The Physicochemical and Functional Properties of Biosurfactants: A Review
Abstract
:1. Introduction
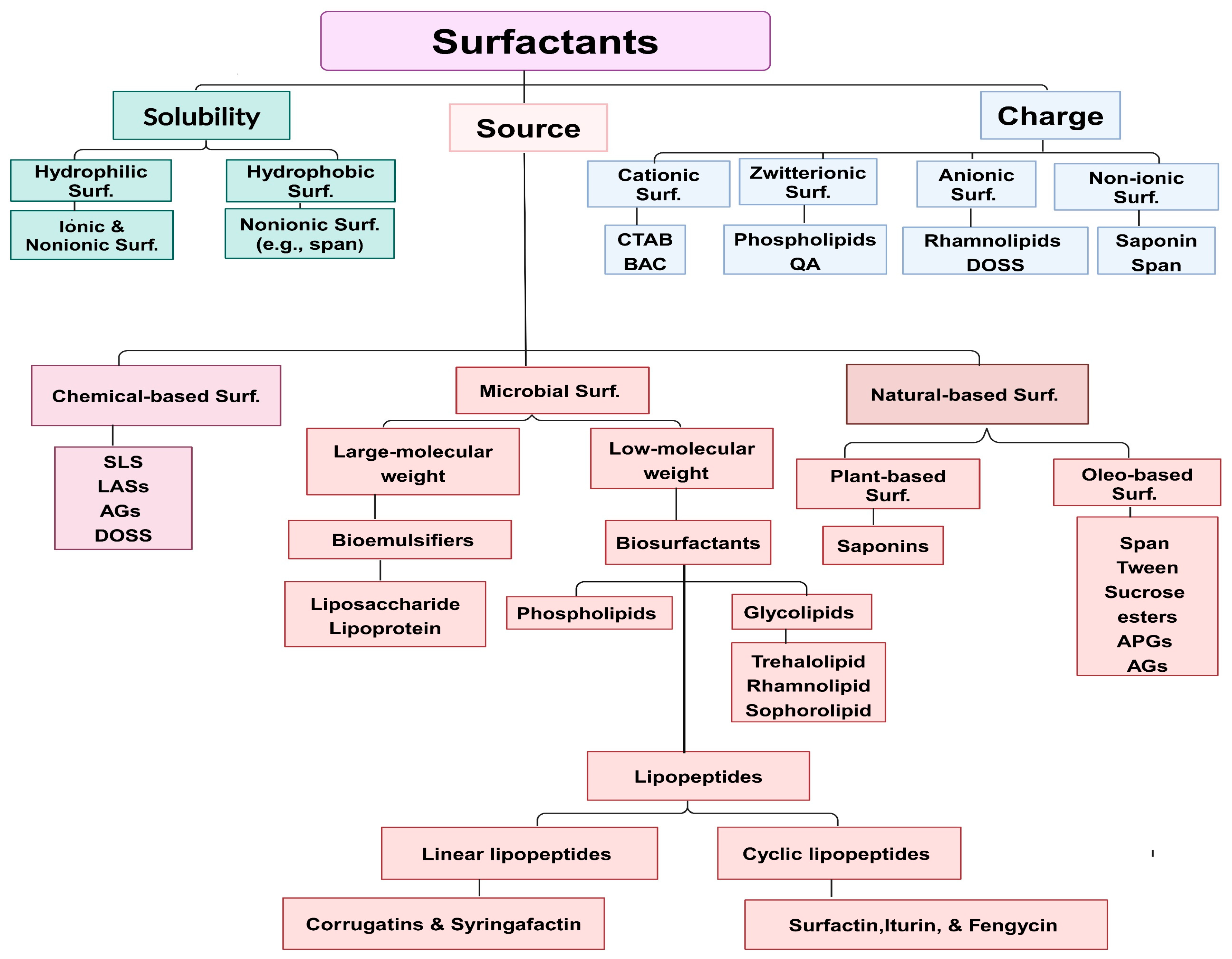
2. Surfactant Classifications
2.1. Synthetic-Based Surfactants
2.2. Natural-Based Surfactants
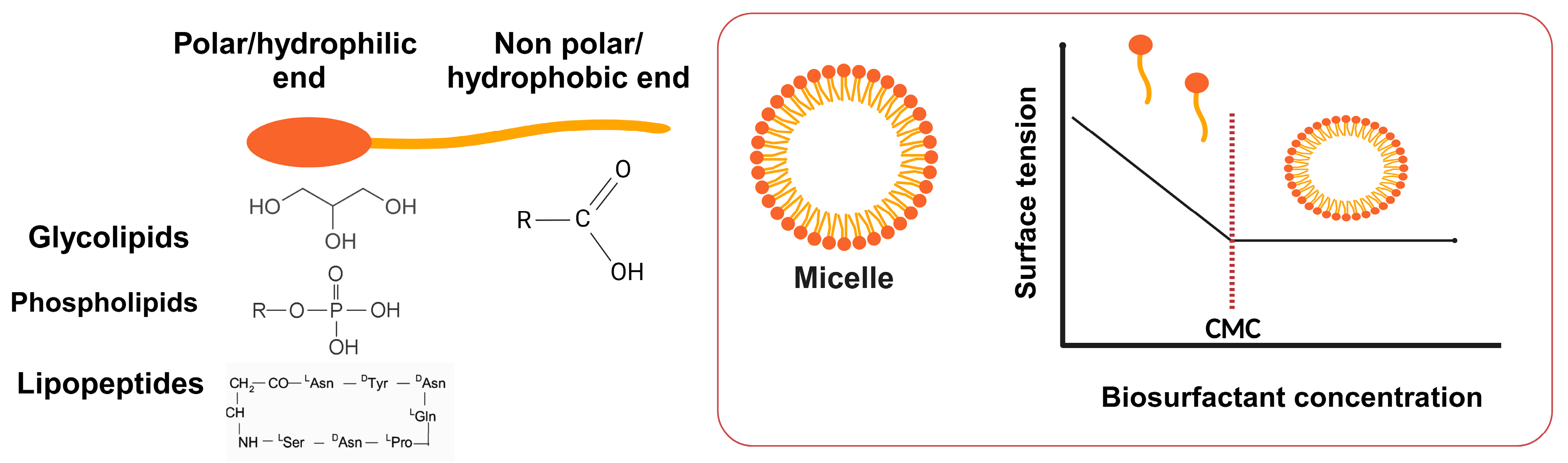
2.2.1. Glycolipids
2.2.2. Lipopeptides
2.2.3. Phospholipids
2.2.4. Glycolipoprotein
3. Physicochemical Properties of Biosurfactants
3.1. Emulsifying Activities
3.2. Surface Tension Reduction
3.3. Biosurfactant Stability
4. Bioactive Properties of Biosurfactants
4.1. Antibacterial Activities
4.2. Anti-Fungal Activities
4.3. Anti-Adhesiveness and Anti-Biofilm Activities
4.4. Antiviral Activity
4.5. Antioxidant Activities
4.6. Antiparasitic Activities
4.7. Anti-Cancer and Anti-Inflammatory Activities
5. Synergetic Effects of Biosurfactants
6. Potential Application of Biosurfactant
| Biosurfactant-Producing Microorganisms | Biosurfactant Types | Types of Food | Functionalities | Ref. |
|---|---|---|---|---|
| Saccharomyces cerevisiae URM 6670 | Glycolipids | Cookie | The biosurfactant replaces the egg yolk, which helps to decrease the concentration of animal fats. The glycolipid showed considerable temperature tolerance from 40 to 400 °C. No considerable changes in the physicochemical parameters of cookies, such as firmness, cohesiveness, and protein and lipid contents | [30] |
| Acinetobacter calcoaceticus RAG-1 | NR | Bread | Used to postpone bread staling by preserving its sensory attributes such as hardness and crumb firmness (at a concentration of 0.5%) | [191] |
| Bacillus cereus UCP 1615 | NR | Cookie | Used to extend the shelf life of the cookie up to 45 days due to a decrease in moisture content without negatively affecting the hardness, cohesiveness, and sponginess of the product | [192] |
| Candida utilis UFPEDA1009 | NR | Cookie | Replacing the isolated biosurfactant with egg yolk in cookie recipes can help reduce the use of animal fat while still maintaining the desired textural elements such as cohesiveness, firmness, and adhesiveness | [193] |
| Candida bombicola URM 3718 | Glycolipidic | Cupcake (emulsifier) | A suitable replacement for vegetable oils (with high saturated fatty acids) as an emulsifier without any undesirable effects on the physicochemical parameters of the products such as lipid content and energy levels | [194] |
| Candida guilliermondii (UCP0992) | NR | Mayonnaise As emulsifier | The combination of the biosurfactant (0.5%) and guar gum exhibited significant consistency and texture with no presence of pathogenic microorganisms | [195] |
| Saccharomyces cerevisiae URM 6670 | NR | Salad dressing | Used to improve the viscosity properties of the product and remain stable at 4 and 28 °C | [196] |
| Bacillus velezensis BVQ121 | Iturin, surfactin, and fengycin | Food packaging | Used to extend the shelf life of the pigeon eggs up to 10 days through inhibition of the pathogen growth of E. coli, E. ictalurid, and Salmonella typhimurium | [197] |
| Bacillus licheniformis MS48 | Lipopeptides | Cookies | Used to reduce the gluten content in the cookies while improving their textural and sensory properties | [198] |
| Saccharomyces cerevisiae URM6670 | Glycolipids | Muffins | Used to reduce the vegetable oil content with no negative changes in the sensorial parameters of the muffins such as aroma and color | [199] |
| Candida utilis | NR | Salad dressing | Used to enhance the consistency and stability of the salad dressing by adding 7% biosurfactant as an emulsifier | [200] |
| Bacillus cereus UCP 1615 | NR | Cookies | Used to keep the textural profiles and energy values and extend the shelf life of the cookies up to 45 days | [192] |
| Lactococcus lactis LNH70 | Xylolipids | Fruit juice | Used to preserve the juice for 5 days by reducing bacterial growth | [201] |
| Bacillus licheniformis MS48 | Lipopeptides | Yoghurt | Used to enhance the flavor and textural and sensorial characteristics of the final product, as well as to improve the growth and properties of the probiotics (such as Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus) | [202] |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Silva, R.D.F.S.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef]
- Guillén-Navarro, K.; López-Gutiérrez, T.; García-Fajardo, V.; Gómez-Cornelio, S.; Zarza, E.; De la Rosa-García, S.; Chan-Bacab, M. Broad-spectrum antifungal, biosurfactants and bioemulsifier activity of Bacillus subtilis subsp. spizizenii—A potential biocontrol and bioremediation agent in agriculture. Plants 2023, 12, 1374. [Google Scholar] [CrossRef]
- Kosaric, N.; Sukan, F.V. Biosurfactants: Production and Utilization—Processes, Technologies, and Economics; CRC Press: Boca Raton, FL, USA, 2014; Volume 159. [Google Scholar]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant self-assembling and critical micelle concentration: One approach fits all? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1482–1493. [Google Scholar] [CrossRef]
- Nogueira, A.R.; Popi, M.D.C.B.; Moore, C.C.S.; Kulay, L. Environmental and energetic effects of cleaner production scenarios on the Sodium Lauryl Ether Sulfate production chain. J. Clean. Prod. 2019, 240, 118203. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Idris, M.; Abdullah, S.R.S.; Anuar, N. Screening for potential biosurfactant producing bacteria from hydrocarbon—Degrading isolates. Adv. Environ. Biol. 2014, 8, 639–648. [Google Scholar]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and bio-derived surfactants versus microbial biosurfactants in the cosmetic industry: An overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef]
- Lv, J.; Da, R.; Cheng, Y.; Tuo, X.; Wei, J.; Jiang, K.; Monisayo, A.O.; Han, B. Mechanism of antibacterial activity of Bacillus amyloliquefaciens C-1 lipopeptide toward anaerobic Clostridium difficile. BioMed Res. Int. 2020, 2020, 3104613. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, A.; Han, P.; Chen, Z.; Jia, Y. Antibacterial mechanism of brevilaterin B: An amphiphilic lipopeptide targeting the membrane of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2020, 104, 10531–10539. [Google Scholar] [CrossRef]
- Barale, S.S.; Ghane, S.G.; Sonawane, K.D. Purification and characterization of antibacterial surfactin isoforms produced by Bacillus velezensis SK. Amb Express 2022, 12, 7. [Google Scholar] [CrossRef]
- de Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Yadav, A.; Shouche, Y.S.; Aphale, S.; Moghe, A.; Pillai, S.; Arora, A.; Bhadekar, R.K. Studies on biosurfactant from sp BRI 10 isolated from Antarctic sea water. Desalination 2013, 318, 64–71. [Google Scholar] [CrossRef]
- Sakr, E.A.; Ahmed, H.A.E.; Saif, F.A.A. Characterization of low-cost glycolipoprotein biosurfactant produced by Lactobacillus plantarum 60 FHE isolated from cheese samples using food wastes through response surface methodology and its potential as antimicrobial, antiviral, and anticancer activities. Int. J. Biol. Macromol. 2021, 170, 94–106. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Kamthan, M.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef]
- Kang, B.R.; Park, J.S.; Jung, W.-J. Antifungal evaluation of fengycin isoforms isolated from Bacillus amyloliquefaciens PPL against Fusarium oxysporum f. sp. lycopersici. Microb. Pathog. 2020, 149, 104509. [Google Scholar] [CrossRef]
