Comprehensive Analysis Reveals the Difference in Volatile Oil between Bupleurum marginatum var. stenophyllum (Wolff) Shan et Y. Li and the Other Four Medicinal Bupleurum Species
Abstract
:1. Introduction
2. Results
2.1. DNA Barcoding Analysis
2.2. E-Nose Analysis of the Dried Roots
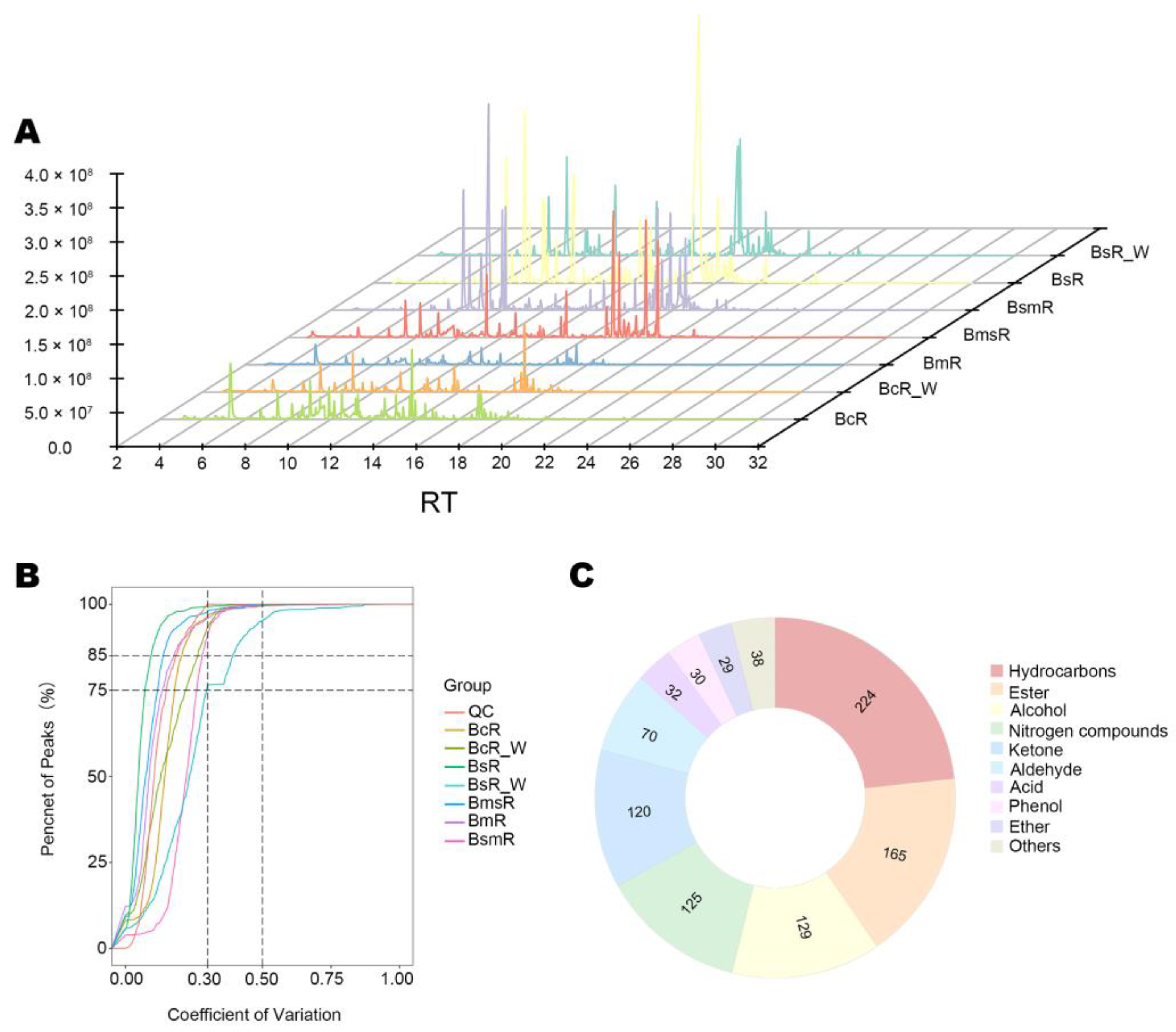
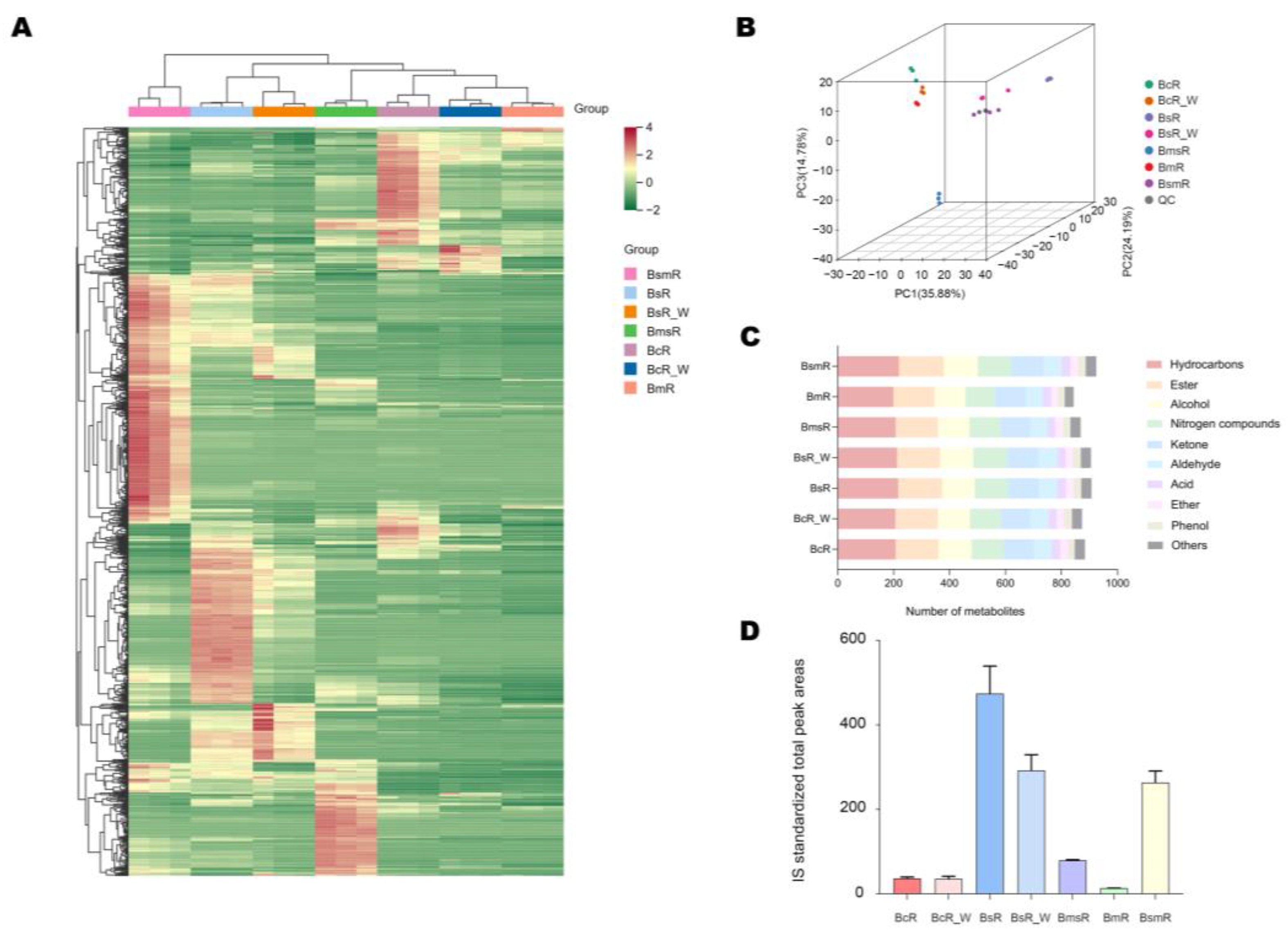
2.3. Metabolomics Profiling of Different Dried Roots
2.4. Identification of Metabolites Responsible for Differences
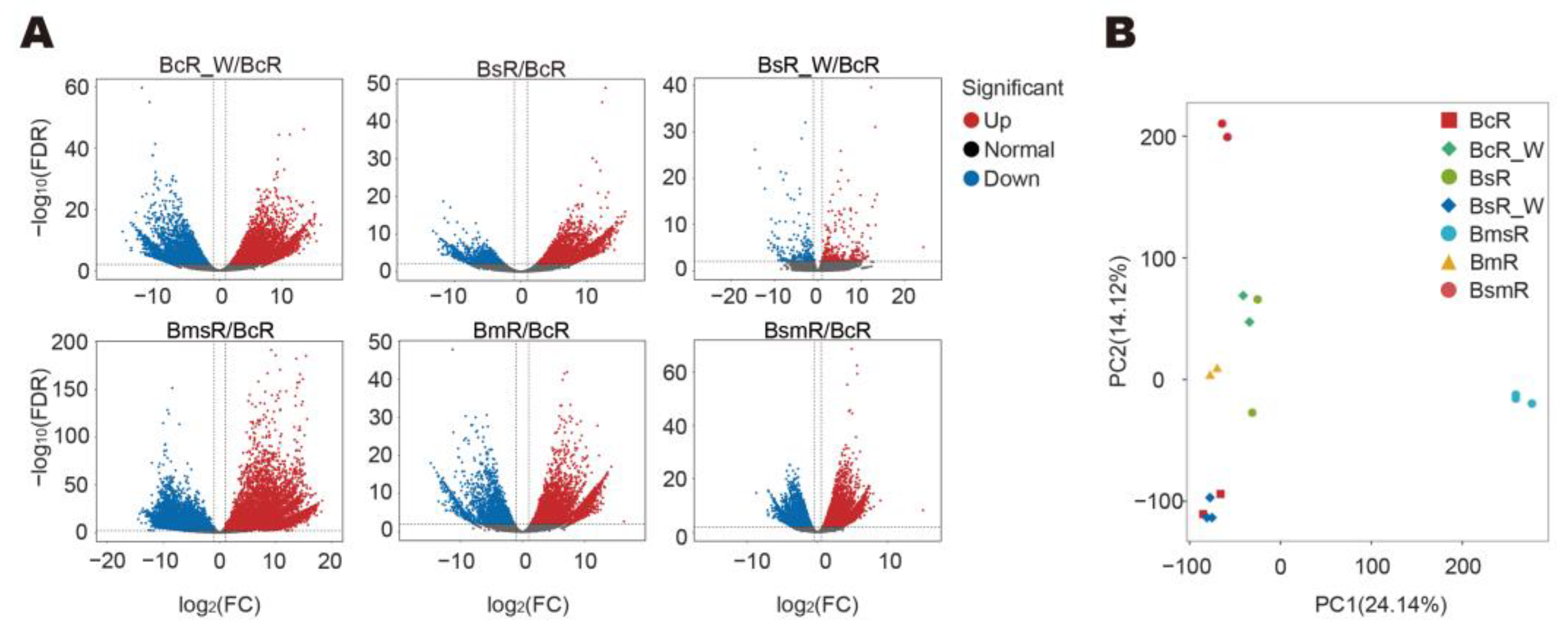
2.5. Transcriptome Sequencing, Assembly, and Annotation of the Fresh Samples
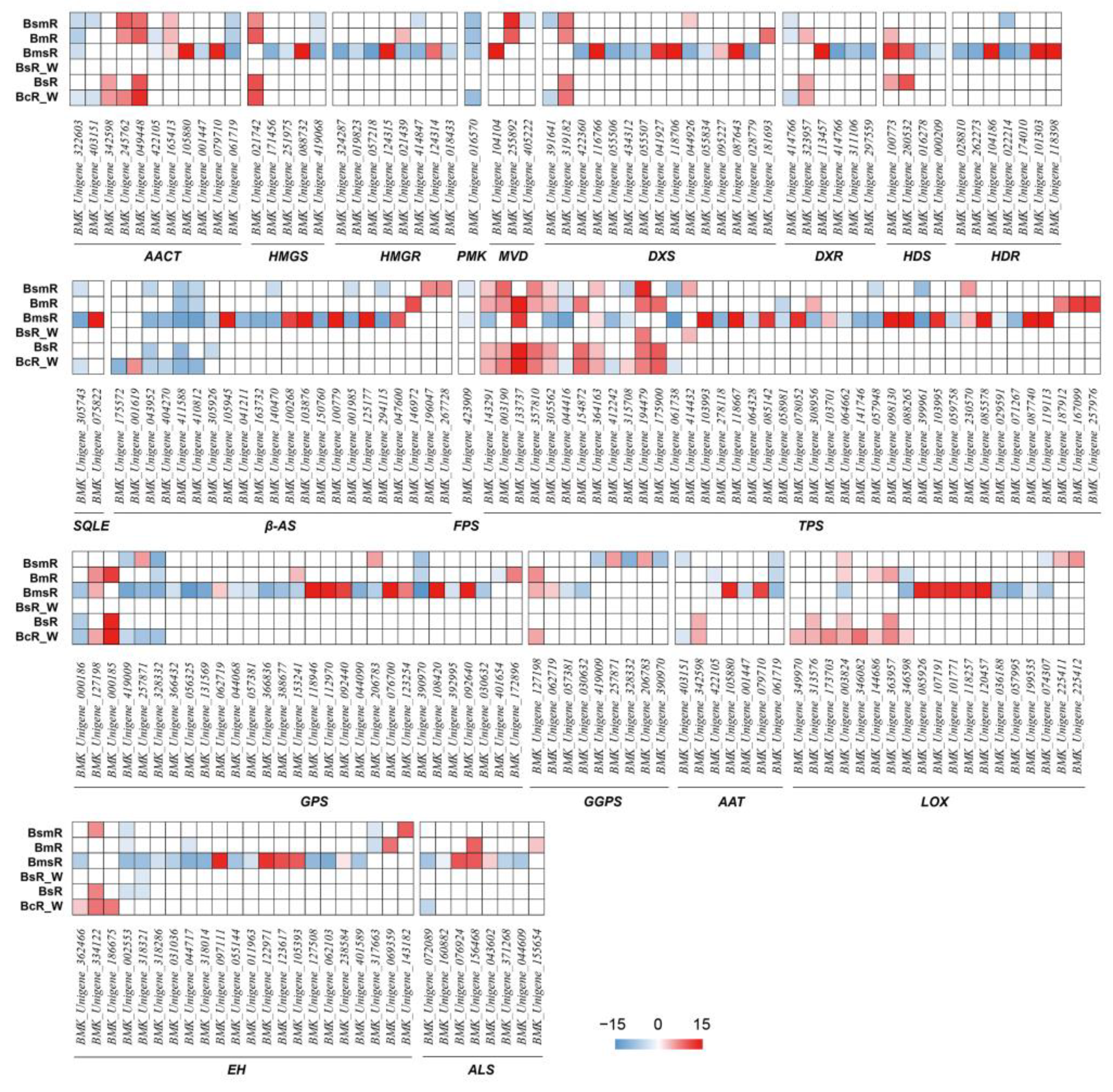
2.6. DEGs Involved in the Synthesis or Metabolism of Volatile Substances in B. marginatum var. stenophyllum
2.7. Microstructure of the Root Cross-Section of the Fresh Samples
3. Discussion
3.1. Substance Basis Difference between B. marginatum var. stenophyllum and Other Four Species
3.2. The Plant Basis Difference between B. marginatum var. stenophyllum and Other Four Species
4. Plant Materials and Methods
4.1. Plant Materials
4.2. DNA Barcoding Analysis
4.3. E-Nose Detection of the Dried Roots
4.4. HS–SPME–GC–MS Detection of Volatiles
4.5. Statistical Analysis
4.6. Transcriptome Analysis
4.7. Gene Expression Analysis
4.8. Observation of the Microstructure of the Root Cross-Section
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teng, L.; Guo, X.; Ma, Y.; Xu, L.; Wei, J.; Xiao, P. A comprehensive review on traditional and modern research of the genus Bupleurum (Bupleurum L., Apiaceae) in recent 10 years. J. Ethnopharmacol. 2023, 306, 116129. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Gao, Z.; Sui, C.; Ji, H.; Jiang, J.; Xinwei, G.; Wei, J. Transcriptome profiling of Bupleurum chinense DC. root provides new insights into the continuous inflorescence removal induced improvements to root growth and saikosaponin biosynthesis. Ind. Crops Prod. 2021, 160, 113085. