S.; Penn, R.L. Controlling Cu2ZnSnS4 (CZTS) phase in microwave solvothermal synthesis. J. Mater. Chem. A 2017, 5, 23179–23189. [Google Scholar] [CrossRef]
- Broge, N.L.N.; Bertelsen, A.D.; Søndergaard-Pedersen, F.; Iversen, B.B. Facile Solvothermal Synthesis of Pt–Ir–Pd–Rh–Ru–Cu–Ni–Co High-Entropy Alloy Nanoparticles. Chem. Mater. 2023, 35, 144–153. [Google Scholar] [CrossRef]
- Rambhujun, N.; Aguey-Zinsou, K.-F. Halide-free Grignard reagents for the synthesis of superior MgH2 nanostructures. Int. J. Hydrogen Energy 2021, 46, 28675–28685. [Google Scholar] [CrossRef]
- Akram, H.; Mateos-Pedrero, C.; Gallegos-Suarez, E.; Chafik, T.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of surfactant concentration on the morphology of MoxSy nanoparticles prepared by a solvothermal route. Green Process. Synth. 2017, 6, 161–171. [Google Scholar] [CrossRef]
- Kuchumov, B.M.; Shevtsov, Y.V.; Semyannikov, P.P.; Filatov, E.S.; Igumenov, I.K. Pulsed MO CVD Processes of MgO Layer Deposition from Mg(thd)2. ECS Trans. 2009, 25, 927–934. [Google Scholar] [CrossRef]
- Park, G.-D.; Yang, J.H.; Lee, K.-H.; Kim, H.-J.; Lee, S.-H.; Kang, J.; Yun, Y.-S.; Lee, M.-H. Ultra-high corrosion resistance of Al-Mg-Si film on steel sheet formed by PVD Mg coating and heat treatment. Corros. Sci. 2021, 192, 109829. [Google Scholar] [CrossRef]
- Xuan, W.; Ye, Z.; Han, D.; Shi, J.; Chen, J.; Kang, J. Multiscale simulation of physical vapor deposition. Mater. Sci. Eng. B 2023, 295, 116596. [Google Scholar] [CrossRef]
- Jarvis, J.S.; Li, Z.; Wang, Z.; Liu, L.; Chang, L.-Y.; Alagumalai, A.; Song, H. Inhibiting platinum sintering in direct ethane dehydrogenation and nonoxidative methane activation reactions by sequential sulfide layered chemical vapor deposition. Chem. Eng. J. 2024, 488, 151080. [Google Scholar] [CrossRef]
- Switzer, J.A.; Hodes, G. Electrodeposition and chemical bath deposition of functional nanomaterials. MRS Bull. 2010, 35, 743–750. [Google Scholar] [CrossRef]
- Anastasiadou, D.; Janssen, J.T.; Hensen, E.J.; Figueiredo, M.C. A Study of Cu-Rh Electro-deposition. Chem. Eur. 2023, 10, e202200842. [Google Scholar]
- Wakayama, H. Hydrogen storage of a mechanically milled carbon material fabricated by plasma chemical vapor deposition. Full-Nanotub. Carbon Nanostructures 2020, 28, 841–845. [Google Scholar] [CrossRef]
- Jin, R.; Chen, G.; Xu, H.; Chen, D. Solvothermal synthesis and growth mechanism of Sb2Se3 nanoplates with electrochemical hydrogen storage ability. Int. J. Hydrogen Energy 2013, 38, 10971–10977. [Google Scholar] [CrossRef]
- Jinzhe, L.; Lider, A.M.; Kudiiarov, V.N. An overview of progress in Mg-based hydrogen storage films. Chin. Phys. B 2019, 28, 098801. [Google Scholar] [CrossRef]
- Wu, G.; Zeng, X.; Ding, W.; Guo, X.; Yao, S. Characterization of ceramic PVD thin films on AZ31 magnesium alloys. Appl. Surf. Sci. 2006, 252, 7422–7429. [Google Scholar] [CrossRef]
- Kennelley, K.J.; Vargas, J.M.; Lohstreter, W.D.; Sykes, J.M.; Ison, A.J. Magnesium-hydride formation kinetics: Temperature-dependent grain growth. J. Mater. Sci. 2007, 42, 1979–1987. [Google Scholar]
