Research Progress on the Degradation of Organic Pollutants in Wastewater via Ultrasound/Periodate Systems: A Review
Abstract
1. Introduction
2. Reaction Mechanism of US/PI System for Degradation of Organic Pollutants in Wastewater
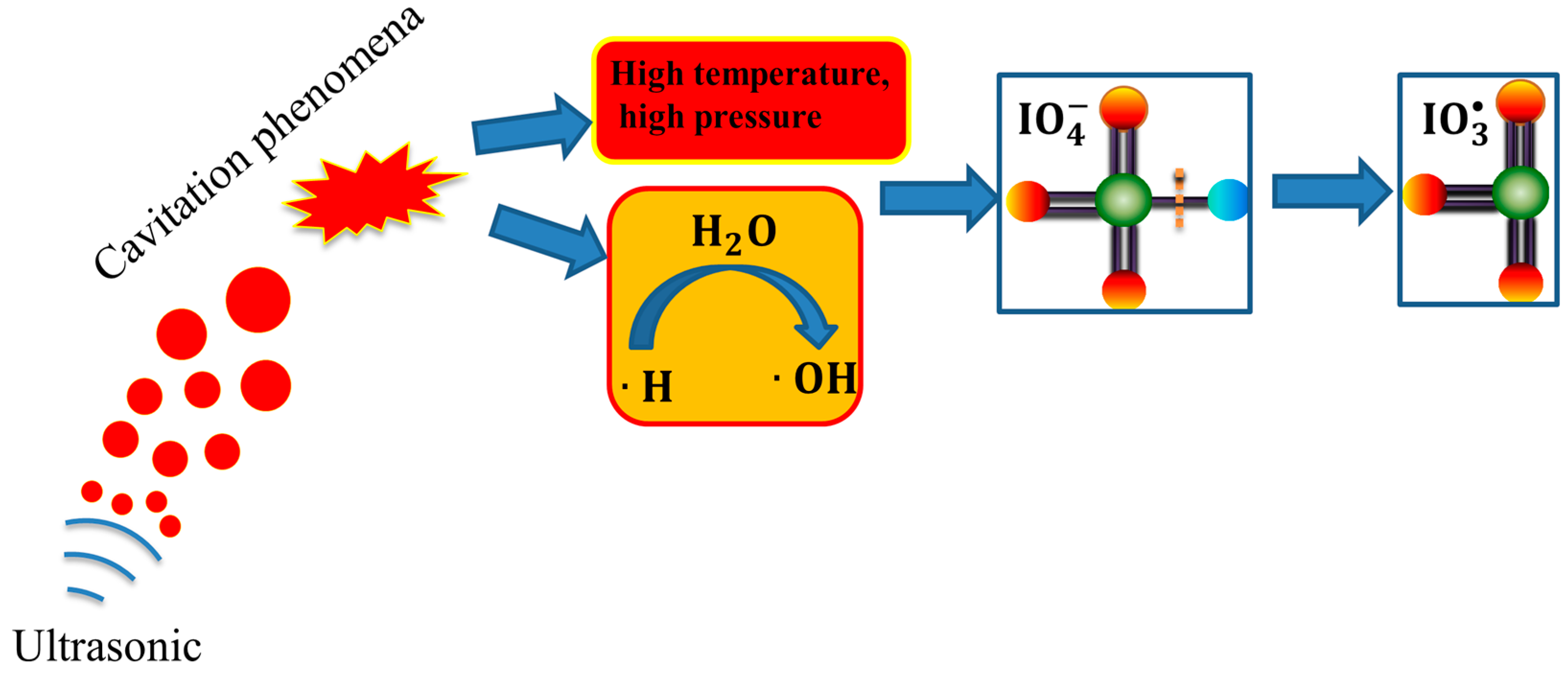
2.1. The Formation Mechanism of Oxidative Free Radicals in US/PI System
2.2. Degradation Mechanisms of Different Organic Compounds in the US/PI System
3. Key Determinants of the US/PI System Is Performance
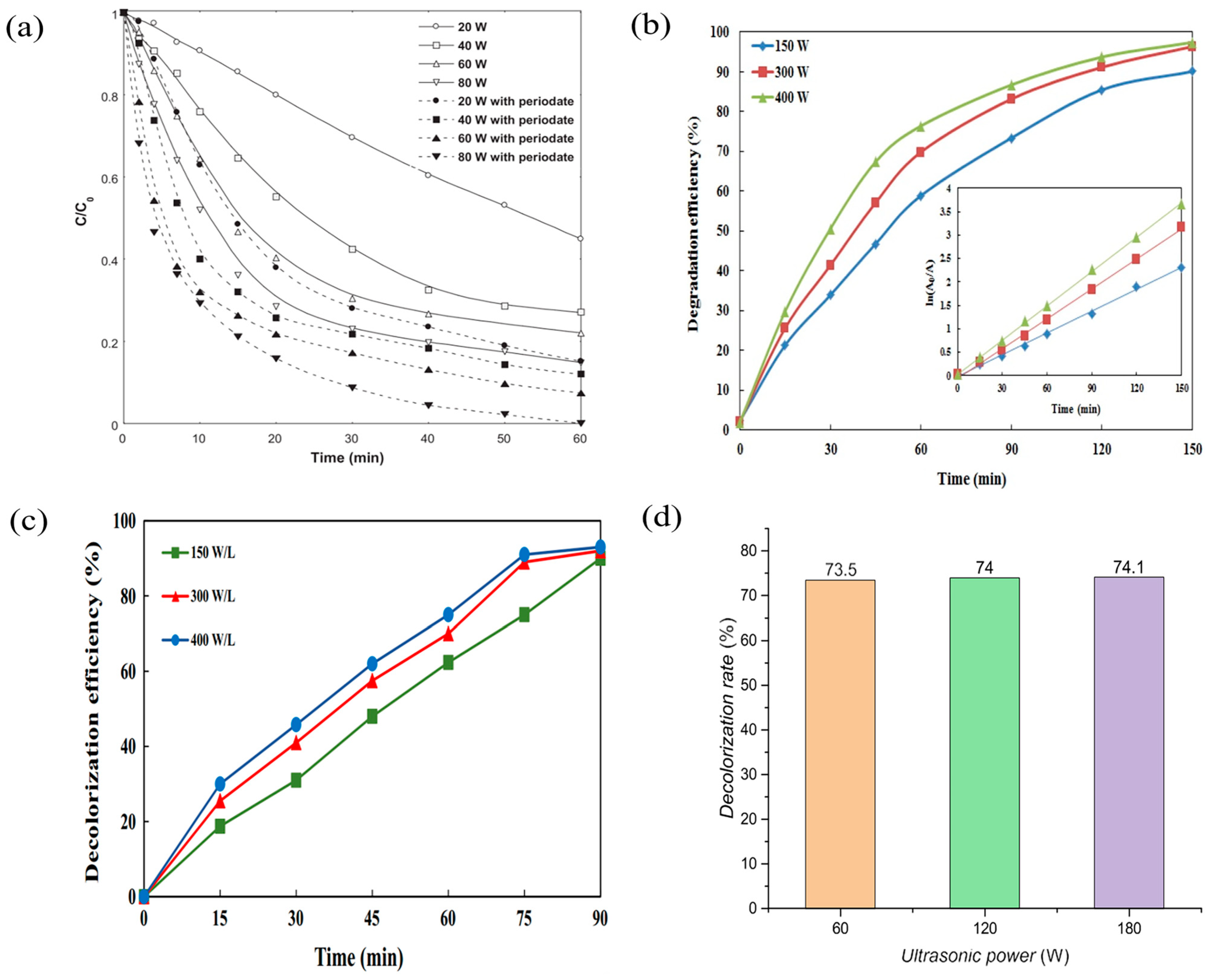
3.1. Ultrasonic Power Intensity
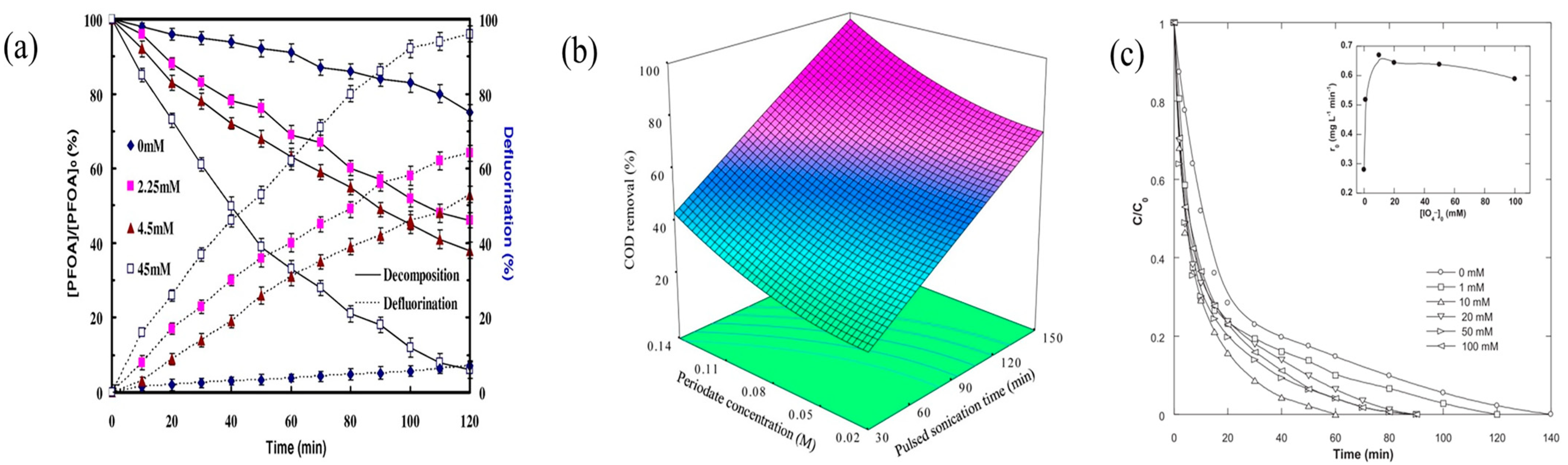
3.2. PI concentration Levels
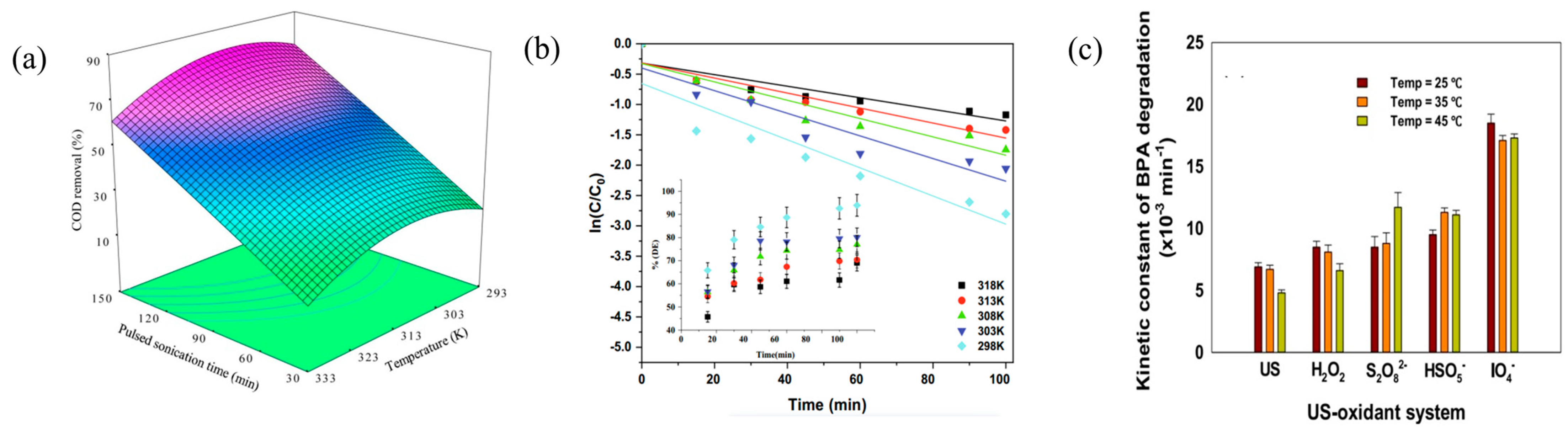
3.3. Temperature
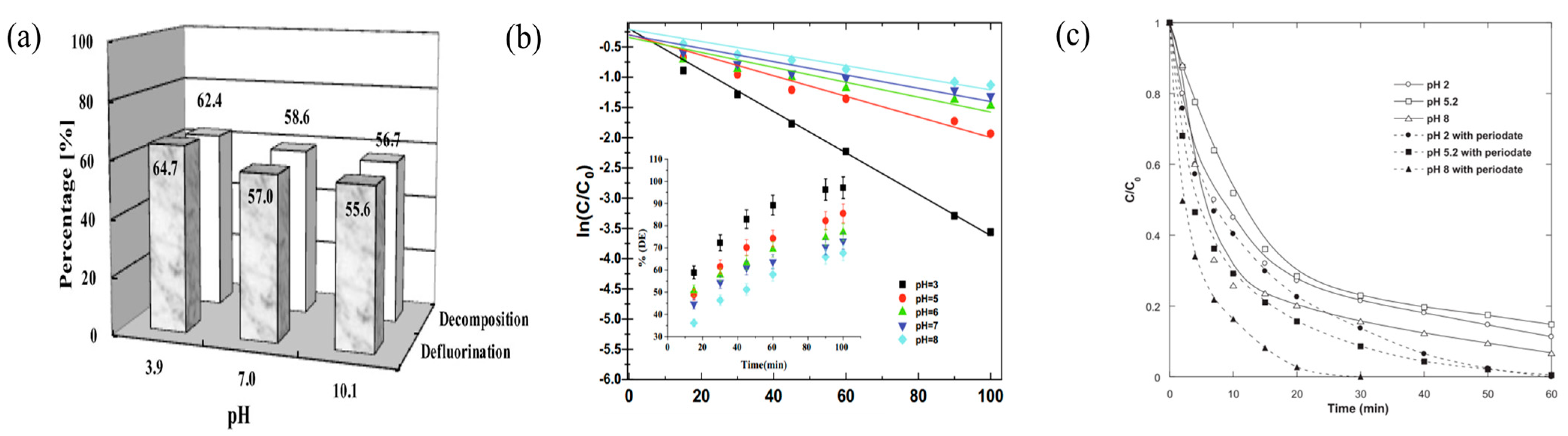
3.4. Solution pH Value
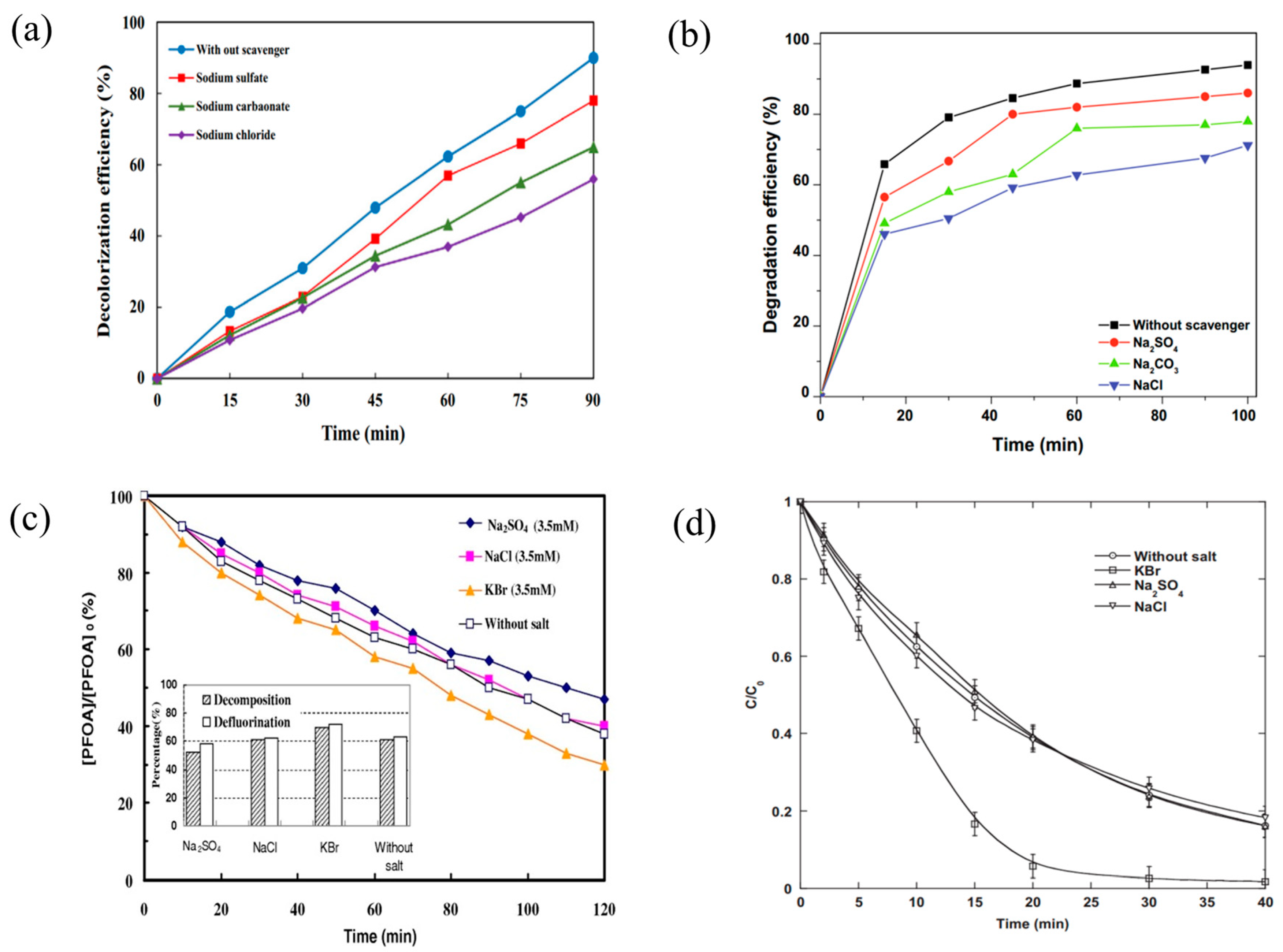
3.5. Presence of Concurrent Inorganic Ions
3.6. Dissolved Organic Matter
3.7. Others
4. Efficacy of Organic Compound Degradation under the US/PI System
5. Concluding Remarks and Future Perspectives
- (a)