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L. Research strategy and prospects for the genomics-driven targeted breeding of Chinese herbal medicine varieties. Chin. Sci. Bull. 2024, 69, 499–509. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Bai, M.; Peng, T.; Li, H.; Xu, H.J.; Guo, G.; Bai, H.; Rong, N.; Sahu, S.K.; et al. Soil conditions and the plant microbiome boost the accumulation of monoterpenes in the fruit of Citrus reticulata ‘Chachi’. Microbiome 2023, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Ma, Y.; Xu, L.; Yang, C.; Peng, D.; Guo, X.; Wei, J. Quantitative analysis of multi-components by single marker method combined with UPLC-PAD fingerprint analysis based on saikosaponin for discrimination of Bupleuri Radix according to geographical origin. Front. Chem. 2023, 11, 1309965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kang, Y.; Guo, Y.; Guo, X.; Li, X.; Cai, R.; Gao, Y.; Qi, Y. Bioactive indicators related to actions of relieving the exterior and reducing the fever of Bupleuri Radix and their correlation with content of saikosaponin a and saikosaponin d. Mod. Chin. Med. 2024, 26, 37–48. [Google Scholar]
- Chen, B.; Zou, H.; Meng, X.; Wang, L.; Hao, X.; Kang, X.; Wang, C.; Zhang, X. Prediction of distribution pattern and change of suitable areas of Bupleurum chinense and Bupleurum scorzonerifolium under climate change in China. Acta Ecol. Sin. 2022, 42, 8471–8482. [Google Scholar]
- Yu, X.; Miao, Z.; Zhang, L.; Zhu, L.; Sheng, H. Extraction, purification, structure characteristics, biological activities and pharmaceutical application of Bupleuri Radix Polysaccharide: A review. Int. J. Biol. Macromol. 2023, 237, 124146. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Zheng, S.; Yu, H.; Wang, X.; Yang, B.; et al. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: A systematic review. Biomed. Pharmacother. 2020, 131, 110679. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Wang, N.; Shi, Y. Chemical constituents of aerial part of Bupleurum marginatum. J. Plant Resour. Environ. 2007, 16, 71–73. [Google Scholar]