- Kourmentza, K.; Gromada, X.; Michael, N.; Degraeve, C.; Vanier, G.; Ravallec, R.; Coutte, F.; Karatzas, K.A.; Jauregi, P. Antimicrobial activity of lipopeptide biosurfactants against foodborne pathogen and food spoilage microorganisms and their cytotoxicity. Front. Microbiol. 2021, 11, 561060. [Google Scholar] [CrossRef]
- Kang, B.R.; Park, J.S.; Jung, W.-J. Antiviral activity by lecithin-induced fengycin lipopeptides as a potent key substrate against Cucumber mosaic virus. Microb. Pathog. 2021, 155, 104910. [Google Scholar] [CrossRef]
- Giugliano, R.; Buonocore, C.; Zannella, C.; Chianese, A.; Palma Esposito, F.; Tedesco, P.; De Filippis, A.; Galdiero, M.; Franci, G.; de Pascale, D. Antiviral Activity of the Rhamnolipids Mixture from the Antarctic Bacterium Pseudomonas gessardii M15 against Herpes Simplex Viruses and Coronaviruses. Pharmaceutics 2021, 13, 2121. [Google Scholar] [CrossRef]
- Bharitkar, Y.; Bathini, S.; Ojha, D.; Ghosh, S.; Mukherjee, H.; Kuotsu, K.; Chattopadhyay, D.; Mondal, N.B. Antibacterial and antiviral evaluation of sulfonoquinovosyldiacylglyceride: A glycolipid isolated from Azadirachta indica leaves. Lett. Appl. Microbiol. 2014, 58, 184–189. [Google Scholar] [CrossRef]
- Gharaie, S.; Ohadi, M.; Hassanshahian, M.; Shakibaie, M.; Shahriary, P.; Forootanfar, H. Glycolipopeptide biosurfactant from Bacillus pumilus SG: Physicochemical characterization, optimization, antibiofilm and antimicrobial activity evaluation. 3 Biotech 2023, 13, 321. [Google Scholar] [CrossRef]
- Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F.; et al. Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from lactobacillus rhamnosus with its physicochemical and functional properties. Antibiotics 2021, 10, 1546. [Google Scholar] [CrossRef]
- de Souza Freitas, F.; Coelho de Assis Lage, T.; Ayupe, B.A.L.; de Paula Siqueira, T.; de Barros, M.; Tótola, M.R. Bacillus subtilis TR47II as a source of bioactive lipopeptides against Gram-negative pathogens causing nosocomial infections. 3 Biotech 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Krupanidhi, S.; Rohini, K.; Mikkili, I.; Kodali, V. Evaluation of anti-cancer, anti-microbial and anti-biofilm potential of biosurfactant extracted from an Acinetobacter M6 strain. J. King Saud Univ. Sci. 2020, 23, 223–227. [Google Scholar] [CrossRef]
- Cheffi, M.; Maalej, A.; Mahmoudi, A.; Hentati, D.; Marques, A.M.; Sayadi, S.; Chamkha, M. Lipopeptides production by a newly Halomonas venusta strain: Characterization and biotechnological properties. Bioorg. Chem. 2021, 109, 104724. [Google Scholar] [CrossRef]
- Jemil, N.; Ben Ayed, H.; Manresa, A.; Nasri, M.; Hmidet, N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. 2017, 17, 144. [Google Scholar] [CrossRef]
- Ciurko, D.; Czyżnikowska, Ż.; Kancelista, A.; Łaba, W.; Janek, T. Sustainable production of biosurfactant from agro-industrial oil wastes by Bacillus subtilis and its potential application as antioxidant and ACE inhibitor. Int. J. Mol. Sci. 2022, 23, 10824. [Google Scholar] [CrossRef]
- Giri, S.; Ryu, E.; Sukumaran, V.; Park, S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Potential food application of a biosurfactant produced by Saccharomyces cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020, 8, 434. [Google Scholar] [CrossRef]
- Duarte, C.; Gudina, E.J.; Lima, C.F.; Rodrigues, L.R. Effects of biosurfactants on the viability and proliferation of human breast cancer cells. AMB Express 2014, 4, 40. [Google Scholar] [CrossRef]
- Ankulkar, R.; Chavan, S.; Aphale, D.; Chavan, M.; Mirza, Y. Cytotoxicity of di-rhamnolipids produced by Pseudomonas aeruginosa RA5 against human cancerous cell lines. 3 Biotech 2022, 12, 323. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef]
- Vakil, H.; Sethi, S.; Fu, S.; Stanek, A.; Wallner, S.; Gross, R.; Bluth, M. Sophorolipids decrease pulmonary inflammation in a mouse asthma model. In Laboratory Investigation; Nature Publishing Group: New York, NY, USA, 2010; p. 392A. [Google Scholar]
- Park, S.Y.; Kim, J.-H.; Lee, S.J.; Kim, Y. Involvement of PKA and HO-1 signaling in anti-inflammatory effects of surfactin in BV-2 microglial cells. Toxicol. Appl. Pharmacol. 2013, 268, 68–78. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; da Silva, R.D.F.S.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green surfactants (biosurfactants): A petroleum-free substitute for sustainability-comparison, applications, market, and future prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Sobrino-Figueroa, A. Toxic effect of commercial detergents on organisms from different trophic levels. Environ. Sci. Pollut. Res. 2018, 25, 13283–13291. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.J.; Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chan, D.J.C.; Bilal, M.; Munawaroh, H.S.H.; Show, P.L. Recent advances of biosurfactant for waste and pollution bioremediation: Substitutions of petroleum-based surfactants. Environ. Res. 2022, 212, 113126. [Google Scholar] [CrossRef]
- Al Ghatta, A.; Aravenas, R.C.; Wu, Y.; Perry, J.M.; Lemus, J.; Hallett, J.P. New biobased sulfonated anionic surfactants based on the esterification of furoic acid and fatty alcohols: A green solution for the replacement of oil derivative surfactants with superior proprieties. ACS Sustain. Chem. Eng. 2022, 10, 8846–8855. [Google Scholar] [CrossRef]
- Ivanova, V.I.; Stanimirova, R.D.; Danov, K.D.; Kralchevsky, P.A.; Petkov, J.T. Sulfonated methyl esters, linear alkylbenzene sulfonates and their mixed solutions: Micellization and effect of Caions. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 519, 87–97. [Google Scholar] [CrossRef]
- Van Ginkel, C. Ultimate biodegradation of ingredients used in cleaning agents. In Handbook for Cleaning/Decontamination of Surfaces; Elsevier: Amsterdam, The Netherlands, 2007; pp. 655–694. [Google Scholar]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Juang, Y.-P.; Liang, P.-H. Biological and pharmacological effects of synthetic saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin surfactants used in drug delivery systems: A new application for natural medicine components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437. [Google Scholar] [CrossRef] [PubMed]
- Tippel, J.; Reim, V.; Rohn, S.; Drusch, S. Colour stability of lutein esters in liquid and spray dried delivery systems based on Quillaja saponins. Food Res. Int. 2016, 87, 68–75. [Google Scholar] [CrossRef]
- Kapadia Sanket, G.; Yagnik, B. Current trend and potential for microbial biosurfactants. Asian J. Exp. Biol. Sci. 2013, 4, 1–8. [Google Scholar]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef]
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Rahman, P.K.; Mayat, A.; Harvey, J.G.H.; Randhawa, K.S.; Relph, L.E.; Armstrong, M.C. Biosurfactants and bioemulsifiers from marine algae. In The role of Microalgae in Wastewater Treatment; Springer Nature Singapore Pte Limited: Singapore, 2019; pp. 169–188. [Google Scholar]
- Hassan, Z. The biosurfactant and antimicrobial activity of lactic acid bacteria isolated from different sources of fermented foods. Asian J. Pharm. Res. Dev. 2018, 6, 60–73. [Google Scholar]
- Xiong, Z.R.; Cobo, M.; Whittal, R.M.; Snyder, A.B.; Worobo, R.W. Purification and characterization of antifungal lipopeptide produced by Bacillus velezensis isolated from raw honey. PLoS ONE 2022, 17, e0266470. [Google Scholar] [CrossRef]
- Soleiman Meiguni, F.; Imanparast, S.; Salimi, F.; Nemati, F. The probiotic biosurfactant from Levilactobacillus brevis strain f20 isolated from a diary product with potential food applications. Food Biotechnol. 2022, 36, 394–411. [Google Scholar] [CrossRef]
