Radical Cyclization-Initiated Difunctionalization Reactions of Alkenes and Alkynes
Abstract
1. Introduction
2. Second Functionalization with X via Atom-Transfer Radical Cyclization
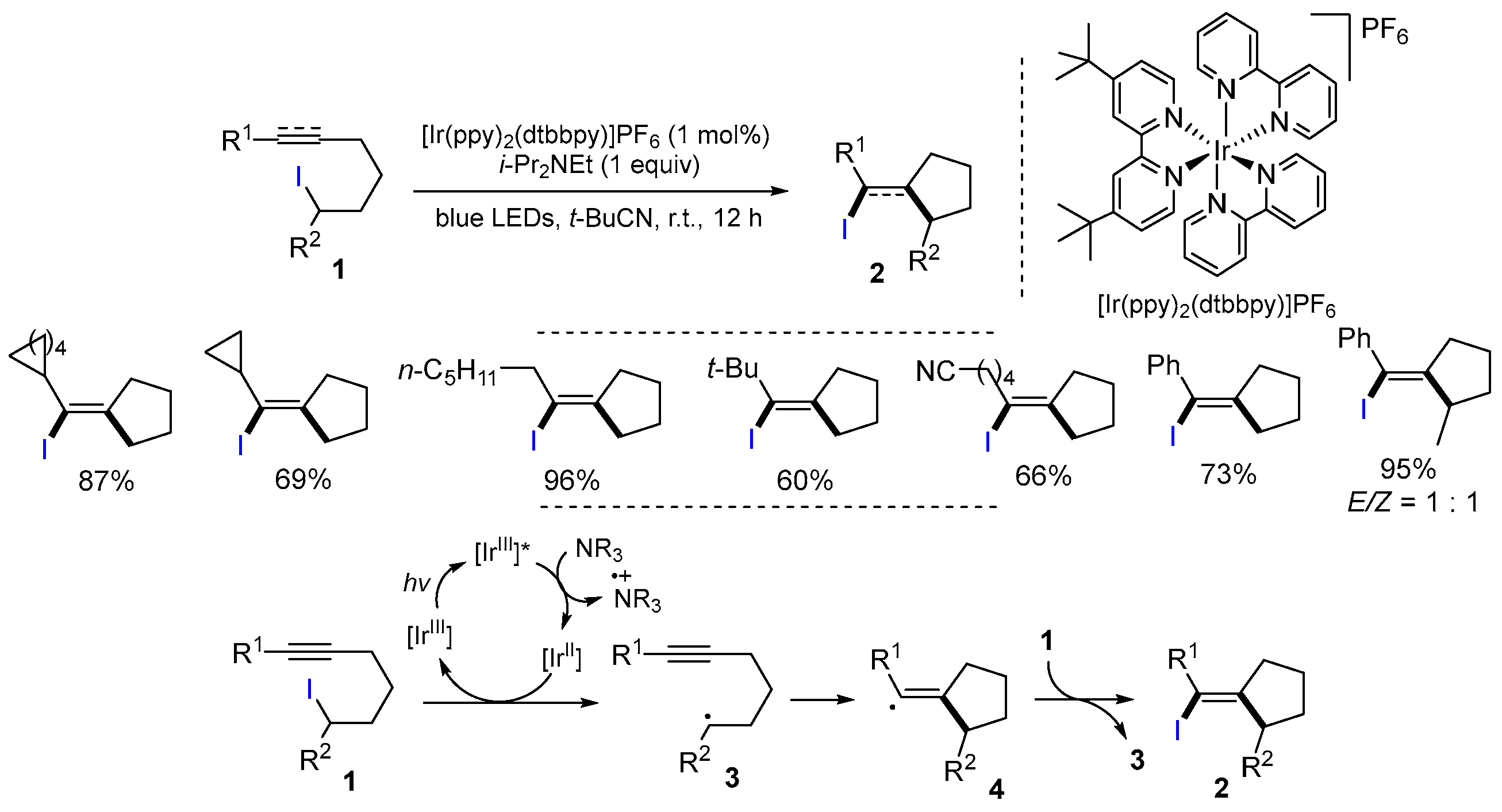
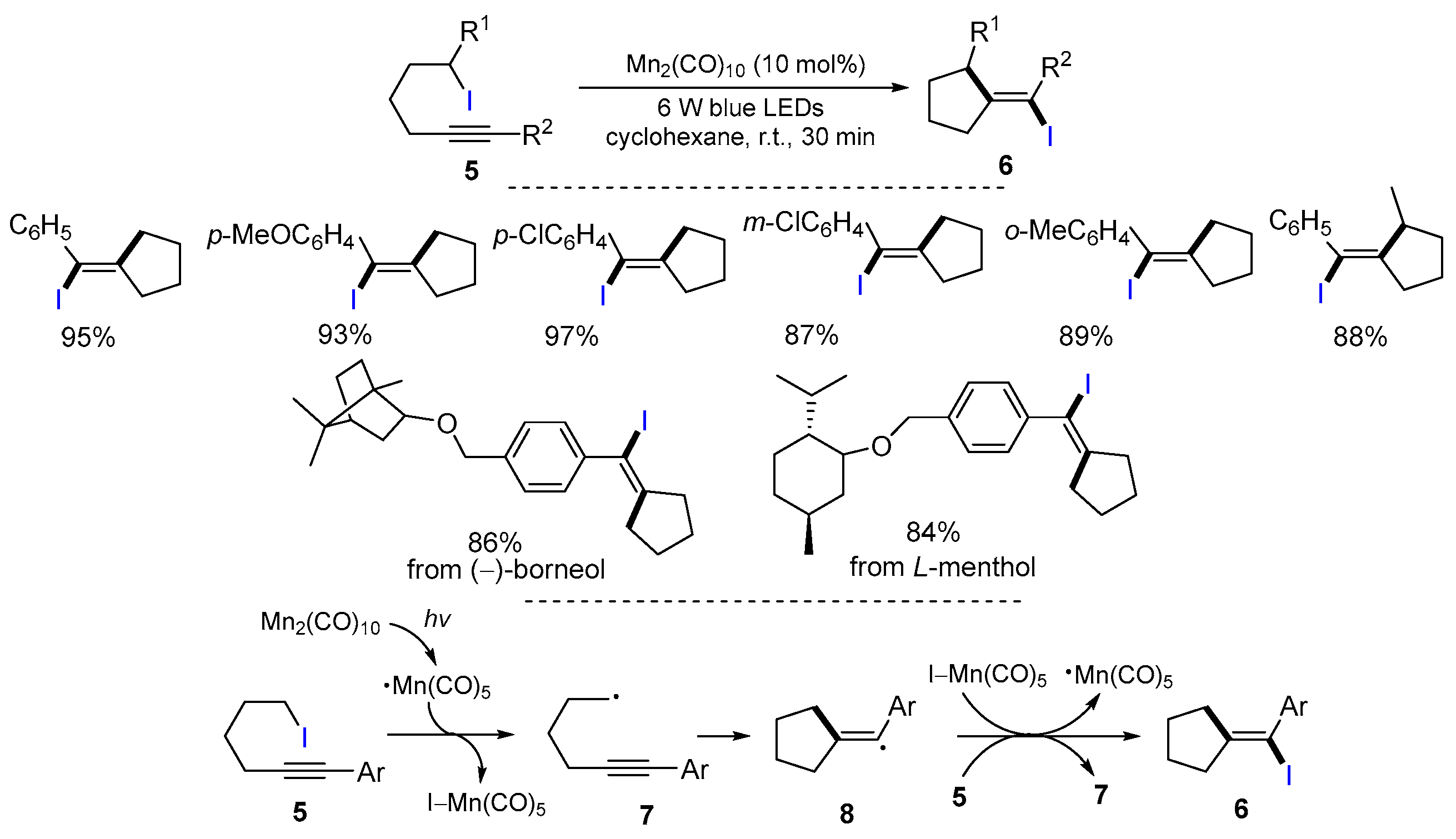
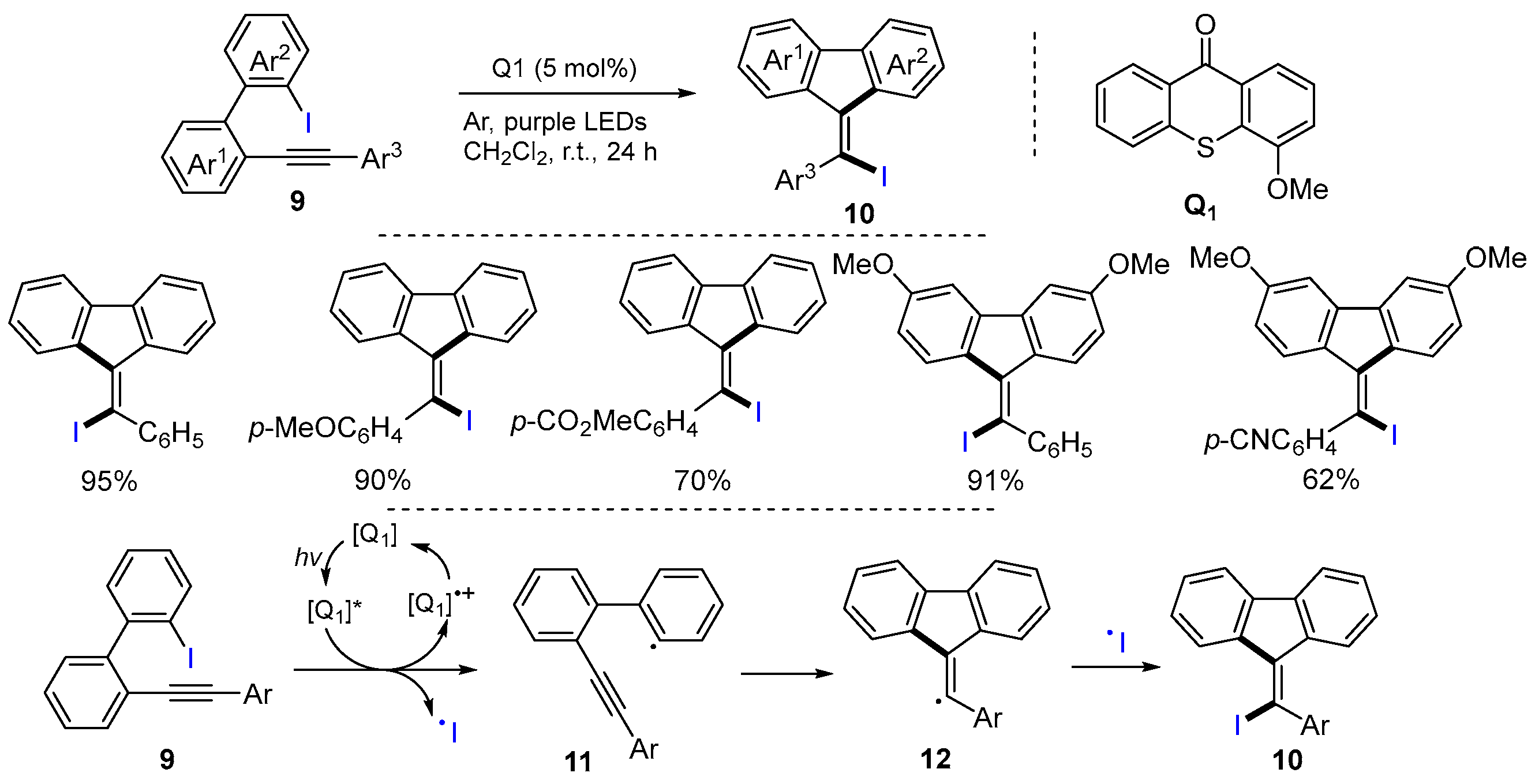
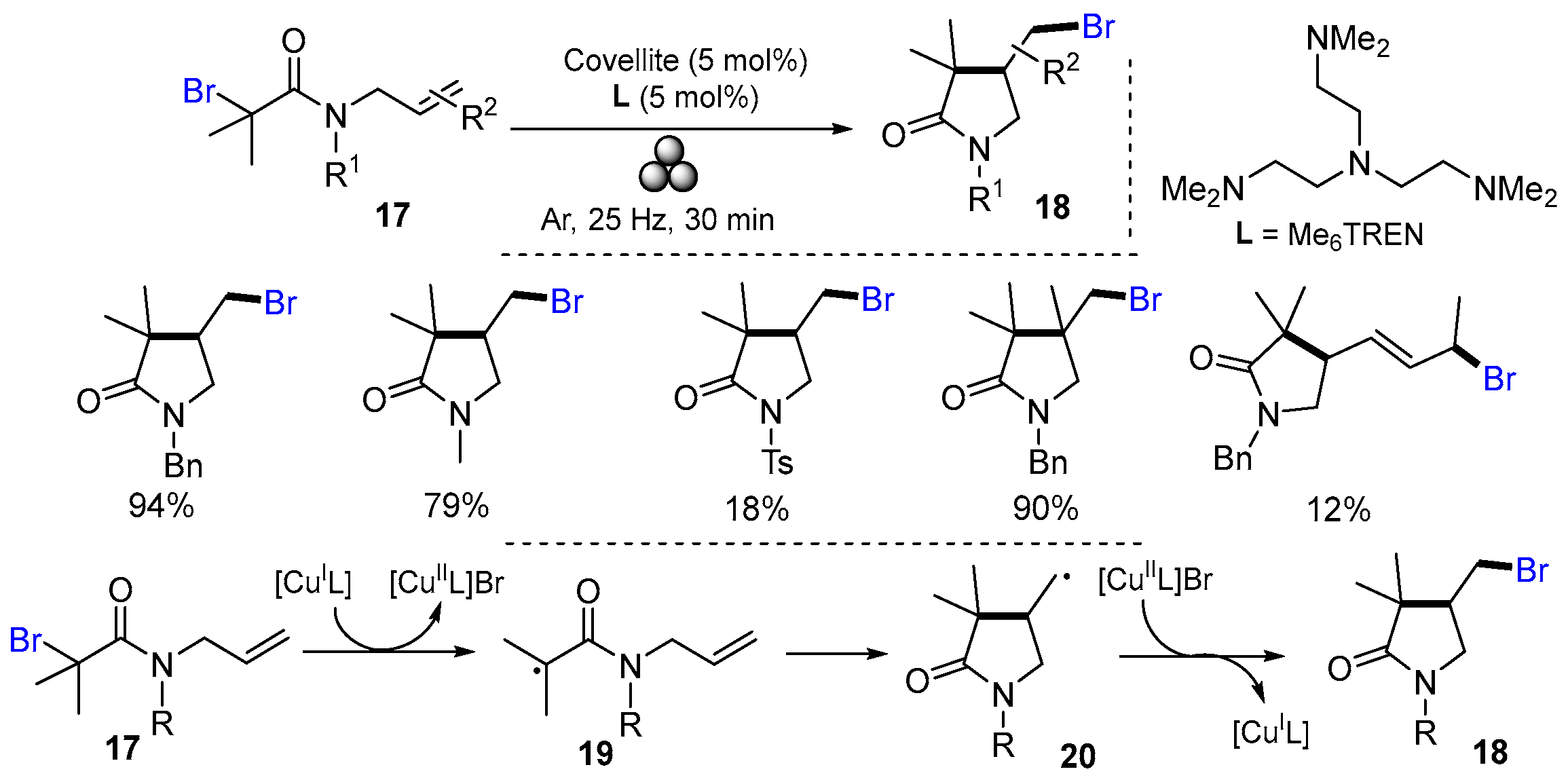
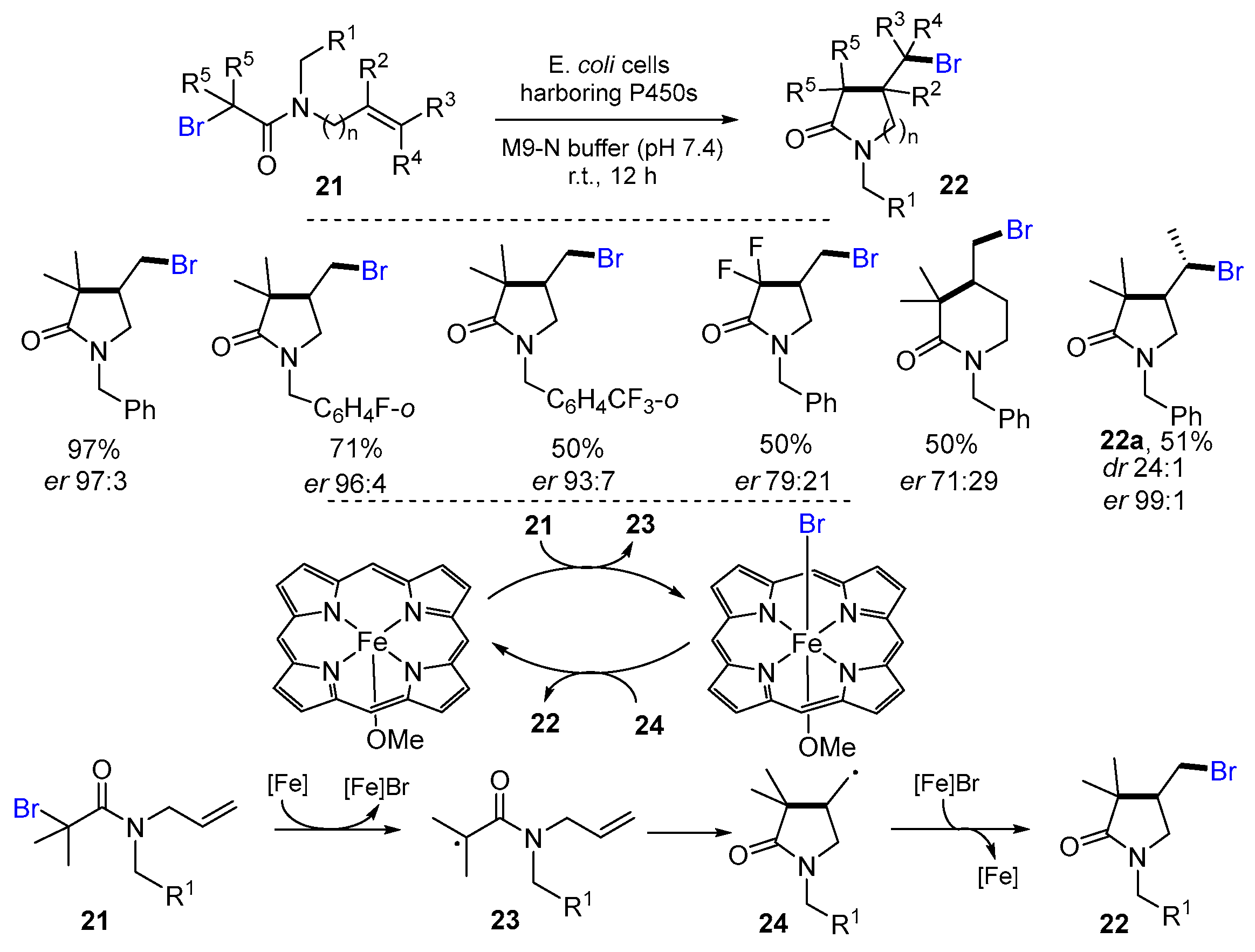
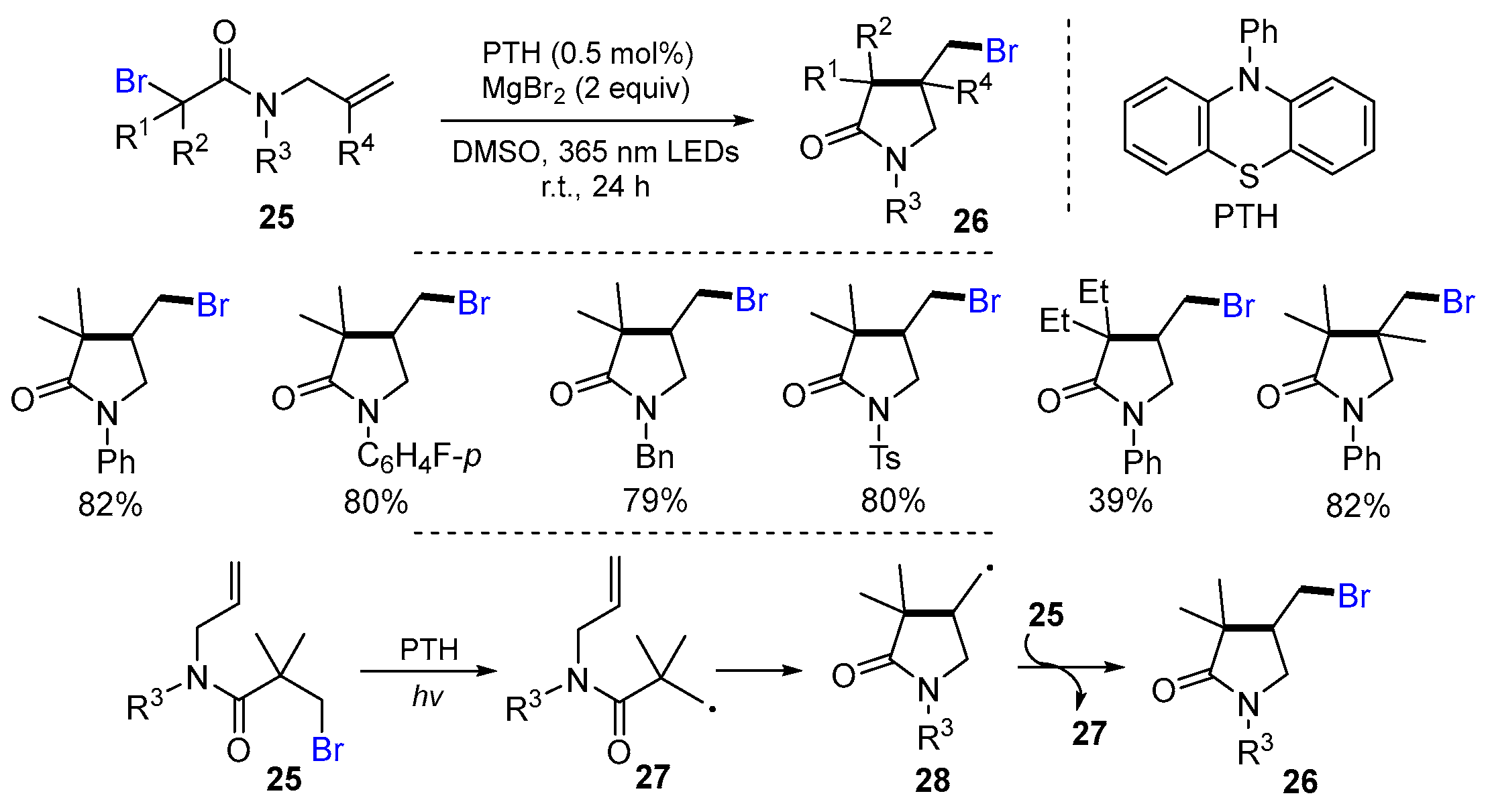
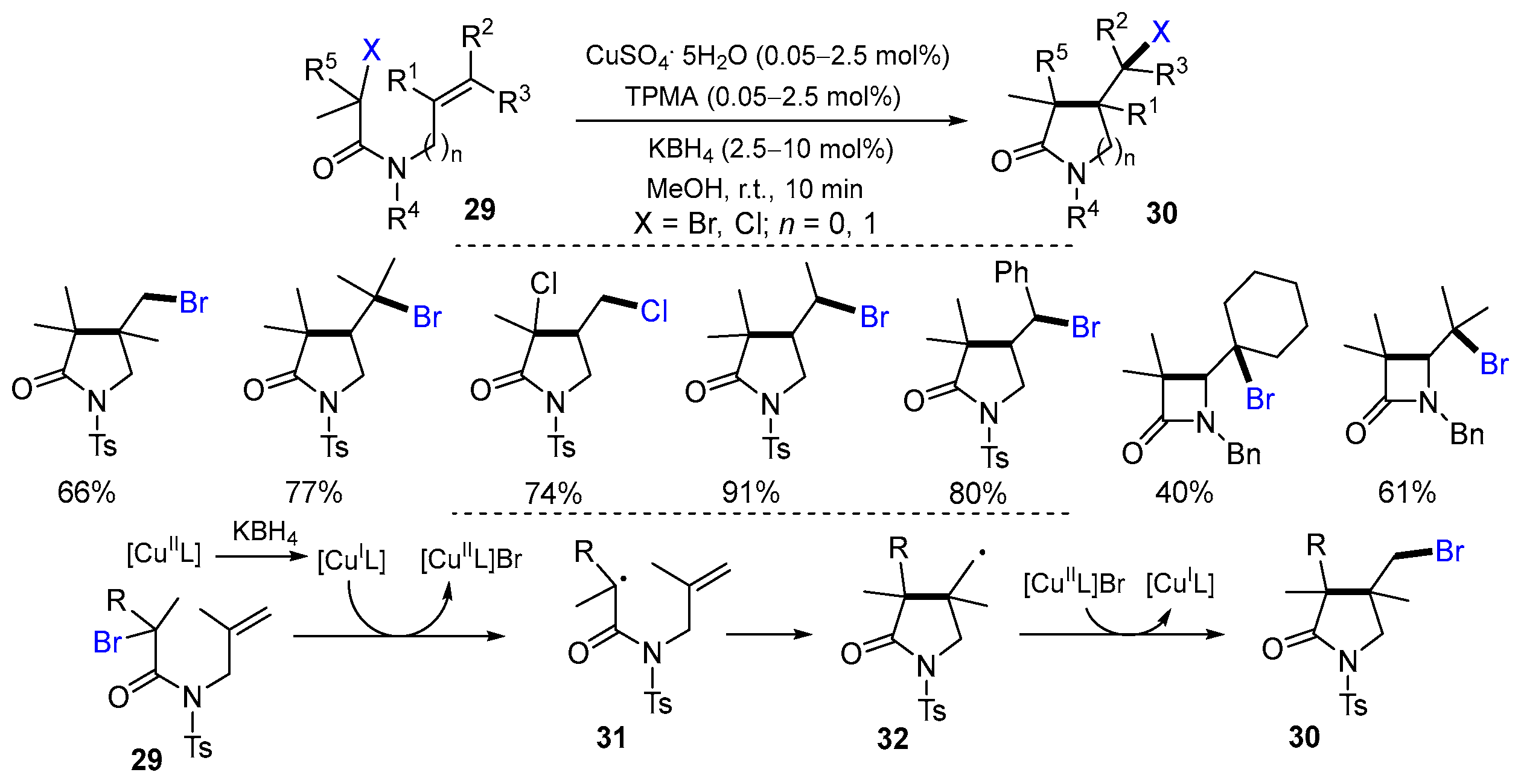
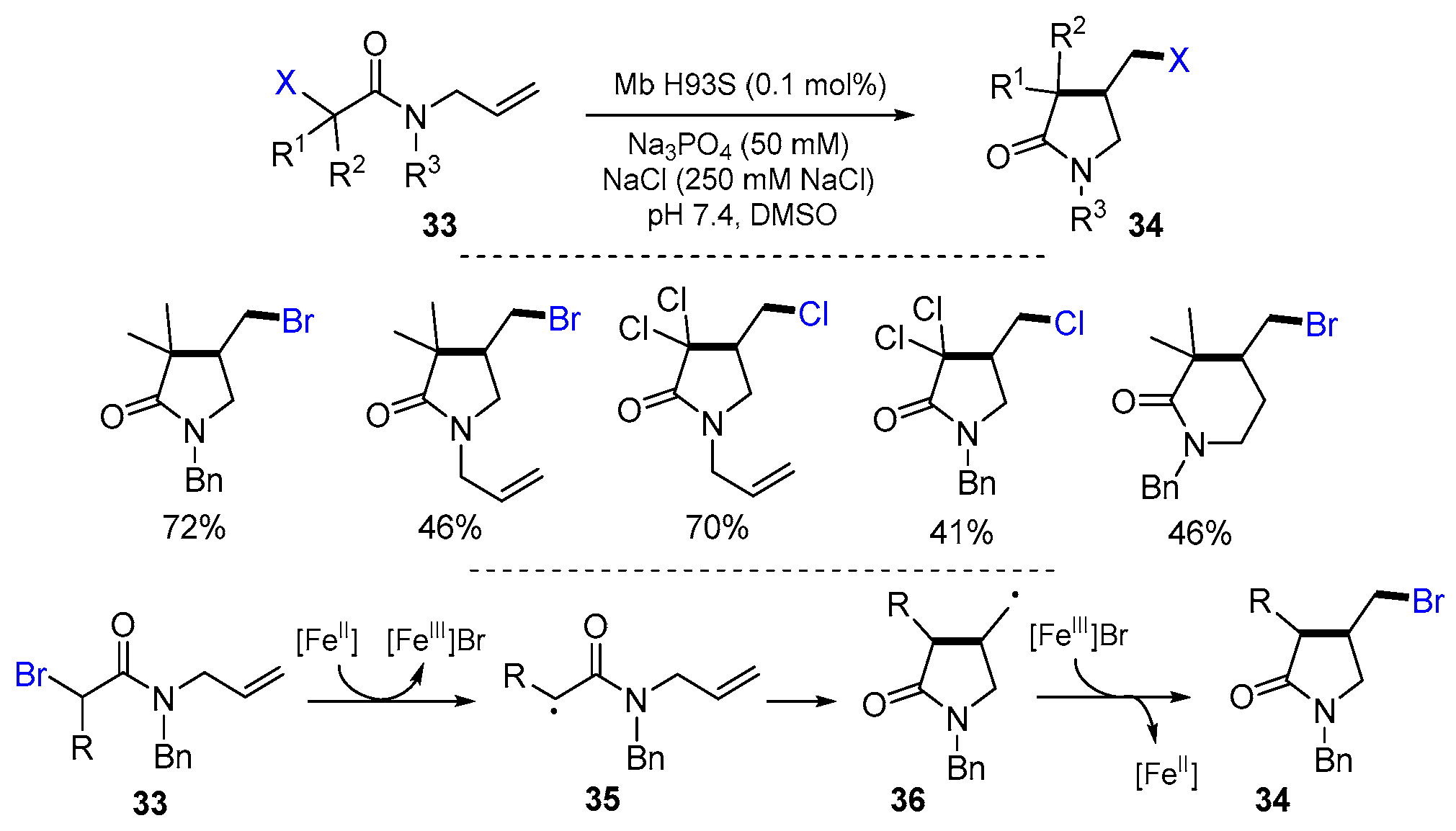
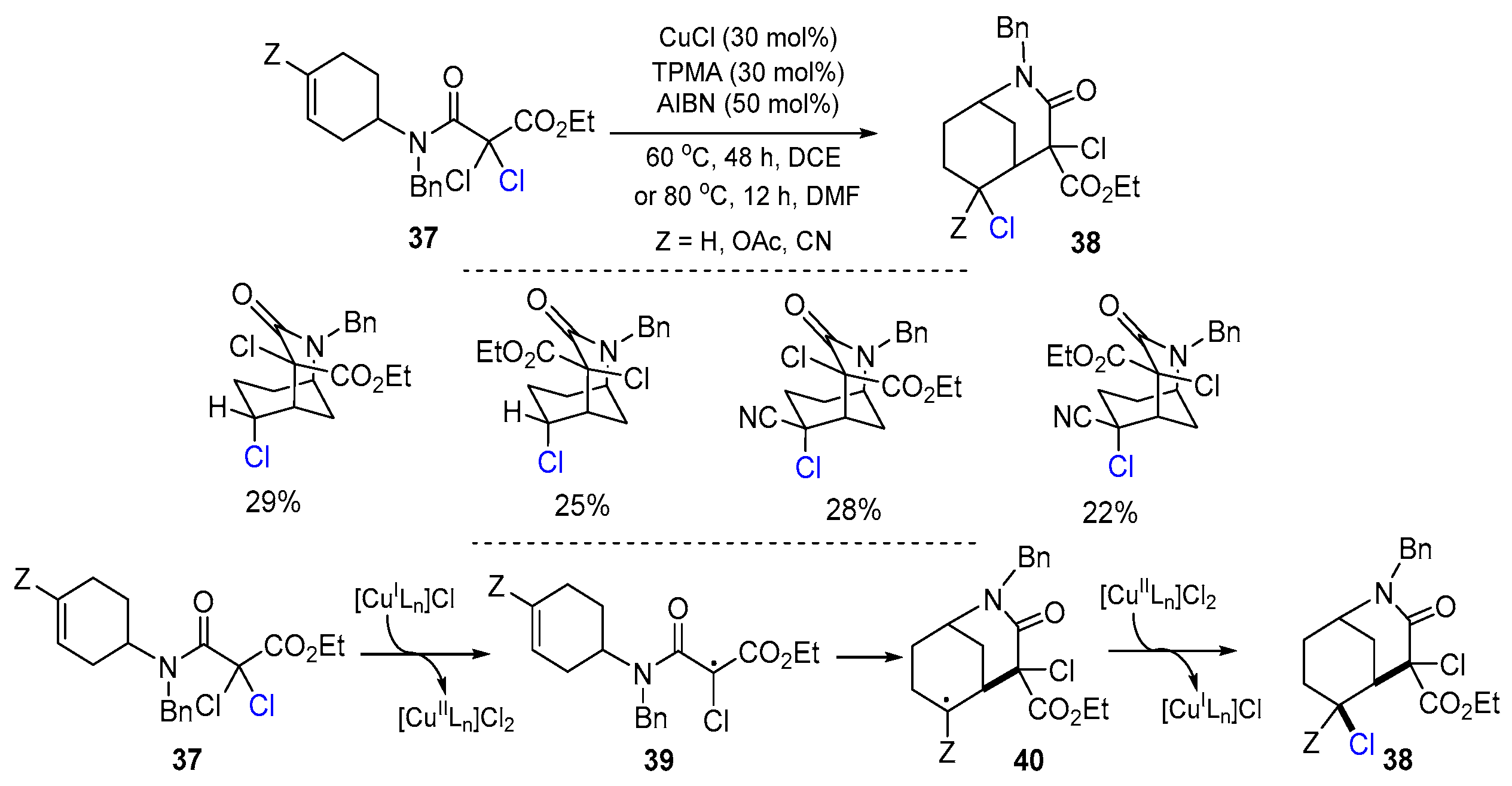
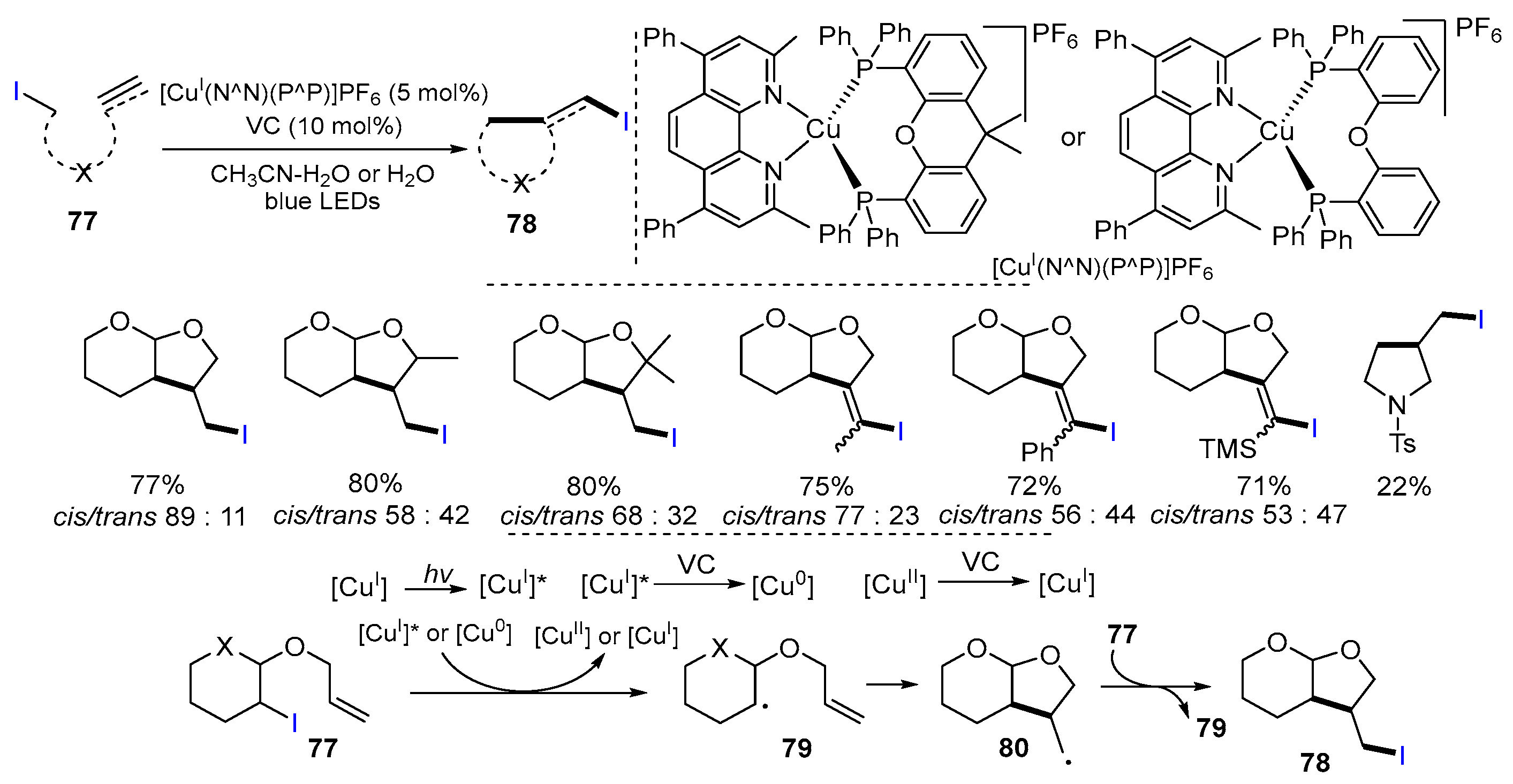
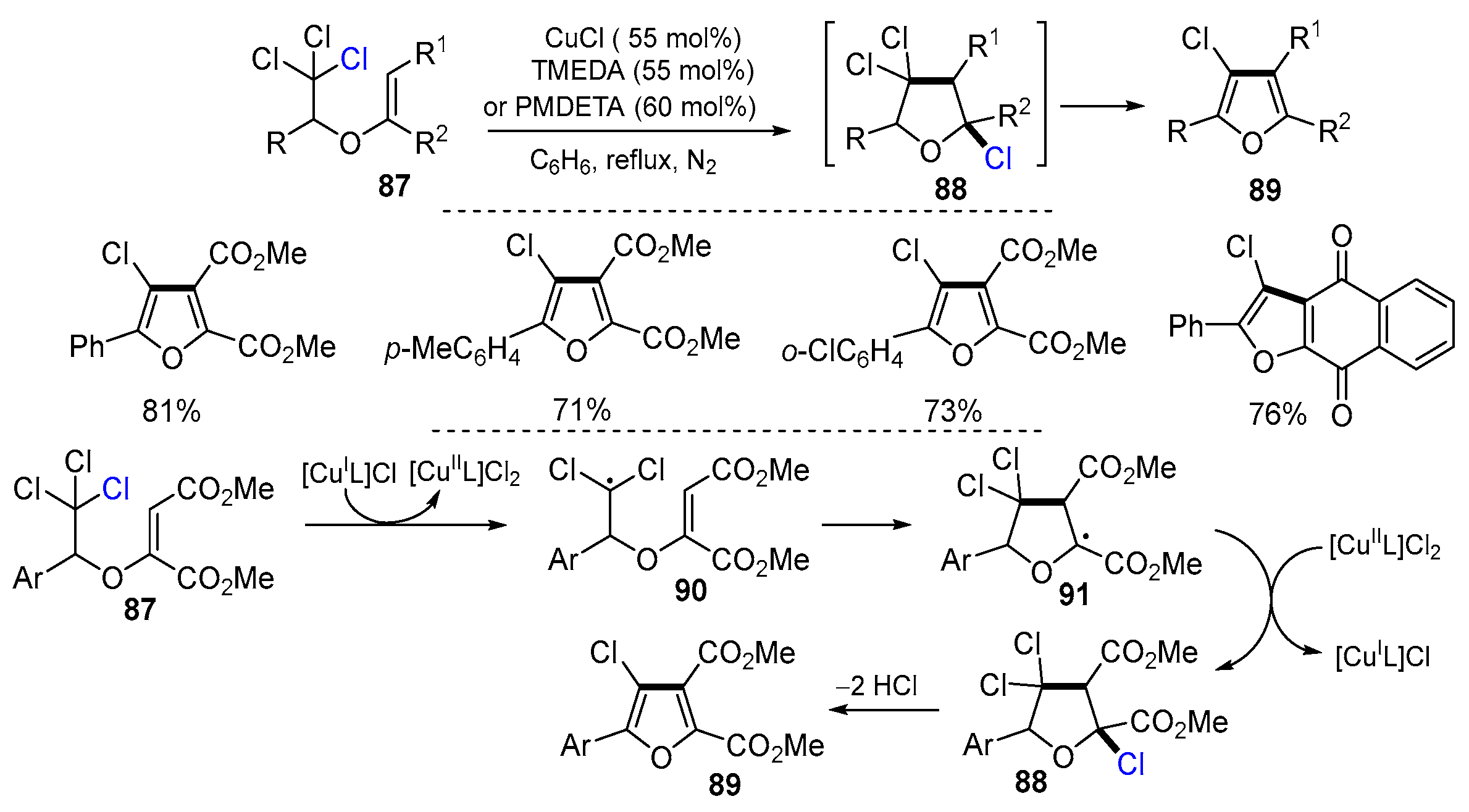
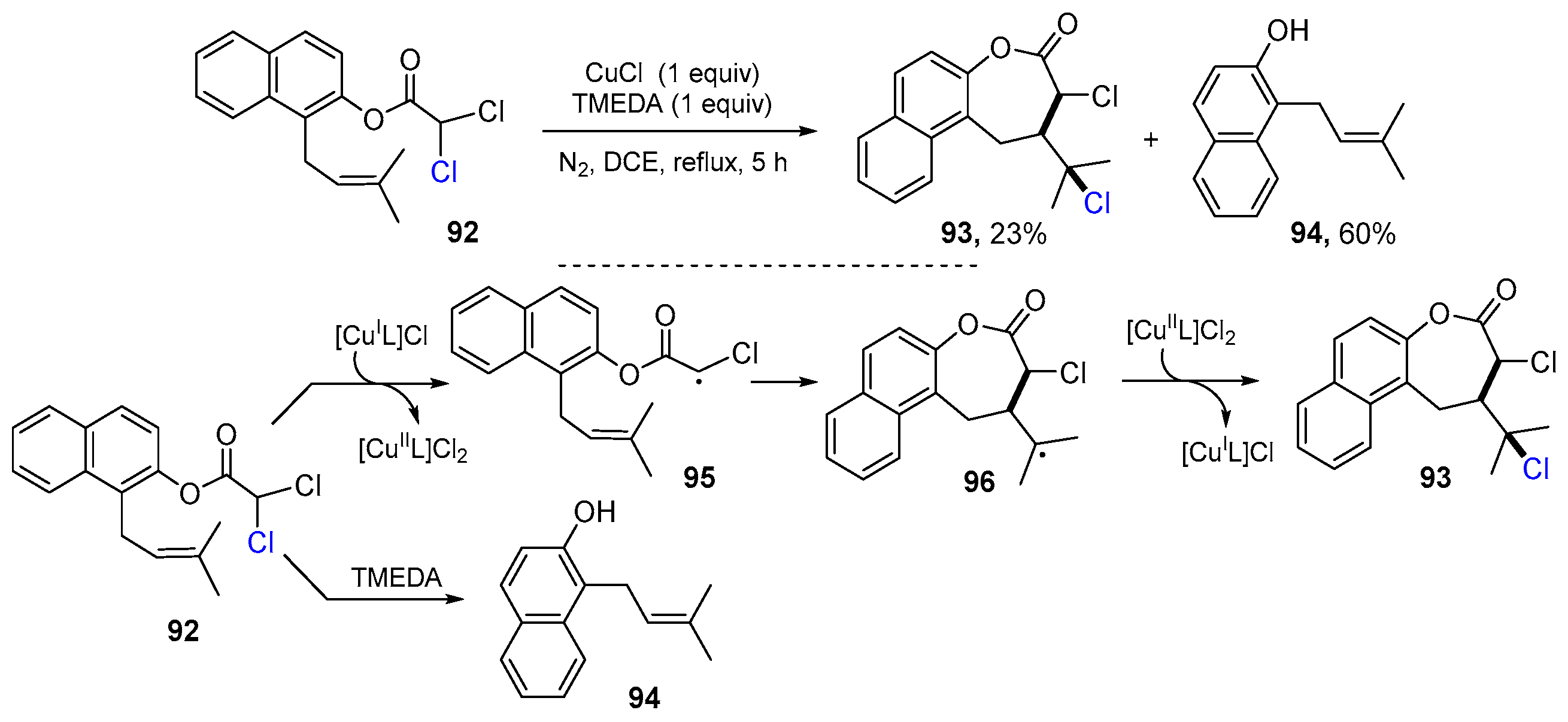
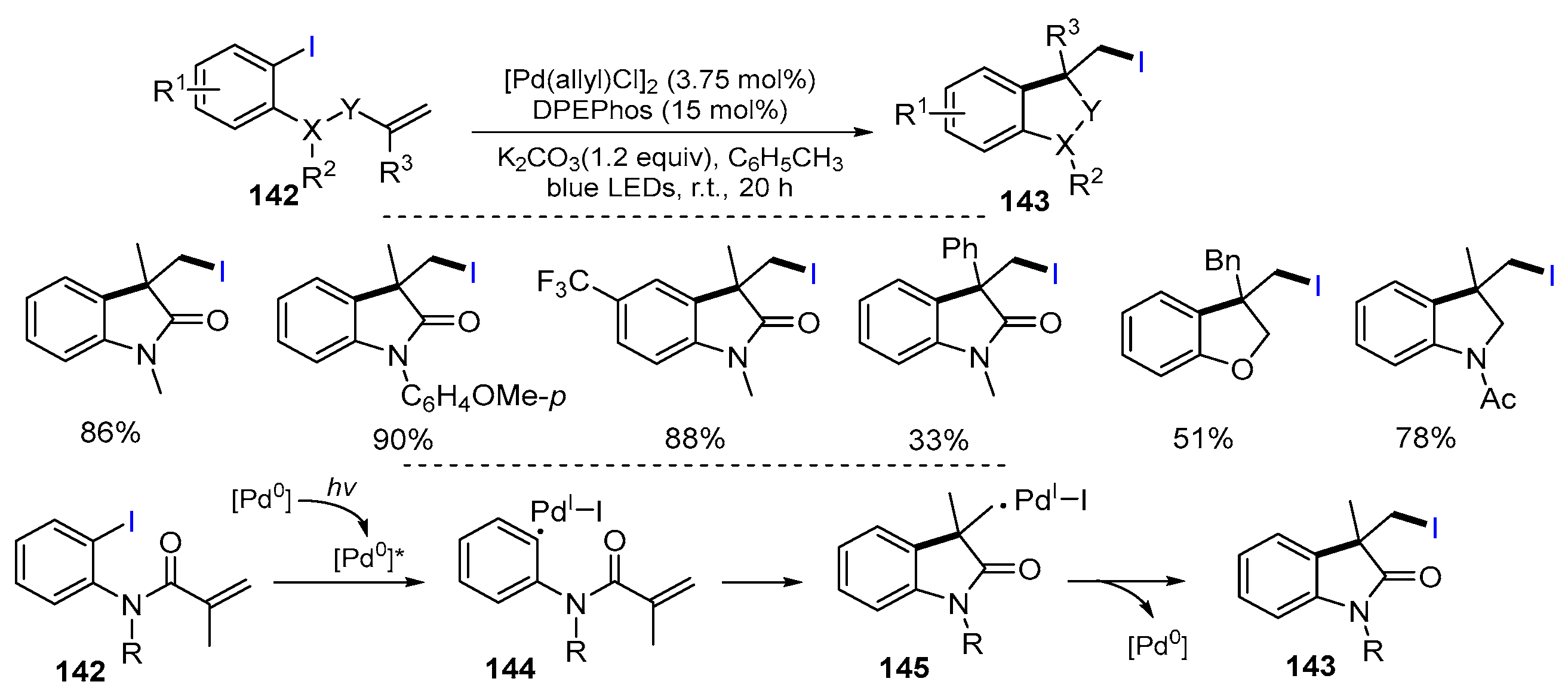
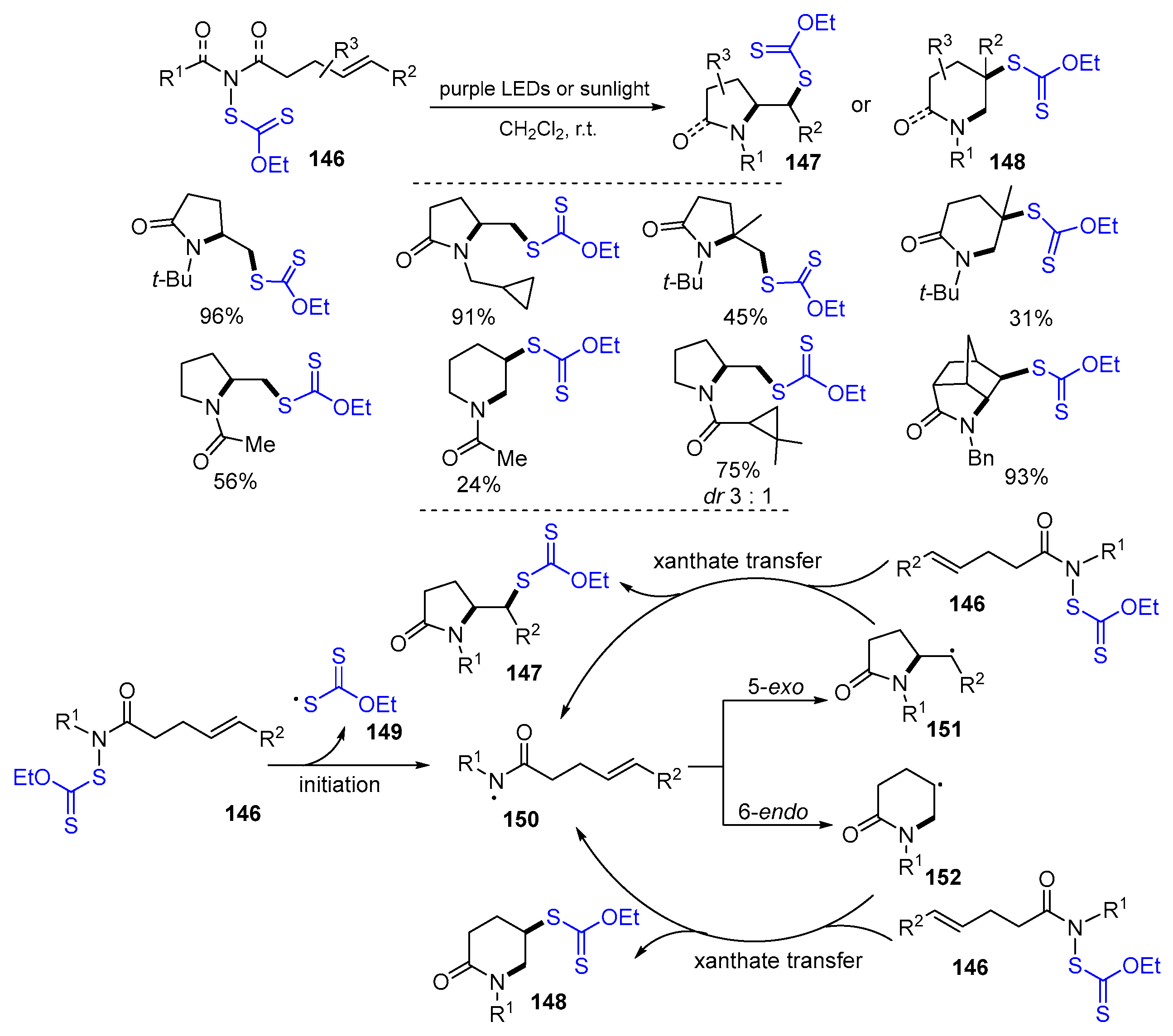
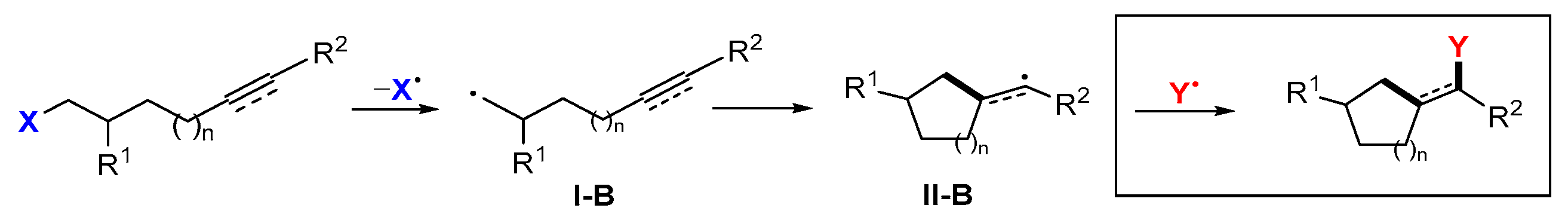
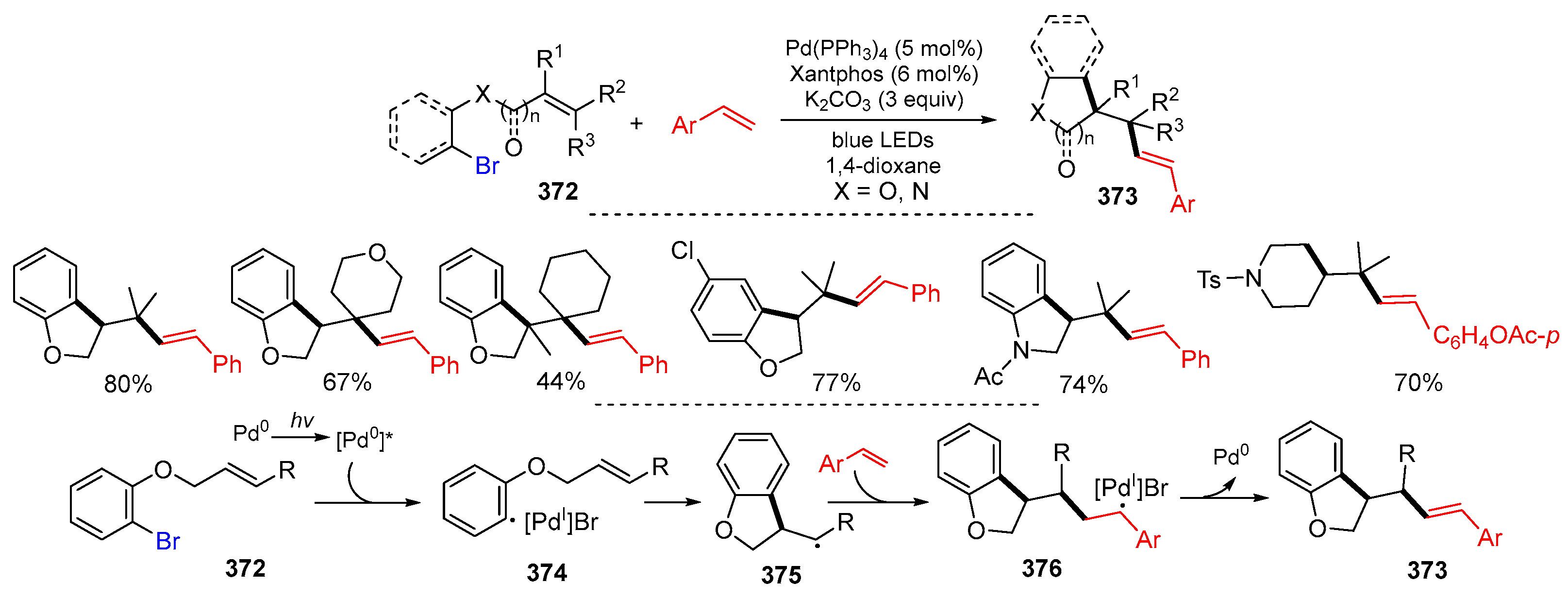
2.1. Halo Alkenes or Alkynes as Substrates
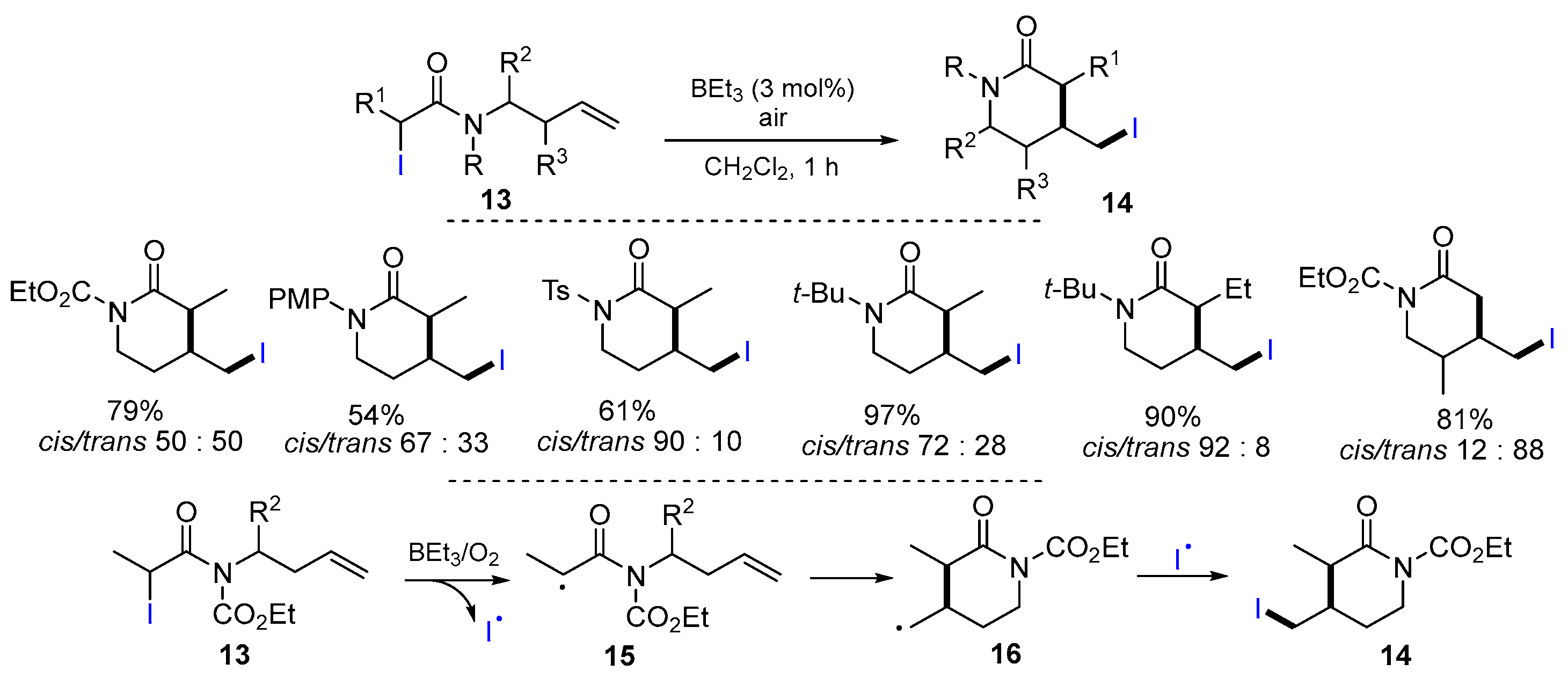
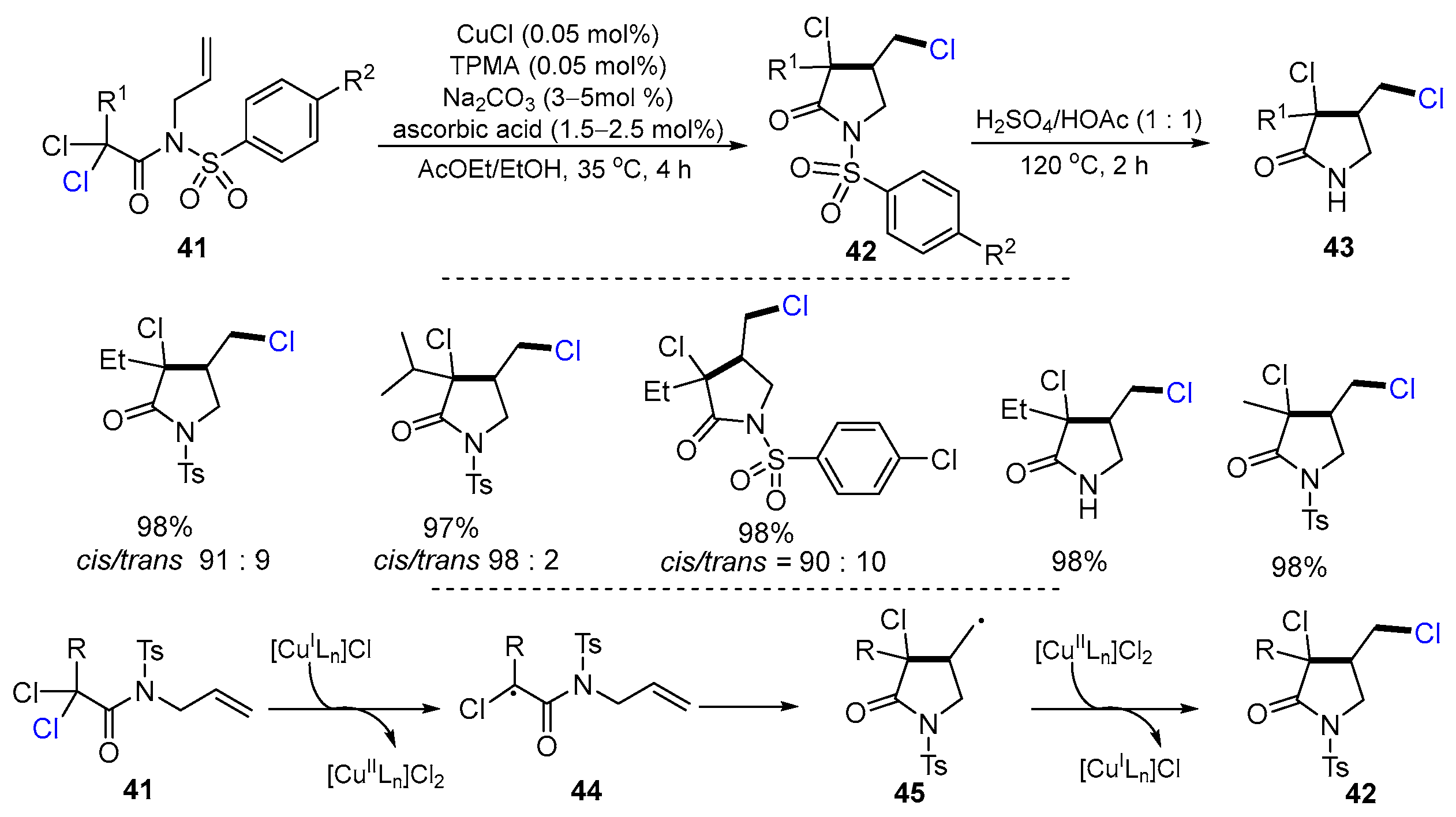
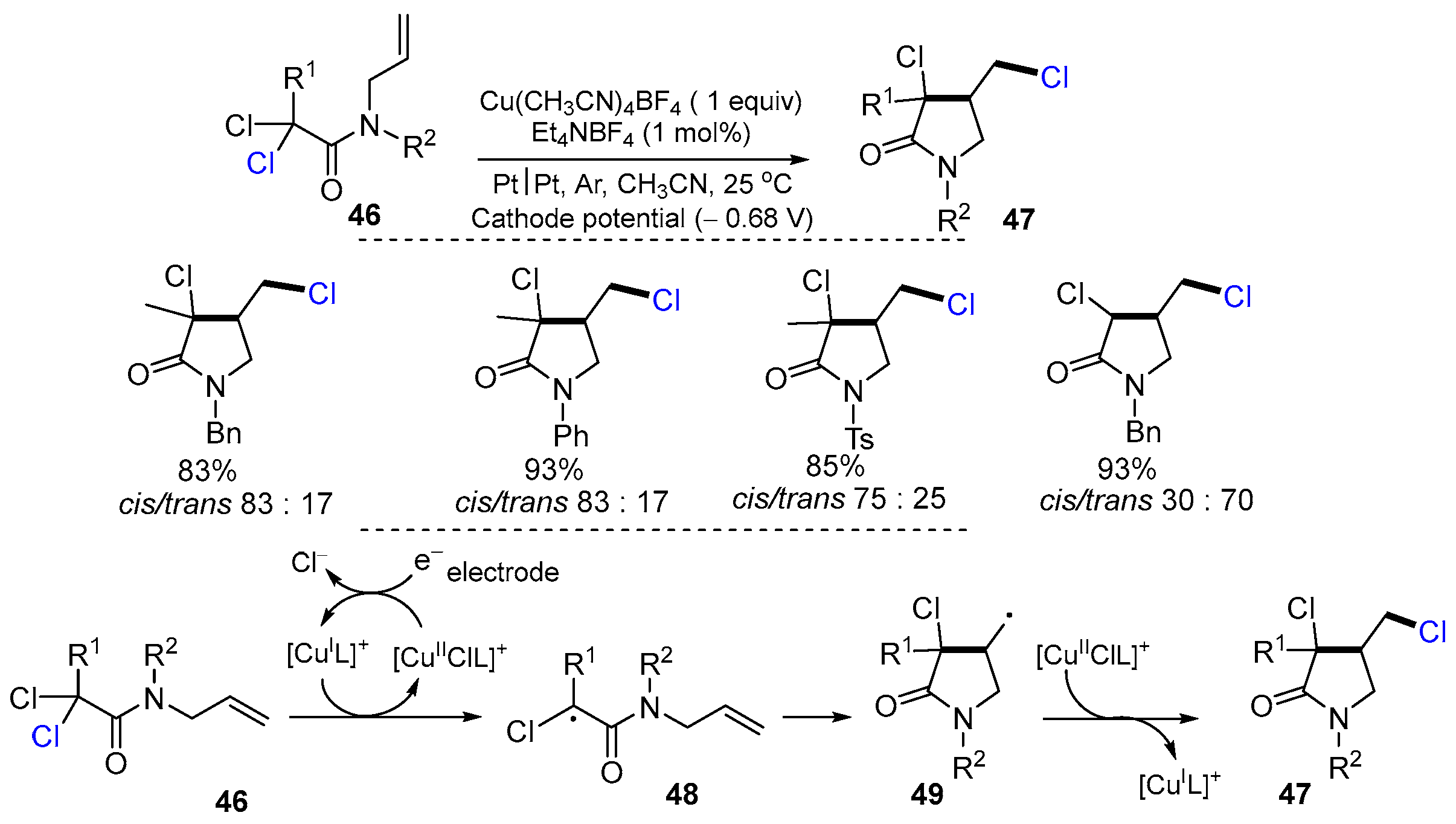
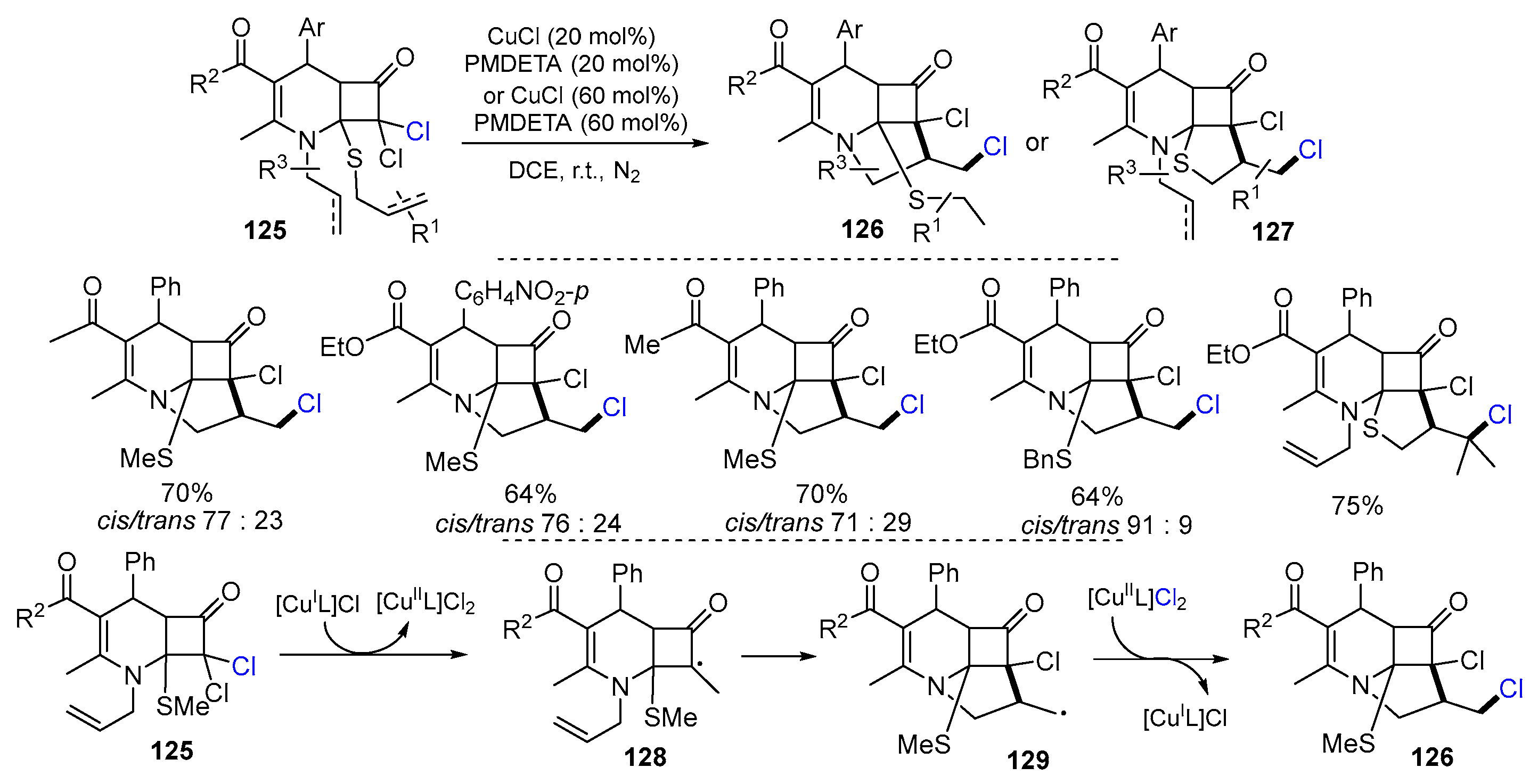
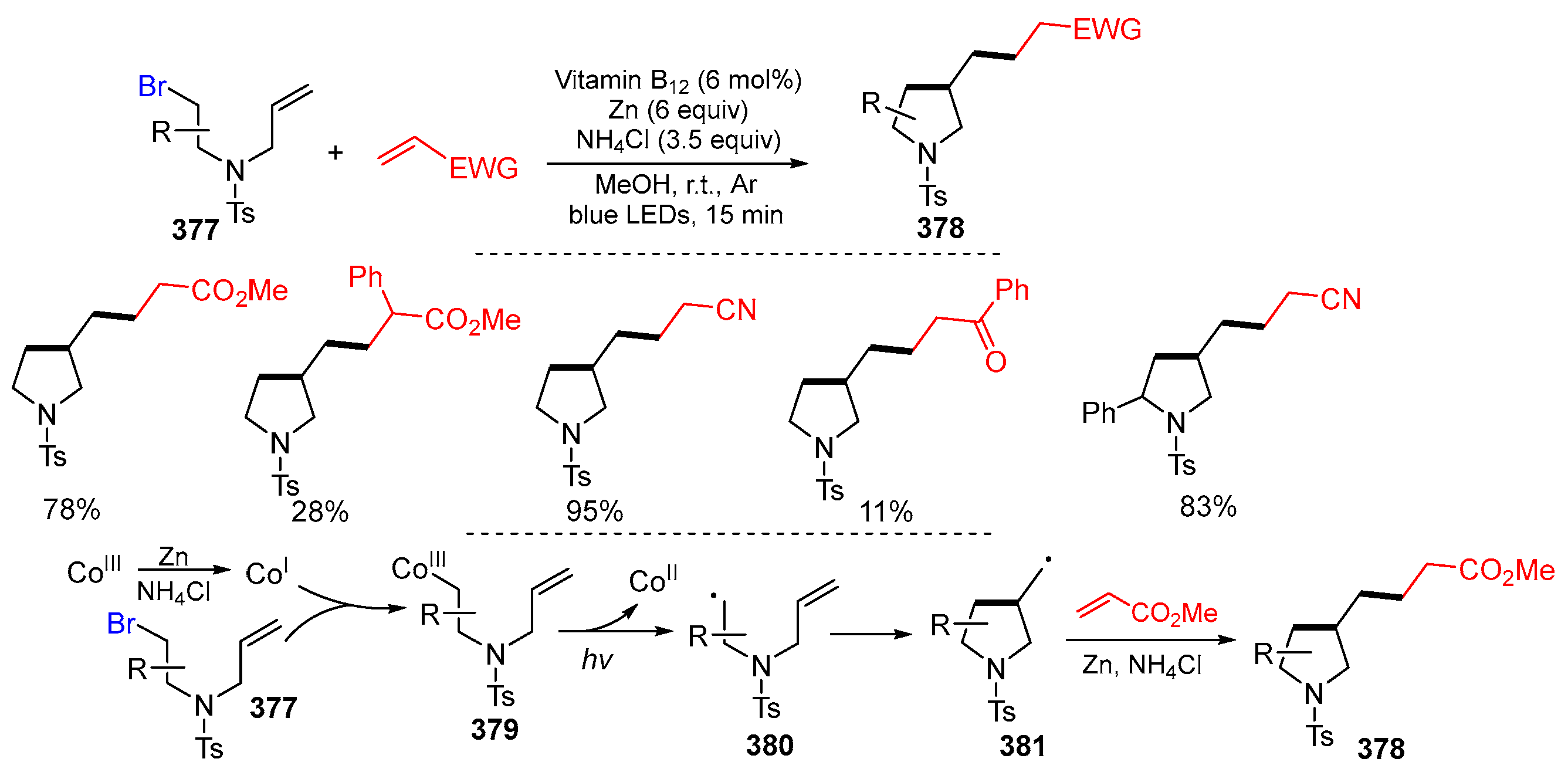
2.2. N-Allyl-haloacetamides as Substrates
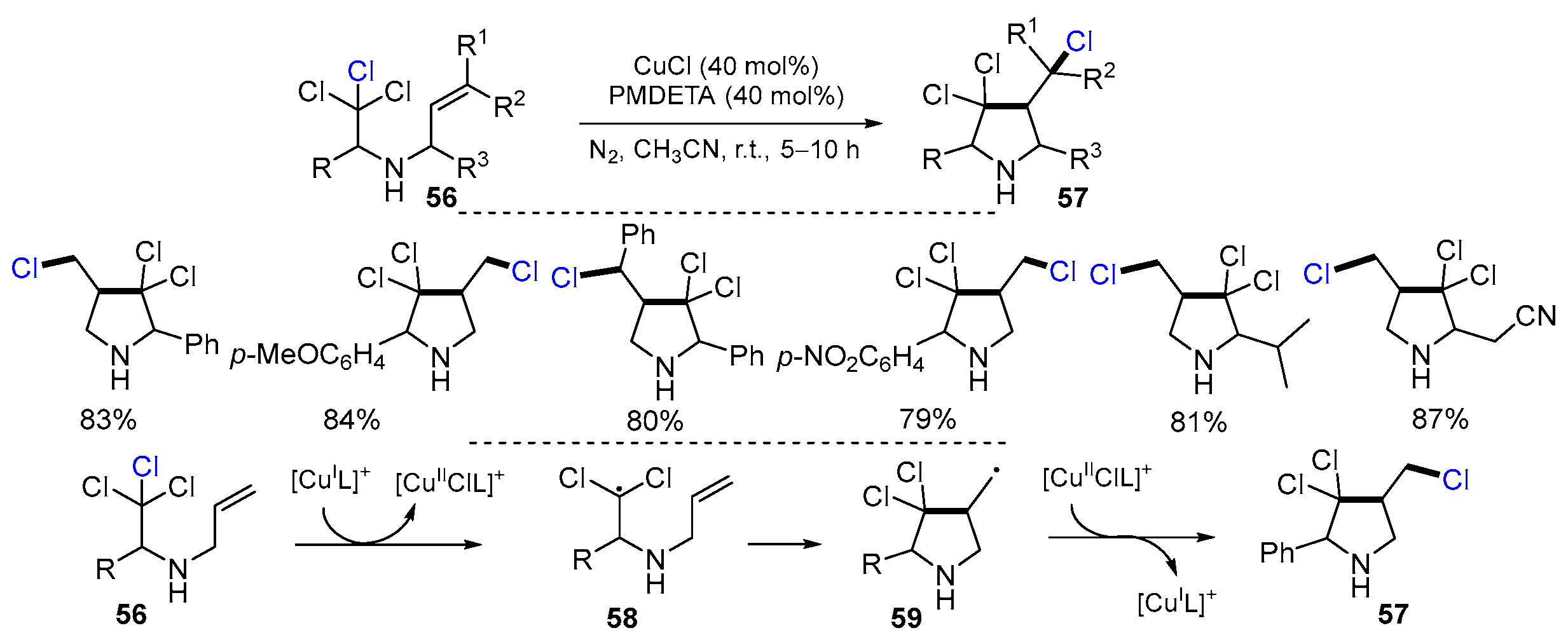
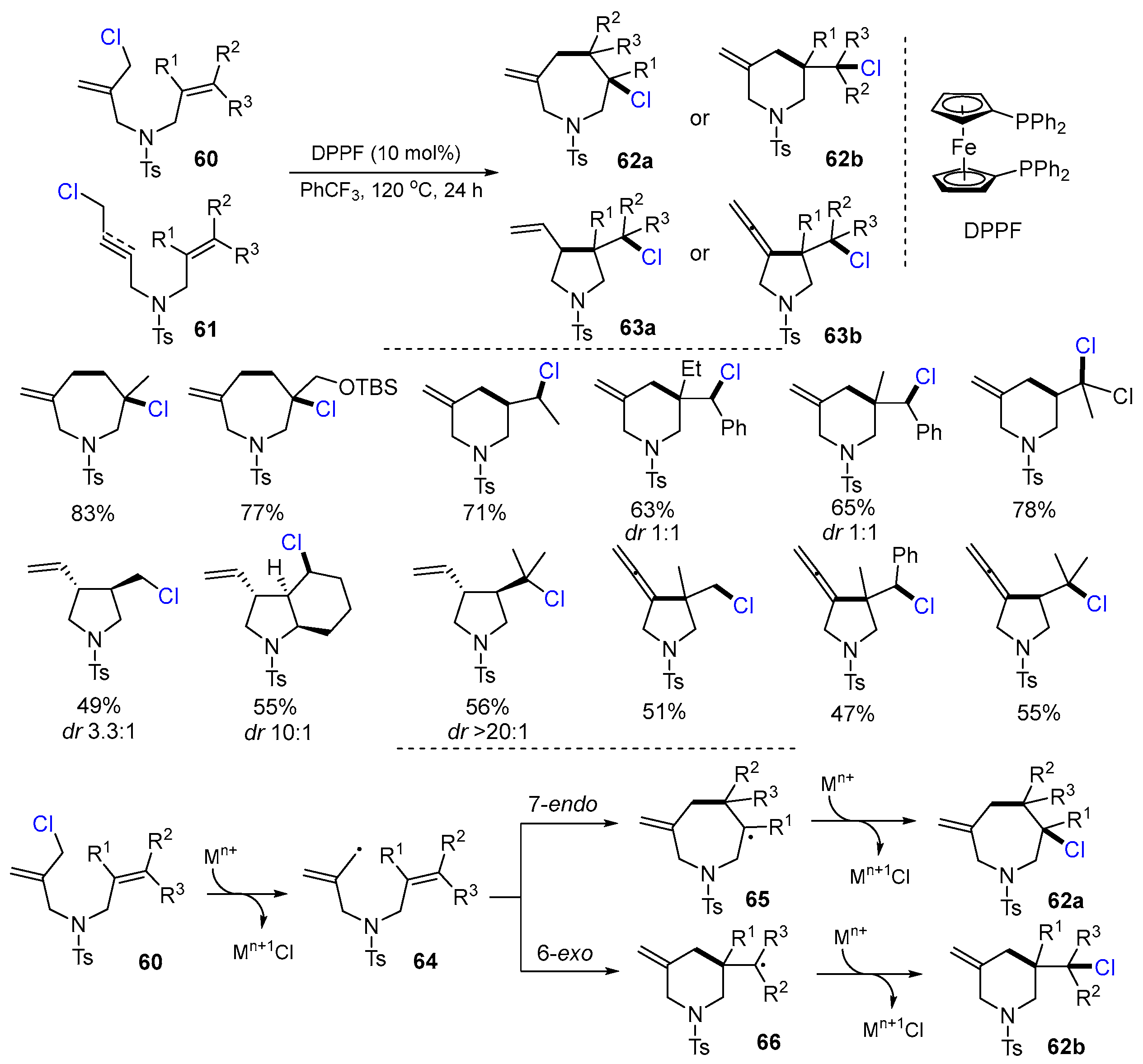
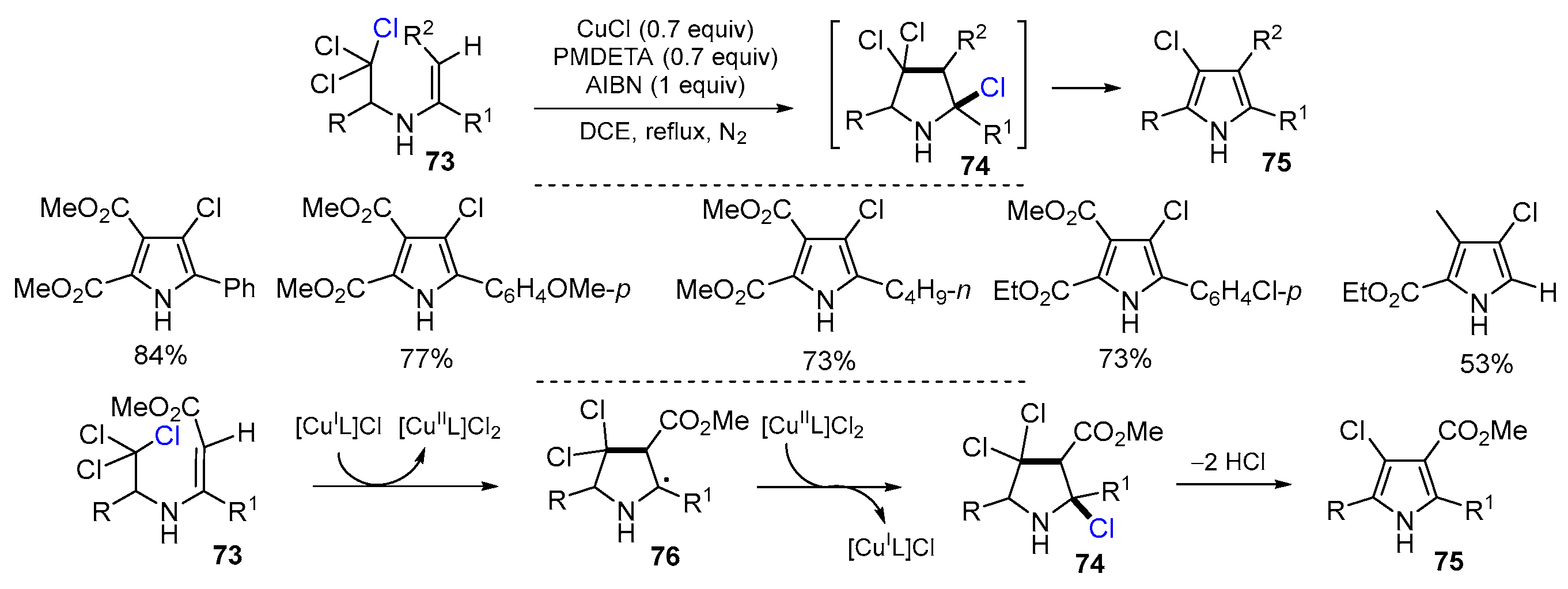
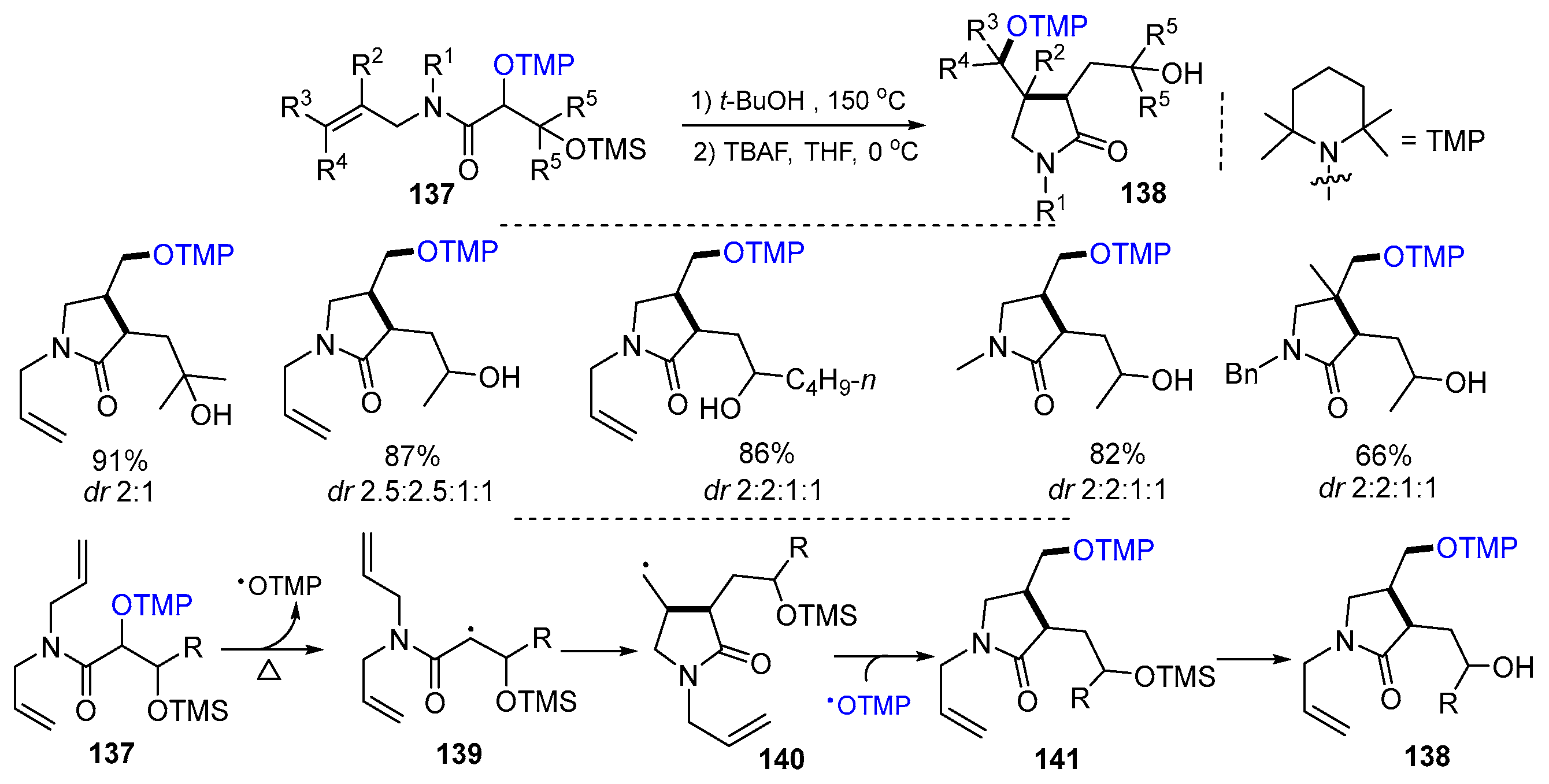
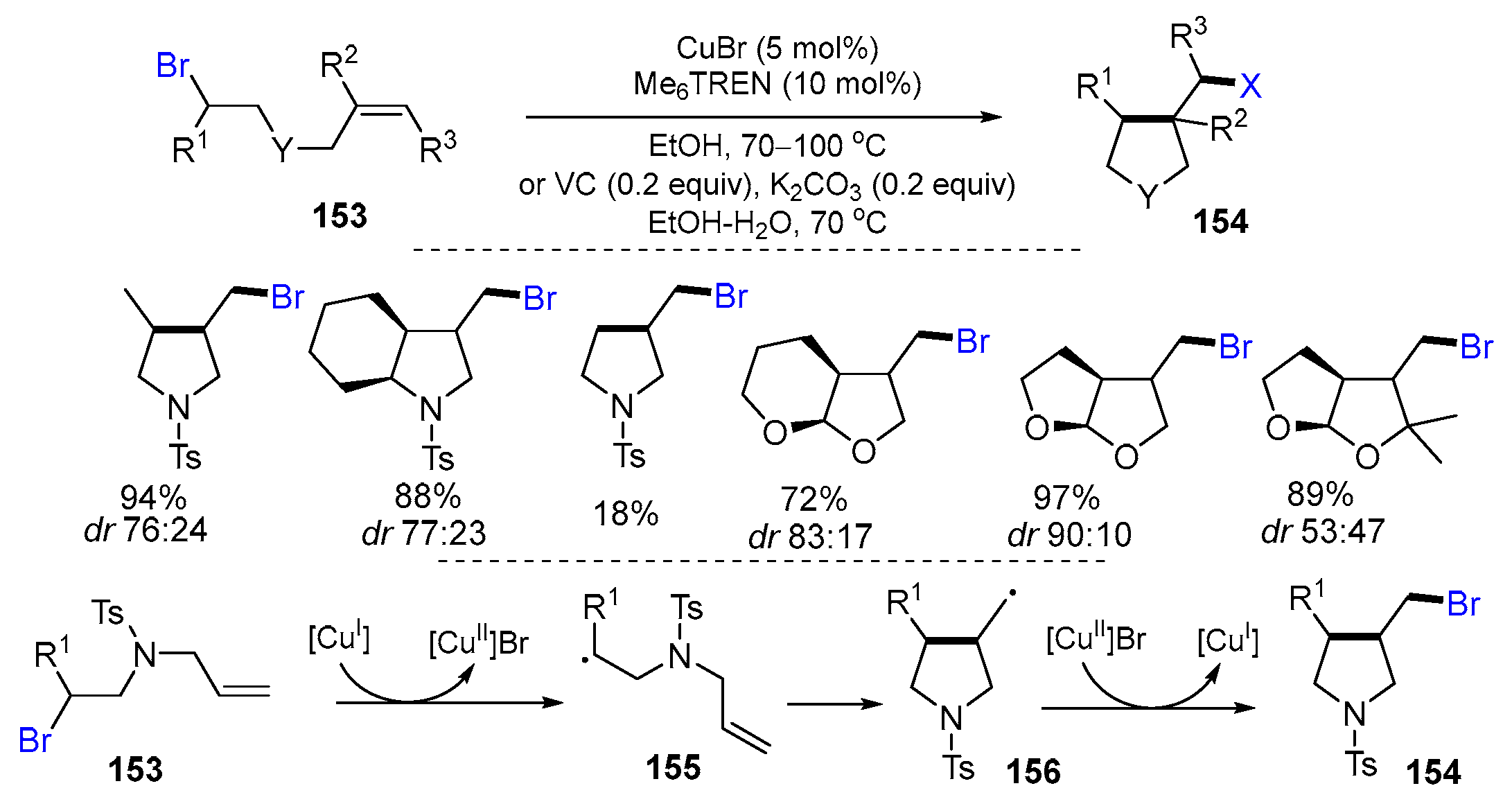
2.3. N-Allyl-haloamines as Substrates
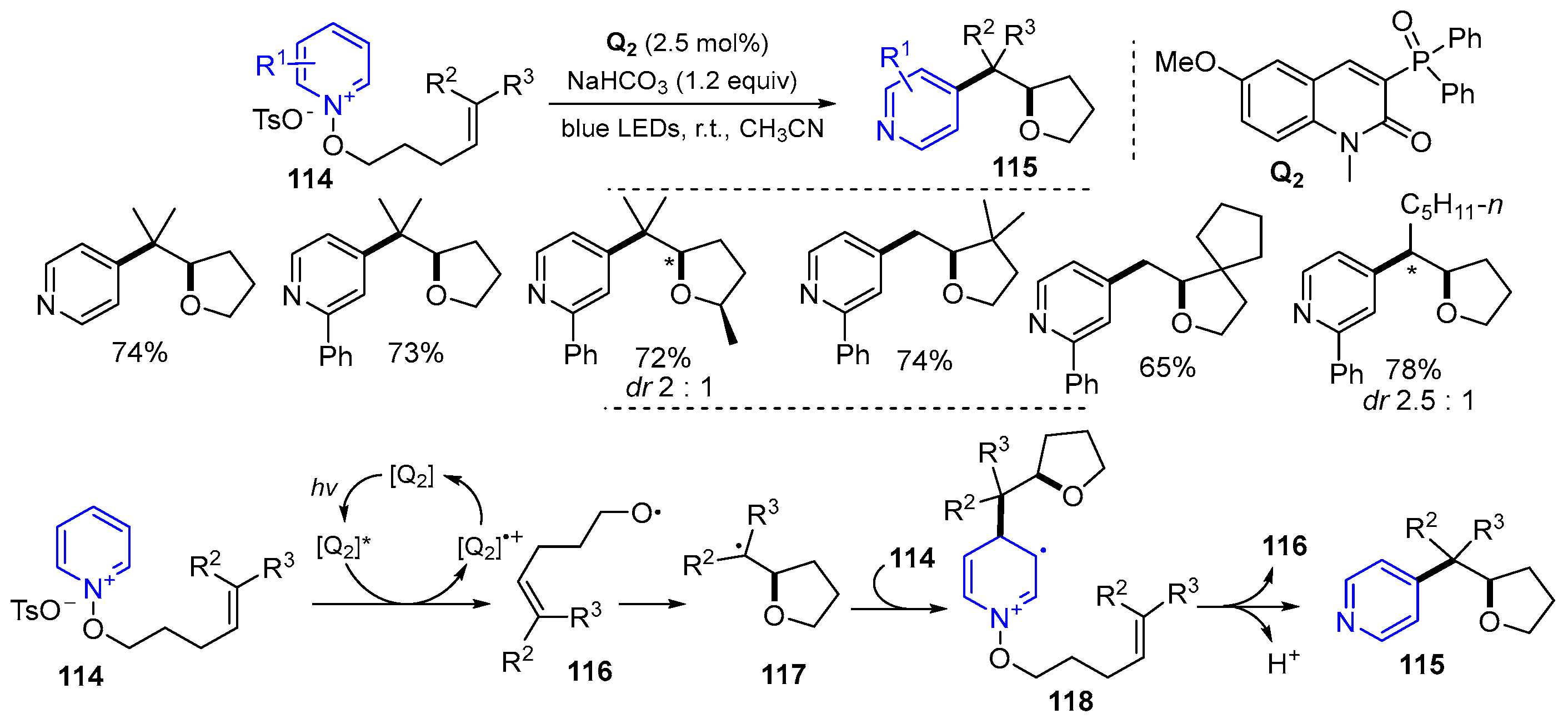
2.4. O-Allyl-halo Ethers as Substrates
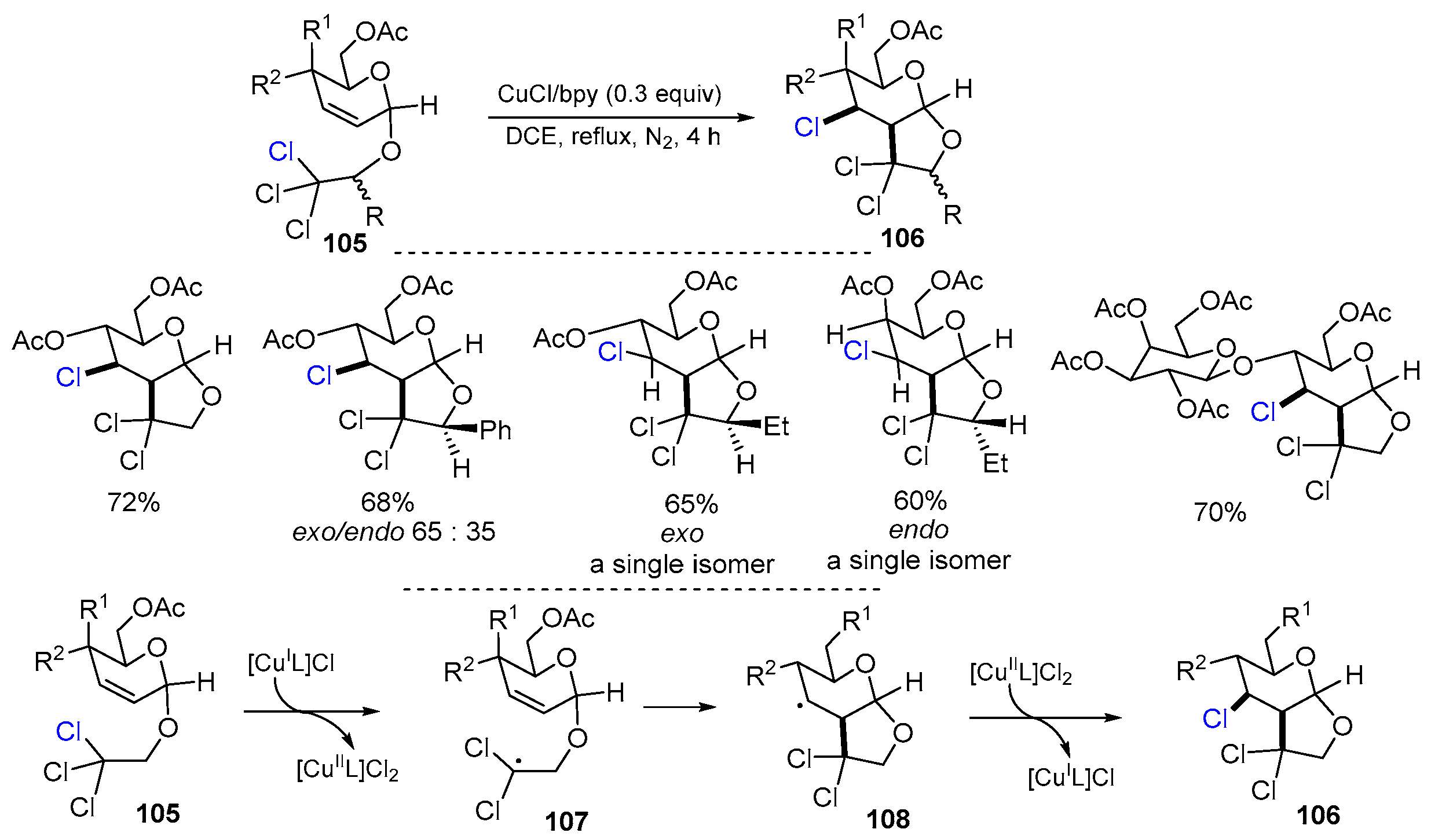
2.5. O-Allyl-halo-hemiacetal Acetates as Substrates
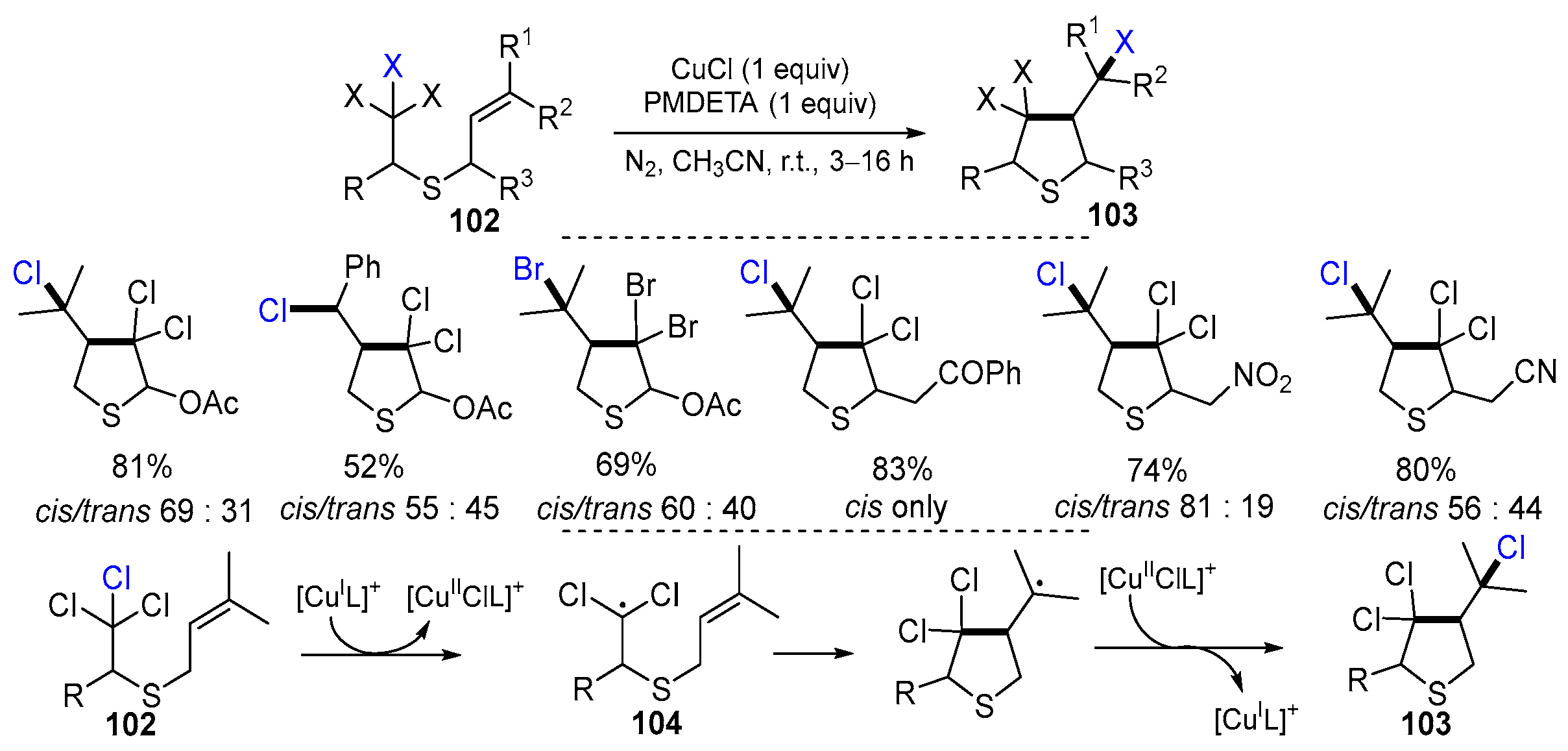
2.6. Other Vinyl Derivatives as Substrates
3. Second Functionalization with Y via Radical Coupling
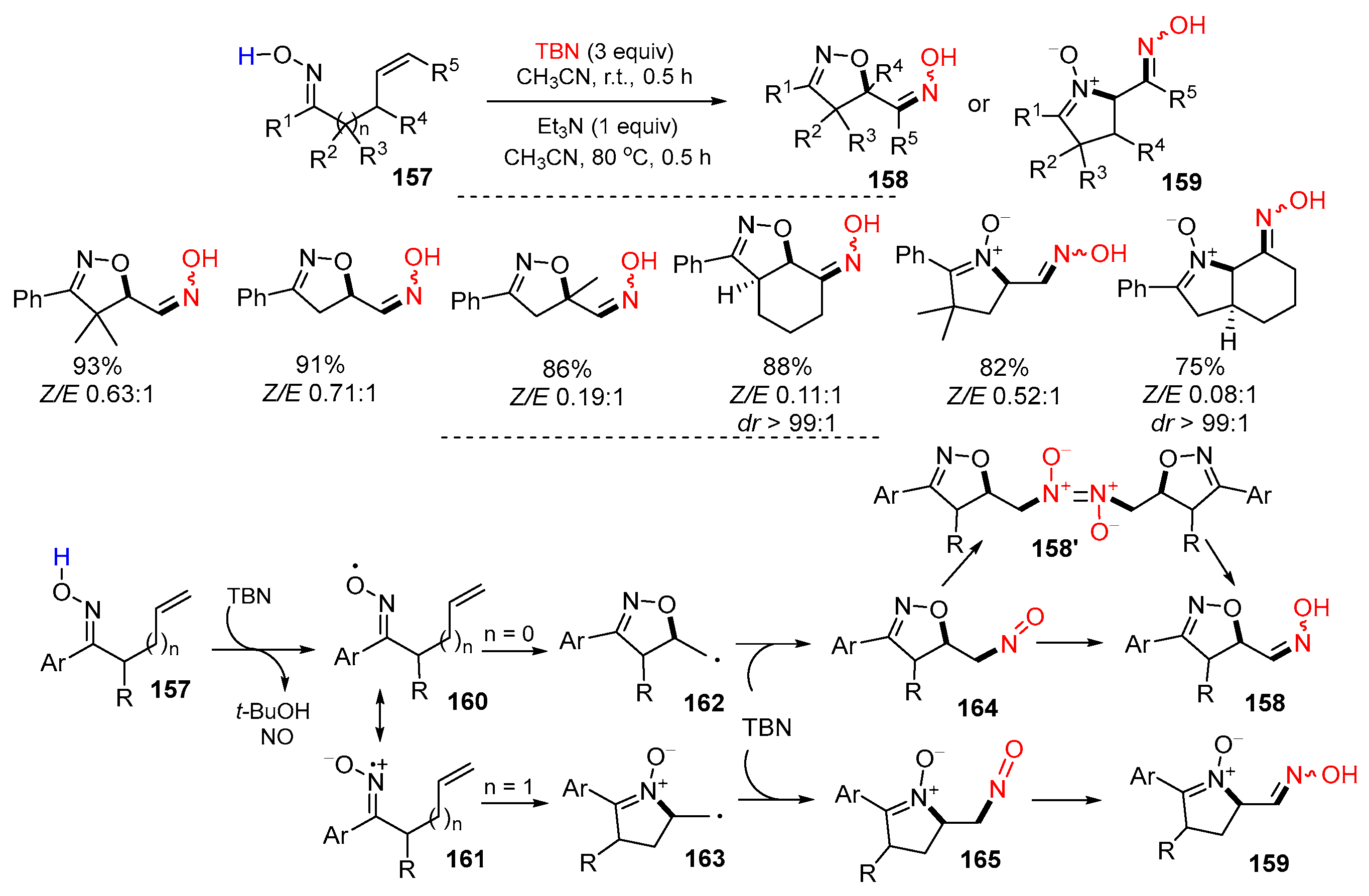
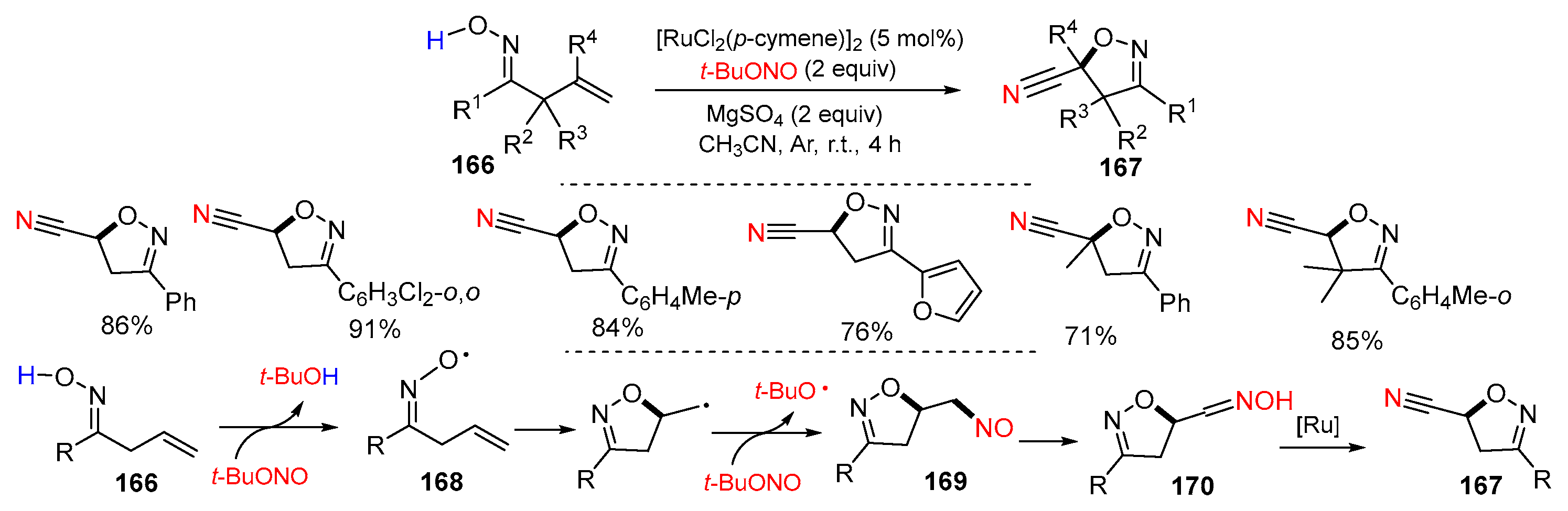
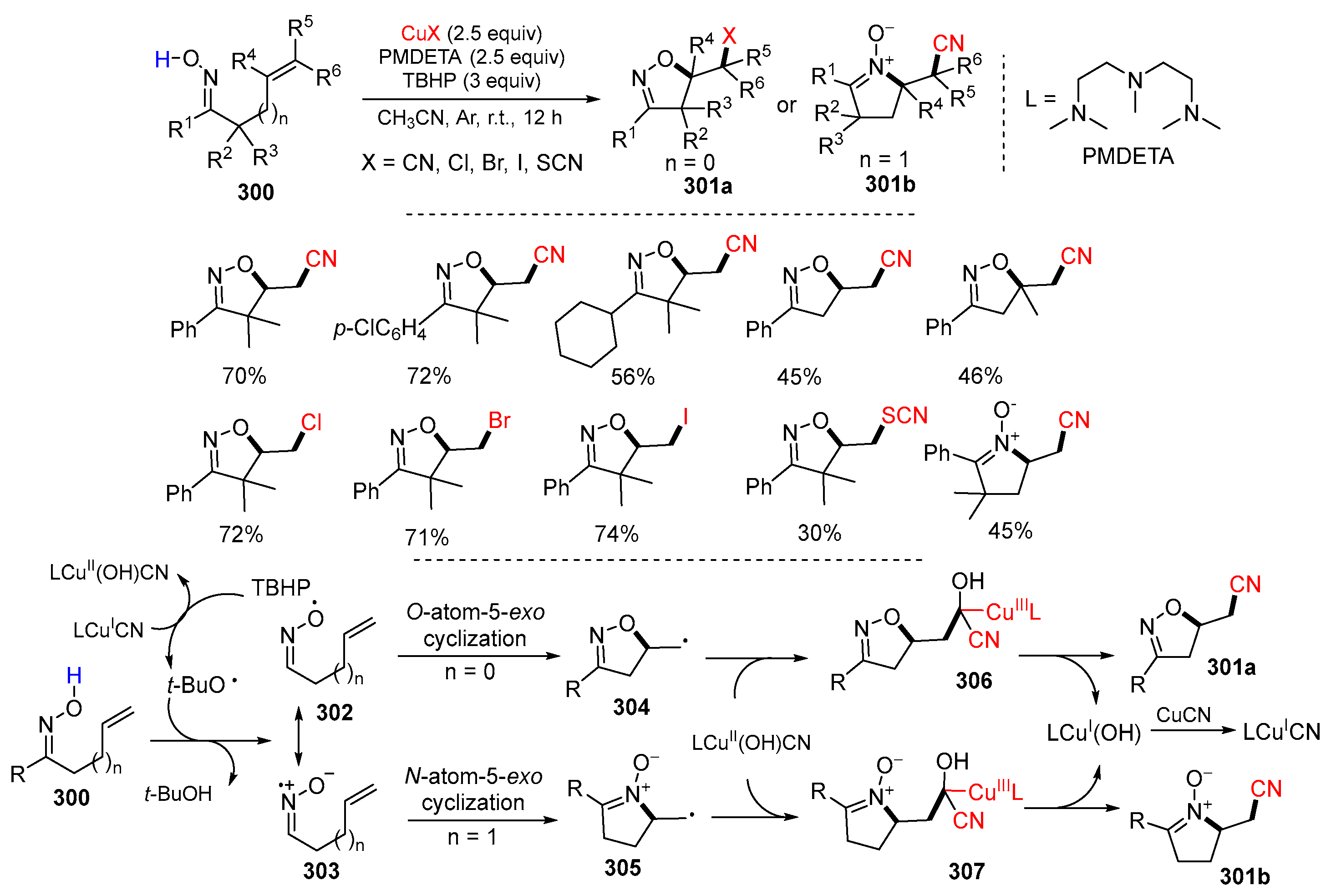
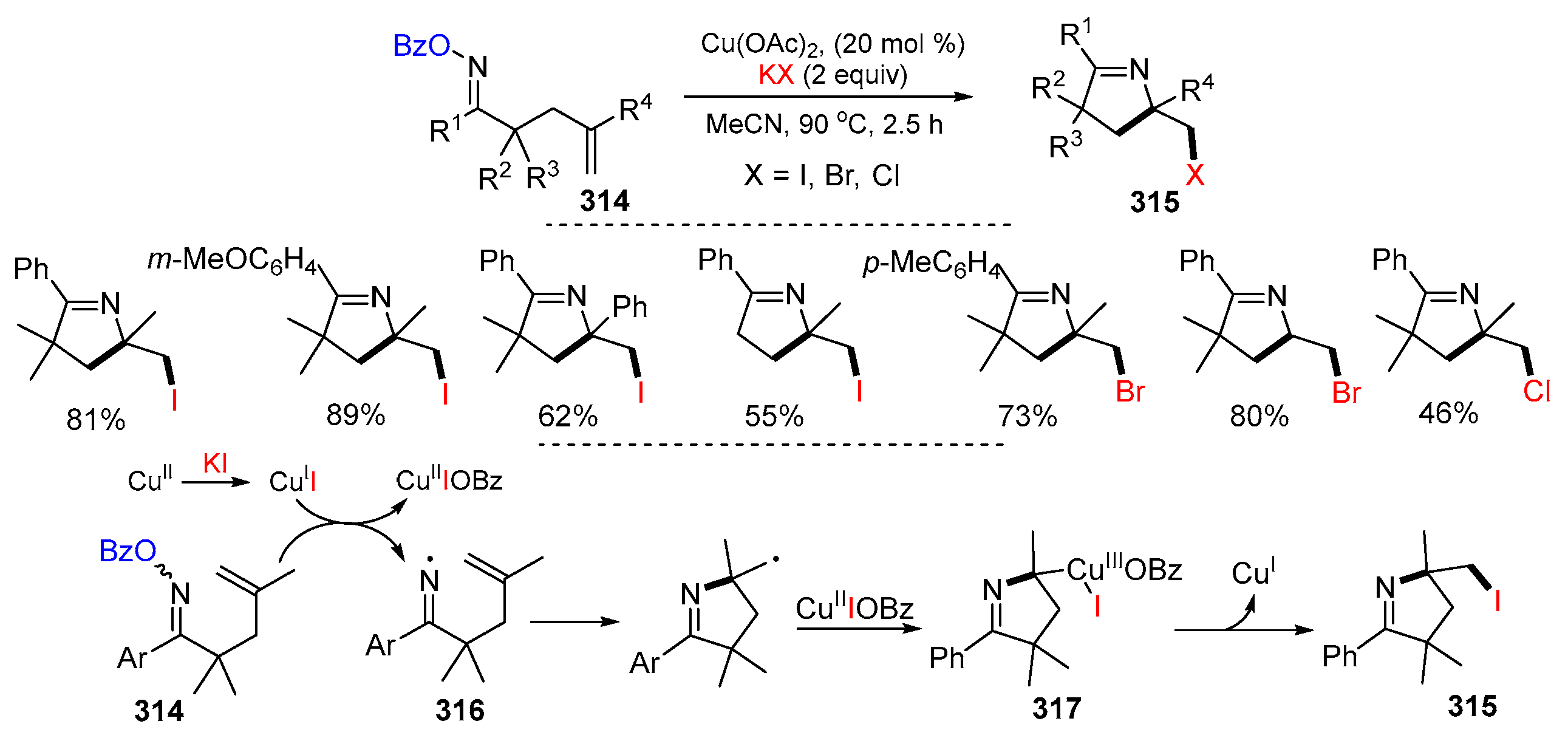
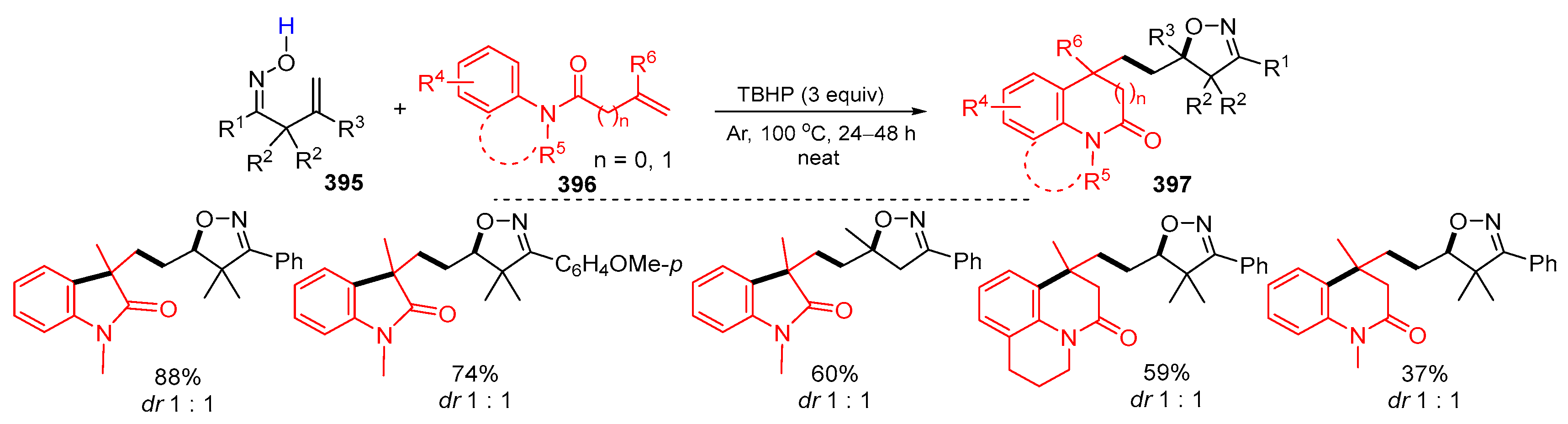
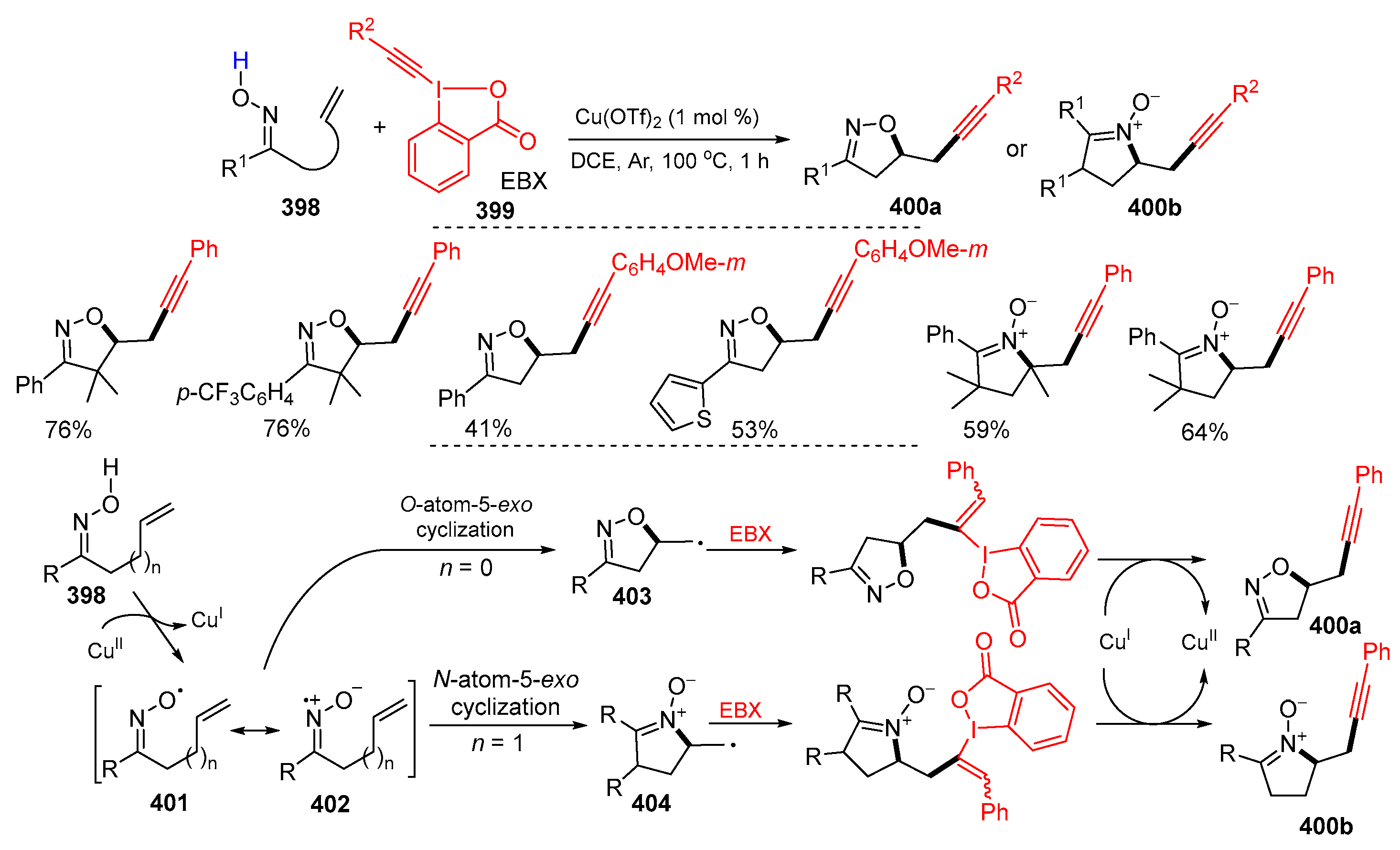
3.1. Alkenyl Oximes as Substrates
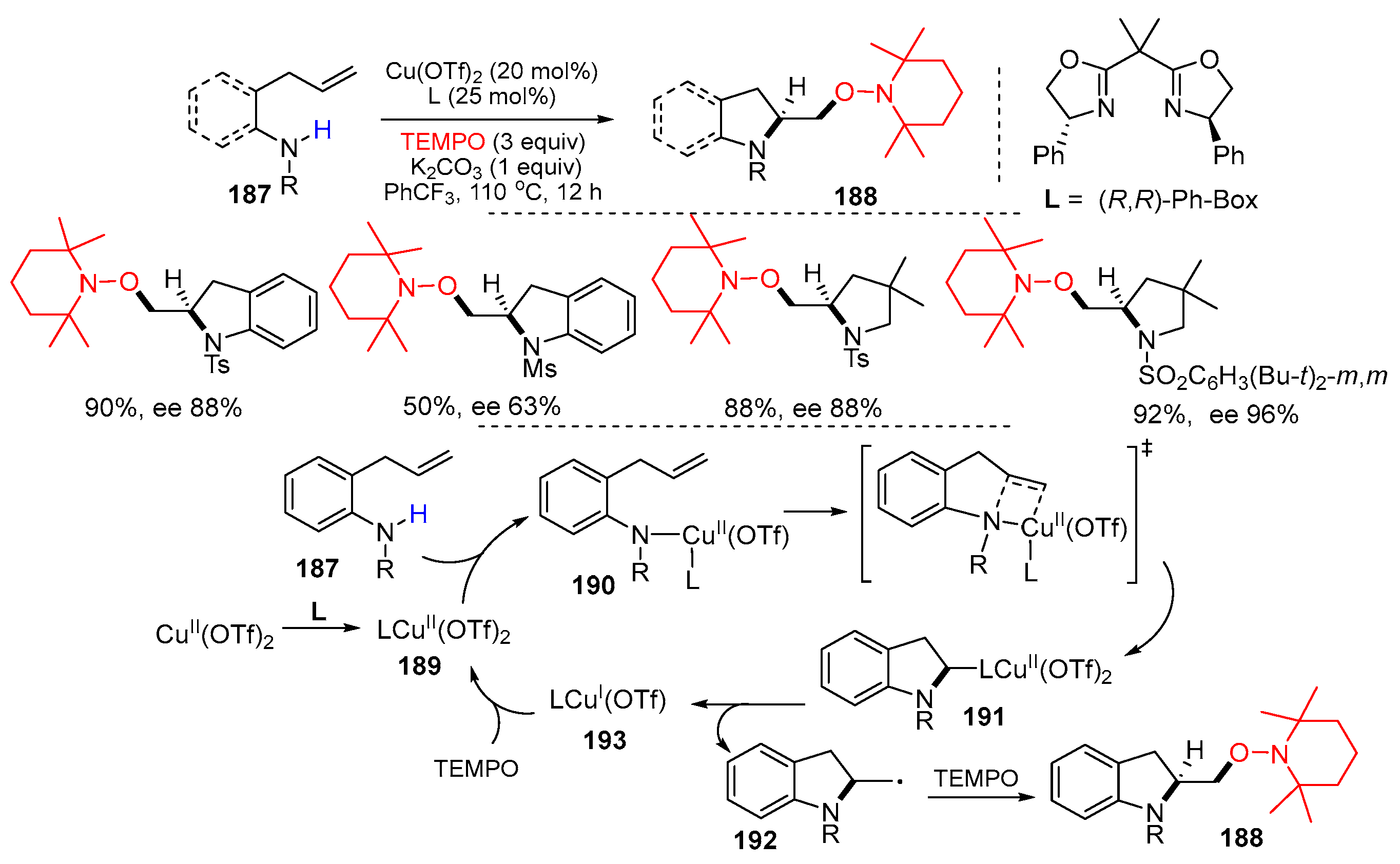
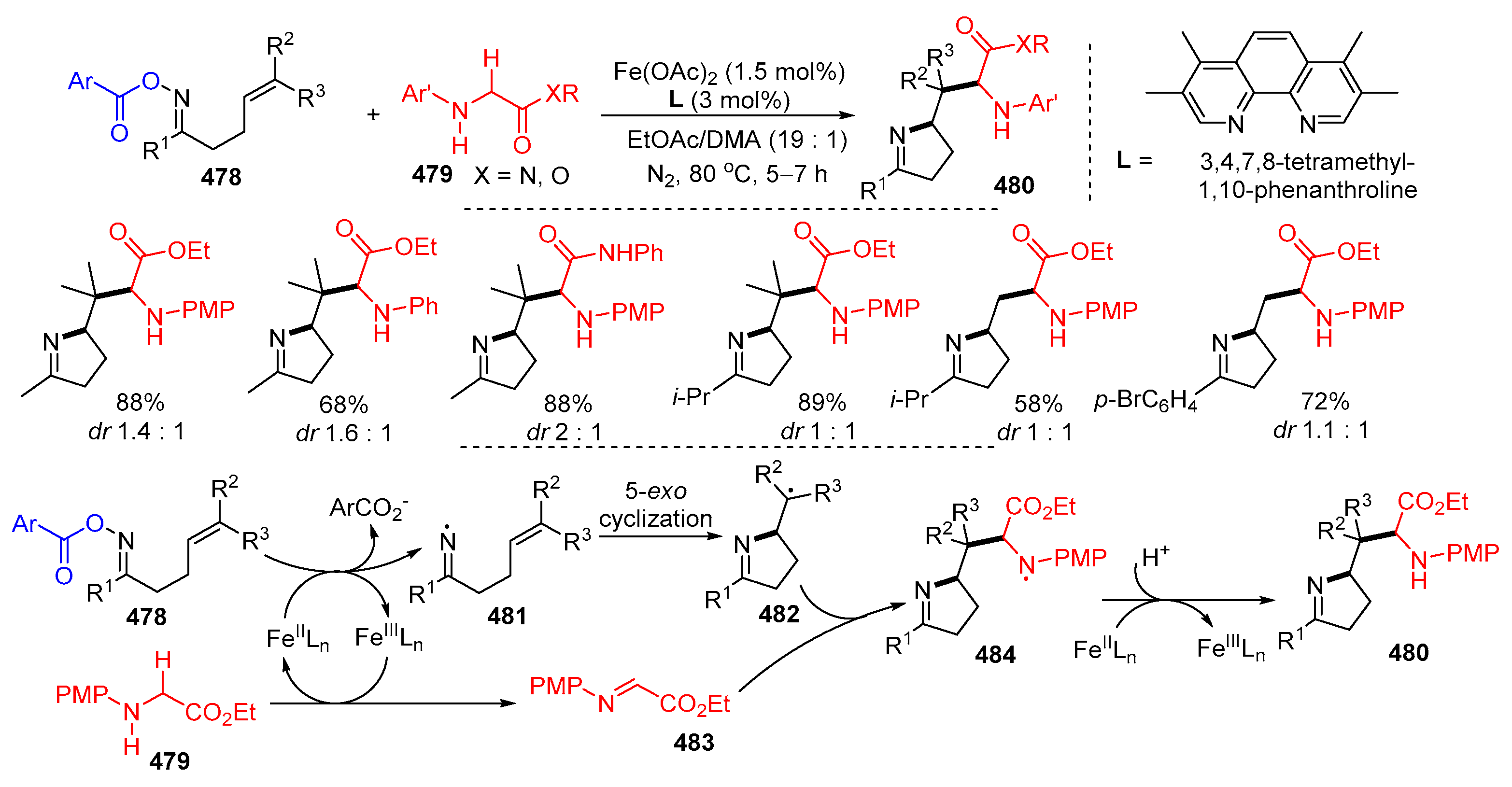
3.2. Amino-Substituted Alkenes, Alkynes, and Allenes as Substrates
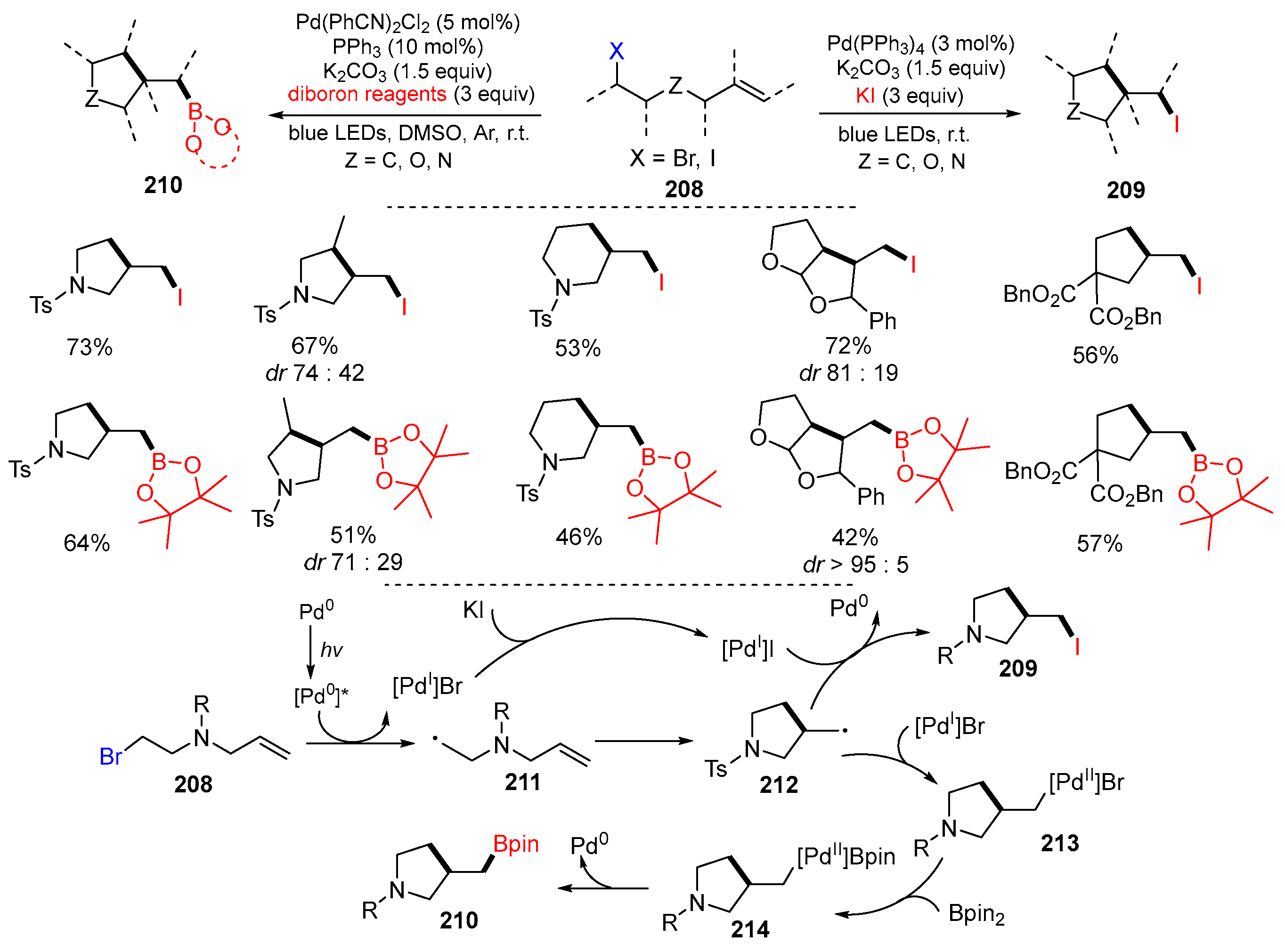
3.3. Halo-Substituted Alkenes, Alkynes and Allenes as Substrates
3.4. Other Functionalized Alkenes and Allenes as Substrates
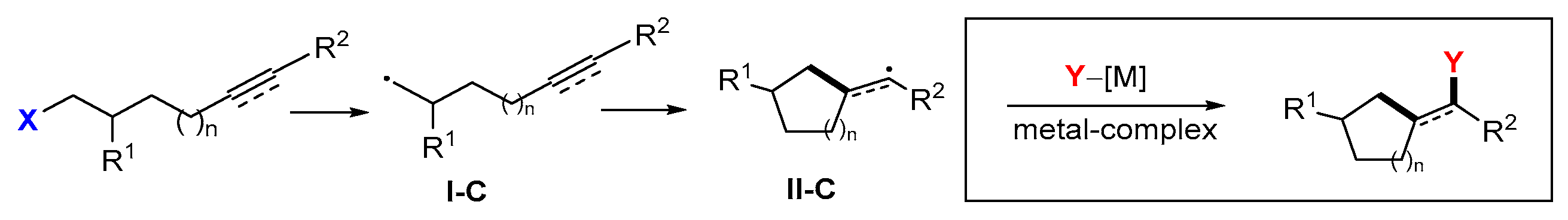
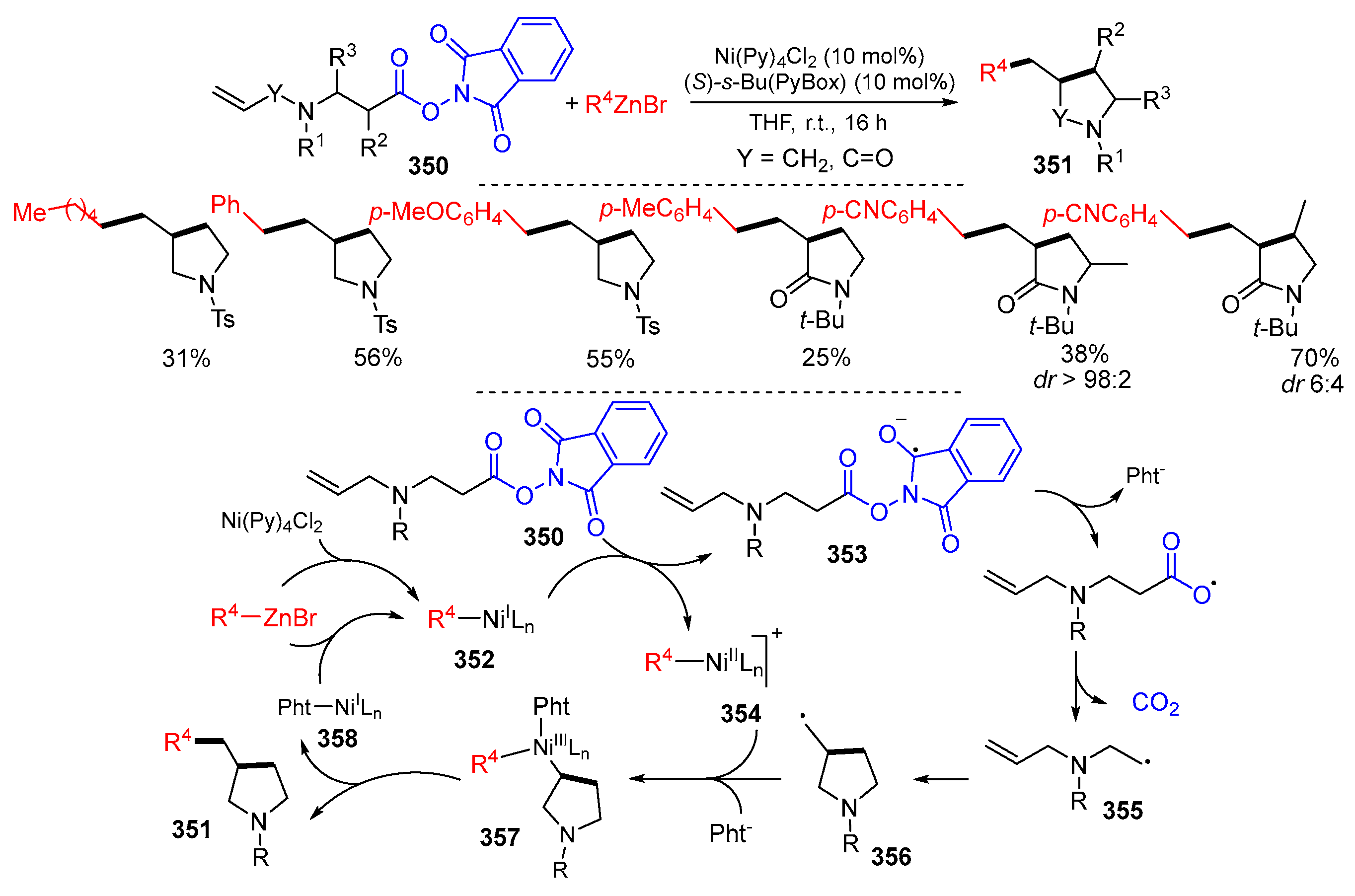
4. Second Functionalization with Metal Complexes
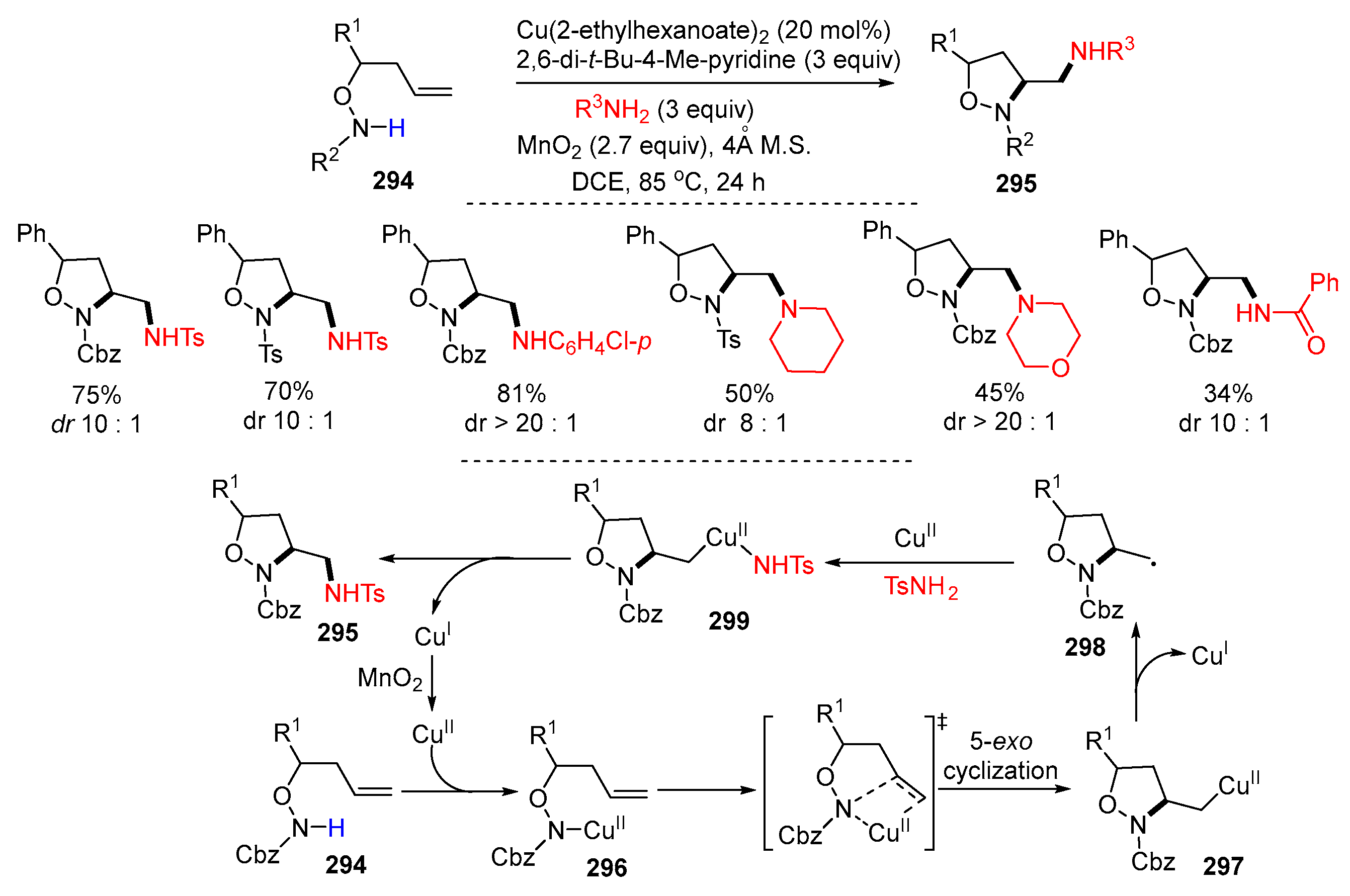
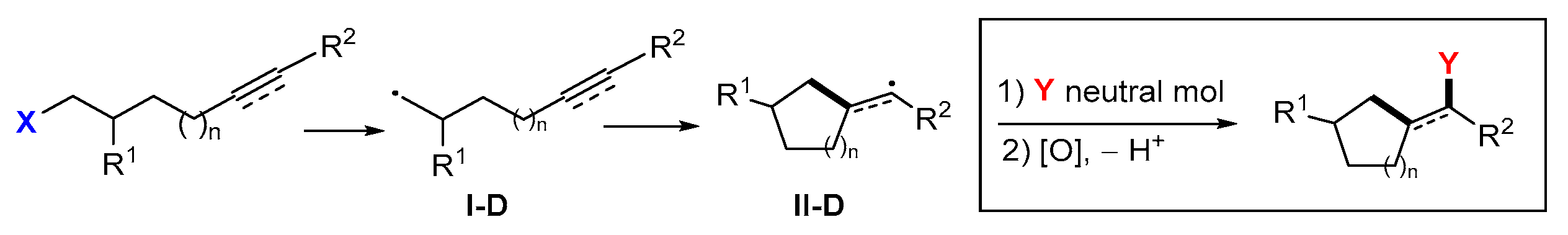
5. Second Functionalization with Y of Neutral Molecules
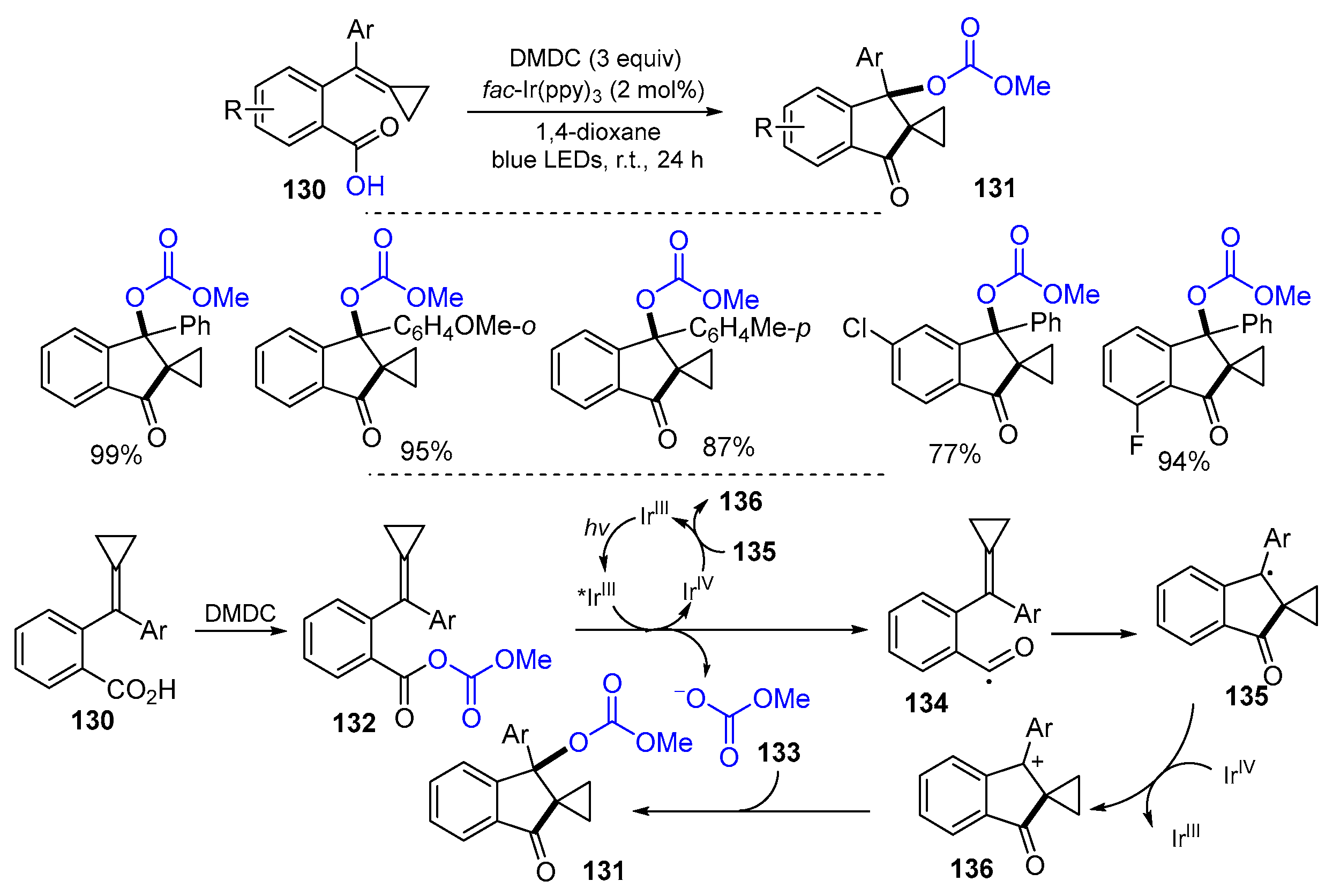
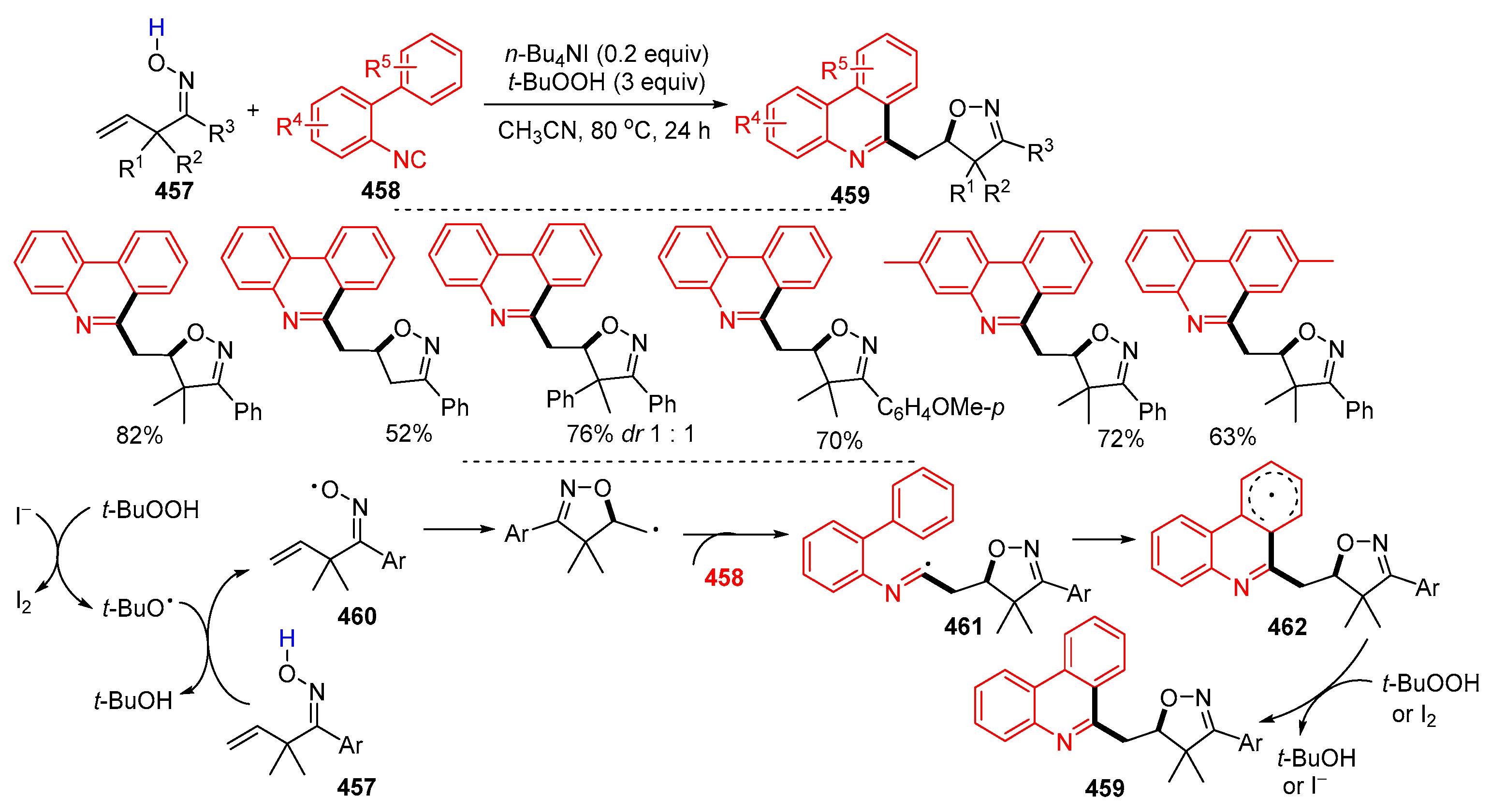
5.1. Alkenes, Alkynes and Arenes as Y
5.2. O2 as Y
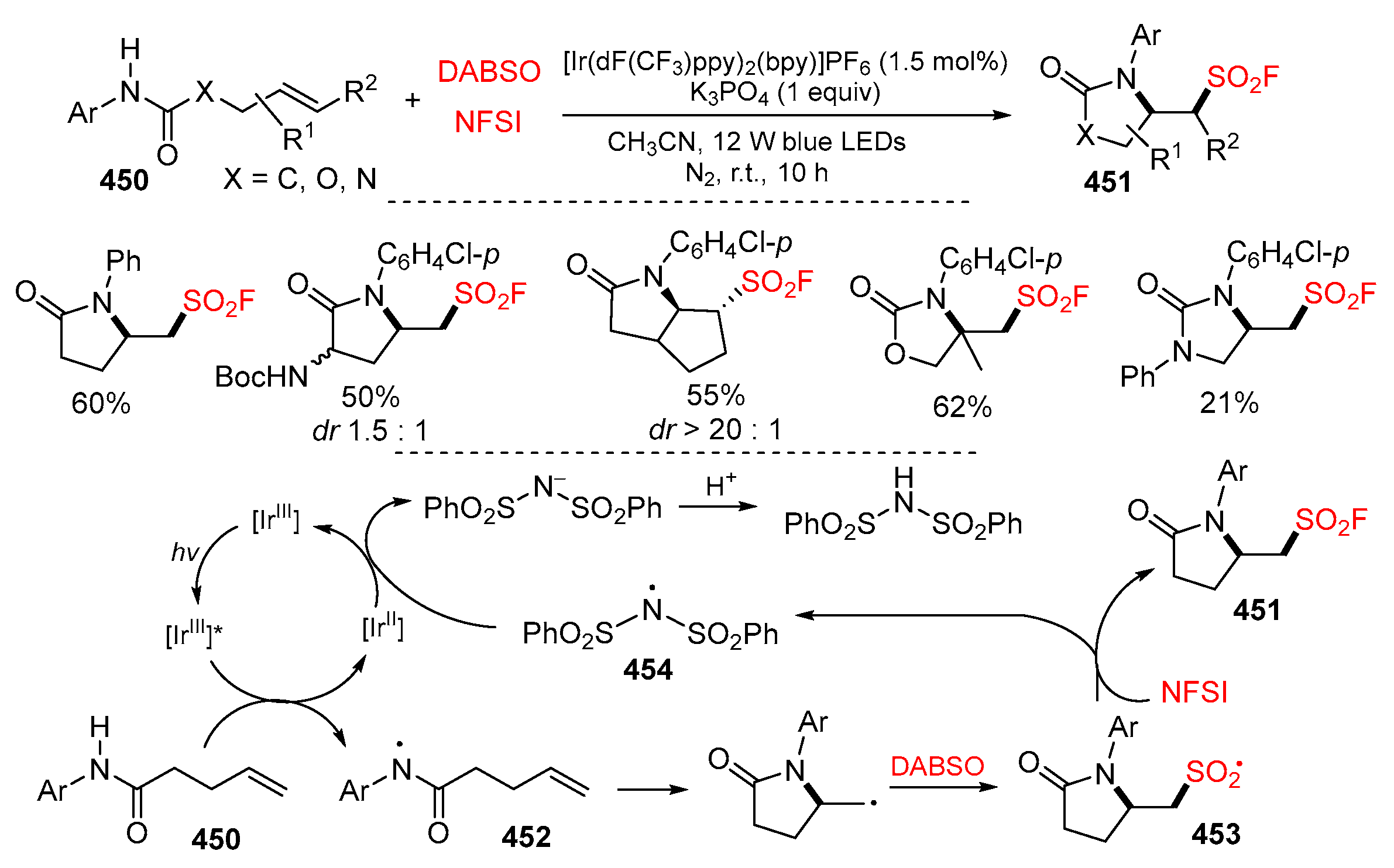
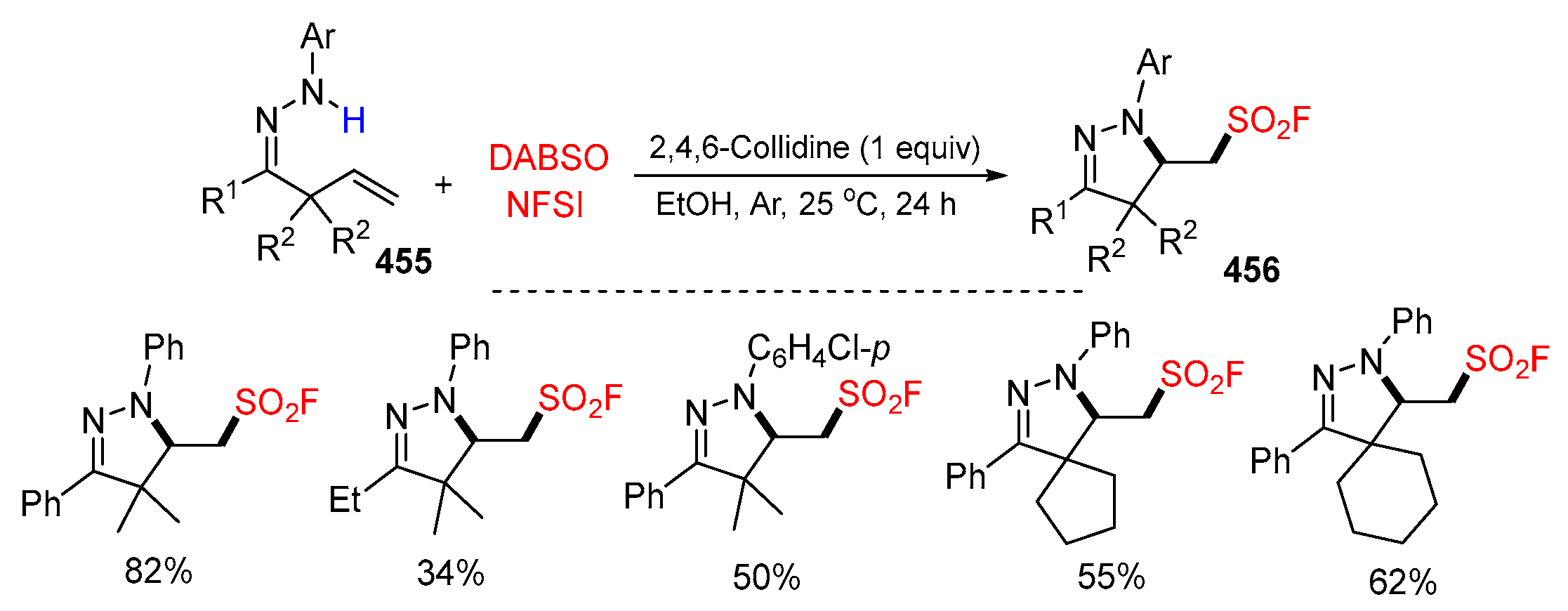
5.3. SO2 from DABSO as Y
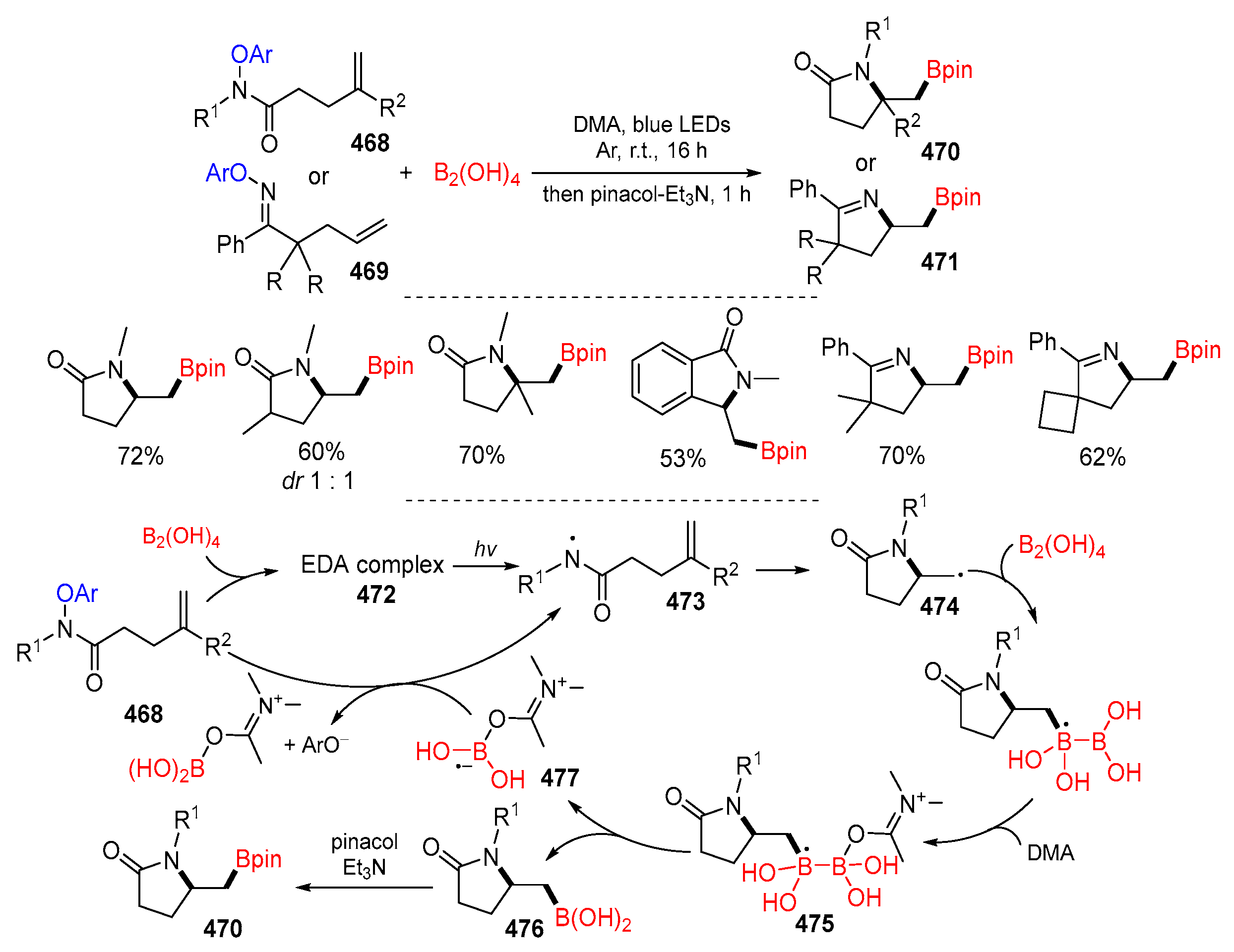
5.4. Y from Other Neutral Molecules
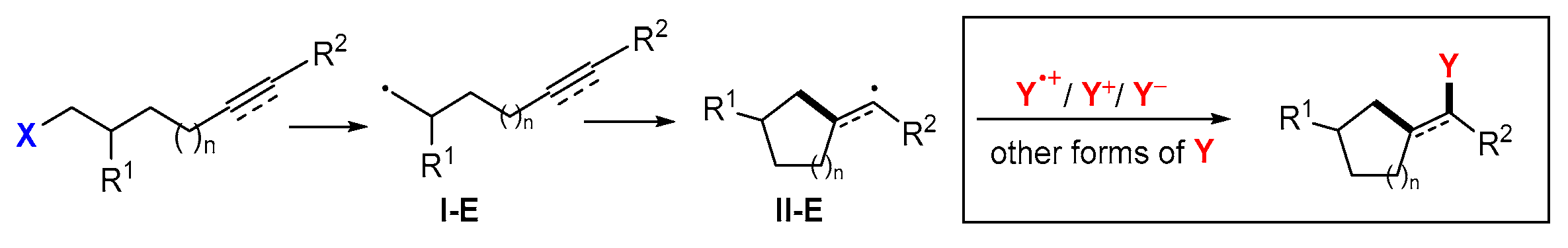
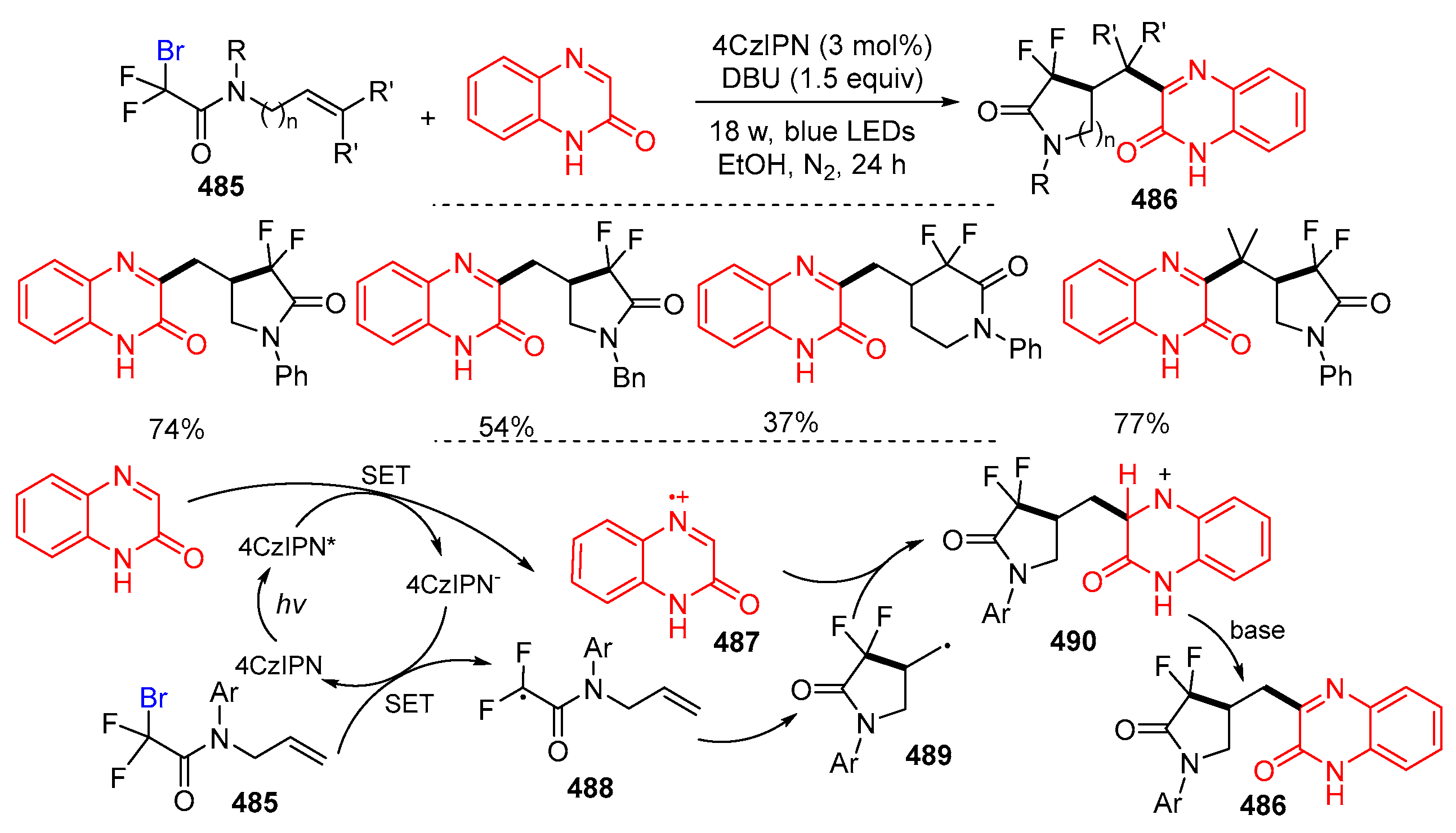
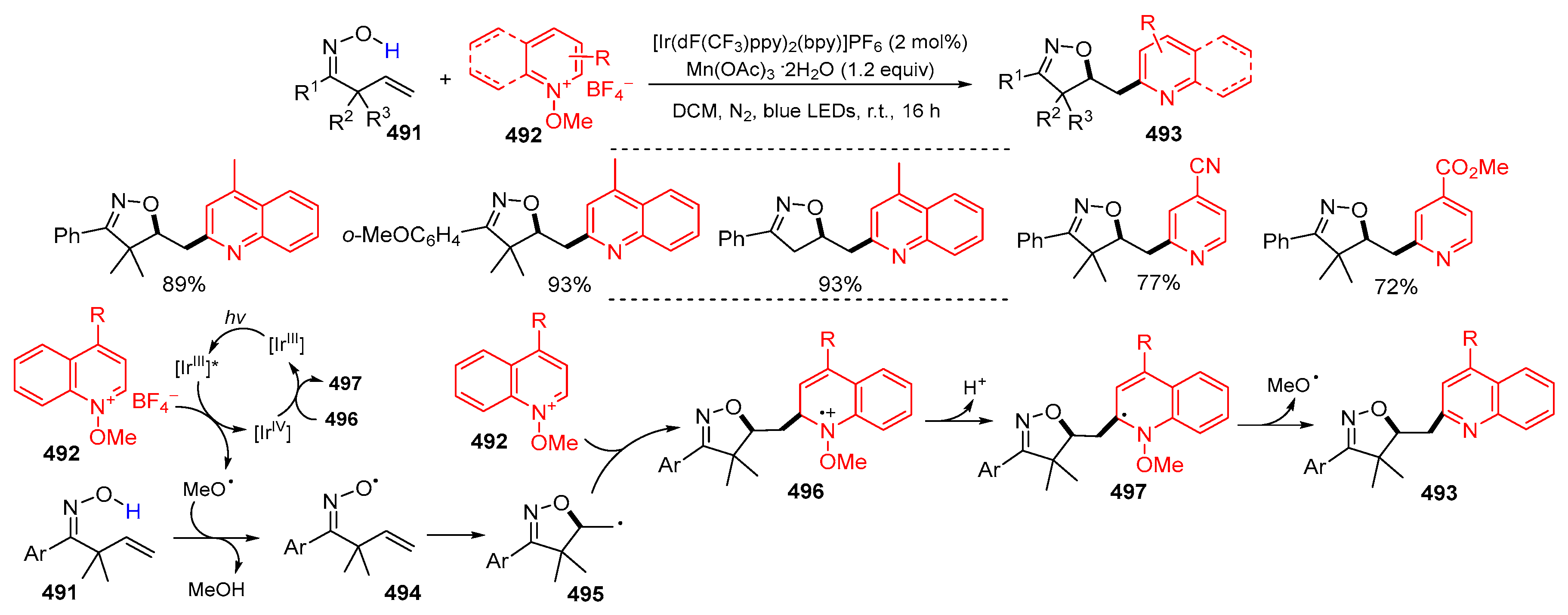
6. Second Functionalization with Other Forms of Y (Y•+, Y+, and Y−)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VC | ascorbic acid |
| ATRC | atom-transfer radical cyclization |
| BNPA | 1,1-binaphthyl-2,2-diyl hydrogen-phosphate |
| TBHP | tert-butyl hydroperoxide |
| TBN | tert-butyl nitrite |
| DTBP | di-tert-butyl peroxide |
| NCS | N-chlorosuccinimide |
| DABSO | 1,4-diazoniabicyclo[2.2.2]-octane-1,4-disulfinate |
| DLP | dilauroyl peroxide |
| DMA | dimethylacetamide |
| EDA | electron donor acceptor |
| NFSI | N-fluorobenzenesulfonimide |
| NHC | N-heterocyclic carbene |
| HFIP | hexafluoro-iso-propanol |
| DMDC | methyl dicarbonate |
| PMDETA | pentamethyldiethylenetriamine |
| 1,10-Phen | 1,10-phenanthroline |
| PCET | proton-coupled electron transfer |
| RVC | reticulated vitreous carbon |
| SET | single electron transfer |
| SED | super electron donor |
| 4CzIPN | 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile |
| TMEDA | tetramethylethylenediamine |
| TEMPO | (2,2,6,6-tetramethylpiperidin-1-yl)-oxy |
| TCCA | trichloroisocyanuric acid |
| Me6TREN | tris [2-(dimethyl-amino)ethyl]amine |
| TPMA | tris(2-pyridylmethyl)amine |
References
- Chatgilialoglu, C.; Studer, A. (Eds.) Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Chichester, UK, 2012. [Google Scholar]