- Fang, W.; Yang, Y.J.; Guo, B.L.; Cen, S. Anti-influenza triterpenoid saponins (saikosaponins) from the roots of Bupleurum marginatum var. stenophyllum. Bioorg. Med. Chem. Lett. 2017, 27, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Y.; Peng, D.; Li, M.; Ji, H.; Guo, X.; Wei, J. General situation of Bupleurum marginatum var. stenophyllum. Mod. Chin. Med. 2023, 10, 2229–2233. [Google Scholar]
- Zhang, T.; Zhou, J.; Wang, Q. HPLC Analysis of Flavonoids from the Aerial Parts of Bupleurum Species. Chin. J. Nat. Med. 2010, 8, 107–113. [Google Scholar] [CrossRef]
- Luo, Y. System Identification of Four Kinds of Bupleurum Seeds; Hubei University of Chinese Medicine: Wuhan, China, 2018. [Google Scholar]
- Wang, Z.; Sun, J.; Pan, T.; Zhang, F.; Xia, H. Antipyretic mechanism of Chrysanthemum morifolium essential oil on fever New Zealand rabbits model induced by endotoxin. Lishizhen Med. Mater. Medica Res. 2018, 29, 2053–2056. [Google Scholar]
- Yang, J.; Song, J.; Hu, R.; Hua, H.; Li, L. Effect of Essential Oil of Ligusticum chuanxiong Hort on the Expressions of COX-2 in Hypothalamus of Fever Rat. Lishizhen Med. Mater. Medica Res. 2009, 20, 315–316. [Google Scholar]
- Jin, G.; Li, B.; Wang, S. Research on the Mechanism, the Effects and Material Foundation of ChaiHu in Relieving Fever. West. J. Tradit. Chin. Med. 2014, 2, 20–22. [Google Scholar]
- Sun, P.; Li, Y.; Wei, S.; Zhao, T.; Wang, Y.; Song, C.; Xue, L.; Wang, F.; Xiao, L.; Wu, J.; et al. Pharmacological Effects and Chemical Constituents of Bupleurum. Mini Rev. Med. Chem. 2018, 19, 34–55. [Google Scholar] [CrossRef]