- It is advisable to employ innovative experimental designs and precise instrumentation, such as experimental segmentation and variable control, to differentiate and accurately quantify the contributions of thermal decomposition processes and free radical reactions. Concurrently, there is an urgent need to develop new technological approaches for the direct identification of various free radicals within the US/PI system, such as employing electron spin resonance (ESR) spectroscopy. Furthermore, these advancements will enable a comprehensive elucidation of the mechanisms by which free radicals degrade organic pollutants.
- (b)
- Although mineralization levels can initially be gauged using Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD), the toxicity of intermediates produced during degradation processes holds significant importance. The discernible scarcity of research focusing on biotoxicity and bioindicators like Biochemical Oxygen Demand (BOD) heralds significant opportunities for future investigations.
- (c)
- The degradation of organic pollutants via the US/PI system involves an inherently intricate and multifaceted mechanism. While this manuscript has delved into aspects of free radical oxidation and ultrasonic pyrolysis, numerous unexplored facets remain, necessitating systematic investigation through further experimental research.
- (d)
- While the US/PI system has demonstrated superior efficacy in the degradation of organic pollutants relative to traditional methodologies, its elevated economic cost remains a significant impediment to widespread adoption. Consequently, identifying strategies to mitigate costs while preserving the system’s high efficiency constitutes a crucial research trajectory for the advancement of US/PI technologies.
- (e)
- Currently, the application of the US/PI system is constrained by operating conditions, including ultrasound frequency and intensity, as well as PI concentration. Future research should explore optimizing these conditions to maximize degradation efficiency, while also considering the balance between cost and energy consumption.