- Chai, X.; Xin, Y.; He, B.; Zhang, F.; Xie, H.; Tian, H. High-efficient Electrodeposition of Magnesium alloy-based Anodes for Ultra-stable Rechargeable Magnesium-ion Batterie. Nanoscale 2024, 16, 9123–9135. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yin, B.; Wang, J.; Sun, J.; Tong, Y.; Li, L.; Wang, R. Theoretical and experimental studies of sol-gel electrodeposition on magnesium alloy. Surf. Interface Anal. 2021, 53, 432–439. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic mechanism of transition-metal compounds on Mg hydrogen sorption reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, G.; Guo, Z.; Huang, Z.; Liu, H.; Yu, X. Porous Ni nanofibers with enhanced catalytic effect on the hydrogen storage performance of MgH2. J. Mater. Chem. A 2015, 3, 15843–15848. [Google Scholar] [CrossRef]
- Varin, R.; Li, S.; Wronski, Z.; Morozova, O.; Khomenko, T. The effect of sequential and continuous high-energy impact mode on the mechano-chemical synthesis of nanostructured complex hydride Mg2FeH2. J. Alloys Compd. 2005, 390, 282–296. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Biswas, K. Effect on de-hydrogenation efficiency on doping of rare earth elements (Pr, Nd, Gd, Dy) in MgH2—A density functional theory study. Int. J. Hydrogen Energy 2017, 42, 1012–1017. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbæk, D.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Zhou, Z. p-Block elements for catalysis. npj Comput. Mater. 2021, 7, 209. [Google Scholar] [CrossRef]
- Karnbrock, S.B.H.; Alcarazo, M. Cooperation between p-Block Elements and Redox-Active Ligands: Stoichiometric and Catalytic Transformations. Chem.–A Eur. J. 2024, 30, e202302879. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yuan, T.; Yang, J.; Gao, M.; Pan, H.; Liu, Y.; Zheng, S. In situ formation of Al3Ti, MgF2 and Al and their superior synergetic effects on reversible hydrogen storage of MgH2. Catal. Today 2018, 318, 107–112. [Google Scholar] [CrossRef]
- Verma, S.K.; Mishra, S.S.; Mukhopadhyay, N.K.; Yadav, T.P. Superior catalytic action of high-entropy alloy on hydrogen sorption properties of MgH2. Int. J. Hydrogen Energy 2024, 50, 749–762. [Google Scholar] [CrossRef]
- Liang, G.; Schulz, R. Synthesis of Mg-Ti alloy by mechanical alloying. J. Mater. Sci. 2003, 38, 1179–1184. [Google Scholar] [CrossRef]
- Leiva, D.; Floriano, R.; Huot, J.; Jorge, A.; Bolfarini, C.; Kiminami, C.; Ishikawa, T.; Botta, W. Nanostructured MgH2 prepared by cold rolling and cold forging. J. Alloys Compd. 2011, 509, S444–S448. [Google Scholar] [CrossRef]
- Fátay, D.; Spassov, T.; Delchev, P.; Ribárik, G.; Révész, Á. Microstructural development in nanocrystalline MgH2 during H-absorption/desorption cycling. Int. J. Hydrogen Energy 2007, 32, 2914–2919. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Yu, X.; Zhao, H.; Shao, H. Geometrical effect in Mg-based metastable nano alloys with BCC structure for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 29291–29296. [Google Scholar] [CrossRef]
- Yang, F.; Li, Y.-G.; Wei, Y.-H.; Wei, H.; Yan, Z.-Y.; Hou, L.-F. Electrochemical synthesis of a surface-porous Mg70.5Al29.5 eutectic alloy in a neutral aqueous NaCl solution. Appl. Surf. Sci. 2018, 435, 1246–1248. [Google Scholar] [CrossRef]