- Fan, S. Introduction to the preparation method of Chaihu injection. Chin. Pharm. J. 1958, 10, 494. [Google Scholar]
- Qu, X.; Hu, S.; Li, T.; Zhang, J.; Wang, B.; Liu, C. Metabolomics Analysis Reveals the Differences between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Front. Plant Sci. 2022, 13, 933849. [Google Scholar] [CrossRef]
- Yu, D.; Wang, W.; Huo, J.; Zhuang, Y.; Chen, Y.; Du, X. Study on molecular mechanism of volatiles variation during Bupleurum scorzonerifolium root development based on metabolome and transcriptome analysis. Front. Plant Sci. 2023, 14, 1159511. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.; Xu, H.; Zhao, R.; Hu, Q. Comparative Study on Volatile Oils among Bupleuri radix Species and Habitats: Yields, Chemical Characterization and Antipyretic Activities. Chem. Biodivers. 2022, 19, e202200549. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Liu, X.; Lin, Y.; Zhang, H. Study of antipyretic active ingredients in Bupleurum chinense volatile oil. Chin. J. Pharm. Anal. 2013, 33, 1202–1209. [Google Scholar]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.; Coelho, G.L.A.; Loula, Y.K.S.F.; Landim, B.L.S.; Lima, C.N.F.; de Sousa Machado, S.T.; Lopes, M.J.P.; Gomes, A.D.S.; da Costa, J.G.M.; de Menezes, I.R.A.; et al. Hypoglycemic, Hypolipidemic, and Anti-Inflammatory Effects of Beta-Pinene in Diabetic Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 8173307. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, N.; Zhang, P. Determination of Hexaldehyde in Chaihu oral liquid by headspace gas chromatography. Tradit. Chin. Med. Res. 2015, 28, 76–78. [Google Scholar]
- Ma, Y.; Hua, W. Multiple components determination and GC-MS analysis of volatile oil from Buplewri Radix. Chin. Tradit. Pat. Med. 2013, 35, 2699–2702. [Google Scholar]
- Yang, D.; Zhang, D.; Wang, C.; Wang, C. Determination of Hexaldehyde in Chaiqin soft capsules. Guide China Med. 2015, 13, 46–47. [Google Scholar]