- Zarf, S.Z. Radical Reactions in Organic Synthesis; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Si, Y.-F.; Lv, Q.-Y.; Yu, B. Radical cascade reactions of β,γ-unsaturated hydrazones/oximes. Adv. Synth. Catal. 2021, 363, 4640–4666. [Google Scholar] [CrossRef]
- Coussanes, G.; Vila, X.; Diaba, F.; Bonjoch, J. Radical cyclization of trichloroacetamides: Synthesis of lactams. Synthesis 2017, 49, 1481–1499. [Google Scholar] [CrossRef]
- Huang, M.-H.; Hao, W.-J.; Jiang, B. Recent advances in radical-enabled bicyclization and annulation/1,n-bifunctionalization reactions. Chem. Asian J. 2018, 13, 2958–2977. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.-Q.; Tian, B.-L.; Yang, H.; Wei, Z.-H.; Yang, S.-H.; Zhou, M.-Y.; Ding, C.-Z.; Wang, Y.-L.; Gao, J.-H.; Wang, S.-J.; et al. Visible light induced palladium-catalyzed reactions involving halogenated hydrocarbon (RX). Mol. Catal. 2023, 541, 113073. [Google Scholar] [CrossRef]
- Bag, D.; Koura, H.; Sawant, S.D. Photo-induced 1,2-carbohalofunctionalization of C–C multiple bonds via ATRA pathway. Org. Biomol. Chem. 2020, 18, 8278–8293. [Google Scholar] [CrossRef] [PubMed]
- Leitch, J.A.; Browne, D.L. Mechanoredox chemistry as an emerging strategy in synthesis. Chem. Eur. J. 2021, 27, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Tian, Y.; Wu, J.; Yan, Q.; Zheng, L.; Ren, Y.; Zhao, J.; Li, Z. Iron-promoted free radical cascade difunctionalization of unsaturated benzamides with silanes. Chem. Commun. 2020, 56, 12656–12659. [Google Scholar] [CrossRef] [PubMed]
- Bag, D.; Mahajan, S.; Sawant, S.D. Transition-metal-catalyzed carbohalogenative 1,2-difunctionalization of C−C multiple bonds. Adv. Synth. Catal. 2020, 362, 3948–3970. [Google Scholar] [CrossRef]
- Clark, A.J. Copper catalyzed atom transfer radical cyclization reactions. Eur. J. Org. Chem. 2016, 2016, 2231–2243. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Li, J.; Xiao, J.; Dong, Y.; Liu, H. Recent advances on palladium radical involved reactions. ACS Catal. 2015, 5, 6111–6137. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Xiao, Y.-T.; Yang, Y.-Z.; Song, R.-J.; Li, J.-H. Recent advances in silver-mediated radical difunctionalization of alkenes. ChemCatChem 2020, 12, 5312–5329. [Google Scholar] [CrossRef]
- Siu, J.C.; Fu, N.K.; Lin, S. Catalyzing electrosynthesis: A homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 2020, 53, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Fang, G.C.; Gu, Q.S.; Liu, X.Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020, 49, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.-W.; Wang, N.-X.; Xing, Y. Recent advances in radical difunctionalization of simple alkenes. Eur. J. Org. Chem. 2017, 2017, 5821–5851. [Google Scholar] [CrossRef]
- Bao, X.Z.; Li, J.; Jiang, W.; Huo, C.D. Radical-mediated difunctionalization of styrenes. Synthesis 2019, 51, 4507–4530. [Google Scholar] [CrossRef]
- Lin, J.; Song, R.J.; Hu, M.; Li, J.H. Recent advances in the intermolecular oxidative difunctionalization of alkenes. Chem. Rec. 2019, 19, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, G.; Zhao, Y.F. Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 589–596. [Google Scholar] [CrossRef]
- Koike, T.; Akita, M. A versatile strategy for difunctionalization of carbon–carbon multiple bonds by photoredox catalysis. Org. Chem. Front. 2016, 3, 1345–1349. [Google Scholar] [CrossRef]
- Besset, T.; Poisson, T.; Pannecoucke, X. Direct vicinal difunctionalization of alkynes: An efficient approach towards the synthesis of highly functionalized fluorinated alkenes. Eur. J. Org. Chem. 2015, 2015, 2765–2789. [Google Scholar] [CrossRef]
- Coppola, G.A.; Pillitteri, S.; Van der Eycken, E.V.; You, S.-L.; Sharma, U.K. Multicomponent reactions and photo/electrochemistry join forces: Atom economy meets energy efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hu, W.; Zhang, W. Difunctionalization of alkenes and alkynes via intermolecular radical and nucleophilic additions. Molecules 2021, 26, 105. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Q.; Zhang, W. Remote radical 1,3-, 1,4-, 1,5-, 1,6-and 1,7-difunctionalization reactions. Molecules 2023, 28, 3027. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Yao, H.; Zhang, W. Difunctionalization of dienes, enynes and related compounds via sequential radical addition and cyclization reactions. Molecules 2023, 28, 1145. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cornella, J.; Juliá-Hernández, F.; Martin, R. Visible-light-promoted atom transfer radical cyclization of unactivated alkyl iodides. ACS Catal. 2017, 7, 409–412. [Google Scholar] [CrossRef]