- Akintayo, S.O.; Treinen, C.; Vahidinasab, M.; Pfannstiel, J.; Bertsche, U.; Fadahunsi, I.; Oellig, C.; Granvogl, M.; Henkel, M.; Lilge, L. Exploration of surfactin production by newly isolated Bacillus and Lysinibacillus strains from food-related sources. Lett. Appl. Microbiol. 2022, 75, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Soltanighias, T.; Singh, A.E.; Satpute, S.K.; Banpurkar, A.G.; Koolivand, A.; Rahi, P. Assessment of biosurfactant-producing bacteria from oil contaminated soils and their hydrocarbon degradation potential. Environ. Sustain. 2019, 2, 285–296. [Google Scholar] [CrossRef]
- Perfumo, A.; Smyth, T.; Marchant, R.; Banat, I. Production and roles of biosurfactants and bioemulsifiers in accessing hydrophobic substrates. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1501–1512. [Google Scholar]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—A review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Subsanguan, T.; Khondee, N.; Rongsayamanont, W.; Luepromchai, E. Formulation of a glycolipid: Lipopeptide mixture as biosurfactant-based dispersant and development of a low-cost glycolipid production process. Sci. Rep. 2022, 12, 16353. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanidou, V.; Alimpoumpa, D.; Papadaki, A.; Lappa, I.; Alexopoulos, K.; Kopsahelis, N. Cheese whey utilization for biosurfactant production: Evaluation of bioprocessing strategies using novel Lactobacillus strains. Biomass Convers. Biorefinery 2022, 12, 4621–4635. [Google Scholar] [CrossRef]
- Sundaram, T.; Govindarajan, R.K.; Vinayagam, S.; Krishnan, V.; Nagarajan, S.; Gnanasekaran, G.R.; Baek, K.-H.; Rajamani Sekar, S.K. Advancements in biosurfactant production using agro-industrial waste for industrial and environmental applications. Front. Microbiol. 2024, 15, 1357302. [Google Scholar] [CrossRef] [PubMed]
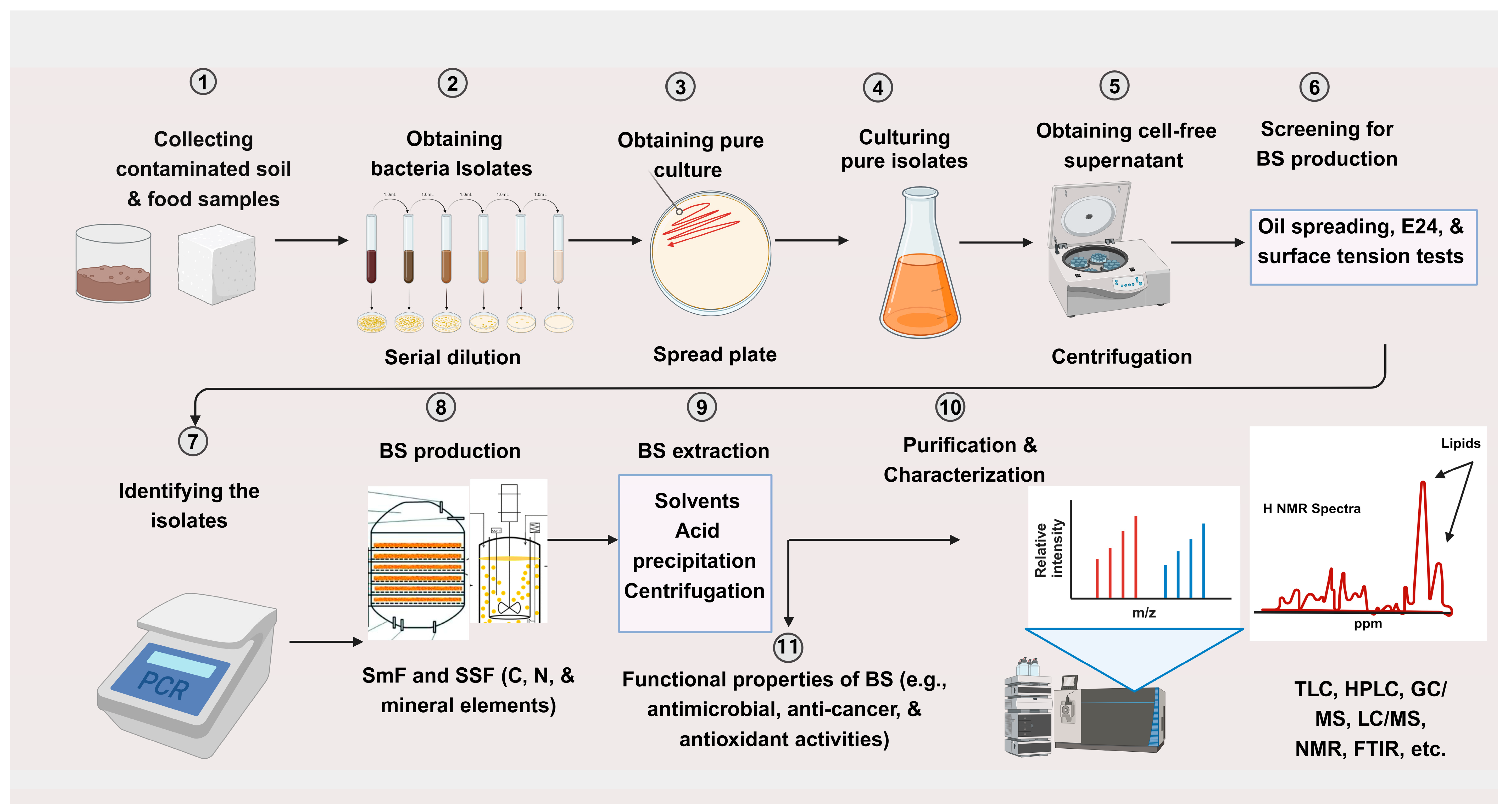
- Trindade, M.; Sithole, N.; Kubicki, S.; Thies, S.; Burger, A. Screening strategies for biosurfactant discovery. In Biosurfactants for the Biobased Economy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 17–52. [Google Scholar]
- Marchant, R.; Banat, I.M. Protocols for measuring biosurfactant production in microbial cultures. In Hydrocarbon and Lipid Microbiology Protocols: Activities and Phenotypes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 119–128. [Google Scholar]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef]
- Favaro, G.; Bogialli, S.; Di Gangi, I.M.; Nigris, S.; Baldan, E.; Squartini, A.; Pastore, P.; Baldan, B. Characterization of lipopeptides produced by Bacillus licheniformis using liquid chromatography with accurate tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2237–2252. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Lee, D.-H.; Lee, J.-H.; Haque, M.A.; Cho, K.-M. Five surfactin isomers produced during Cheonggukjang fermentation by Bacillus pumilus HY1 and their properties. Molecules 2021, 26, 4478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, L.; Ding, J.; Wang, M.; Ge, R.; Zhao, H.; Zhang, B.; Fan, J. Natural antimicrobial lipopeptides secreted by Bacillus spp. and their application in food preservation, a critical review. Trends Food Sci. Technol. 2022, 127, 26–37. [Google Scholar] [CrossRef]
- Liu, J.-F.; Mbadinga, S.M.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. Int. J. Mol. Sci. 2015, 16, 4814–4837. [Google Scholar] [CrossRef] [PubMed]
- Šegota, S.; Heimer, S.; Težak, Đ. New catanionic mixtures of dodecyldimethylammonium bromide/sodium dodecylbenzenesulphonate/water: I. Surface properties of dispersed particles. Colloids Surf. Physicochem. Eng. Aspects 2006, 274, 91–99. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Zehra, S.; Aslam, J. Biosurfactants: Types, sources, and production. In Advancements in Biosurfactants Research; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–24. [Google Scholar]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Lavania, M.; Lal, B. Biosurfactant: An emerging tool for the petroleum industries. Front. Microbiol. 2023, 14, 1254557. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.A.T.; Simões, L.A.; Dias, D.R. Comparison of biodegradability, and toxicity effect of biosurfactants with synthetic surfactants. In Advancements in Biosurfactants Research; Springer: Berlin/Heidelberg, Germany, 2023; pp. 117–136. [Google Scholar]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and applications in drug delivery, biotechnology and ecotoxicology. Bioengineering 2021, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Rhamnolipids. Available online: https://www.jeneilbiotech.com/biosurfactant/rhamnolipids (accessed on 30 January 2024).
- Abdel-Mawgoud, A.M.; Stephanopoulos, G. Simple glycolipids of microbes: Chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechnol. 2018, 3, 3–19. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320. [Google Scholar] [CrossRef]
- Guzmán Solís, E.; Ortega Gómez, F.; González Rubio, R. Exploring the world of rhamnolipids: A critical review of their production, interfacial properties, and potential application. Curr. Opin. Colloid Interface Sci. 2024, 69, 101780. [Google Scholar] [CrossRef]
- Bartal, A.; Vigneshwari, A.; Bóka, B.; Vörös, M.; Takács, I.; Kredics, L.; Manczinger, L.; Varga, M.; Vágvölgyi, C.; Szekeres, A. Effects of different cultivation parameters on the production of surfactin variants by a Bacillus subtilis strain. Molecules 2018, 23, 2675. [Google Scholar] [CrossRef]
- Soccol, V.T.; Pandey, A.; Resende, R.R. Current Developments in Biotechnology and Bioengineering: Human and Animal Health Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Sani, A.; Qin, W.-Q.; Li, J.-Y.; Liu, Y.-F.; Zhou, L.; Yang, S.-Z.; Mu, B.-Z. Structural diversity and applications of lipopeptide biosurfactants as biocontrol agents against phytopathogens: A review. Microbiol. Res. 2023, 278, 127518. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Mazumdar, S.; Pandey, A.; Kanwar, S.S. Pseudomonas lipopeptide: An excellent biomedical agent. MedComm–Biomater. Appl. 2023, 2, e27. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Zhu, J.; Lu, Q.; Cryle, M.J.; Zhang, Y.; Yan, F. Structural diversity, biosynthesis, and biological functions of lipopeptides from Streptomyces. Nat. Prod. Rep. 2023, 40, 557–594. [Google Scholar] [CrossRef]
- Götze, S.; Stallforth, P. Structure, properties, and biological functions of nonribosomal lipopeptides from Pseudomonads. Nat. Prod. Rep. 2020, 37, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Júnior, R.A.; de Faria Poloni, J.; Pinto, É.S.M.; Dorn, M. Interdisciplinary overview of lipopeptide and protein-containing biosurfactants. Genes 2023, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Nikam, R.; Gaikwad, S.; Sapre, V.; Kaur, J. Biosurfactant: Types, detection methods, importance and applications. Indian J. Microbiol. Res. 2016, 3, 5–10. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Pons, R.; Espuny, M.; Aranda, F.; Teruel, J.; Manresa, A.; Ortiz, A.; Marqués, A. Isolation and partial characterization of a biosurfactant mixture produced by Sphingobacterium sp. isolated from soil. J. Colloid Interface Sci. 2011, 361, 195–204. [Google Scholar] [CrossRef]
- Mouafo, H.T.; Sokamte, A.T.; Mbawala, A.; Ndjouenkeu, R.; Devappa, S. Biosurfactants from lactic acid bacteria: A critical review on production, extraction, structural characterization and food application. Food Biosci. 2022, 46, 101598. [Google Scholar] [CrossRef]