- Cao, Z. Comparison of antipyretic and anti-inflammatory effects of Bupleurum smithii H. Wolff and Bupleurum chinense DC. Tradit. Chin. Med. Res. 2009, 22, 15–17. [Google Scholar]
- Zhang, J.; Wu, X.; Qiu, J.; Zhang, L.; Zhang, Y.; Qiu, X.; Huang, Z.; Xu, W. Comprehensive Comparison on the Chemical Profile of Guang Chen Pi at Different Ripeness Stages Using Untargeted and Pseudotargeted Metabolomics. J. Agric. Food Chem. 2020, 68, 8483–8495. [Google Scholar] [CrossRef]
- Zhou, T.; Luo, C.; Zhang, S.; Huang, Z.; Tang, Y. Study on dynamic changes of chemical constituents during different drying processes of Salvia miltiorrhiza. China J. Chin. Mater. Medica 2019, 44, 4634–4640. [Google Scholar]
- D’Auria, M.; Racioppi, R. The Effect of Drying of the Composition of Volatile Organic Compounds in Rosmarinus officinalis, Laurus nobilis, Salvia officinalis and Thymus serpyllum. A HS-SPME-GC-MS Study. J. Essent. Oil Bear. Plants 2015, 18, 1209–1223. [Google Scholar] [CrossRef]
- Pachura, N.; Zimmer, A.; Grzywna, K.; Figiel, A.; Szumny, A.; Łyczko, J. Chemical investigation on Salvia officinalis L. Affected by multiple drying techniques—The comprehensive analytical approach (HS-SPME, GC-MS, LC-MS/MS, GC-O and NMR). Food Chem. 2022, 397, 133802. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Aly, M.S.A.; Alsuqub, A.N.A.; Khan, R.A. Drying Induced Impact on Composition and Oil Quality of Rosemary Herb, Rosmarinus Officinalis Linn. Molecules 2020, 25, 2830. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Q.; Chai, Z.; Ma, W. Inhibition of cis-3-hexenal on lipoxygenase and its elicitation on the production of astragalosides and isoflavonoids by adventitious roots of Astragalus mongholicus. Ind. Crops Prod. 2023, 203, 117090. [Google Scholar] [CrossRef]