- Weng, W.-Z.; Liang, H.; Liu, R.-Z.; Ji, Y.-X.; Zhang, B. Visible-light-promoted manganese-catalyzed atom transfer radical cyclization of unactivated alkyl iodides. Org. Lett. 2019, 21, 5586–5590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Guo, H. Light-induced intramolecular iodine-atom transfer radical addition of alkyne: An approach from aryl iodide to alkenyl iodide. Org. Lett. 2019, 21, 9133–9137. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Fang, X.; Wang, Z.; Liu, K.; Li, C. Stereoselectivity of 6-exo cyclization of α-carbamoyl radicals. J. Org. Chem. 2016, 81, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, F.; Schumacher, C.; Wotruba, H.; Hernández, J.G.; Bolm, C. The use of copper and vanadium mineral ores in catalyzed mechanochemical carbon–carbon bond formations. ACS Sustain. Chem. Eng. 2020, 8, 7262–7266. [Google Scholar] [CrossRef]
- Schumacher, C.; Hernández, J.G.; Bolm, C. Electro-mechanochemical atom transfer radical cyclizations using piezoelectric BaTiO3. Angew. Chem. Int. Ed. 2020, 59, 16357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chin, M.; Fu, Y.; Liu, P.; Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 2021, 374, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Nakashima, Y.; Hirata, G.; Nishikata, T. Atom-transfer radical cyclization of α-bromocarboxamides under organophotocatalytic conditions. Tetrahedron Lett. 2021, 69, 152952. [Google Scholar] [CrossRef]
- Clark, A.J.; Collis, A.E.C.; Fox, D.J.; Halliwell, L.L.; James, N.; O’Reilly, R.K.; Parekh, H.; Ross, A.; Sellars, A.B.; Willcock, H.; et al. Atom-transfer cyclization with CuSO4/KBH4: A formal “activators generated by electron transfer” process also applicable to atom-transfer polymerization. J. Org. Chem. 2012, 77, 6778–6788. [Google Scholar] [CrossRef] [PubMed]
- Lubskyy, A.; Guo, C.; Chadwick, R.J.; Petri-Fink, A.; Bruns, N.; Pellizzoni, M.M. Engineered myoglobin as a catalyst for atom transfer radical cyclisation. Chem. Commun. 2022, 58, 10989–10992. [Google Scholar] [CrossRef] [PubMed]
- Diaba, F.; Martínez-Laporta, A.; Bonjoch, J. Chlorine atom transfer radical 6-exo cyclizations of carbamoyldichloroacetate-tethered alkenes, enol acetates and α,β-unsaturated nitriles leading to morphans. Eur. J. Org. Chem. 2014, 2014, 2371–2378. [Google Scholar] [CrossRef]
- Clark, A.J.; Cornia, A.; Felluga, F.; Gennaro, A.; Ghelfi, F.; Isse, A.A.; Menziani, M.C.; Muniz-Miranda, F.; Roncaglia, F.; Spinelli, D. Arylsulfonyl groups: The best cyclization auxiliaries for the preparation of ATRC γ-lactams can be acidolytically removed. Eur. J. Org. Chem. 2014, 2014, 6734–6745. [Google Scholar] [CrossRef]
- Isse, A.A.; Visonà, G.; Ghelfi, F.; Roncaglia, F.; Gennaro, A. Electrochemical approach to copper-catalyzed reversed atom transfer radical cyclization. Adv. Synth. Catal. 2015, 357, 782–792. [Google Scholar] [CrossRef]
- Ram, R.N.; Soni, V.K. Copper(I)-catalyzed synthesis of chlorinated tetrahydropyridazin-3-ones and 1,2-diazepan-3-ones from N-allyl-N′-trichloroacetylhydrazines. Adv. Synth. Catal. 2016, 358, 276–282. [Google Scholar] [CrossRef]
- Ram, R.N.; Gupta, D.K. Direct and stereoselective synthesis of diversely substituted 2,4-trans-(NH)-pyrrolidines by copper(I)-catalyzed radical cyclization at low temperature. Adv. Synth. Catal. 2016, 358, 3254–3264. [Google Scholar] [CrossRef]
- Hou, L.; Zhou, Z.; Wang, D.; Zhang, Y.; Chen, X.; Zhou, L.; Hong, Y.; Liu, W.; Hou, Y.; Tong, X. DPPF-catalyzed atom-transfer radical cyclization via allylic radical. Org. Lett. 2017, 19, 6328–6331. [Google Scholar] [CrossRef]
- Sadanandan, S.; Gupta, D.K. Changing stereoselectivity and regioselectivity in copper(I)-catalyzed 5-exo cyclization by chelation and rigidity in aminoalkyl radicals: Synthesis towards diverse bioactive N-heterocycles. New J. Chem. 2020, 44, 3350–3365. [Google Scholar] [CrossRef]
- Ram, R.N.; Sadanandan, S.; Gupta, D.K. β,β,β-Trichloroethyl-NH-enamine as viable system for 5-endo-trig radical cyclization via multifaceted CuI−CuII redox catalysis: Single step synthesis of multi-functionalized NH-pyrroles. Adv. Synth. Catal. 2019, 361, 5661–5676. [Google Scholar] [CrossRef]