- Song, Z.; Luo, W.; Zhu, Y. The surface effect on the mechanical behavior of MG nanowires: A molecular dynamic simulation. J. Non-Cryst. Solids 2023, 606, 122224. [Google Scholar] [CrossRef]
- Moshtaghi, S.; Hamadanian, M.; Amiri, O.; Goli, M.; Salavati-Niasari, M. Controllable synthesis and characterization of Mg2SiO4 nanostructures via a simple hydrothermal route using carboxylic acid as capping agent and their photocatalytic performance for photodegradation of azo dyes. RSC Adv. 2021, 11, 21588–21599. [Google Scholar] [CrossRef] [PubMed]
- Ghahari, M.; Mostafavi, K. Synthesis of Mg2SiO4:Dy3+ nanoparticles by hydrothermal method and investigation of their thermo and photo luminescence properties. Mater. Res. Bull. 2016, 77, 48–53. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, M.; Li, B.; Liu, Y.; Zhuang, J.; Zhao, X.; Xue, M.; Wang, L.; Liu, Y.; Tao, X. Developing hydrothermal fabrication and energy storage applications for MTeMoO6 (M = Zn, Mg, Mn). J. Supercrit. Fluids 2021, 171, 105187. [Google Scholar] [CrossRef]
- El Khouja, O.; Boukhoubza, I.; Derkaoui, I.; Assahsahi, I.; Achehboune, M.; Talbi, A.; Khaaissa, Y.; Makha, M.; Touhami, M.E.; Nouneh, K. Investigation of structural and optical properties of Mg doped ZnS thin films prepared by Mist-CVD technique: Experimental and theoretical aspects. Mater. Chem. Phys. 2024, 313, 128707. [Google Scholar] [CrossRef]
- Wen, J.; de Rango, P.; Allain, N.; Laversenne, L.; Grosdidier, T. Improving hydrogen storage performance of Mg-based alloy through microstructure optimization. J. Power Sources 2020, 480, 228823. [Google Scholar] [CrossRef]
- Rizo-Acosta, P.; Cuevas, F.; Latroche, M. Hydrides of early transition metals as catalysts and grain growth inhibitors for enhanced reversible hydrogen storage in nanostructured magnesium. J. Mater. Chem. A 2019, 7, 23064–23075. [Google Scholar] [CrossRef]
- Huang, L.J.; Wang, H.; Ouyang, L.Z.; Zhu, M.; Lin, H.J. Decorating crystalline YFe2–xAlx on the Mg60La10Ni20Cu10 amorphous alloy as “hydrogen pump” to realize fast de/hydrogenation. J. Mater. Sci. Technol. 2024, 173, 72–79. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Lupescu, S.; Benchea, M.; Vizureanu, P. Preliminary Microstructural and Microscratch Results of Ni-Cr-Fe and Cr3C2-NiCr Coatings on Magnesium Substrate. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012024. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Yao, B.; Wang, R.; Dong, C. Laser cladding of eutectic-based Ti–Ni–Al alloy coating on magnesium surface. Surf. Coat. Technol. 2010, 205, 189–194. [Google Scholar] [CrossRef]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar] [CrossRef]
- Jiru, W.G.; Sankar, M.R.; Dixit, U.S. Laser Surface Alloying of Copper, Manganese, and Magnesium with Pure Aluminum Substrate. J. Mater. Eng. Perform. 2016, 25, 1172–1181. [Google Scholar] [CrossRef]
- Ansari, M.; Ramezani, H.; Yari, S.; Soltani, R. Pulsed Nd:YAG laser surface alloying of AZ31 magnesium with nickel for improved wear and corrosion resistance. J. Laser Appl. 2015, 28, 012013. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Z.; Yu, B.; Ruan, Q.; Chu, P.K. Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy. Coatings 2020, 10, 313. [Google Scholar] [CrossRef]