- Kenchanmane Raju, S.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. 2018, 276, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Ning, X.; Chen, S.; Zhong, L.; Guo, C.; Yang, Y.; Liu, J.; Tang, M.; Liao, G.; Wang, X.; et al. Differences in fruit yields and essential oil contents and composition among natural provenances of Litsea cubeba in China and their relationships with main habitat factors. Ind. Crops Prod. 2023, 194, 116285. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.; Jing, T.; Wang, J.; Lu, M.; Pan, Y.; Du, W.; Zhao, C.; Bao, Z.; Zhao, W.; et al. (Z)-3-Hexenol integrates drought and cold stress signaling by activating abscisic acid glucosylation in tea plants. Plant Physiol. 2023, 193, 1491–1507. [Google Scholar] [CrossRef]
- Pi, X.; Chang, N.; Zhou, Z.; Li, Y.; Zhang, X. CsFAD2 and CsFAD5 are key genes for C18:2 fatty acid pathway-mediated cold tolerance in tea (Camellia sinensis). Environ. Exp. Bot. 2023, 210, 105317. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, F.; Hao, F.; Ye, H.; Yue, M.; Woeste, K.; Zhao, P.; Zhang, S. Pan-genome and transcriptome analyses provide insights into genomic variation and differential gene expression profiles related to disease resistance and fatty acid biosynthesis in eastern black walnut (Juglans nigra). Hortic. Res. 2023, 10, uhad015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, M.; Chen, X.; Liu, G.; Zhang, Z.; Tan, Q.; Hu, Y.; Fan, Y.; Liu, Y.; Zhu, T.; et al. Chromosome-Level Genome Assembly of Bupleurum chinense DC Provides Insights Into the Saikosaponin Biosynthesis. Front. Genet. 2022, 13, 878431. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ling, X.Y.; Zhou, X.F.; Chen, Y.X.; Wang, T.T.; Lin, X.J.; Zhao, Y.Y.; Ye, Y.S.; Huang, L.X.; Sun, Y.W.; et al. Comparing genomes of Fructus Amomi-producing species reveals genetic basis of volatile terpenoid divergence. Plant Physiol. 