- Yang, X.; Yu, W. Promoting effect of water on light and phenanthroline-diphosphine Cu(I) complex-initiated iodine atom transfer cyclisation. Chem. Commun. 2022, 58, 11693–11696. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Okita, Y.; Morita, T.; Yoshimi, Y. An approach to the synthesis of naphtho[b]furans from allyl bromonaphthyl ethers employing sequential photoinduced radical cyclization and dehydrohalogenation reactions. Tetrahedron Lett. 2014, 55, 3355–3357. [Google Scholar] [CrossRef]
- Ram, R.N.; Gupta, D.K.; Soni, V.K. Copper(I)/ligand-catalyzed 5-endo radical cyclization-aromatization of 2,2,2-trichloroethyl vinyl ethers: Synthesis of 2,3-difunctionalized 4-chlorofurans. J. Org. Chem. 2016, 81, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Tittal, R.K. Synthesis and molecular crystal of 3-chloro-2-(1-chloro-1-methyl-ethyl)-2,3-dihydro-1H-naphtho [2,1-b]oxepin-4-one. J. Mol. Struct. 2018, 1156, 621–626. [Google Scholar] [CrossRef]
- Roncaglia, F.; Ghelfi, F.; Felluga, F.; Poppi, V. Simultaneous deprotection–oxidation of cyclic hemiacetals: A fine ending for a Ueno–Stork ATRC to dichloro-γ-lactones. Tetrahedron Lett. 2014, 55, 2865–2868. [Google Scholar] [CrossRef]
- Ram, R.N.; Gupta, D.K.; Soni, V.K. Copper(I)-promoted synthesis of highly substituted and functionalized tetrahydrothiophenes. Eur. J. Org. Chem. 2016, 2016, 3434–3440. [Google Scholar] [CrossRef]
- Ram, R.N.; Kumar, N.; Gupta, D.K. Substrate-controlled diastereoselective synthesis of sugar-based chlorinated perhydrofuro [2,3-b]pyrans via copper(I)-catalyzed radical cyclization. Adv. Synth. Catal. 2017, 359, 432–442. [Google Scholar] [CrossRef]
- Ratushnyy, M.; Parasram, M.; Wang, Y.; Gevorgyan, V. Palladium-catalyzed atom-transfer radical cyclization at remote unactivated C(sp3)−H sites: Hydrogen-atom transfer of hybrid vinyl palladium radical intermediates. Angew. Chem. Int. Ed. 2018, 57, 2712–2715. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.; Mathi, G.R.; Kima, I.; Hong, S. Visible-light-induced cascade radical ring-closure and pyridylation for the synthesis of tetrahydrofurans. Green Chem. 2019, 21, 2082–2087. [Google Scholar] [CrossRef]
- Zidan, M.; McCallum, T.; Swann, R.; Barriault, L. Formal bromine atom transfer radical addition of nonactivated bromoalkanes using photoredox gold catalysis. Org. Lett. 2020, 22, 8401–8406. [Google Scholar] [CrossRef] [PubMed]
- Dawra, N.; Ram, R.N.; Thakur, S.K. Synthesis of chlorinated, heteroatom-rich and differently fused tricyclic β-lactams by Cu(I)-catalyzed halogen atom transfer radical cyclization. Chem. Sel. 2020, 5, 6204–6215. [Google Scholar] [CrossRef]
- Wei, H.-Z.; Wei, Y.; Shi, M. Intramolecular difunctionalization of methylenecyclopropanes tethered with carboxylic acid by visible-light photoredox catalysis. Org. Chem. Front. 2021, 8, 4527–4532. [Google Scholar] [CrossRef]
- Klychnikov, M.K.; Pohl, R.; Císařová, I.; Jahn, U. α,γ-Dioxygenated amides via tandem Brook rearrangement / radical oxygenation reactions and their application to syntheses of γ-lactams. Beilstein J. Org. Chem. 2021, 17, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.D.; Durant, A.G.; Reid, C.M.; Jans, C.; Arora, R.; Lautens, M. Pd(0)/blue light promoted carboiodination reaction-evidence for reversible C–I bond formation via a radical pathway. J. Am. Chem. Soc. 2022, 144, 20554–20560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, H.; Liu, Y.; Wang, H.; Yan, Q.; Wang, W.; Chen, F. Visible-light-promoted xanthate-transfer cyclization reactions of unactivated olefins under photocatalyst- and additive-free conditions. J. Org. Chem. 2022, 87, 15582–15597. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, J.; Wu, S.; Yu, W. Copper-mediated bromine atom transfer radical cyclisation of unactivated alkyl bromides. Chem. Commun. 2023, 59, 8993–8996. [Google Scholar] [CrossRef]