- Kano, E.; Uzuhashi, J.; Kobayashi, K.; Ishikawa, K.; Sawabe, K.; Narita, T.; Sierakowski, K.; Bockowski, M.; Ohkubo, T.; Kachi, T.; et al. Impact of Sequential N Ion Implantation on Extended Defects and Mg Distribution in Mg Ion-Implanted GaN. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2024, 2400074. [Google Scholar] [CrossRef]
- So, S.H.; Ha, S.; Min, C.G.; Lee, Y.-S.; Park, C.R. Correction: Effects of nitrogen plasma treatments on hydrogen storage capacity of microporous carbon at room temperature and its feasibility as a hydrogen storage material. Carbon Lett. 2023, 33, 1027–1034. [Google Scholar] [CrossRef]
- Çakmak, G.; Károly, Z.; Mohai, I.; Öztürk, T.; Szépvölgyi, J. The processing of Mg–Ti for hydrogen storage; mechanical milling and plasma synthesis. Int. J. Hydrogen Energy 2010, 35, 10412–10418. [Google Scholar] [CrossRef]
- Fatimah, S.; Khoerunnisa, F.; Kwon, J.; Kim, Y.; Ko, Y. Inorganic-metallic bilayer on Mg alloy via wet and dry plasma treatments. Surf. Coat. Technol. 2019, 360, 56–63. [Google Scholar] [CrossRef]
- Wang, S.; Yong, H.; Yao, J.; Ma, J.; Liu, B.; Hu, J.; Zhang, Y. Influence of the phase evolution and hydrogen storage behaviors of Mg-RE alloy by a multi-valence Mo-based catalyst. J. Energy Storage 2023, 58, 106397. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, L.; Xuan, W.; Ji, Z. Surface doping of the LaMg3 alloy with nano-cobalt particles for promoting the hydrogenation properties through magnetron sputtering. Appl. Surf. Sci. 2019, 466, 673–678. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Zhao, Y.; Wang, S.; Chen, Y.; Liu, B.; Hu, J.; Zhang, Y. Phase evolution, hydrogen storage thermodynamics, and kinetics of ternary Mg98Ho1.5Fe0.5 alloy. J. Rare Earths 2023, in press. [Google Scholar] [CrossRef]
- Lin, H.; Ouyang, L.; Wang, H.; Liu, J.; Zhu, M. Phase transition and hydrogen storage properties of melt-spun Mg3LaNi0.1 alloy. Int. J. Hydrogen Energy 2012, 37, 1145–1150. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Zhang, L.; Zhang, L. Synthesis and hydrogen storage properties of Mg2Ni-type alloys. J. Rare Earths 2012, 30, 898–902. [Google Scholar]
- Li, L.R.; Chen, W.; Liu, X.H.; Li, Q. Morphology and electrochemical hydrogen storage properties of melt-spun Mg–Ce–Ni–Al alloys. Int. J. Hydrogen Energy 2009, 34, 8389–8394. [Google Scholar] [CrossRef]
- Ouyang, L.; Cao, Z.; Wang, H.; Hu, R.; Zhu, M. Application of dielectric barrier discharge plasma-assisted milling in energy storage materials—A review. J. Alloys Compd. 2017, 691, 422–435. [Google Scholar] [CrossRef]
- Selvam, P.; Yvon, K. Synthesis of Mg2FeH6, Mg2CoH5 and Mg2NiH4 by high-pressure sintering of the elements. Int. J. Hydrogen Energy 1991, 16, 615–617. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Fang, F.; Sun, D.; Zhang, Q. Hydrogen storage properties of in situ synthesized Mg-5 wt% TiC nano-composite by mechanical milling. J. Alloys Compd. 2017, 708, 169–178. [Google Scholar]