2023, 193, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L.; et al. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol. Plant 2022, 15, 189–202. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Yang, Q.; Wang, Q.; Ma, B.; Li, Z.; Cheng, W.; Tang, H.; Feng, S.; Wang, Z. Metabolomic profiling of M. speciosa champ at different growth stages. Food Chem. 2021, 376, 131941. [Google Scholar] [CrossRef]




| No. | Retention Time (min) | Compounds | Molecular Formula | Type |
|---|---|---|---|---|
| 1 | 9.37 | 2-Methyltetrahydrothiophen-3-one | C5H8OS | Sulfur compounds |
| 2 | 17.23 | Dehydrodihydroionone | C13H20O | Ketones |
| 3 | 18.72 | (Z)-γ-bisabolene | C15H24 | Hydrocarbons |
| 4 | 18.71 | Geranyl isobutyrate | C14H24O2 | Esters |
| 5 | 18.73 | Cubebanol | C15H26O | Alcohols |
| 6 | 18.75 | (-)-α-Alaskene | C15H24 | Hydrocarbons |
| 7 | 19.27 | N-Benzyloxy-2-carbomethoxyaziridine | C11H13NO3 | Nitrogen compounds |
| 8 | 19.27 | 4-Hydroxyphenylacetic acid | C8H8O3 | Acids |
| 9 | 16.90 | Cedrene | C15H24 | Hydrocarbons |
| 10 | 17.22 | 4-Hydroxybenzyl alcohol | C7H8O2 | Alcohols |
| 11 | 18.72 | 4′-Methoxypropiophenone | C10H12O2 | Ketones |
| 12 | 15.02 | (2-Nitroethyl)benzene | C8H9NO2 | Nitrogen compounds |
| 13 | 19.27 | 4-Aminobenzoic acid | C7H7NO2 | Acids |
| 14 | 19.27 | 1-(4-Methoxyphenyl)-3-methyl-1-pentanone | C13H18O2 | Ketones |
| 15 | 17.23 | Geosmin | C12H22O | Alcohols |
| 16 | 16.90 | 2-methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane | C15H24 | Hydrocarbons |
| 17 | 16.90 | α-Gurjunene | C15H24 | Hydrocarbons |
| 18 | 17.96 | Acoradiene | C15H24 | Hydrocarbons |
| 19 | 19.27 | 8,14-Cedranoxide | C15H24O | Ethers |
| 20 | 17.23 | Myosmine | C9H10N2 | Nitrogen compounds |
| Species | Abbreviation | Type | Producing Area | Latitude and Longitude | Altitude |
|---|---|---|---|---|---|