- Peng, X.-X.; Deng, Y.-J.; Yang, X.-L.; Zhang, L.; Yu, W.; Han, B. Iminoxyl radical-promoted dichotomous cyclizations: Efficient oxyoximation and aminooximation of alkenes. Org. Lett. 2014, 16, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-J.; Chen, B.-Y.; Wang, Y.-Q.; Zhang, X.-W. Ruthenium-catalyzed direct transformation of alkenyl oximes to 5-cyanated isoxazolines: A cascade approach based on non-stabilized radical intermediate. Eur. J. Org. Chem. 2018, 2018, 1342–1346. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Xiao, Z.-F.; Wang, M.-M.; Zhuang, Y.-J.; Kang, Y.-B. Transition-metal-free oxychlorination of alkenyl oximes: In situ generated radicals with tert-butyl nitrite. Org. Biomol. Chem. 2016, 14, 7275–7281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Su, Y.; Wang, K.-H.; Wu, L.; Chang, B.; Shi, Y.; Huang, D.; Hu, Y. Trichloroisocyanuric acid promoted cascade cyclization/trifluoromethylation of allylic oximes: Synthesis of trifluoromethylated ioxazolines. Org. Lett. 2017, 19, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, Y.; Qian, L.; Gao, Y.; Wang, X.; Yan, X.; Xu, X. General 5-halomethyl isoxazoline synthesis enabled by copper-catalyzed oxyhalogenation of alkenes. J. Org. Chem. 2019, 84, 12656–12663. [Google Scholar] [CrossRef] [PubMed]
- Paderes, M.C.; Keister, J.B.; Chemler, S.R. Mechanistic analysis and optimization of the copper-catalyzed enantioselective intramolecular alkene aminooxygenation. J. Org. Chem. 2013, 78, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Folgueiras-Amador, A.A.; Philipps, K.; Guilbaud, S.; Poelakker, J.; Wirth, T. An easy-to-machine electrochemical flow microreactor: Efficient synthesis of isoindolinone and flow functionalization. Angew. Chem. Int. Ed. 2017, 56, 15446–15450. [Google Scholar] [CrossRef]
- Azzi, E.; Ghigo, G.; Sarasino, L.; Parisotto, S.; Moro, R.; Renzi, P.; Deagostino, A. Photoinduced chloroamination cyclization cascade with N-chlorosuccinimide: From N-(allenyl)sulfonylamides to 2-(1-chlorovinyl)pyrrolidines. J. Org. Chem. 2023, 88, 6420–6433. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-W.; Chen, X.; Zhou, Z.-Z.; Liang, Y.-M. Visible-light-induced palladium-catalyzed carbocyclization of unactivated alkyl bromides with alkenes involving C–I or C–B coupling. J. Org. Chem. 2020, 85, 9301–9312. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Huang, T.; Shi, B.; Cao, W.; Yu, C.; Li, T.; Zhang, K.; Yao, C. Synthesis of 2-pyrrolidinone derivatives via N-heterocyclic carbene catalyzed radical tandem cyclization/coupling reactions. Org. Chem. Front. 2023, 10, 2695–2700. [Google Scholar] [CrossRef]
- Dong, X.; Hu, Y.; Xiao, T.; Zhou, L. Synthesis of 2-trifluoromethyl indoles via visible-light induced intramolecular radical cyclization. RSC Adv. 2015, 5, 39625–39629. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, F.; Guo, M.; Wang, G.; Tang, X.; Zhao, W. Switchable synthetic strategy toward trisubstituted and tetrasubstituted exocyclic alkenes. Org. Lett. 2018, 20, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Si, X.; Rominger, F.; Hashmi, A.S.K. Visible-light-induced radical carbo-cyclization/gem-diborylation through triplet energy transfer between a gold catalyst and aryl iodides. J. Am. Chem. Soc. 2020, 142, 10485–10493. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ding, A.; Zhang, Y.; Guo, H. Photochemical synthesis of 1,4-dicarbonyl bifluorene compounds via oxidative radical coupling using TEMPO as the oxygen atom donor. J. Org. Chem. 2021, 86, 3656–3666. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, M.; Yu, K.; Liu, C.; Liu, Z.; Duan, S.; Chen, W.; Yang, X.; Zhang, H.; Walsh, P.J. Synthesis of benzofuran derivatives through cascade radical cyclization/intermolecular coupling of 2-azaallyls. Angew. Chem. Int. Ed. 2019, 58, 2826–2830. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Li, M.; Deng, G.; Liu, C.; Wang, J.; Liu, Z.; Zhang, H.; Yang, X.; Walsh, P.J. An efficient route to isochromene derivatives via cascade radical cyclization and radical-radical coupling. Adv. Synth. Catal. 2019, 361, 4354–4359. [Google Scholar] [CrossRef]
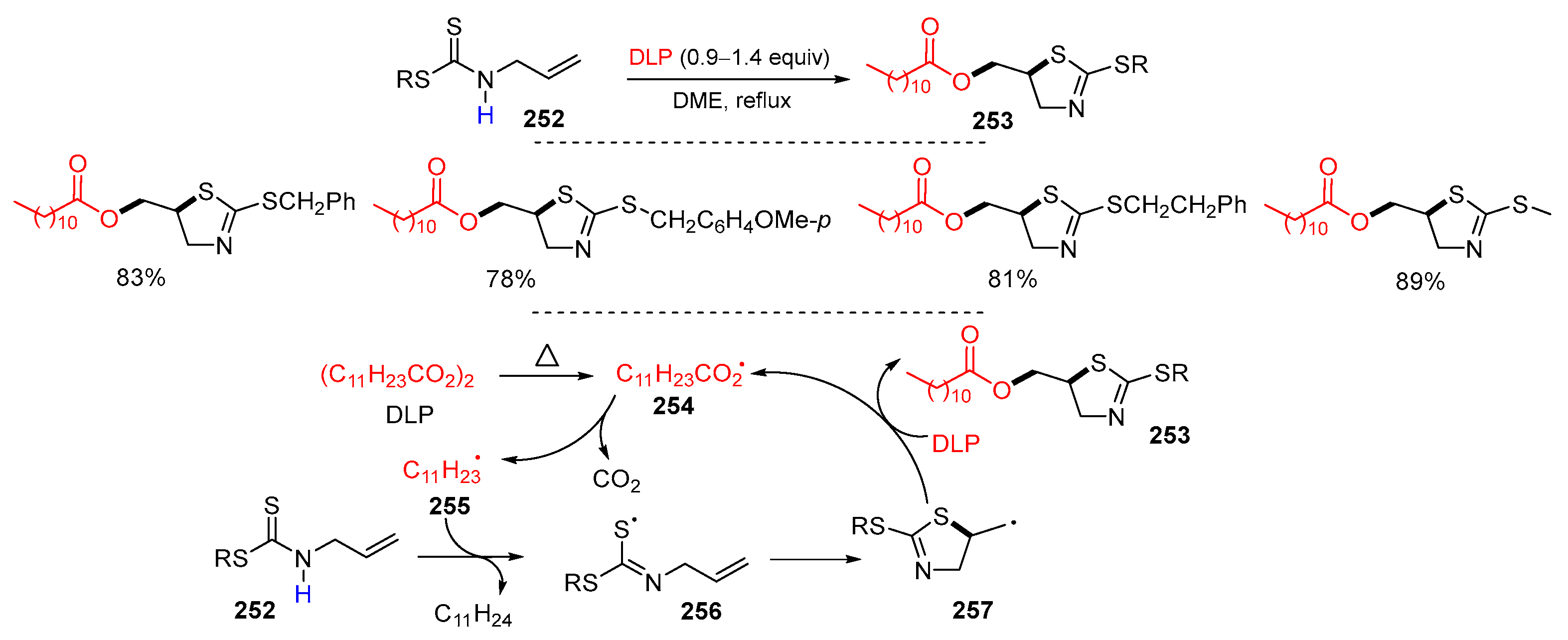
- Kakaeia, S.; Xu, J. Synthesis of (2-alkylthiothiazolin-5-yl)methyl dodecanoates via tandem radical reaction. Org. Biomol. Chem. 2013, 11, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
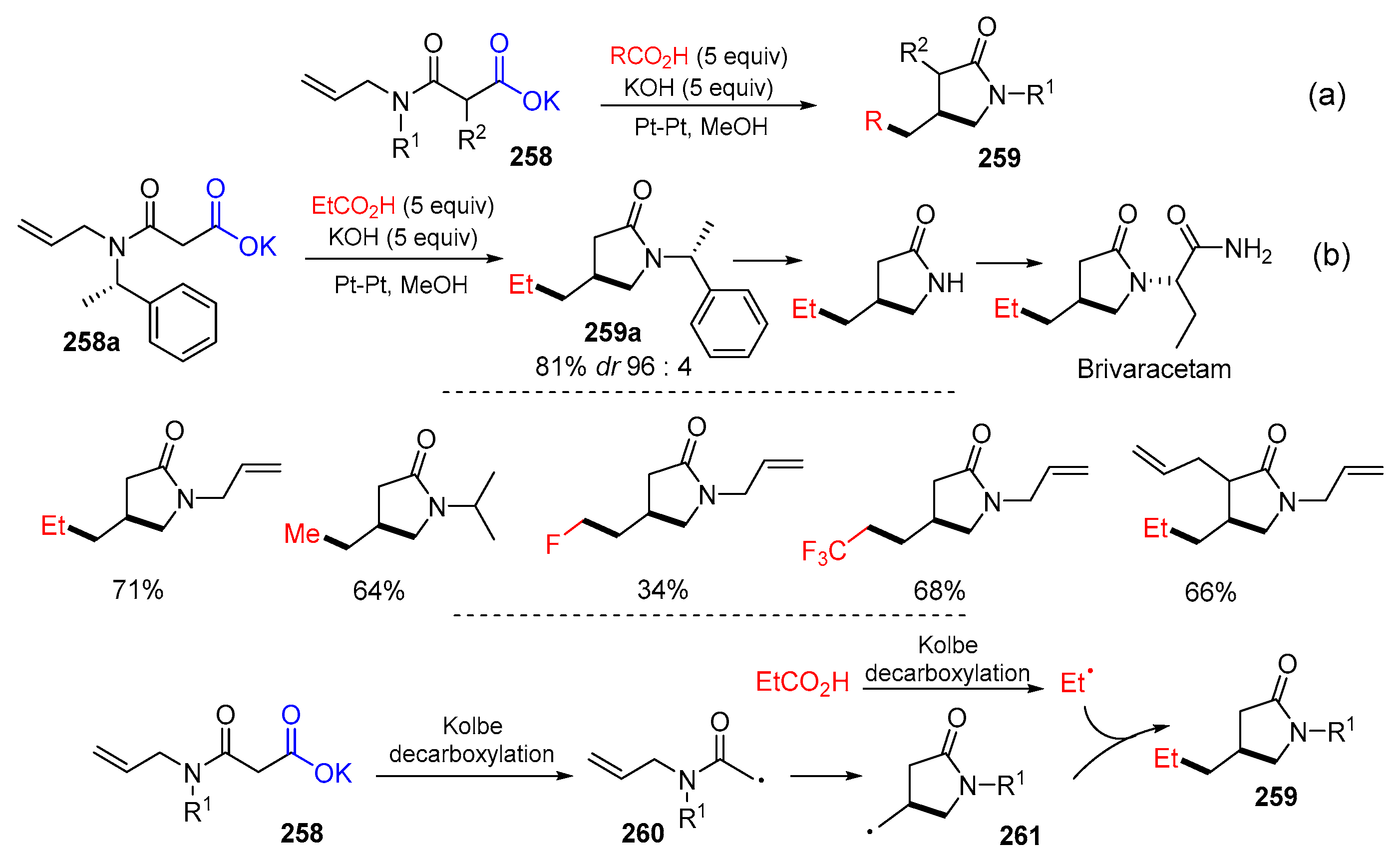
- Quertenmont, M.; Goodall, I.; Lam, K.; Markó, I.; Riant, O. Kolbe anodic decarboxylation as a green way to access 2-pyrrolidinones. Org. Lett. 2020, 22, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Quertenmont, M.; Toussaint, F.C.; Defrance, T.; Lam, K.; Markó, I.E.; Riant, O. Continuous flow electrochemical oxidative cyclization and successive functionalization of 2-pyrrolidinones. Org. Process Res. Dev. 2021, 25, 2631–2638. [Google Scholar] [CrossRef]