- Walter, V.; Syldatk, C.; Hausmann, R. Screening concepts for the isolation of biosurfactant producing microorganisms. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Poon, S.; Clarke, A.E.; Schultz, C.J. Structure–function analysis of the emulsifying and interfacial properties of apomyoglobin and derived peptides. J. Colloid Interface Sci. 1999, 213, 193–203. [Google Scholar] [CrossRef] [PubMed]
- de Faria, A.F.; Teodoro-Martinez, D.S.; de Oliveira Barbosa, G.N.; Vaz, B.G.; Silva, Í.S.; Garcia, J.S.; Tótola, M.R.; Eberlin, M.N.; Grossman, M.; Alves, O.L. Production and structural characterization of surfactin (C14/Leu7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochem. 2011, 46, 1951–1957. [Google Scholar] [CrossRef]
- Peele, K.A.; Ch, V.R.; Kodali, V.P. Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech 2016, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, F.; Pradilla, D.; Cruz, J.C.; Alvarez, O. Emerging emulsifiers: Conceptual basis for the identification and rational design of peptides with surface activity. Int. J. Mol. Sci. 2021, 22, 4615. [Google Scholar] [CrossRef] [PubMed]
- Adheeb Usaid, A.; Premkumar, J.; Ranganathan, T. Emulsion and it’s applications in food processing—A review. Int. J. Eng. Res. Appl. 2014, 4, 241–248. [Google Scholar]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The formation, stabilization and separation of oil–water emulsions: A review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Durval, I.J.B.; da Silva, I.A.; Sarubbo, L.A. Application of microbial biosurfactants in the food industry. In Microbial Biosurfactants: Preparation, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–10. [Google Scholar]
- Lima, R.A.; Andrade, R.F.; RodrÃguez, D.M.; Araújo, H.W.; Santos, V.P.; Campos-Takaki, G.M. Production and characterization of biosurfactant isolated from Candida glabrata using renewable substrates. Afr. J. Microbiol. Res. 2017, 11, 237–244. [Google Scholar]
- Ahamed, M.I.; Prasad, R. (Eds.) Microbial Biosurfactants: Preparation, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Luna, J.M.; Rufino, R.D.; Campos-Takaki, G.M.; Sarubbo, L.A. Properties of the Biosurfactant Produced by Cultivated in Low-Cost Substrates. Ibic2012 Int. Conf. Ind. Biotechnol. 2012, 27, 67–72. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Production and applications of lipopeptide biosurfactant for bioremediation and oil recovery by CN2. Biochem. Eng. J. 2015, 101, 168–178. [Google Scholar] [CrossRef]
- Pandey, R.; Krishnamurthy, B.; Singh, H.P.; Batish, D.R. Evaluation of a glycolipopepetide biosurfactant from Aeromonas hydrophila RP1 for bioremediation and enhanced oil recovery. J. Clean. Prod. 2022, 345, 131098. [Google Scholar] [CrossRef]
- Wu, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Determining the critical micelle concentration of surfactants by a simple and fast titration method. Anal. Chem. 2019, 92, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.; Behnke, T.; Resch-Genger, U. Determination of the critical micelle concentration of neutral and ionic surfactants with fluorometry, conductometry, and surface tension—A method comparison. J. Fluoresc. 2018, 28, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, Y.; Cao, M.; Wang, J.; Lu, J.R.; Xu, H. Rational design, properties, and applications of biosurfactants: A short review of recent advances. Curr. Opin. Colloid Interface Sci. 2020, 45, 57–67. [Google Scholar] [CrossRef]
- Sharma, S.; Pandey, L.M. Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour. Technol. 2020, 307, 123261. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Padhan, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Clouding behaviour in surfactant systems. Adv. Colloid Interface Sci. 2011, 162, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rub, M.A.; Hoque, M.A.; Khan, M.A.; Asiri, A.M. Clouding and thermodynamic behaviours of nonionic surfactant: Effects of cefixime trihydrate drug and different electrolytes. J. Mol. Liq. 2020, 312, 113366. [Google Scholar] [CrossRef]
- Zhen, C.; Ge, X.F.; Lu, Y.T.; Liu, W.Z. Chemical structure, properties and potential applications of surfactin, as well as advanced strategies for improving its microbial production. AIMS Microbiol. 2023, 9, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xue, R.; Liu, S.; Xu, N.; Xin, F.; Zhang, W.; Jiang, M.; Dong, W. High di-rhamnolipid production using Pseudomonas aeruginosa KT1115, separation of mono/di-rhamnolipids, and evaluation of their properties. Front. Bioeng. Biotechnol. 2019, 7, 245. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaabouni, S.; Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Safari, P.; Hosseini, M.; Lashkarbolooki, M.; Ghorbani, M.; Najafpour Darzi, G. Evaluation of surface activity of rhamnolipid biosurfactants produced from rice bran oil through dynamic surface tension. J. Pet. Explor. Prod. Technol. 2023, 13, 2139–2153. [Google Scholar] [CrossRef]
- Prashant; Tomar, S.K.; Singh, R.; Gupta, S.C.; Arora, D.K.; Joshi, B.K.; Kumar, D. Phenotypic and genotypic characterization of lactobacilli from Churpi cheese. Dairy Sci. Technol. 2009, 89, 531–540. [Google Scholar] [CrossRef]
- Ahn, C.; Morya, V.K.; Kim, E.-K. Tuning surface-active properties of bio-surfactant sophorolipids by varying fatty-acid chain lengths. Korean J. Chem. Eng. 2016, 33, 2127–2133. [Google Scholar] [CrossRef]
- White, D.; Hird, L.; Ali, S. Production and characterization of a trehalolipid biosurfactant produced by the novel marine bacterium Rhodococcus sp., strain PML026. J. Appl. Microbiol. 2013, 115, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Zafar, A.; Wali, H.; Siddique, M.P.; Qazi, M.A.; Naeem, A.H.; Malik, Z.A.; Ahmed, S. Low-cost production and application of lipopeptide for bioremediation and plant growth by Bacillus subtilis SNW3. AMB Express 2021, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yang, F.; Ye, S.; Li, J.; Shen, F.; Ding, Y. Characterization of lipopeptide produced by Bacillus altitudinis Q7 and inhibitory effect on Alternaria alternata. J. Basic Microbiol. 2023, 63, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Wang, Y.L. Structure-activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Pérez, L.; García, M.T.; Pinazo, A.; Pérez-Matas, E.; Hafidi, Z.; Bautista, E. Cationic Surfactants Based on Arginine-Phenylalanine and Arginine-Tryptophan: Synthesis, Aggregation Behavior, Antimicrobial Activity, and Biodegradation. Pharmaceutics 2022, 14, 2602. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial activity, in vitro cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Shi, D.; Yang, Y.; Zhang, Y.; Zhu, W. Cationic gemini surfactants containing both amide and ester groups: Synthesis, surface properties and antibacterial activity. J. Mol. Liq. 2020, 299, 112248. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef]
- Mouafo, T.H.; Mbawala, A.; Ndjouenkeu, R. Effect of Different Carbon Sources on Biosurfactants’ Production by Three Strains of Lactobacillus spp. BioMed Res. Int. 2018, 2018, 5034783. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Fink, J.; Merrifield, R.; Mauzerall, D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 5072–5076. [Google Scholar] [CrossRef] [PubMed]