- Li, Q.; Jiang, L.J.; Fu, Y.; Guo, S.; Zhou, H.Y.; Wang, L.B.; Yu, X.B. Synthesis of TiC/Mg composites with enhanced hydrogen storage kinetics. Dalton Trans. 2011, 40, 4131–4137. [Google Scholar]
- Li, S.; Varin, R.A.; Morozova, O.; Khomenko, T. Controlled mechano-chemical synthesis of nanostructured ternary complex hydride Mg2FeH6 under low-energy ball milling. J. Alloys Compd. 2004, 384, 231–248. [Google Scholar] [CrossRef]
- Boruah, B.; Kalita, B. Alloying ratio versus cluster size for reversible hydrogen storage in 3d transition metal doped small Mg clusters: Dispersion corrected DFT study. J. Energy Storage 2023, 72, 108217. [Google Scholar] [CrossRef]
- Zeng, K.; Klassen, T.; Oelerich, W.; Bormann, R. Thermodynamic analysis of the hydriding process of Mg–Ni alloys. J. Alloys Compd. 1999, 283, 213–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, F.; Guemou, S.; Yu, H.; Zhang, L.; Wang, Y. Constructing Mg2Co–Mg2CoH5 nano hydrogen pumps from LiCoO2 nanosheets for boosting the hydrogen storage property of MgH2. Dalton Trans. 2022, 51, 16195–16205. [Google Scholar] [CrossRef]
- Tao, S.X.; Notten, P.H.; van Santen, R.A.; Jansen, A.P. DFT studies of hydrogen storage properties of Mg0.75Ti0.25. J. Alloys Compd. 2011, 509, 210–216. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Cai, F.; Xu, F.; Wei, W.; Guo, J.; Lan, Z. Catalytic effects of V and V2O5 on hydrogen storage property of Mg17Al12 alloy. Int. J. Hydrogen Energy 2018, 43, 14578–14583. [Google Scholar] [CrossRef]
- Qi, Y.; Sheng, P.; Li, J.; Zhang, X.; Zhang, W.; Guo, S.; Zhang, Y. Improved hydrogen storage thermodynamics and kinetics of La–Ce–Mg–Ni alloy by ball milling. J. Phys. Chem. Solids 2023, 179, 111417. [Google Scholar] [CrossRef]
- Bambhaniya, K.G.; Grewal, G.S.; Shrinet, V.; Singh, N.L.; Govindan, T.P. Fast hydriding Mg–Zr–Mn–Ni alloy compositions for high capacity hydrogen storage application. Int. J. Hydrogen Energy 2012, 37, 3671–3676. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Yang, C.; Jiang, Q. Clarifying the capacity deterioration mechanism sheds light on the design of ultra-long-life hydrogen storage alloys. Chem. Eng. J. 2018, 352, 325–332. [Google Scholar] [CrossRef]
- Han, Z.; Wu, Y.; Yu, H.; Zhou, S. Location-dependent effect of nickel on hydrogen dissociation and diffusion on Mg (0001) surface: Insights into hydrogen storage material design. J. Magnes. Alloys 2022, 10, 1617–1630. [Google Scholar] [CrossRef]
- Dong, S.; Li, C.; Wang, J.; Liu, H.; Ding, Z.; Gao, Z.; Yang, W.; Lv, W.; Wei, L.; Wu, Y.; et al. The “burst effect” of hydrogen desorption in MgH2 dehydrogenation. J. Mater. Chem. A 2022, 10, 22363–22372. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, H.; Zhang, M.; Ye, Y.; Wang, K.; Zhou, Y.; Zhu, Y.; Zhang, Y. Synergistic effect of Pd single atoms and clusters on the de/re-hydrogenation performance of MgH2. J. Mater. Sci. Technol. 2024, 191, 49–62. [Google Scholar] [CrossRef]
- Vyas, D.; Jain, P.; Khan, J.; Kulshrestha, N.; Jain, A.; Jain, I.P. Effect of Ni on hydrogen storage properties of Mg-Mn-Ni alloys. Int. J. Hydrogen Energy 2013, 38, 7695–7700. [Google Scholar]