- Singh, J.; Nelson, T.J.; Mansfield, S.A.; Nickel, G.A.; Cai, Y.; Jones, D.D.; Small, J.E.; Ess, D.H.; Castle, S.L. Microwave- and thermally promoted iminyl radical cyclizations: A versatile method for the synthesis of functionalized pyrrolines. J. Org. Chem. 2022, 87, 16250–16262. [Google Scholar] [CrossRef]
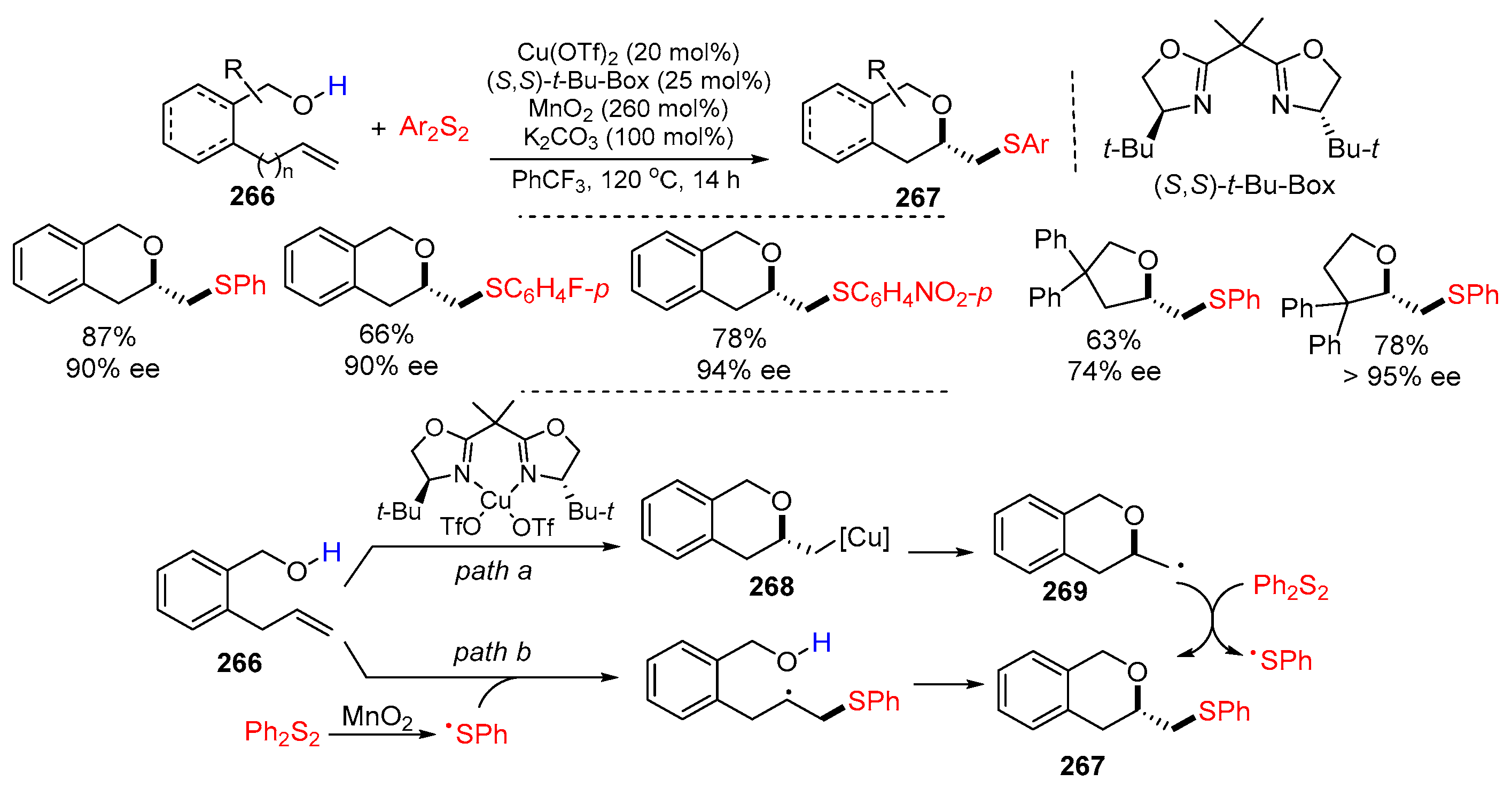
- Burde, A.S.; Chemler, S.R. Copper-catalyzed enantioselective oxysulfenylation of alkenols: Synthesis of arylthiomethyl-substituted cyclic ethers. ACS Catal. 2022, 12, 7559–7564. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Liao, H.; Qin, H.; Wang, Y.; Li, Y. Access to benzocyclic boronates via light-promoted intramolecular arylborylation of alkenes. J. Org. Chem. 2023, 88, 6237–6246. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-X.; Zhang, C.-L.; Gao, Z.-H.; Ye, S. Iminoacylation of alkenes via photoredox N-heterocyclic carbene catalysis. Org. Lett. 2023, 25, 855–860. [Google Scholar] [CrossRef]
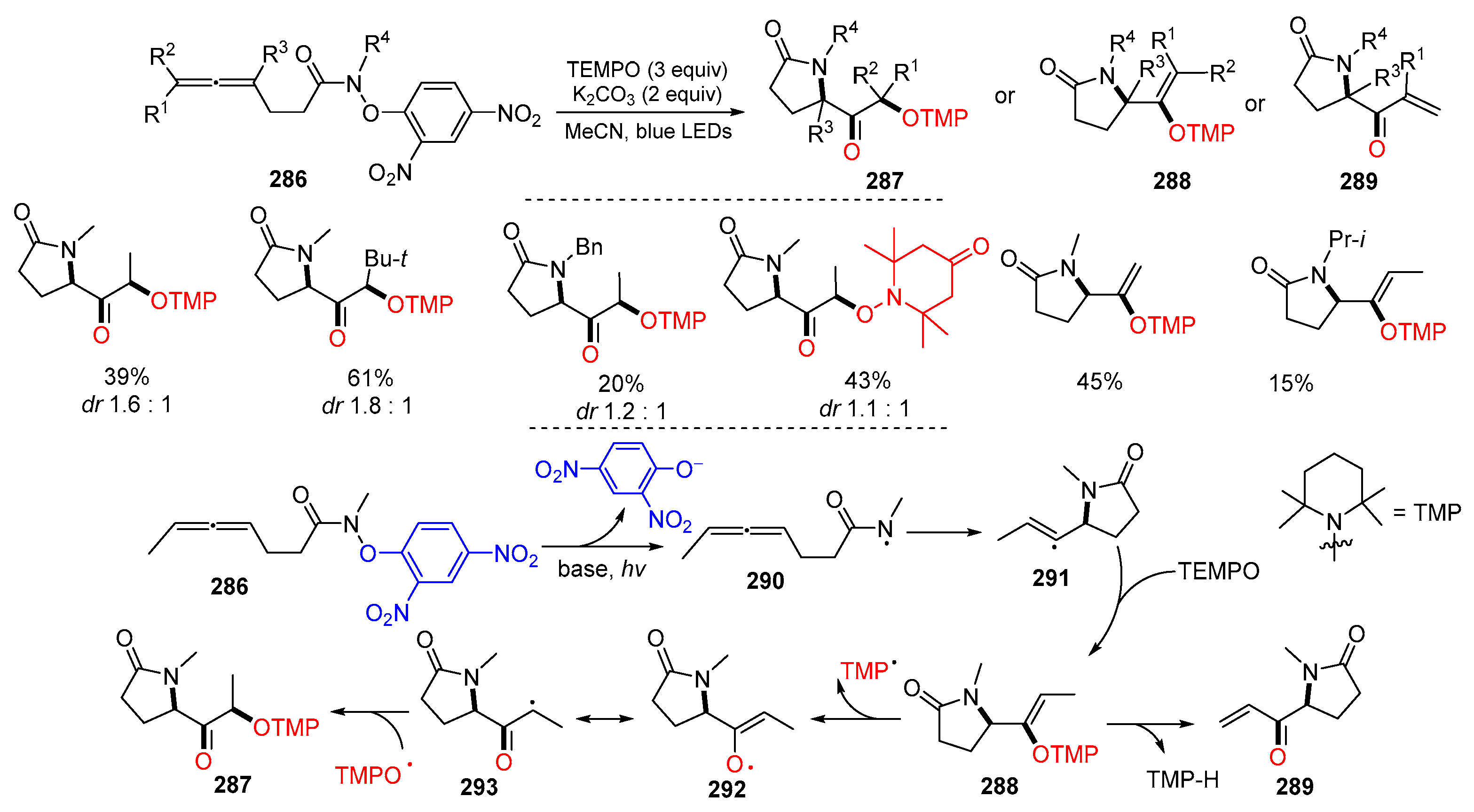
- Ward, R.M.; Schomaker, J.M. Allene trifunctionalization via amidyl radical cyclization and TEMPO trapping. J. Org. Chem. 2021, 86, 8891–8899. [Google Scholar] [CrossRef] [PubMed]
- Khoder, Z.M.; Wong, C.E.; Chemler, S.R. Stereoselective synthesis of isoxazolidines via copper-catalyzed alkene diamination. ACS Catal. 2017, 7, 4775–4779. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, F.-F.; Zhang, M.; Liu, R.-H.; Yu, W.; Han, B. Iminoxyl radical-promoted oxycyanation and aminocyanation of unactivated alkenes: Synthesis of cyano-featured isoxazolines and cyclic nitrones. Org. Lett. 2017, 19, 3255–3258. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Ren, P.-X.; Qi, L.; Chen, M.; Lu, Y.-L.; Zhao, J.-Y.; Liu, R.; Chen, J.-M.; Li, W. Copper-mediated aminoazidation, aminohalogenation, and aminothiocyanation of β,γ-unsaturated hydrazones: Synthesis of versatile functionalized pyrazolines. Org. Lett. 2018, 20, 4411–4415. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ding, J.; Wang, Y.; Shi, X.; Jiao, D.; Zhu, B. Copper-catalyzed iminohalogenation of γ, δ-unsaturated oxime esters with halide salts: Synthesis of 2-halomethyl pyrrolines. Tetrahedron Lett. 2019, 60, 151000. [Google Scholar] [CrossRef]
- Sun, Q.; Yoshikai, N. Cobalt-catalyzed tandem radical cyclization/C–C coupling initiated by directed C–H activation. Org. Lett. 2019, 21, 5238–5242. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-D.; Li, X.-R.; Wang, Y.-W.; Chu, X.-Q.; Rao, W.; Xu, H.; Han, G.-Z.; Shen, Z.-L. Indium-mediated difunctionalization of iodoalkyl-tethered unactivated alkenes via an intramolecular cyclization and an ensuing palladium-catalyzed cross-coupling reaction with aryl halides. Org. Chem. Front. 2020, 7, 2703–2709. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; He, H.; Xu, N.; Fang, Q.; Guo, K.; Cao, S.; Shi, Y.; Zhu, Y. Copper-catalyzed domino cyclization/thiocyanation of unactivated olefins: Access to SCN-containing pyrazolines. Adv. Synth. Catal. 2020, 362, 248–254. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.-P.; Li, J.-H.; Wang, Q.-A. External-oxidant-free amino-benzoyloxylation of unactivated alkenes of unsaturated ketoximes with O-benzoylhydroxylamines. Chem. Commun. 2021, 57, 5215–5218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ngo, D.T.; Nagib, D.A. Regioselective radical amino-functionalizations of allyl alcohols via dual catalytic cross-coupling. ACS Catal. 2021, 11, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Meervelt, L.V.; Van der Eycken, E.V.; Sharma, U.K. Visible-light-driven palladium-catalyzed radical tandem dearomatization of indoles with unactivated alkenes. Org. Lett. 2022, 24, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Carmona, J.C.; Román, R.S.; Buñuel, E.; Cárdenas, D.J. Nickel-catalyzed cascade cyclization-Negishi coupling of redox active esters for the synthesis of pyrrolidines. Eur. J. Org. Chem. 2022, 2022, e202200992. [Google Scholar] [CrossRef]
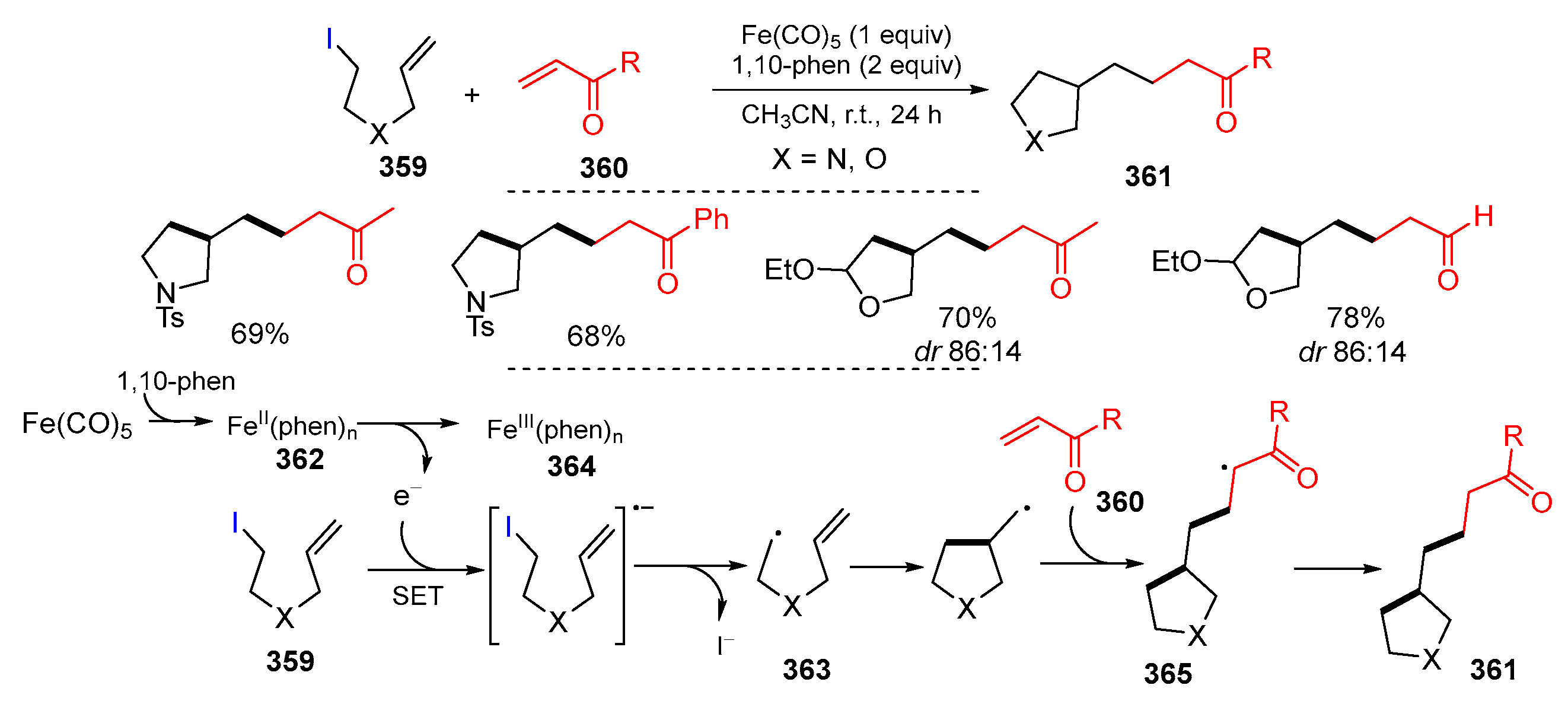