- Kagan, B.L.; Selsted, M.E.; Ganz, T.; Lehrer, R.I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 1990, 87, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential agents for controlling cellular communication, motility, and antagonism. Front. Mol. Biosci. 2021, 8, 727070. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Maier, E.; Benz, R.; Hancock, R.E. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 1999, 38, 7235–7242. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.D.; Palmer, M. The action mechanism of daptomycin. Biorg. Med. Chem. 2016, 24, 6253–6268. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, P.; Yadav, V.; Pathak, A. A review on daptomycin; the first US-FDA approved lipopeptide antibiotics. J. Sci. Innov. Res. 2013, 2, 970–980. [Google Scholar]
- Deris, Z.Z.; Swarbrick, J.D.; Roberts, K.D.; Azad, M.A.; Akter, J.; Horne, A.S.; Nation, R.L.; Rogers, K.L.; Thompson, P.E.; Velkov, T.; et al. Probing the penetration of antimicrobial polymyxin lipopeptides into gram-negative bacteria. Bioconjug. Chem. 2014, 25, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Polymyxin derivatives that sensitize Gram-negative bacteria to other antibiotics. Molecules 2019, 24, 249. [Google Scholar] [CrossRef]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, L.; Zhong, J.; Guan, W.; Lu, C. Lighting up the interactions between bacteria and surfactants with aggregation-induced emission characteristics. Mater. Chem. Front. 2017, 1, 1829–1835. [Google Scholar] [CrossRef]
- Chebbi, A.; Elshikh, M.; Haque, F.; Ahmed, S.; Dobbin, S.; Marchant, R.; Sayadi, S.; Chamkha, M.; Banat, I.M. Rhamnolipids from Pseudomonas aeruginosa strain W10; as antibiofilm/antibiofouling products for metal protection. J. Basic Microbiol. 2017, 57, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shao, D.; Jiang, C.; Shi, J.; Li, Q.; Huang, Q.; Rajoka, M.S.R.; Yang, H.; Jin, M. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Johri, B. Antimicrobial lipopeptides of Bacillus: Natural weapons for biocontrol of plant pathogens. In Microorganisms in Sustainable Agriculture and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 91–111. [Google Scholar]
- Zhang, B.; Dong, C.J.; Shang, Q.M.; Han, Y.Z.; Li, P.L. New insights into membrane-active action in plasma membrane of fungal hyphae by the lipopeptide antibiotic bacillomycin L. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Yang, C.; Bao, X.; Chen, F.; Guo, X. Strategies for controlling biofilm formation in food industry. Grain Oil Sci. Technol. 2022, 5, 179–186. [Google Scholar] [CrossRef]
- JB, A.; RR, V. Effect of small chain N acyl-homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J. Food Sci. Technol. 2016, 53, 3609–3614. [Google Scholar]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An overview of biofilm formation–combating strategies and mechanisms of action of antibiofilm agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef]
- de Araujo, L.V.; Guimarães, C.R.; da Silva Marquita, R.L.; Santiago, V.M.; de Souza, M.P.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Tabbene, O.; Di Grazia, A.; Azaiez, S.; Ben Slimene, I.; Elkahoui, S.; Alfeddy, M.N.; Casciaro, B.; Luca, V.; Limam, F.; Mangoni, M.L. Synergistic fungicidal activity of the lipopeptide bacillomycin D with amphotericin B against pathogenic Candida species. FEMS Yeast Res. 2015, 15, fov022. [Google Scholar] [CrossRef]
- Englerova, K.; Bedlovicova, Z.; Nemcova, R.; Kiraly, J.; Madar, M.; Hajduckova, V.; Stykova, E.; Mucha, R.; Reiffova, K. Bacillus amyloliquefaciens-Derived Lipopeptide Biosurfactants Inhibit Biofilm Formation and Expression of Biofilm-Related Genes of Staphylococcus aureus. Antibiotics 2021, 10, 1252. [Google Scholar] [CrossRef]
- Carvalho, D.; Menezes, R.; Chitolina, G.Z.; Kunert-Filho, H.C.; Wilsmann, D.E.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.S.; do Nascimento, V.P. Antibiofilm activity of the biosurfactant and organic acids against foodborne pathogens at different temperatures, times of contact, and concentrations. Braz. J. Microbiol. 2022, 53, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Crovella, S.; de Freitas, L.C.; Zupin, L.; Fontana, F.; Ruscio, M.; Pena, E.P.N.; Pinheiro, I.O.; Calsa Junior, T. Surfactin bacterial antiviral lipopeptide blocks in vitro replication of SARS-CoV-2. Appl. Microbiol. 2022, 2, 680–687. [Google Scholar] [CrossRef]
- Huang, X.Q.; Lu, Z.X.; Zhao, H.Z.; Bie, X.M.; Lu, F.X.; Yang, S.J. Antiviral activity of antimicrobial lipopeptide from Bacillus subtilis fmbj against Pseudorabies virus, porcine parvovirus, newcastle disease virus and infectious bursal disease virus in vitro. Int. J. Pept. Res. Ther. 2006, 12, 373–377. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Özel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Olsztynska, S.; Komorowska, M. Biomedical Engineering: Trends, Research and Technologies; BoD–Books on Demand: London, UK, 2011. [Google Scholar]
- Khodavirdipour, A.; Chamanrokh, P.; Alikhani, M.Y.; Alikhani, M.S. Potential of Bacillus subtilis against SARS-CoV-2–a sustainable drug development perspective. Front. Microbiol. 2022, 13, 718786. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Philip, E.; Madhavan, A.; Binod, P.; Awasthi, M.K.; Pandey, A.; Sindhu, R. Applications of biosurfactant as an antioxidant in foods. In Applications of Next Generation Biosurfactants in the Food Sector; Elsevier: Amsterdam, The Netherlands, 2023; pp. 391–401. [Google Scholar]
- Shao, C.; Liu, L.; Gang, H.; Yang, S.; Mu, B. Structural diversity of the microbial surfactin derivatives from selective esterification approach. Int. J. Mol. Sci. 2015, 16, 1855–1872. [Google Scholar] [CrossRef] [PubMed]
- Tabbene, O.; Gharbi, D.; Slimene, I.B.; Elkahoui, S.; Alfeddy, M.N.; Cosette, P.; Mangoni, M.L.; Jouenne, T.; Limam, F. Antioxidative and DNA protective effects of bacillomycin D-like lipopeptides produced by b38 strain. Appl. Biochem. Biotechnol. 2012, 168, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Zouari, R.; Moalla-Rekik, D.; Sahnoun, Z.; Rebai, T.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Evaluation of dermal wound healing and in vitro antioxidant efficiency of Bacillus subtilis SPB1 biosurfactant. Biomed. Pharmacother. 2016, 84, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Jemil, N.; Ouerfelli, M.; Almajano, M.P.; Elloumi-Mseddi, J.; Nasri, M.; Hmidet, N. The conservative effects of lipopeptides from Bacillus methylotrophicus DCS1 on sunflower oil-in-water emulsion and raw beef patties quality. Food Chem. 2020, 303, 125364. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of lipopeptide biosurfactant by a marine Nesterenkonia sp. and its application in food industry. Front. Microbiol. 2017, 8, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Wu, C.M.; Chen, W.J.; Hua, K.F.; Liu, J.R.; Cheng, Y.H. Effectiveness of Bacillus licheniformis-fermented products and their derived antimicrobial lipopeptides in controlling coccidiosis in broilers. Animals 2021, 11, 3576. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.P.; Audisio, M.C.; Sabate, D.C.; Ibarguren, C.; Medici, S.K.; Sarlo, E.G.; Garrido, P.M.; Eguaras, M.J. Effect of bacterial metabolites on microsporidian Nosema ceranae and on its host Apis mellifera. Parasitol. Res. 2010, 107, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Ohtomo, K.; Kurisawa, N.; Shiota, I.; Rahmawati, Y.; Jeelani, G.; Nozaki, T.; Suenaga, K. Isolation, structure determination, and total synthesis of hoshinoamide c, an antiparasitic lipopeptide from the marine cyanobacterium caldora penicillata. J. Nat. Prod. 2021, 84, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Soussi, S.; Essid, R.; Karkouch, I.; Saad, H.; Bachkouel, S.; Aouani, E.; Limam, F.; Tabbene, O. Effect of lipopeptide-loaded chitosan nanoparticles on Candida albicans adhesion and on the growth of Leishmania major. Appl. Biochem. Biotechnol. 