- Zhang, Y.; Li, B.; Ren, H.; Li, X.; Qi, Y. Enhanced hydrogen storage properties of Mg–Ni–Ce composite by melt spinning followed by crystallization treatment. Mater. Chem. Phys. 2010, 124, 795–799. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Pluengphon, P.; Bovornratanaraks, T.; Tsuppayakorn-Aek, P.; Pinsook, U.; Inceesungvorn, B. High-pressure phases induce H-vacancy diffusion kinetics in TM-doped MgH2: Ab initio study for hydrogen storage improvement. Int. J. Hydrogen Energy 2019, 44, 21948–21954. [Google Scholar] [CrossRef]
- Chong, M.; Karkamkar, A.; Autrey, T.; Orimo, S.I.; Jalisatgi, S.; Jensen, C.M. Reversible dehydrogenation of mag-nesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem. Commun. 2011, 47, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-J.; Dou, Z.-H.; Zhang, T.-A.; Meng, D.-L.; Fan, Y.-Y. Separation of metal ions and resource utilization of magnesium from saline lake brine by membrane electrolysis. Sep. Purif. Technol. 2020, 251, 117316. [Google Scholar] [CrossRef]
- Neatu, S.; Neatu, F.; Chirica, I.M.; Borbath, I.; Talas, E.; Tompos, A.; Somacescu, S.; Osiceanu, P.; Folgado, M.A.; Chaparro, A.M.; et al. Recent progress in electrocatalysts and electrodes for portable fuel cells. J. Mater. Chem. A 2021, 9, 17065–17128. [Google Scholar] [CrossRef]
- Ma, J.; Wang, G.; Li, Y.; Ren, F.; Volinsky, A.A. Electrochemical performance of Mg-air batteries based on AZ series magnesium alloys. Ionics 2019, 25, 2201–2209. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Hou, Q.; Guo, X.; Xu, G. Improvement of Mg-Based Hydrogen Storage Materials by Metal Catalysts: Review and Summary. ChemistrySelect 2021, 6, 8809–8829. [Google Scholar] [CrossRef]
- Xie, X.; Hou, C.; Chen, C.; Sun, X.; Pang, Y.; Zhang, Y.; Yu, R.; Wang, B.; Du, W. First-principles studies in Mg-based hydrogen storage Materials: A review. Energy 2020, 211, 118959. [Google Scholar] [CrossRef]
- Ruetschi, P.; Meli, F.; Desilvestro, J. Nickel-metal hydride batteries. The preferred batteries of the future? J. Power Sources 1995, 57, 85–91. [Google Scholar] [CrossRef]
- Poupin, L.; Humphries, T.D.; Paskevicius, M.; Buckley, C.E. A thermal energy storage prototype using sodium magnesium hydride. Sustain. Energy Fuels 2019, 3, 985–995. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Lacasta, A.; Giro-Paloma, J.; Chimenos, J.; Haurie, L.; Formosa, J. Magnesium phosphate cements formulated with low grade magnesium oxide incorporating phase change materials for thermal energy storage. Constr. Build. Mater. 2017, 155, 209–216. [Google Scholar] [CrossRef]
- Albert, R.; Wagner, C.; Urbanczyk, R.; Felderhoff, M. Effective thermal conductivity of dimagnesium iron hexahydride (Mg2FeH6) for heat storage applications. Appl. Phys. A 2022, 129, 62. [Google Scholar] [CrossRef]
- Pistidda, C.; Bergemann, N.; Wurr, J.; Rzeszutek, A.; Møller, K.; Hansen, B.; Garroni, S.; Horstmann, C.; Milanese, C.; Girella, A.; et al. Hydrogen storage systems from waste Mg alloys. J. Power Sources 2014, 270, 554–563. [Google Scholar] [CrossRef]