- Hwang, J.Y.; Baek, J.H.; Shin, T.I.; Shin, J.H.; Oh, J.W.; Kim, K.P.; You, Y.; Kang, E.J. Single-electron-transfer strategy for reductive radical cyclization: Fe(CO)5 and phenanthroline system. Org. Lett. 2016, 18, 4900–4903. [Google Scholar] [CrossRef] [PubMed]
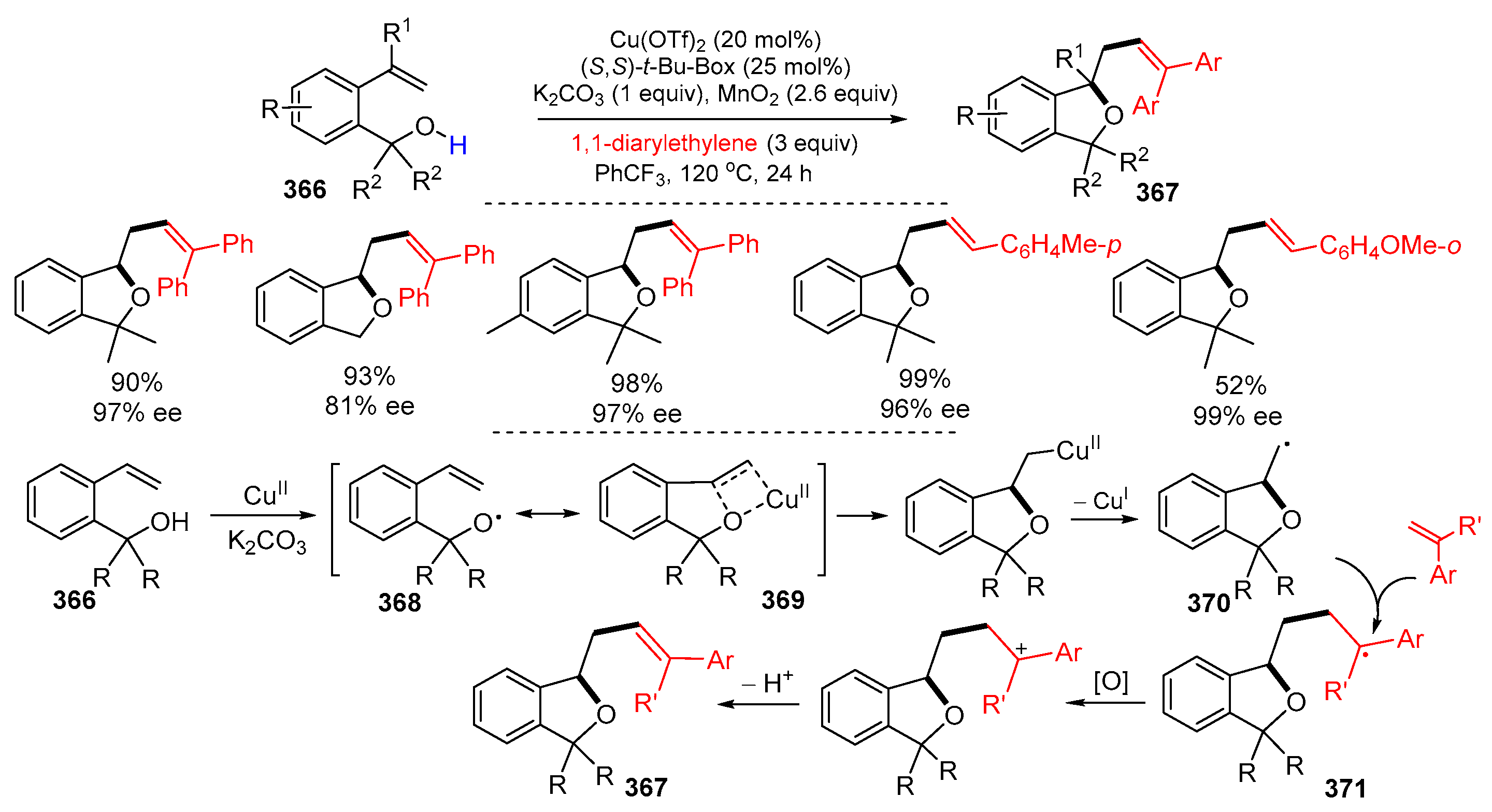
- Chen, D.; Chemler, S.R. Synthesis of phthalans via copper-catalyzed enantioselective cyclization/carboetherification of 2-vinylbenzyl alcohols. Org. Lett. 2018, 20, 6453–6456. [Google Scholar] [CrossRef] [PubMed]
- Koy, M.; Bellotti, P.; Katzenburg, F.; Daniliuc, C.G.; Glorius, F. Synthesis of all-carbon quaternary centers by palladium-catalyzed olefin dicarbofunctionalization. Angew. Chem. Int. Ed. 2020, 59, 2375–2379. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Wincenciuk, A.; Drapała, O.; Gryko, D. Vitamin B12-catalyzed dicarbofunctionalization of bromoalkenes under visible light irradiation. Synthesis 2021, 53, 1645–1653. [Google Scholar] [CrossRef]
- Li, P.-S.; Teng, Q.-Q.; Chen, M. Photoinduced radical cascade domino Heck coupling of N-aryl acrylamide with vinyl arenes enabled by palladium catalysis. Chem. Commun. 2023, 59, 10620–10623. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-Y.; Yang, X.-L.; Jia, P.-P.; Zhang, M.; Han, B. Hydrazonyl radical-participated tandem reaction: A strategy for the synthesis of pyrazoline-functionalized oxindoles. Org. Lett. 2015, 17, 6022–6025. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Long, Y.; Chen, F.; Han, B. Synthesis of isoxazoline-featured oxindoles by iminoxyl radical-promoted cascade oxyalkylation/alkylarylation of alkenes. Org. Chem. Front. 2016, 3, 184–189. [Google Scholar] [CrossRef]
- Han, W.-J.; Wang, Y.-R.; Zhang, J.-W.; Chen, F.; Zhou, B.; Han, B. Cu-catalyzed oxyalkynylation and aminoalkynylation of unactivated alkenes: Synthesis of alkynyl-featured isoxazolines and cyclic nitrones. Org. Lett. 2018, 20, 2960–2963. [Google Scholar] [CrossRef] [PubMed]
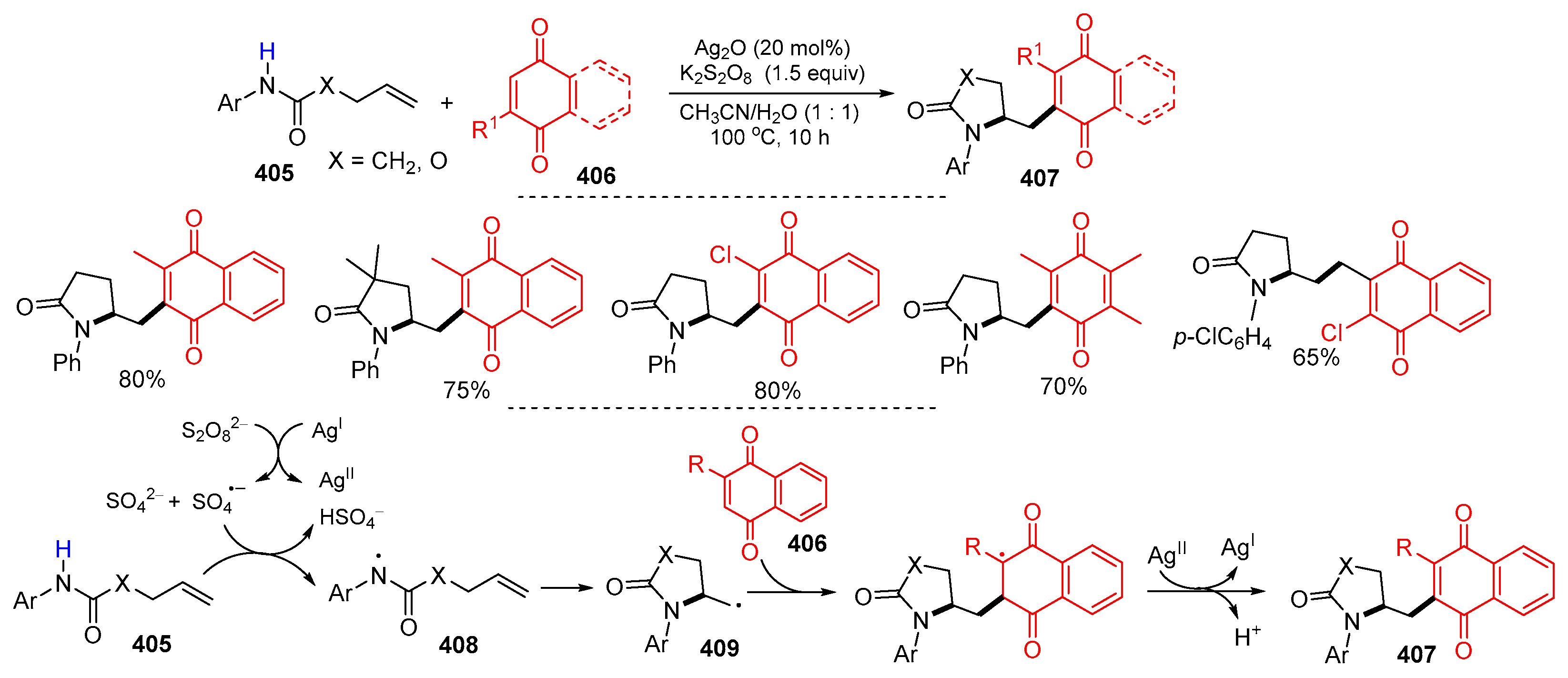
- Fang, Z.; Xie, L.; Wang, L.; Zhang, Q.; Li, D. Silver-catalyzed cascade cyclization and functionalization of N-aryl-4-pentenamides: An efficient route to γ-lactam-substituted quinone derivatives. RSC Adv. 2022, 12, 26776–26780. [Google Scholar] [CrossRef] [PubMed]
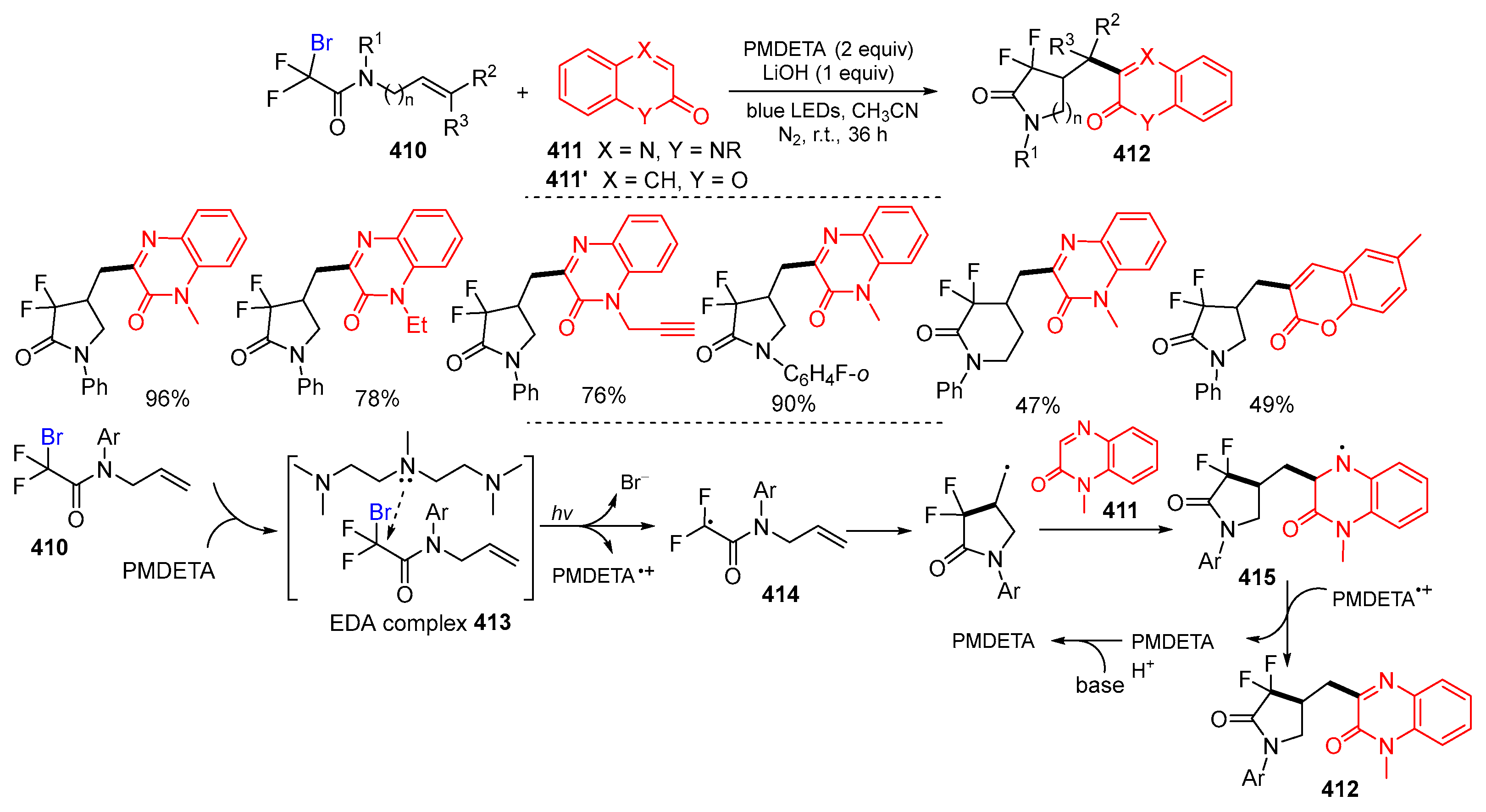
- Zhao, W.; Xuan, L.; Liu, Y.; Yin, J.; Wang, H.; Yan, Q.; Wang, W.; Huang, J.; Chen, F. Photoinduced tandem radical cyclization/heteroarylation of N-allylbromodifluoroacetamides with quinoxalin-2(1H)-ones or coumarins under metal- and photocatalyst-free conditions. New J. Chem. 2023, 47, 10744–10750. [Google Scholar] [CrossRef]
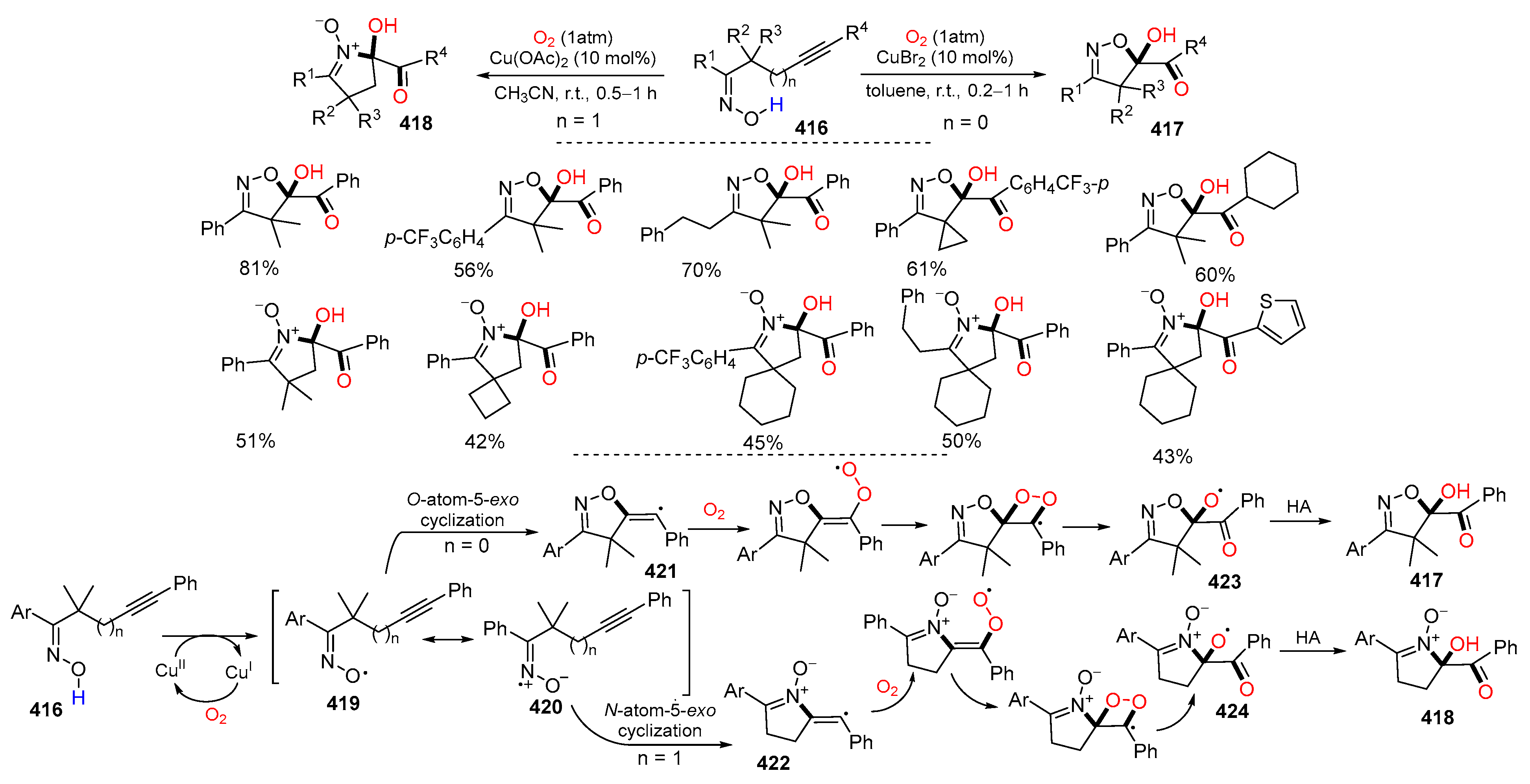
- Peng, X.-X.; Wei, D.; Han, W.-J.; Chen, F.; Yu, W.; Han, B. Dioxygen activation via Cu-catalyzed cascade radical reaction: An approach to isoxazoline/cyclic nitrone-featured α-ketols. ACS Catal. 2017, 7, 7830–7834. [Google Scholar] [CrossRef]
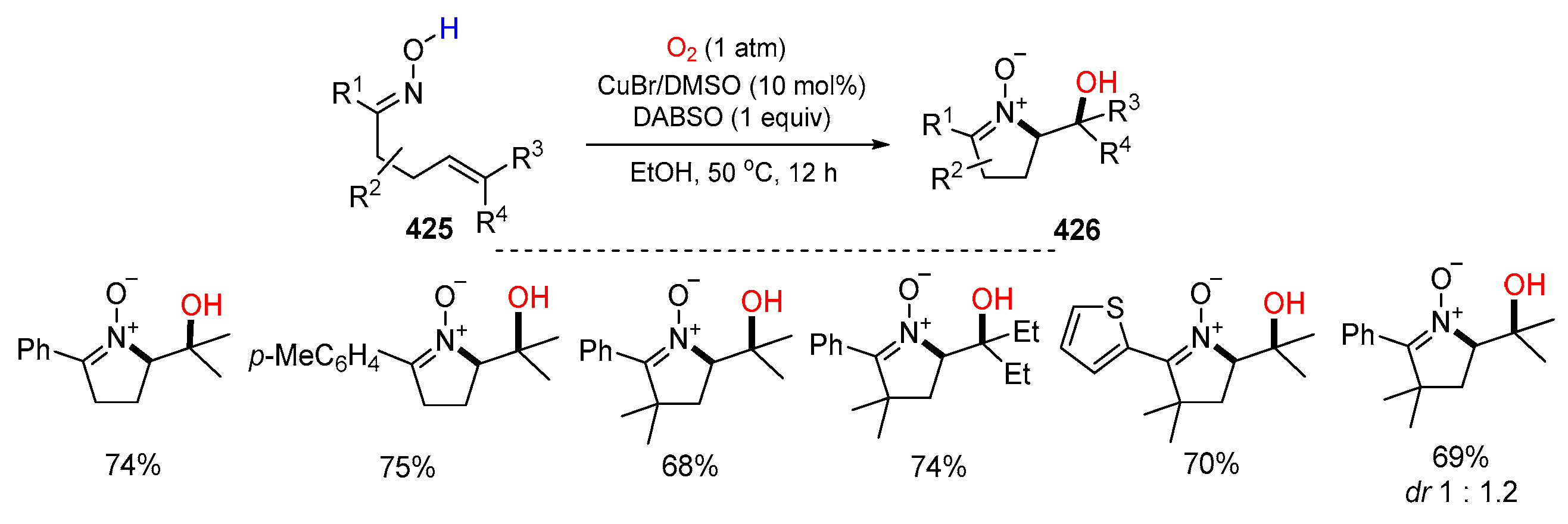
- Liu, X.-D.; Wang, Q.-A.; Zhu, Y.-P.; Peng, Z.-H.; Li, J.-H. Copper-catalyzed aerobic hydroxyamination of alkenes of unsaturated keto oximes in EtOH toward cyclic nitrones. Green Chem. 2022, 24, 2476–2482. [Google Scholar] [CrossRef]
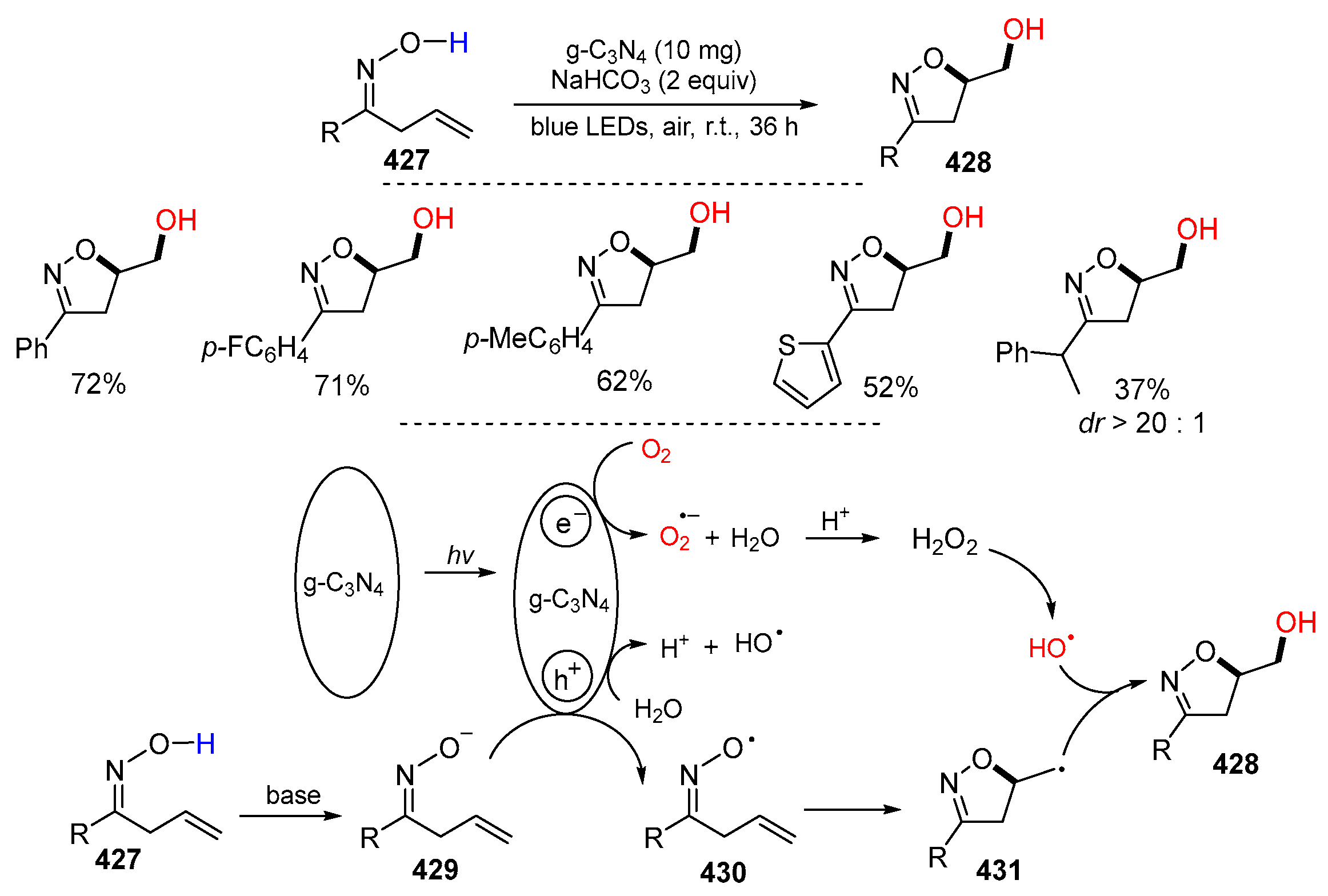
- Fu, X.-Y.; Si, Y.-F.; Qiao, L.-P.; Zhao, Y.-F.; Chen, X.-L.; Yu, B. Visible light-promoted recyclable carbon nitride-catalyzed dioxygenation of β,γ-unsaturated oximes. Adv. Synth. Catal. 2022, 364, 574–580. [Google Scholar] [CrossRef]
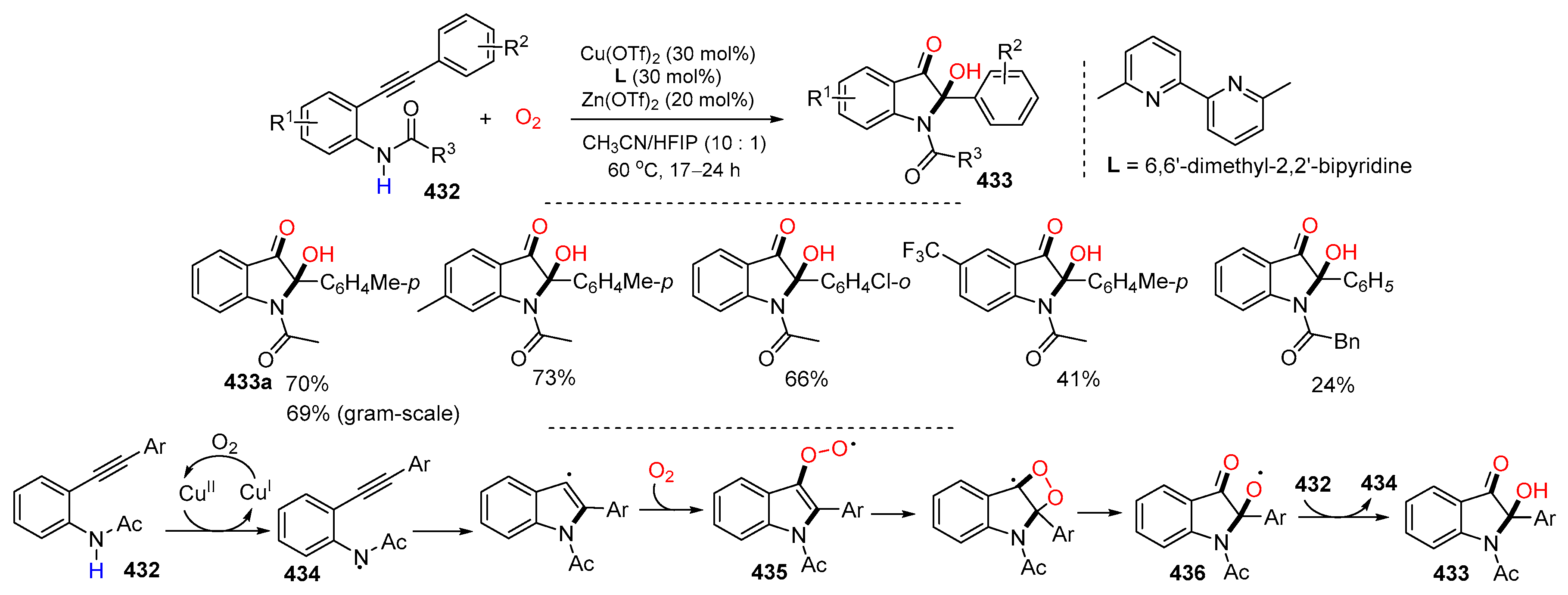
- Sun, W.; Cui, X.; Qu, J.; Cai, X.; Hu, J.; Xiong, Z.; Guo, S.; Xu, J.; Chen, W.-H.; Wu, J.-Q. Facile access to 2-hydroxy-2-substituted indole-3-ones via a copper-catalyzed oxidative cyclization of 2-arylethynylanilines. Chem. Commun. 2023, 59, 7228–7231. [Google Scholar] [CrossRef]
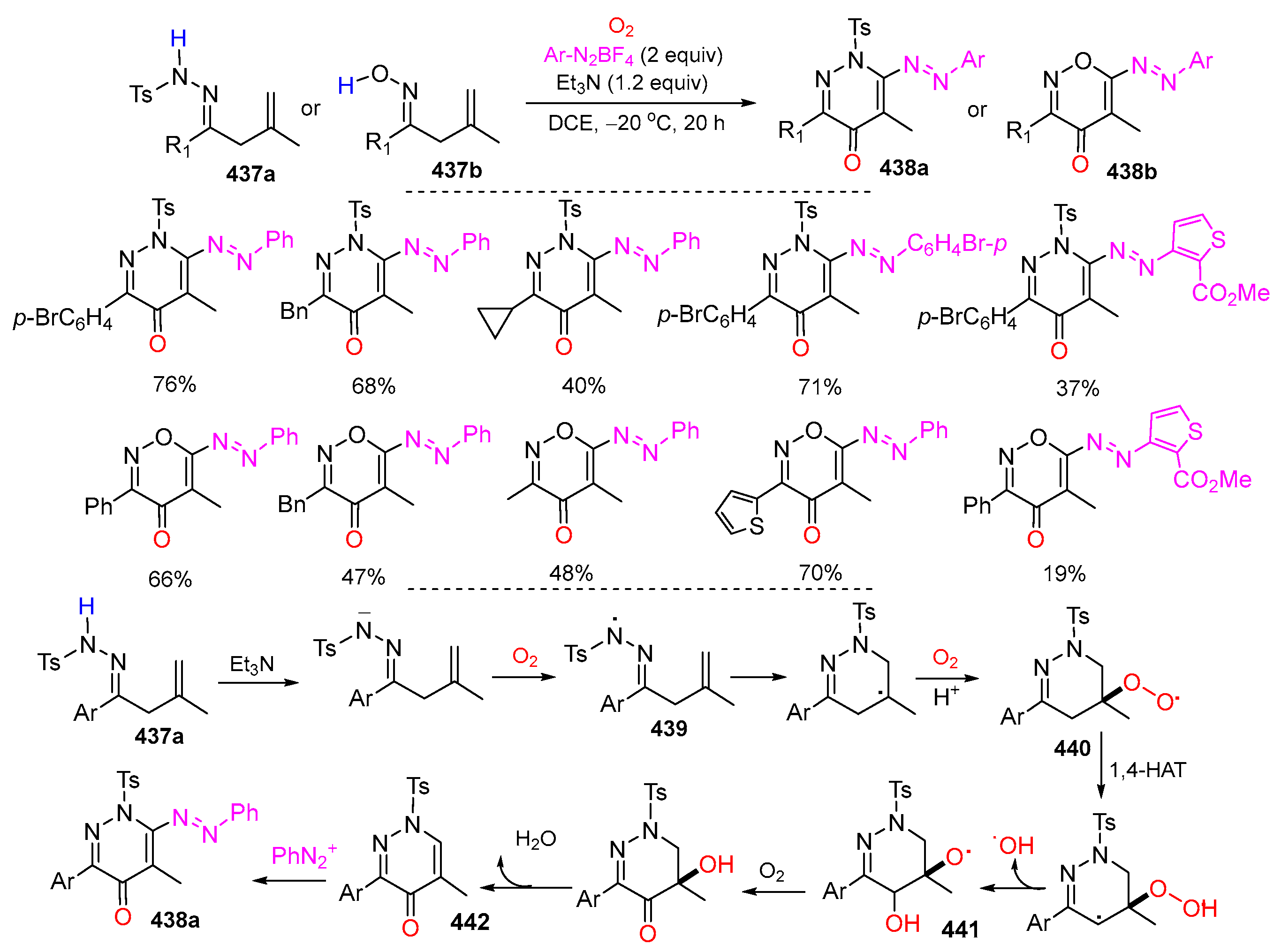
- Qi, Z.; Wen, S.; Liu, Z.; Jiang, D. Oxygen-promoted 6-endo-trig cyclization of β,γ-unsaturated hydrazones/ketoximes with diazonium tetrafluoroborates for pyridazin-4(1H)-ones/oxazin-4(1H)-ones. Org. Lett. 2023, 25, 6110–6115. [Google Scholar] [CrossRef]
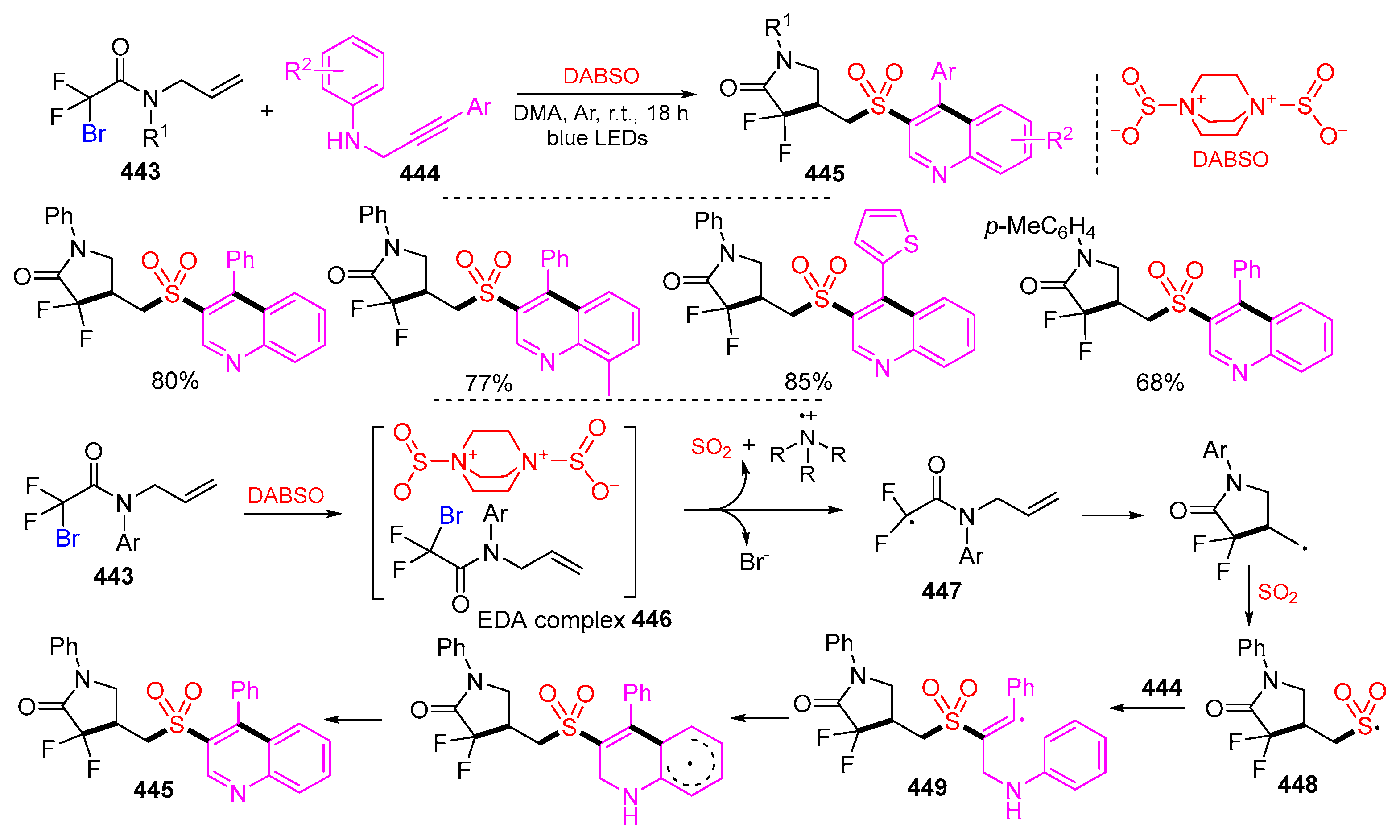