2021, 193, 3732–3752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, L.; Guo, L.; Sun, H.; Huang, Q.; Shao, D.; Jiang, C.; Shi, J. Effects of Bacillus subtilis iturin A on HepG2 cells in vitro and vivo. AMB Express 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, C.; Wang, Y.; Liu, D.; Lv, Y.; Lv, F.; Lu, Z. Fengycin inhibits the growth of the human lung cancer cell line 95D through reactive oxygen species production and mitochondria-dependent apoptosis. Anti-Cancer Drugs 2013, 24, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Wang, A.H.; Wang, C.L.; Mao, D.Z.; Lu, M.F.; Cui, Y.Q.; Jiao, R.Z. Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem. Biol. Interact. 2010, 183, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Feng, Y.; Ren, J.; Jing, D.; Wang, C. Anti-tumor role of Bacillus subtilis fmbJ-derived fengycin on human colon cancer HT29 cell line. Neoplasma 2016, 63, 215–222. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Lei, S.; Shao, D.; Jiang, C.; Shi, J.; Zhang, Y.; Liu, L.; Lei, S.; Sun, H. Iturin A-like lipopeptides from Bacillus subtilis trigger apoptosis, paraptosis, and autophagy in Caco-2 cells. J. Cell. Physiol. 2019, 234, 6414–6427. [Google Scholar] [CrossRef]
- Gang, H.Z.; Bian, P.C.; He, X.; He, X.; Bao, X.; Mu, B.Z.; Li, Y.; Yang, S.Z. Mixing of surfactin, an anionic biosurfactant, with alkylbenzene sulfonate, a chemically synthesized anionic surfactant, at the n-decane/water interface. J. Surfactants Deterg. 2021, 24, 445–457. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Moniruzzaman, M.; Sivapragasam, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. A binary mixture of a biosurfactant and an ionic liquid surfactant as a green dispersant for oil spill remediation. J. Mol. Liq. 2019, 280, 111–119. [Google Scholar] [CrossRef]
- Dey, G.; Bharti, R.; Das, A.K.; Sen, R.; Mandal, M. Resensitization of Akt induced docetaxel resistance in breast cancer by ‘Iturin A’a lipopeptide molecule from marine bacteria Bacillus megaterium. Sci. Rep. 2017, 7, 17324. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gou, Z.; Wen, Y.; Luo, Q.; Huang, Z. Marine compounds targeting the PI3K/Akt signaling pathway in cancer therapy. Biomed. Pharmacother. 2020, 129, 110484. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Su, W.; Zhang, X.; Yang, S.; Zhu, Y.; Liu, X. Self-assembly of sophorolipid and eugenol into stable nanoemulsions for synergetic antibacterial properties through alerting membrane integrity. Colloids Surf. B. Biointerfaces 2024, 234, 113749. [Google Scholar] [CrossRef]
- Karamchandani, B.M.; Maurya, P.A.; Dalvi, S.G.; Waghmode, S.; Sharma, D.; Rahman, P.K.; Ghormade, V.; Satpute, S.K. Synergistic activity of rhamnolipid biosurfactant and nanoparticles synthesized using fungal origin chitosan against phytopathogens. Front. Bioeng. Biotechnol. 2022, 10, 917105. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Huang, L.; Xiu, J.; Yi, L.; Zhang, Y.; Wu, B. Study on the thermal washing of oily sludge used by rhamnolipid/sophorolipid binary mixed bio-surfactant systems. Ecotoxicol. Environ. Saf. 2022, 240, 113696. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Imura, T.; Taira, T. Synergy in the microbial cyclic lipopeptide/petroleum-derived surfactant mixture can reduce the total surfactant consumption. ACS Sustain. Chem. Eng. 2023, 11, 5115–5121. [Google Scholar] [CrossRef]
- Sur, S.; Grossfield, A. Effects of cholesterol on the mechanism of fengycin, a biofungicide. Biophys. J. 2022, 121, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Wolanski, A.; Shore, V. Context and Background. In Tugendhat and Christie: The Law of Privacy and the Media; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Nitschke, M.; Silva, S.S.E. Recent food applications of microbial surfactants. Crit. Rev. Food Sci. Nutr. 2018, 58, 631–638. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, Q.; Gao, H.; Lai, H.; Wang, P. Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb. Cell Factories 2016, 15, 168. [Google Scholar] [CrossRef]
- Greenwell, M.; Sarker, M.; Rahman, P.K. Biosurfactant production and biodegradation of leather dust from tannery. Open Biotechnol. J. 2016, 10, 312–325. [Google Scholar] [CrossRef]
- Pereira, J.F.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, P. Novel and cost-effective technologies for hydrocarbon bioremediation. In Microbial Action on Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2018; pp. 543–565. [Google Scholar]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. Biosurfactants 2010, 672, 135–145. [Google Scholar]
- Sales da Silva, I.G.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil bioremediation: Overview of technologies and trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouze-Chaabouni, S.; Ghribi, D. Economic production of Bacillus subtilis SPB1 biosurfactant using local agro-industrial wastes and its application in enhancing solubility of diesel. J. Chem. Technol. Biotechnol. 2013, 88, 779–787. [Google Scholar] [CrossRef]
- Hmidet, N.; Ben Ayed, H.; Jacques, P.; Nasri, M. Enhancement of surfactin and fengycin production by Bacillus mojavensis A21: Application for diesel biodegradation. BioMed Res. Int. 2017, 2017, 5893123. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Cameotra, S.S. Efficiency of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 7367–7376. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ngueagni, P.T. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar]
- Ganji, Z.; Beheshti-Maal, K.; Massah, A.; Emami-Karvani, Z. A novel sophorolipid-producing Candida keroseneae GBME-IAUF-2 as a potential agent in microbial enhanced oil recovery (MEOR). FEMS Microbiol. Lett. 2020, 367, fnaa144. [Google Scholar] [CrossRef]
- Sadeghi, H.; Rashedi, H.; Mazaheri Assadi, M.; Seyedin Ardebili, M. Potential application of bioemulsifier RAG-1 as an anti-staling agent in flat bread quality. J. Food Sci. Technol. 2023, 60, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.; Ribeiro, B.G.; Aguiar, J.S.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Application of a biosurfactant produced by Bacillus cereus UCP 1615 from waste frying oil as an emulsifier in a cookie formulation. Fermentation 2021, 7, 189. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; dos Santosb, M.M.; Pintoc, M.I.; Meirac, H.M.; Durvald, I.J.; Guerrab, J.M.; Sarubboc, L.A. Production and optimization of the extraction conditions of the biosurfactant from Candida utilis UFPEDA1009 with potential application in the food industry. CET J.-Chem. Eng. Trans. 2019, 74, 1477–1482. [Google Scholar]
- Silva, I.; Veras, B.; Ribeiro, B. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. PeerJ 2020, 8, 9064. [Google Scholar] [CrossRef] [PubMed]
- Lira, I.R.d.S.; Santos, E.M.d.S.; dos Santos, J.C.; da Silva, R.R.; da Silva, Y.A.; Durval, I.J.B.; Guerra, J.M.; Sarubbo, L.A.; de Luna, J.M. Production of biossurfactant by Candida Guillhermondii and application in a mayonnaise emulsion. Chem. Eng. Trans. 2021, 87, 259–264. [Google Scholar]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Production of a biosurfactant from S. cerevisiae and its application in salad dressing. Biocatal. Agric. Biotechnol. 2022, 42, 102358. [Google Scholar] [CrossRef]