- Hardian, R.; Pistidda, C.; Chaudhary, A.-L.; Capurso, G.; Gizer, G.; Cao, H.; Milanese, C.; Girella, A.; Santoru, A.; Yigit, D.; et al. Waste Mg-Al based alloys for hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 16738–16748. [Google Scholar] [CrossRef]

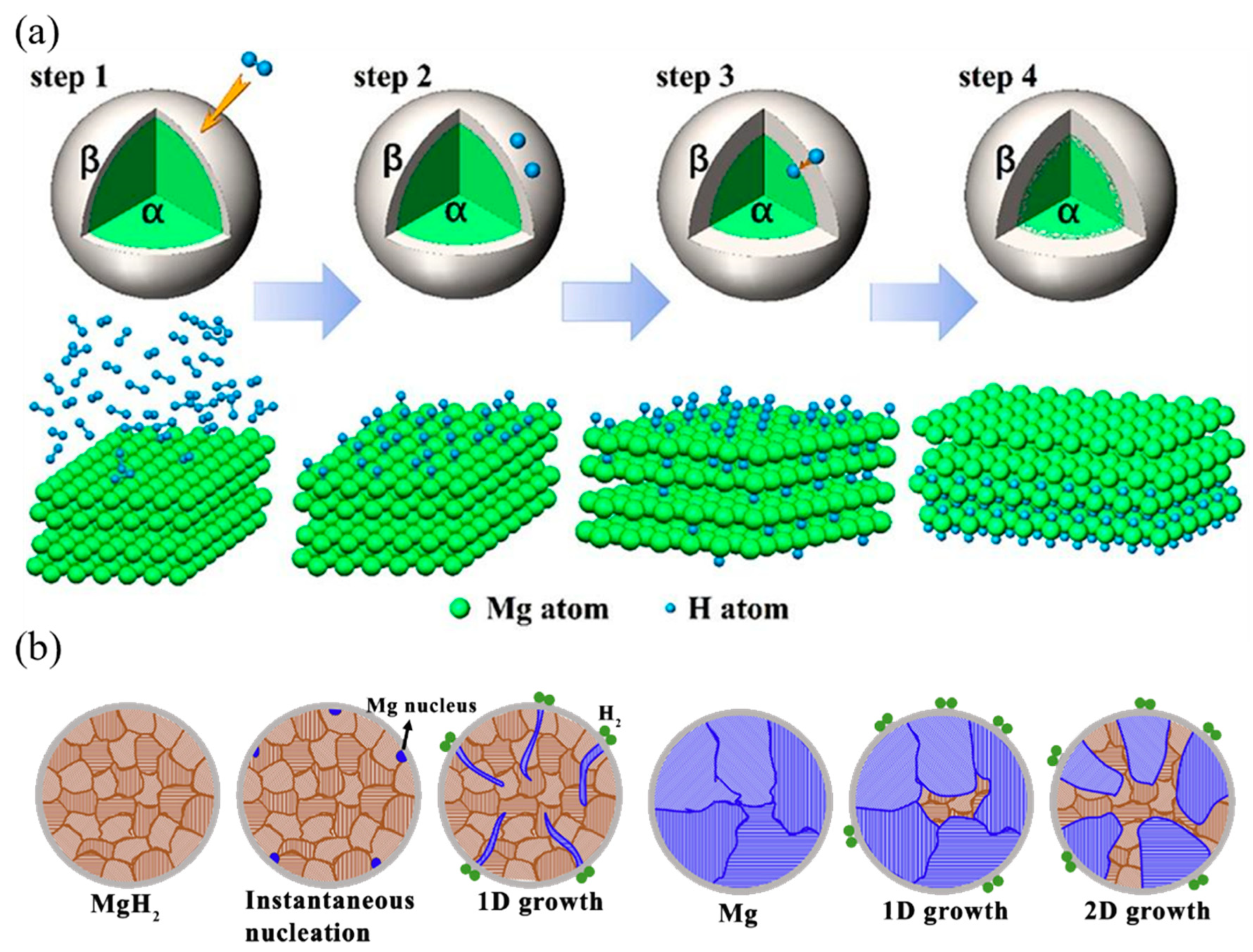

| Alloy System | Composition | Hydrogen Storage Capacity (wt.%) | Desorption Temperature (°C) | Absorption/Desorption Kinetics | Ref. |
|---|---|---|---|---|---|
| Mg-Ni | Mg2Ni | 3.6 | 250–300 | Moderate | [16] |
| Mg2Ni0.8Co0.2 | 3.4 | 270–320 | Fast | [17] | |
| Mg2Ni0.7Mn0.3 | 3.5 | 240–290 | Relatively fast | [18] | |
| Mg-Fe | Mg2FeH6 | 5.5 | 320–350 | Slow | [19] |
| Mg-10wt.%Fe | 6.2 | 330–360 | Relatively slow | [20] | |
| Mg-Co | Mg2CoH5 | 4.5 | 280–320 | Moderate | [21] |
| Mg-5wt.%Ti | 6.8 | 300–340 | Fast | [22] | |
| Mg-Ti | Mg-10wt.%Ti | 6.0 | 250–300 | Fast | [23] |
| Mg-5wt.%Ti-5wt.%Fe | 5.5 | 240–280 | Very fast | [24] | |
| Mg-V | Mg-10wt.%V | 6.5 | 200–250 | Extremely fast | [25] |
| Mg-5wt.%V-5wt.%Ni | 5.8 | 190–240 | Fastest | [26] | |
| Mg-La | Mg-30wt.%La | 5.0 | 250–300 | Fast | [27] |
| Mg-30wt.%La-10%wt.%Ni | 4.8 | 230–280 | Very fast | [28] | |
| Mg-Ce | Mg-30wt.%Ce | 4.8 | 270–320 | Moderate | [29] |
| Mg-30wt.%Ce-10wt.%Co | 4.6 | 260–300 | Relatively fast | [29] |
| Alloy System | Enthalpy ΔH (kJ/mol H2) | Entropy ΔS (kJ/(mol·K)) | Desorption Temperature (°C) | Ref. |
|---|---|---|---|---|
| Mg-Ni | 64.5 | 130.2 | 255 | [30] |
| 62.8 | 126.5 | 246 | [30] | |
| 65.9 | 132.6 | 262 | [31] | |
| Mg-Fe | 77.4 | 137.8 | 320 | [31] |
| 75.6 | 135.2 | 311 | [32] | |
| 79.1 | 140.1 | 326 | [33] | |
| Mg-Co | 75.1 | 135.5 | 310 | [30] |
| 73.5 | 132.8 | 301 | [31] | |
| 76.4 | 137.3 | 318 | [32] | |
| Mg-Ti | 72.3 | 133.1 | 288 | [34] |
| 70.7 | 130.6 | 280 | [34] | |
| 73.5 | 135.0 | 295 | [35] | |
| Mg-Nb | 68.7 | 132.4 | 275 | [35] |
| 67.4 | 129.8 | 267 | [34] | |
| 70.2 | 134.6 | 282 | [35] |
| Alloy System | Milling Speed (rpm) | Ball-to-Powder Ratio | Hydrogen Pressure (MPa) | Milling Time (h) | Milling Atmosphere | Process Control Agent | Milling Ball Material | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mg-Ni | 450 | 50:1 | 1.2 | 25 | H2 | Graphite (1 wt.%) | Tungsten carbide | [57] |
| 400 | 40:1 | 1.0 | 20 | Ar | None | Stainless steel | [60] | |
| 350 | 30:1 | 0.8 | 15 | Vacuum | Stearic acid (2 wt.%) | Zirconia | [64] | |
| Mg-Fe | 400 | 35:1 | 1.0 | 30 | Ar | Graphite (1 wt.%) | Tungsten carbide | [60] |
| 350 | 30:1 | 0.8 | 25 | H2 | None | Stainless steel | [61] | |
| 300 | 35:1 | 0.6 | 20 | Vacuum | Stearic acid (2 wt.%) | Zirconia | [64] | |
| Mg-Co | 500 | 40:1 | 1.5 | 32 | Ar | Graphite (1 wt.%) | Tungsten carbide | [58] |
| 450 | 35:1 | 1.2 | 28 | H2 | None | Stainless steel | [60] | |
| 400 | 30:1 | 1.0 | 24 | Vacuum | Stearic acid (2 wt.%) | Zirconia | [64] | |
| Mg-Ti | 550 | 60:1 | 1.8 | 20 | Ar | Graphite (1 wt.%) | Tungsten carbide | [57] |
| 500 | 50:1 | 1.5 | 15 | H2 | None | Stainless steel | [58] | |
| 450 | 40:1 | 1.2 | 12 | Vacuum | Stearic acid (2 wt.%) | Zirconia | [60] | |
| Mg-Nb | 600 | 55:1 | 2.5 | 15 | Ar | Graphite (1 wt.%) | Tungsten carbide | [65] |
| 550 | 45:1 | 2.0 | 12 | H2 | None | Stainless steel | [66] | |