- Ye, H.; Zhou, L.; Chen, Y.; Tong, H. Visible light driven multicomponent synthesis of difluoroamidosulfonyl quinoline derivatives. Org. Biomol. Chem. 2023, 21, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Yi, J.-T.; Chen, Z.-D.; Zhuang, Q.-C.; Li, Y.-Z.; Lu, G.; Weng, J. Photoredox-catalyzed aminofluorosulfonylation of unactivated olefins. Chem. Sci. 2021, 12, 9359–9365. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-M.; Qi, L.; Cao, T.-Y.; Liu, J.-L.; Du, H.-J.; Dong, Y.-C.; Li, W.; Wang, L.-J. Aminofluorosulfonylation of β,γ-unsaturated hydrazones with sulfur dioxide and N-fluorobenzenesulfonimide: Accessing pyrazoline-functionalized aliphatic sulfonyl fluorides. Org. Lett. 2023, 25, 3910–3915. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Chen, F.; Zhou, N.-N.; Yu, W.; Han, B. Synthesis of isoxazoline-functionalized phenanthridines via iminoxyl radical-participated cascade sequence. Org. Lett. 2014, 16, 6476–6479. [Google Scholar] [CrossRef] [PubMed]
- Micic, N.; Polyzos, A. Radical carbonylation mediated by continuous-flow visible-light photocatalysis: Access to 2,3-dihydrobenzofurans. Org. Lett. 2018, 20, 4663–4666. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Jana, K.; Alasmary, F.A.; Daniliuc, C.G.; Studer, A. Transition-metal-free intramolecular radical aminoboration of unactivated alkenes. Org. Lett. 2021, 23, 7688–7692. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, S.; Xie, Z.; Lu, D.; Gong, Y. An iron-catalysed radical cyclization/C(sp3)–H alkylation cascade between γ,δ-unsaturated oxime esters and glycine derivatives: Access to pyrroline-containing amino acids. Org. Chem. Front. 2023, 10, 382–387. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Chen, Y.; Sun, J.; Wang, J.-Y.; Zhou, M.-D. Visible-light-promoted radical cyclization/arylation cascade for the construction of α,α-difluoro-γ-lactam-fused quinoxalin-2(1H)-ones. Chin. J. Chem. 2022, 40, 713–718. [Google Scholar] [CrossRef]
- Yuan, T.-T.; Chen, J.; Pham, L.N.; Paul, S.; White, L.V.; Li, J.; Lan, P.; Coote, M.L.; Banwell, M.G.; He, Y.-T. Visible light-mediated syntheses of unsymmetrical methylene-bridged bis-heterocycles via an alkoxy radical relay reaction. Org. Chem. Front. 2023, 10, 4649–4657. [Google Scholar] [CrossRef]
- Xu, H.-C.; Campbell, J.M.; Moelle, K.D. Cyclization reactions of anode-generated amidyl radicals. J. Org. Chem. 2014, 79, 379–391. [Google Scholar] [CrossRef] [PubMed]
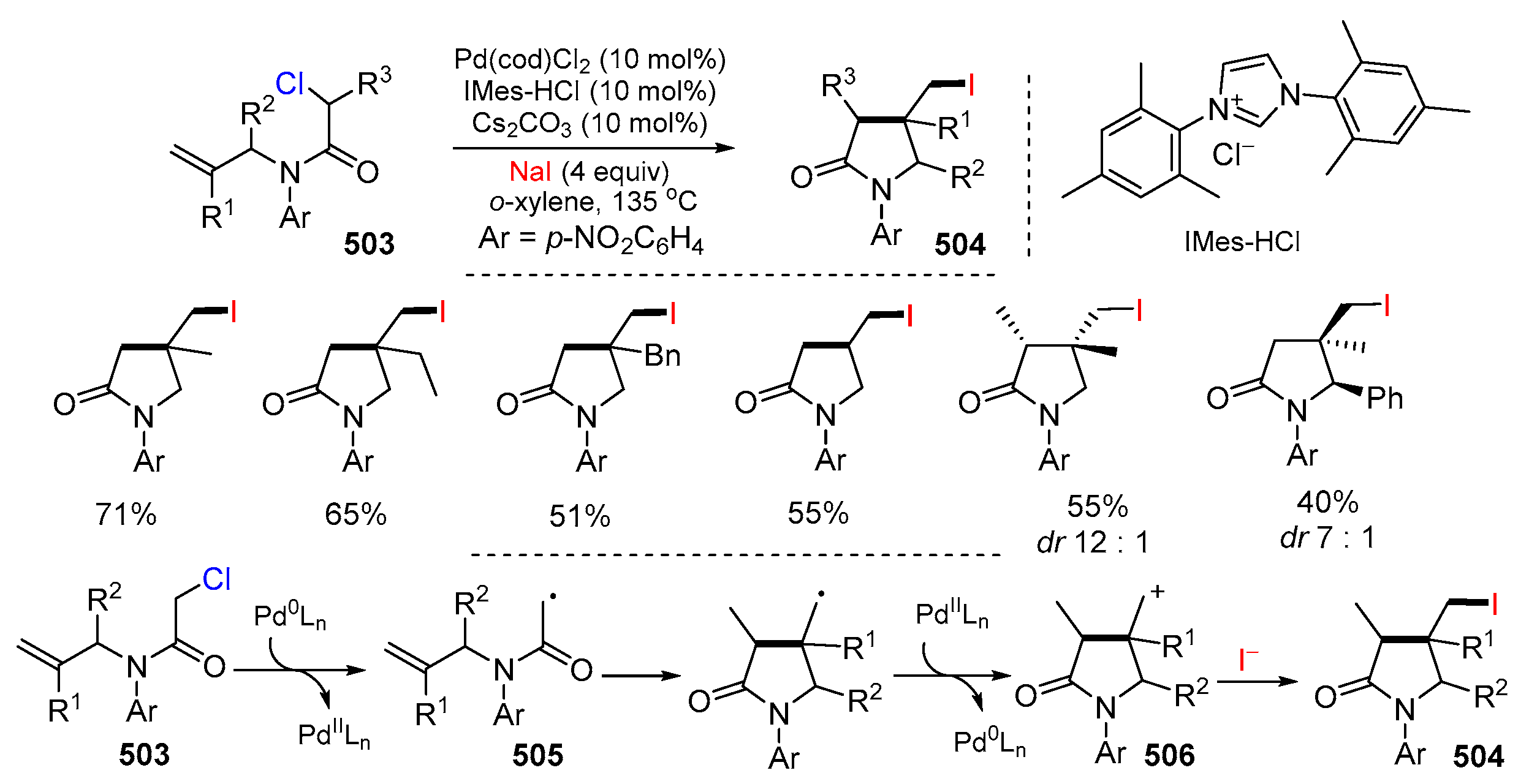
- Liu, Q.; Chen, C.; Tong, X. Pd(0)-catalyzed atom transfer radical cyclization of N-allyl-α-chloroamides: Highly stereoselective synthesis of substituted γ-lactam. Tetrahedron Lett. 2015, 56, 4483–4485. [Google Scholar] [CrossRef]
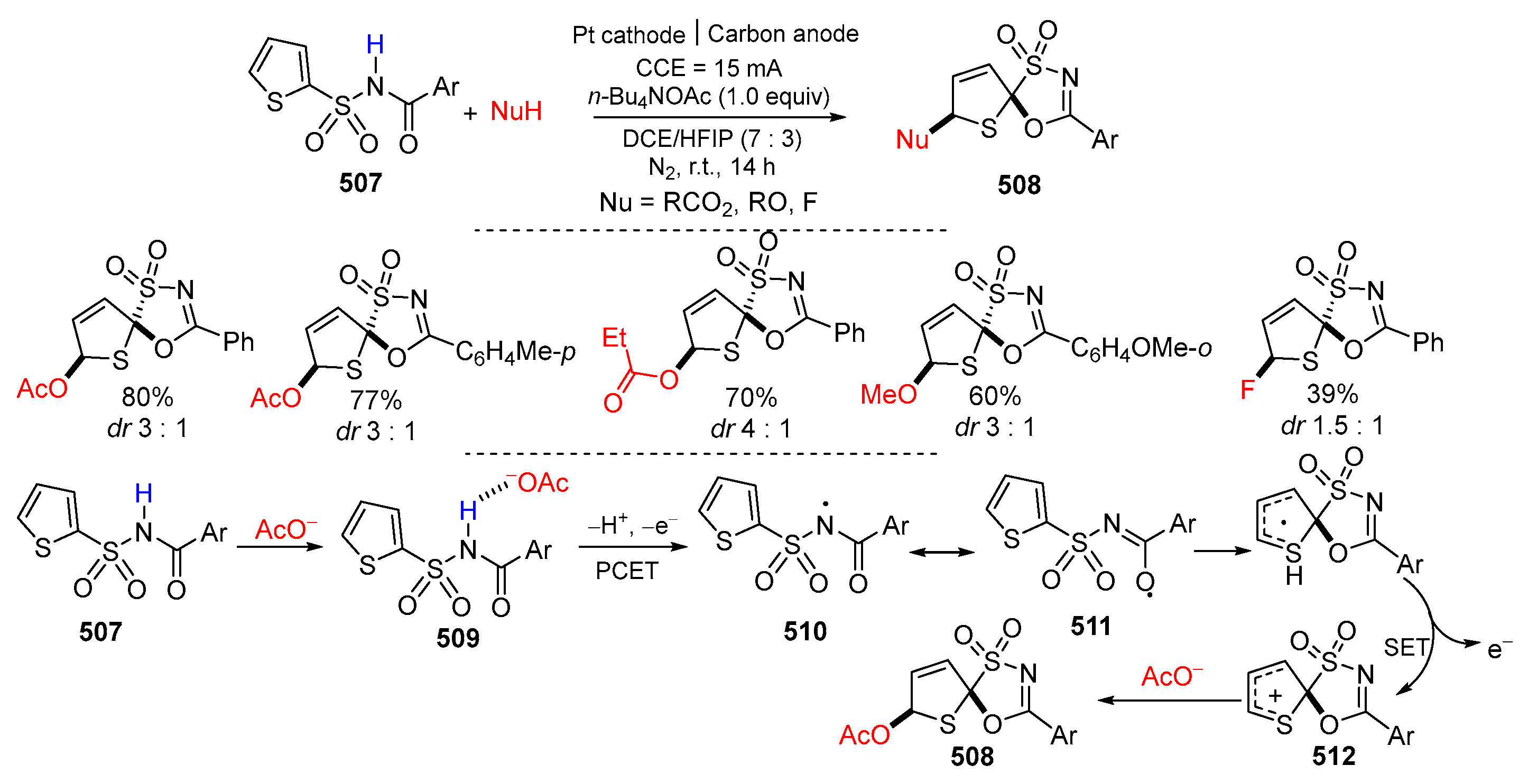
- Shi, Z.; Wang, W.-Z.; Li, N.; Yuan, Y.; Ye, K.-Y. Electrochemical dearomative spirocyclization of N-acyl thiophene-2-sulfonamides. Org. Lett. 2022, 24, 6321–6325. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, L.; Mu, H.; Zhang, Q.; Fang, Z.; Li, D. Synthesis of cyano-substituted γ-lactams through a copper-catalyzed cascade cyclization/cyanation reaction. Org. Biomol. Chem. 2023, 21, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, Y.; Luo, M.-J.; Yang, R.; Bai, J.; Song, X.-R.; Xiao, Q. Metal-free electrochemical oxidative intramolecular cyclization of N-propargylbenzamides: Facile access to oxazole ketals. Org. Chem. Front. 2023, 10, 3786–3791. [Google Scholar] [CrossRef]














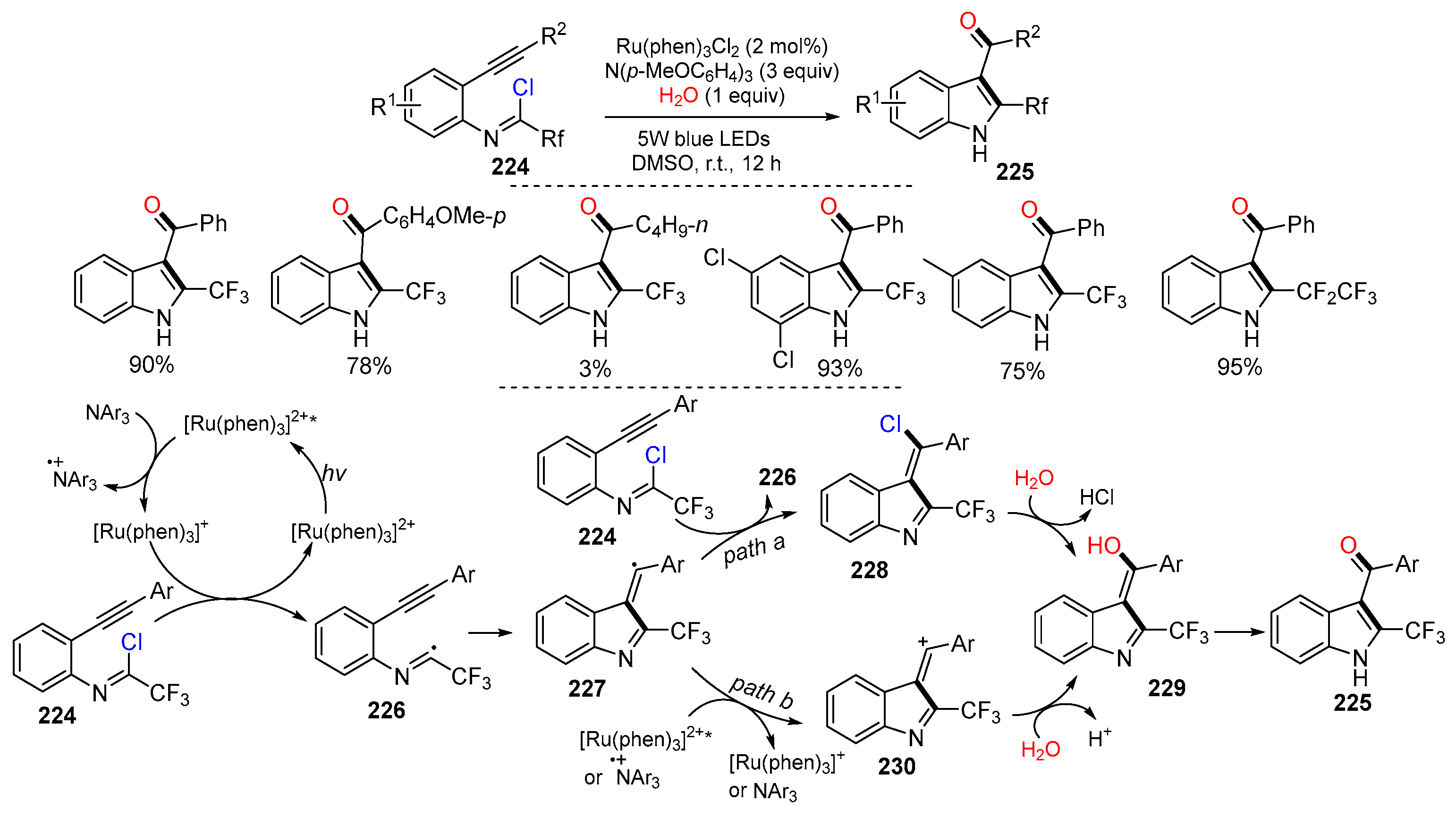

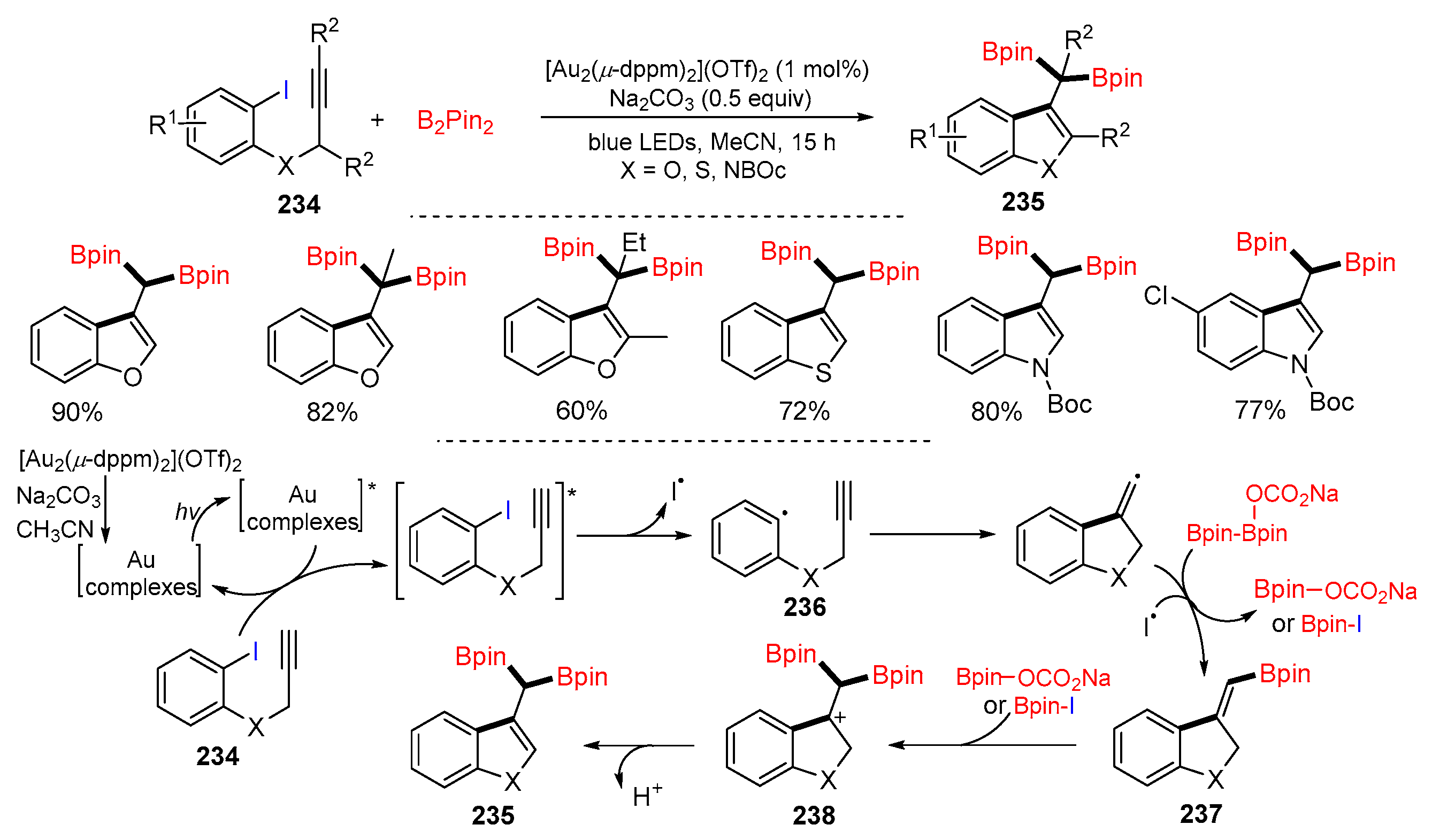
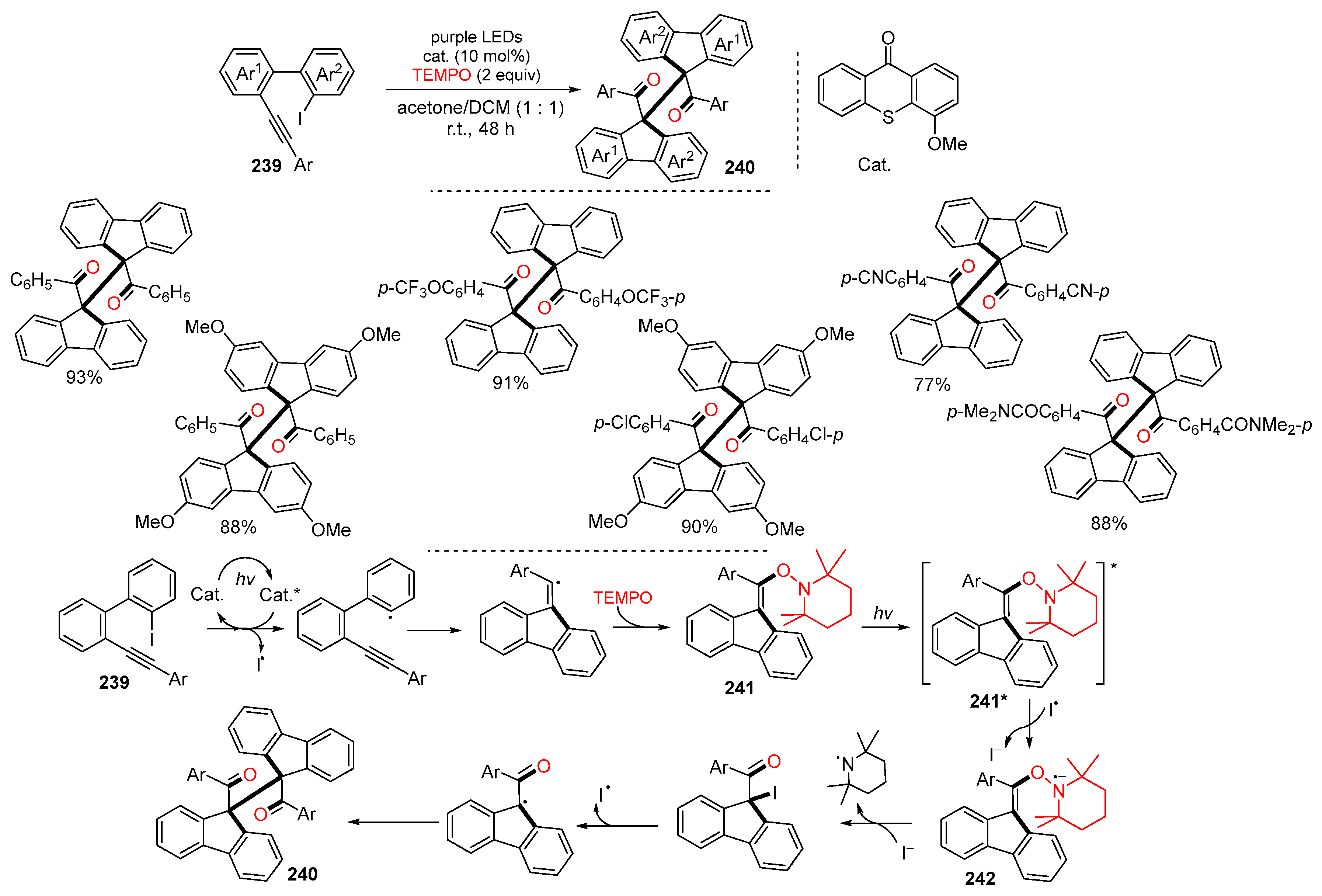
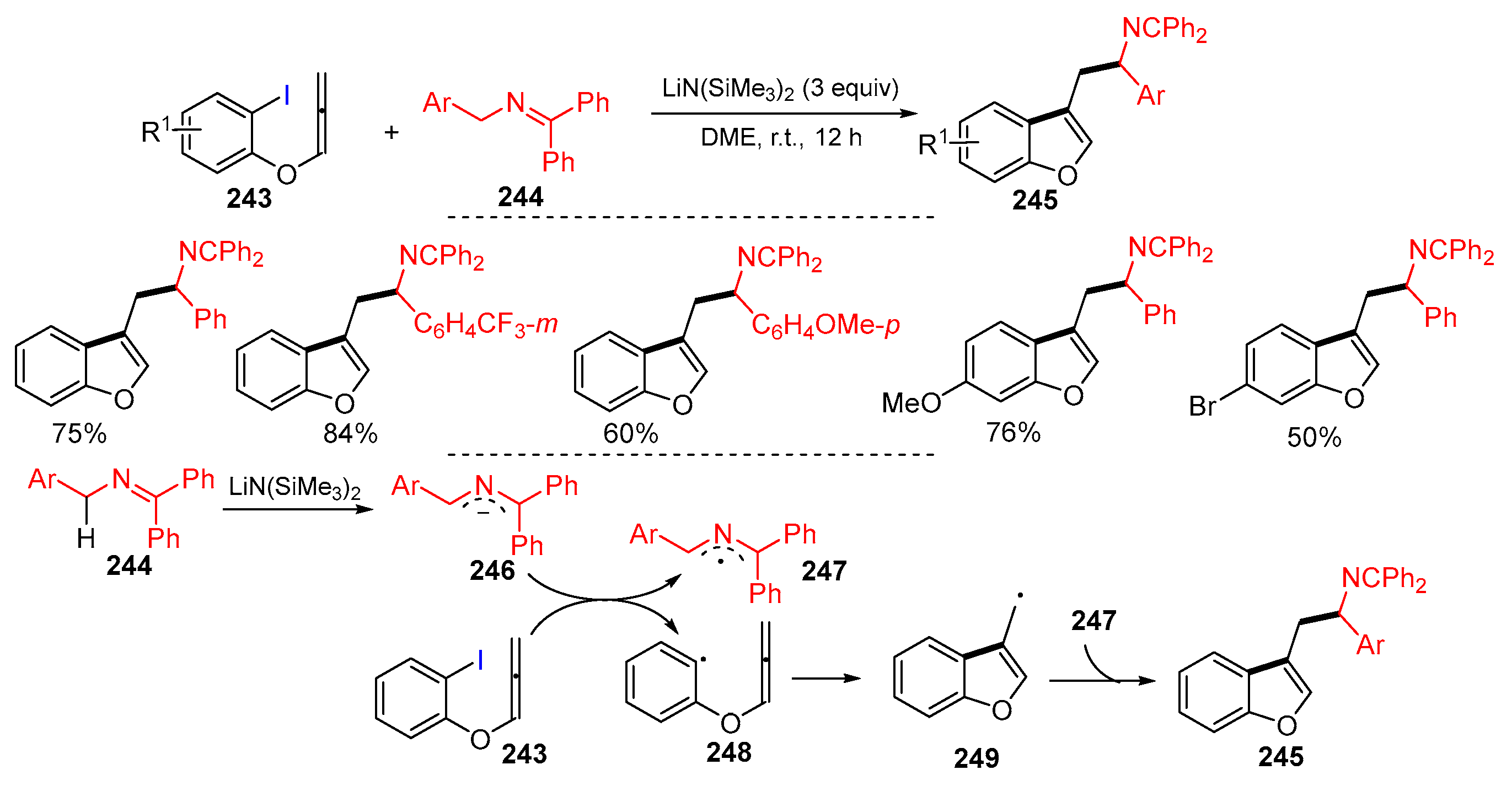





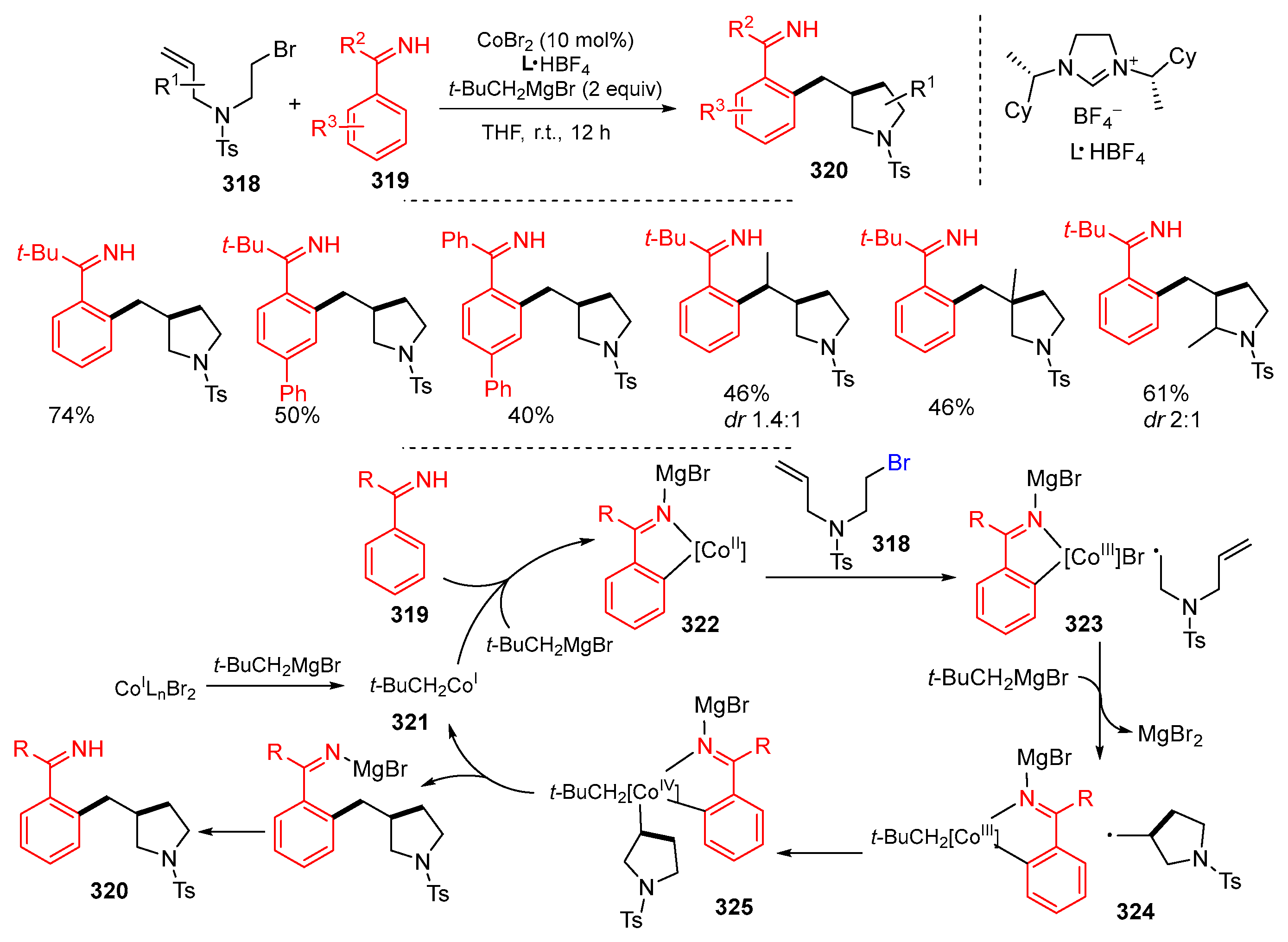
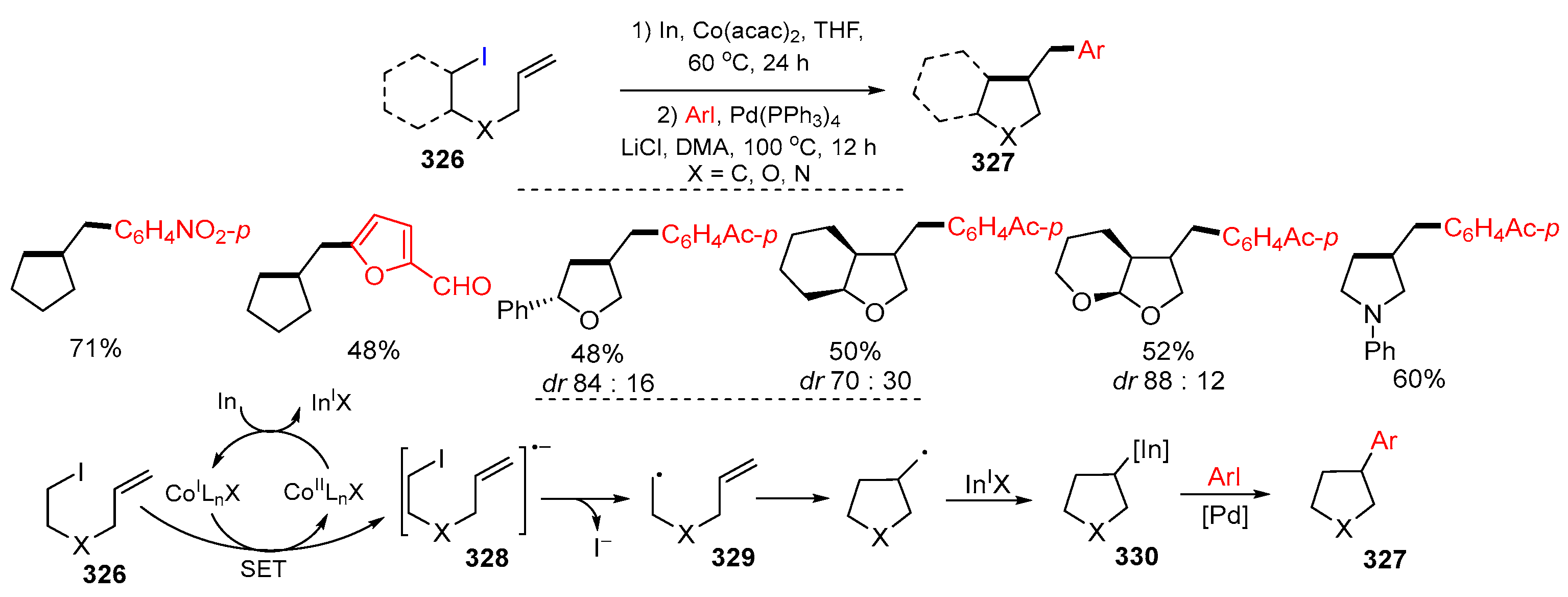
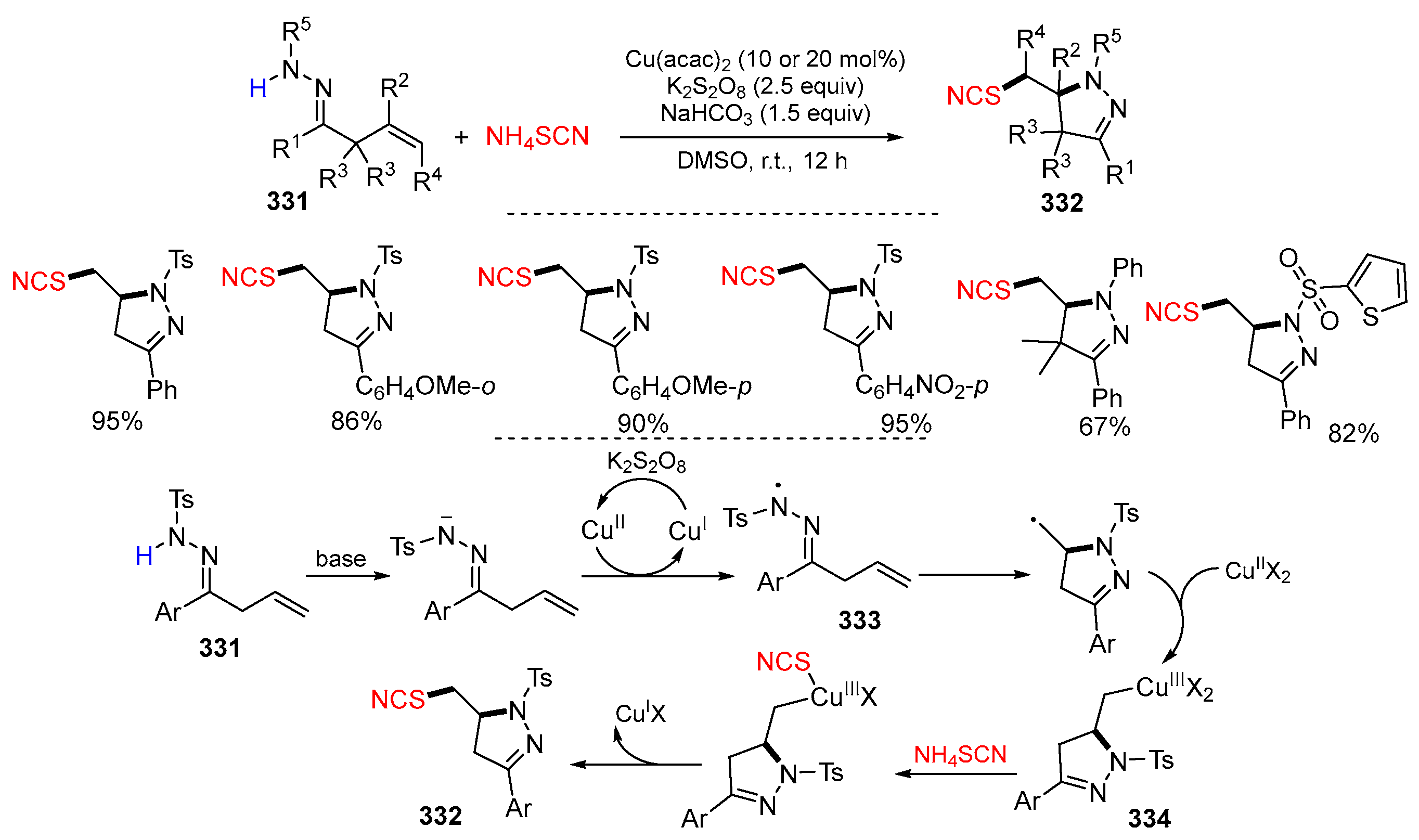

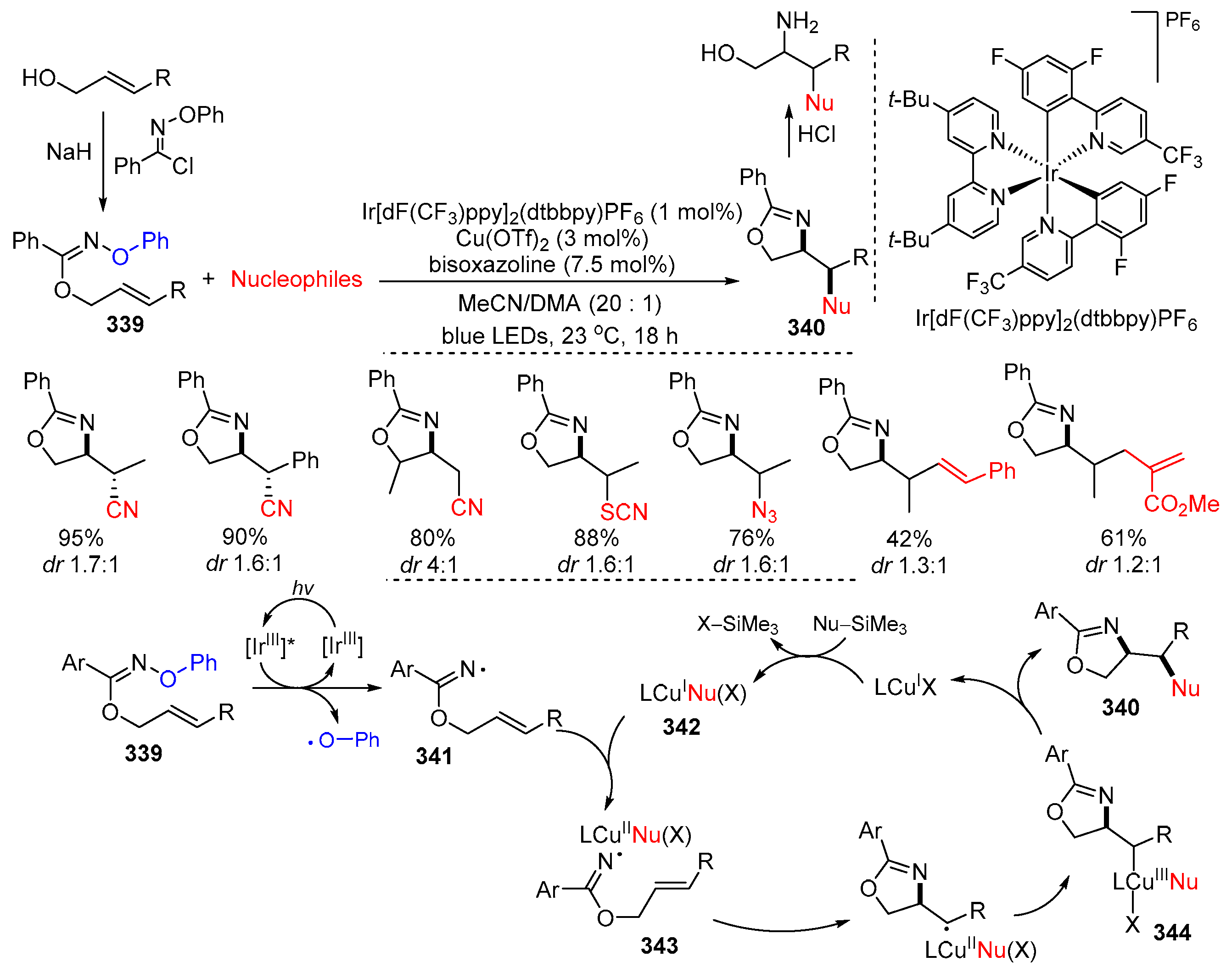







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, S.; Ma, X.; Zhang, W. Radical Cyclization-Initiated Difunctionalization Reactions of Alkenes and Alkynes. Molecules 2024, 29, 2559. https://doi.org/10.3390/molecules29112559
Zhi S, Ma X, Zhang W. Radical Cyclization-Initiated Difunctionalization Reactions of Alkenes and Alkynes. Molecules. 2024; 29(11):2559. https://doi.org/10.3390/molecules29112559
Chicago/Turabian StyleZhi, Sanjun, Xiaoming Ma, and Wei Zhang. 2024. "Radical Cyclization-Initiated Difunctionalization Reactions of Alkenes and Alkynes" Molecules 29, no. 11: 2559. https://doi.org/10.3390/molecules29112559
APA StyleZhi, S., Ma, X., & Zhang, W. (2024). Radical Cyclization-Initiated Difunctionalization Reactions of Alkenes and Alkynes. Molecules, 29(11), 2559. https://doi.org/10.3390/molecules29112559