- Qian, R.; Ji, X.; Xu, X.; Li, S.; Xu, H. Production of Lipopeptides from Bacillus velezensis BVQ121 and Their Application in Chitosan Antibacterial Coating. J. Agric. Food Chem. 2024, 72, 7861–7869. [Google Scholar] [CrossRef]
- Ravindran, A.; Selvin, J.; Seghal Kiran, G. Effective utilization of wheat gluten by lipopeptide biosurfactant in the formulation of cookies. J. Sci. Food Agric. 2023, 103, 4685–4691. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; de Souza Leão, V.L.X.; Guerra, J.M.C.; Sarubbo, L.A. Cookies and muffins containing biosurfactant: Textural, physicochemical and sensory analyses. J. Food Sci. Technol. 2023, 60, 2180–2192. [Google Scholar] [CrossRef]
- Campos, J.; Stamford, T.; Sarubbo, L. Characterization and application of a biosurfactant isolated from Candida utilis in salad dressings. Biodegradation 2019, 30, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nageshwar, L.; Parameshwar, J.; Rahman, P.K.; Banat, I.M.; Hameeda, B. Anti-oxidative property of xylolipid produced by Lactococcus lactis LNH70 and its potential use as fruit juice preservative. Braz. J. Microbiol. 2022, 53, 2157–2172. [Google Scholar] [CrossRef]
- Ravindran, A.; Kiran, G.S.; Selvin, J. Revealing the effect of lipopeptide on improving the probiotics characteristics: Flavor and texture enhancer in the formulated yogurt. Food Chem. 2022, 375, 131718. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef]
- Abdallah, D.B.; Tounsi, S.; Gharsallah, H.; Hammami, A.; Frikha-Gargouri, O. Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ. Sci. Pollut. Res. 2018, 25, 36518–36529. [Google Scholar] [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef]
- Fan, H.; Ru, J.; Zhang, Y.; Wang, Q.; Li, Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017, 199, 89–97. [Google Scholar] [CrossRef]
- Ambrico, A.; Trupo, M. Efficacy of cell free supernatant from Bacillus subtilis ET-1, an Iturin A producer strain, on biocontrol of green and gray mold. Postharvest Biol. Technol. 2017, 134, 5–10. [Google Scholar] [CrossRef]
- Basaid, K.; Chebli, B.; Mayad, E.H.; Furze, J.N.; Bouharroud, R.; Krier, F.; Barakate, M.; Paulitz, T. Biological activities of essential oils and lipopeptides applied to control plant pests and diseases: A review. Int. J. Pest Manag. 2021, 67, 155–177. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A review of germination and early growth as a proxy for plant fitness under petrogenic contamination—Knowledge gaps and recommendations. Sci. Total Environ. 2017, 603, 728–744. [Google Scholar] [CrossRef]
- Schellenberger, R.; Touchard, M.; Clément, C.; Baillieul, F.; Cordelier, S.; Crouzet, J.; Dorey, S. Apoplastic invasion patterns triggering plant immunity: Plasma membrane sensing at the frontline. Mol. Plant Pathol. 2019, 20, 1602–1616. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M.; et al. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef]
- de Paula Siqueira, T.; Barbosa, W.F.; Rodrigues, E.M.; Miranda, F.R.; de Souza Freitas, F.; Martins, G.F.; Tótola, M.R. Rhamnolipids on Aedes aegypti larvae: A potential weapon against resistance selection. 3 Biotech 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W. Daptomycin, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183395. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, L.; Xia, S.; Zhang, T.; Cao, R.; Liang, G.; Li, Y.; Meng, G.; Wang, W.; Shi, W. De novo design of α-helical lipopeptides targeting viral fusion proteins: A promising strategy for relatively broad-spectrum antiviral drug discovery. J. Med. Chem. 2018, 61, 8734–8745. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.H.; Seo, W.T.; Yun, H.D.; Hong, S.Y.; Kim, M.K.; Cho, K.M. The production of surfactin during the fermentation of cheonggukjang by potential probiotic Bacillus subtilis CSY191 and the resultant growth suppression of MCF-7 human breast cancer cells. Food. Chem. 2012, 131, 1347–1354. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, X.; Yang, T.; Shang, J.; Shang, L.; Mai, H.; Qi, G. Immunomodulation therapy of diabetes by oral administration of a surfactin lipopeptide in NOD mice. Vaccine 2014, 32, 6812–6819. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Lipopeptides for vaccine development. Bioconj. Chem. 2021, 32, 1472–1490. [Google Scholar] [CrossRef]
- Bouassida, M.; Fourati, N.; Krichen, F.; Zouari, R.; Ellouz-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 lipopeptides in toothpaste formulation. J. Adv. Res. 2017, 8, 425–433. [Google Scholar] [CrossRef]
- Palanisamy, S.; Ahalliya, R.M.; Rathankumar, A.K.; Saikia, K.; Valan Arasu, M.; Rajapandian, V.; Vellingiri, M. Biosurfactants in Cosmetic Industry. In Multifunctional Microbial Biosurfactants; Springer: Berlin/Heidelberg, Germany, 2023; pp. 341–361. [Google Scholar]
- Pilz, M.; Cavelius, P.; Qoura, F.; Awad, D.; Brück, T. Lipopeptides development in cosmetics and pharmaceutical applications: A comprehensive review. Biotechnol. Adv. 2023, 67, 108210. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.G.; Miastkowska, M.A.; Pulit-Prociak, J.; Dey, P.; Rangarajan, V. Formulation of a stable biocosmetic nanoemulsion using a Bacillus lipopeptide as the green-emulsifier for skin-care applications. J. Dispers. Sci. Technol. 2023, 44, 2045–2057. [Google Scholar] [CrossRef]
- Bueno-Mancebo, J.; Barrena, R.; Artola, A.; Gea, T.; Altmajer-Vaz, D. Surfactin as an ingredient in cosmetic industry: Benefits and trends. Int. J. Cosmet. Sci. 2024. [Google Scholar] [CrossRef]
- Ganesan, N.G.; Rangarajan, V. A kinetics study on surfactin production from Bacillus subtilis MTCC 2415 for application in green cosmetics. Biocatal. Agric. Biotechnol. 2021, 33, 102001. [Google Scholar] [CrossRef]
- Ayed, H.B.; Bardaa, S.; Moalla, D.; Jridi, M.; Maalej, H.; Sahnoun, Z.; Rebai, T.; Jacques, P.; Nasri, M.; Hmidet, N. Wound healing and in vitro antioxidant activities of lipopeptides mixture produced by Bacillus mojavensis A21. Process Biochem. 2015, 50, 1023–1030. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Gaur, V.K.; Gupta, P.; Tripathi, V.; Thakur, R.S.; Regar, R.K.; Patel, D.K.; Manickam, N. Valorization of agro-industrial waste for rhamnolipid production, its role in crude oil solubilization and resensitizing bacterial pathogens. Environ. Technol. Innov. 2022, 25, 102108. [Google Scholar] [CrossRef]
- Pathania, A.S.; Jana, A.K. Utilization of waste frying oil for rhamnolipid production by indigenous Pseudomonas aeruginosa: Improvement through co-substrate optimization. J. Environ. Chem. Eng. 2020, 8, 104304. [Google Scholar] [CrossRef]

| Functional Properties | Biosurfactant Types | Microorganism Isolates | Used Tests | Mechanisms | Results | Ref. |
|---|---|---|---|---|---|---|
| Antibacterial activities | Lipopeptides (surfactin, iturin and fengycin) | Bacillus amyloliquefaciens C-1 | The disc diffusion and the broth microdilution methods, scanning electron microscope, and fluorescence microscope analysis | Destruction of the integrity and permeability of the cell membrane and cell wall leading to cellular death | Considerable inhibitory effects against Clostridium difficile (MIC: 0.75 μg/mL) | [10] |
| Lipopeptides (Brevilaterin B) | Brevibacillus laterosporus S62-9 | The broth microdilution method and transmission electron microscopy | Destruction of the cytoplasmic membrane, subsequent loss of intracellular material, and eventual cell death | Significant inhibitory effects against Listeria monocytogenes (1 μg/mL) | [11] | |