| 500 | 40:1 | 1.8 | 10 | Vacuum | Stearic acid (2 wt.%) | Zirconia | [67] |
| Synthesis Method | Raw Materials | Product Morphology | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Physical vapor deposition | Mg, Ni, etc. | Thin film | Controllable composition and thickness, high purity | Slow deposition rate, high cost | [73] |
| Porous thin film | Large specific surface area, fast kinetics | Easy contamination, poor stability | [81] | ||
| Chemical vapor deposition | Metal–organic sources, H2 | Thin film | Controllable composition, high deposition rate | High temperature required, high precursor cost | [76] |
| Nanowire/rod/tube arrays | Large specific surface area, high storage capacity | Uneven morphology, poor stability | [82] | ||
| Electrodeposition | Mg, Ni-containing solution | Thin film | Fast deposition at room temperature, simple process | Non-uniform composition and thickness | [79] |
| Porous film | Fast hydrogen storage kinetics | Easy cracking, easy oxidation | [83] | ||
| Electroless deposition | Mg-, Ni-containing solution | Powder, thin film | Applicable to complex substrates, low cost | Slow deposition rate, high waste liquid | [84] |
| Coated powder | Dense coating layer, oxidation prevention | Uneven coating | [85] |
| Surface Modification Method | Modifier/Condition | Desorption Peak Temperature Decrease (°C) | Dehydrogenation Capacity Increase at 300 (°C) | Capacity Retention After 50 Cycles (%) | Ref. |
|---|---|---|---|---|---|
| Surface coating | Ni | 45 | 1.5 | 92 | [108] |
| Ti | 52 | 1.8 | 94 | [109] | |
| V | 58 | 2.1 | 96 | [110] | |
| Surface alloying | Ti | 35 | 1.2 | 95 | [110] |
| Fe | 40 | 1.4 | 94 | [112] | |
| Nb | 45 | 1.6 | 97 | [112] | |
| Plasma | Ar/H2 plasma | 35 | 1.2 | 95 | [116] |
| N2 plasma | 38 | 1.3 | 93 | [115] | |
| Ion implantation | Ti ions | 48 | 1.7 | 96 | [113] |
| Fe ions | 55 | 2.0 | 95 | [114] | |
| Fe ions | 40 | 1.3 | 93 | [113] |
| Application Scenario | Operating Temperature | Cycle Life (Times) | Hydrogen Purity (%) | Filling Time (min) | System Weight (kg/kW) | Ref. |
|---|---|---|---|---|---|---|
| Fuel cell vehicle | 25–80 | ≥1000 | ≥99.99 | ≤5 | ≤0.5 | [143] |
| Portable power source | 20–60 | ≥500 | ≥99.9 | ≤10 | ≤1 | [144] |
| Thermochemical heat storage | 300–400 | ≥200 | ≥99 | ≤30 | ≤5 | [145] |
| Backup power for mobile base stations | 20–50 | ≥800 | ≥99.95 | ≤15 | ≤2 | [146] |
| Drone power supply | 0–40 | ≥600 | ≥99.999 | ≤8 | ≤0.8 | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules 2024, 29, 2525. https://doi.org/10.3390/molecules29112525
Xu Y, Zhou Y, Li Y, Hao Y, Wu P, Ding Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules. 2024; 29(11):2525. https://doi.org/10.3390/molecules29112525
Chicago/Turabian StyleXu, Yaohui, Yang Zhou, Yuting Li, Yechen Hao, Pingkeng Wu, and Zhao Ding. 2024. "Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications" Molecules 29, no. 11: 2525. https://doi.org/10.3390/molecules29112525
APA StyleXu, Y., Zhou, Y., Li, Y., Hao, Y., Wu, P., & Ding, Z. (2024). Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules, 29(11), 2525. https://doi.org/10.3390/molecules29112525