| B. chinense DC. | BcR | Cultivated | Chencang District, Baoji City, Shanxi Province, China | 107°37′ E 34°35′ N | 700 m |
| BcR_W | Wild | Chencang District, Baoji City, Shanxi Province, China | 107°37′ E 34°35′ N | 700 m | |
| B. scorzonerifolium Willd. | BsR | Cultivated | Lindian County, Daqing City, Heilongjiang Province, China | 124°87′ E 47°17′ N | 150 m |
| BsR_W | Wild | Lindian County, Daqing City, Heilongjiang Province, China | 124°87′ E 47°17′ N | 150 m | |
| B. marginatum var. stenophyllum (Wolff) Shan et Y. Li | BmsR | Cultivated | Lintao County, Dingxi City, Gansu Province, China | 103°86′ E 35°40′ N | 1800 m |
| B. marginatum Wall. ex DC. | BmR | Cultivated | Rong County, Zigong City, Sichuan Province, China | 104°42′ E 29°45′ N | 350 m |
| B. smithii Wolff | BsmR | Cultivated | Pinshun County, Changzhi City, Shanxi Province, China | 113°44′ E 36°20′ N | 1200 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Guo, X.; Wu, P.; Li, Y.; Zhang, R.; Xu, L.; Wei, J. Comprehensive Analysis Reveals the Difference in Volatile Oil between Bupleurum marginatum var. stenophyllum (Wolff) Shan et Y. Li and the Other Four Medicinal Bupleurum Species. Molecules 2024, 29, 2561. https://doi.org/10.3390/molecules29112561
Ma Y, Guo X, Wu P, Li Y, Zhang R, Xu L, Wei J. Comprehensive Analysis Reveals the Difference in Volatile Oil between Bupleurum marginatum var. stenophyllum (Wolff) Shan et Y. Li and the Other Four Medicinal Bupleurum Species. Molecules. 2024; 29(11):2561. https://doi.org/10.3390/molecules29112561
Chicago/Turabian StyleMa, Yuzhi, Xinwei Guo, Peiling Wu, Yuting Li, Ruyue Zhang, Lijia Xu, and Jianhe Wei. 2024. "Comprehensive Analysis Reveals the Difference in Volatile Oil between Bupleurum marginatum var. stenophyllum (Wolff) Shan et Y. Li and the Other Four Medicinal Bupleurum Species" Molecules 29, no. 11: 2561. https://doi.org/10.3390/molecules29112561