| Lipopeptides (surfactin) | Bacillus velezensis SK | Agar well diffusion assay | NR | The antimicrobial activities of the extracted lipopeptide, specifically against B. cereus and Staphylococcus aureus, were observed to be stable over a wide pH range of 2 to and thermos-stability range of 0 to 80 °C | [12] | |
| Rhamnolipids | NR | The micro-broth dilution method | Their amphiphilic character that permits interaction with phospholipids, modifying cytoplasmic membrane permeability due to pore formation with consequent leakage of cell components and cell death | Gram-positive bacteria are more sensitive (S. aureus) | [13] | |
| Glycolipoprotein | Oceanobacillus sp. | Disk diffusion method | NR | More excellent inhibition zone for E. coli | [14] | |
| Glycolipoprotein | Lactobacillus plantarum 60 FHE | Well diffusion agar method | NR | Greater inhibitory effects on Staphylococcus epidermidis and Micrococcus luteus | [15] | |
| Sophorolipid | Candida spp. | NR | To generate ROS to damage the microbial cell | Considerable antibacterial activities against both Gram-negative and positive pathogens (E. coli and B. subtilis) | [16] | |
| Antifungal activities | Lipopeptide (fengycin and iturin) | Bacillus amyloliquefaciens | Agar disk diffusion test | To inhibit the mycelial growth and sporulation of Colletotrichum gloeosporioides and Fusarium oxysporum | Fusarium oxysporum exhibits higher sensitivity to fengycin compared to iturin, while fengycin demonstrates more potent inhibitory effects against Colletotrichum gloeosporioide | [17] |
| Lipopeptides (mycosubtiliin and surfactin) | Bacillus sp. | Broth microdilution method | NR | Significant antifungal activities against Paecilomyces variotti and Byssochlamys fulva | [18] | |
| Antiviral activities | Lipopeptides (fengycin) | Bacillus amyloliquefaciens PPL | Western blotting | NR | Antiviral activities against Cucumber mosaic virus | [19] |
| Rhamnolipids | Pseudomonas gessardii M15 | Plaque reduction assays PCR, fluorescent microscopy, and Transmission electron microscopy | To solvate and disrupt lipid-based viruses and inhibit the attachment of virus to the host cell | Antiviral activities against HSV-1 and SARS-CoV-2 | [20] | |
| Glycolipids | Azadirachta indica | Plaque reduction assay and Eliza | Anti-viral effects against HSV | Inhibits inflammatory cytokines | [21] | |
| Antibiofilm activities | Glycolipid | Bacillus pumilus SG | Microtiter plate method | 90% P. aeruginosa biofilm reduction | The amphipathic properties of glycolipids can increase bacterial surface hydrophobicity, disrupt lipid packing, alter the cell membrane permeability, and finally reduce microbial adhesion to solid surfaces. | [22] |
| Lactobacillus rhamnosus | Microtiter plate method | ~71% Bacillus subtilis biofilm reduction | Disrupts the integrity of the cell walls and inhibits EPS production | [23] | ||
| Lipopeptides (bacillomycin D) | Bacillus subtilis TR47II | Microtiter plate method | ~90% C. albicans biofilm reduction | Damages biofilm in a concentration-dependent manner | [24] | |
| Glycolipoprotein | Acinetobacter M6 | Microtiter plate method | ~83% MRSA biofilm destruction | NR | [25] | |
| Lipopeptides | Halomonas venusta PHKT | NR | More than 80% biofilm eradication against E. coli, Staphylococcus aureus, and Candida albicans | NR | [26] | |
| Antioxidant activities | Lipopeptides | Bacillus methylotrophicus DCS1 | DPPH | Radical scavenging activities | NR | [27] |
| Bacillus subtilis SPB1 | β-Carotene bleaching assay, DPPH, FRAP, and Ferrous ion chelating assay | Neutralizes free radicals | Enables the reaction of hydrocarbons in the hydrophobic chain with the free radicals | |||
| Bacillus subtilis #309 | DPPH, ABTS, and FRAP | Free radical scavenging capacity | This ability is dependent on the molecular structure of surfactin, specifically regarding the presence of hydroxyl groups, hydrophobic amino acids, and acidic amino acids | [28] | ||
| Bacillus subtilis VSG4 and Bacillus licheniformis VS16 | DPPH and hydroxyl radical scavenging assays | Free radical scavenging capacity | NR | [29] | ||
| Glycolipid | Saccharomyces cerevisiae URM 6670 | DPPH, TAC capacity, Sequestration of Superoxide Ion | Superoxide ion inhibition | The biosurfactant is a potential antioxidant at concentrations above 5000 μg/mL | [30] | |
| Anti-cancer activities | Lipopeptides (surfactin) | Bacillus subtilis 573 | MTS | Significant anti-breast cancer potential | Inhibits cell proliferation (T47D and MDA-MB-231 cell lines) | [31] |
| Glycolipoprotein | Acinetobacter M6 | NR | Significant anti-lung cancer potential | Reduces cell viability and induces cell cycle arrest at the G1 phase | [25] | |
| Rhamnolipids | Pseudomonas aeruginosa RA5 | Sulforhodamine B test | Considerable anticancer activities against breast and leukemia cancer | NR | [32] | |
| Anti-inflammatory activities | glycolipid | Rhodococcus ruber IEGM 231 | Western Blot Analysis | Significant reduction of pro-inflammatory cytokines | Inhibits the production of IL-12, IL-18, and ROS | [33] |
| Sophorolipids | Candida bombicola | Western Blot Analysis | Significant reduction of pro-inflammatory cytokines | Reduces the IgE level, mRNA expression of TLR-2, IL-6, and STAT3 | [34] | |
| Lipopeptides | Staphylococcus aureus | Western blot analysis and Eliza | Significant reduction of pro-inflammatory cytokines | Increases STAT-3 phosphorylation and the expression of heme oxygenase-1 (HO-1) | [35] |
| Biosurfactant Producing Screening | Advantages | Disadvantages |
|---|---|---|
| Oil-spreading assay | Speed Simplicity No required special tools Reliability Minimum samples required | Non-quantitative Time-consuming |
| Microplate assay | Speed Simplicity Accuracy Minimum samples required | Non-quantitative Time-consuming |
| Du-Nouy-Ring method | Simplicity Accuracy Direct measurement | Requires special tools Non-simultaneous measurement Significant samples required |
| Drop collapse assay | Speed Simplicity Reproducibility Qualitative method Minimum samples required | Non-quantitative Difficult observation |
| Emulsifying capacity assay (E24) | Simplicity Accuracy | Requires significant sample size Time-consuming Semi-quantitative |
| CTAB Agar Plate | Semi-quantitative | Difficult observation Restricted biosurfactant detection (e.g., anionic surfactants and glycolipids) |
| Homolysis | Simplicity | Inaccuracy Restricted biosurfactant detection Poor specificity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, S.; Bekhit, A.E.-D.A.; Roohinejad, S.; Vale, J.M.; Agyei, D. The Physicochemical and Functional Properties of Biosurfactants: A Review. Molecules 2024, 29, 2544. https://doi.org/10.3390/molecules29112544
Dini S, Bekhit AE-DA, Roohinejad S, Vale JM, Agyei D. The Physicochemical and Functional Properties of Biosurfactants: A Review. Molecules. 2024; 29(11):2544. https://doi.org/10.3390/molecules29112544
Chicago/Turabian StyleDini, Salome, Alaa El-Din A. Bekhit, Shahin Roohinejad, Jim M. Vale, and Dominic Agyei. 2024. "The Physicochemical and Functional Properties of Biosurfactants: A Review" Molecules 29, no. 11: 2544. https://doi.org/10.3390/molecules29112544
APA StyleDini, S., Bekhit, A. E.-D. A., Roohinejad, S., Vale, J. M., & Agyei, D. (2024). The Physicochemical and Functional Properties of Biosurfactants: A Review. Molecules, 29(11), 2544. https://doi.org/10.3390/molecules29112544