- (f)
- The majority of research on the degradation of organic pollutants in wastewater using the US/PI system remains at the laboratory stage. Future research should investigate scaling up the US/PI system for industrial applications, including treating complex combinations of organic pollutants in real-world wastewater scenarios and assessing the system’s stability and sustainability.
- (g)
- When employing the US/PI system for wastewater treatment, it is crucial to evaluate the by-products generated during the reaction and their potential environmental impacts. Future research should encompass the identification, quantification, and toxicological evaluation of these by-products.
- (h)
- Future research should investigate integrating the US/PI system with other wastewater treatment methodologies, such as biological treatment and adsorption, to enhance treatment efficacy and cost-efficiency. This integrated approach could potentially be more effective in treating recalcitrant or highly concentrated organic pollutants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Lan, S.; Zhu, M. Piezoelectricity Activates Persulfate for Water Treatment: A Perspective. Environ. Sci. Ecotechnol. 2024, 18, 100329. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in Depressants for Flotation Separation of Cu-Fe Sulfide Minerals at Low Alkalinity: A Critical Review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, G.; Zhang, Q.; Zhao, W. Synergistic Activation of Sulfidized Hemimorphite with Copper-Lead Species for Improving Surface Hydrophobicity and Floatability. Sep. Purif. Technol. 2024, 332, 125854. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, W.; Ma, J.; Wen, G.; Liu, Q. Relationship between Acceleration of Hydroxyl Radical Initiation and Increase of Multiple-Ultrasonic Field Amount in the Process of Ultrasound Catalytic Ozonation for Degradation of Nitrobenzene in Aqueous Solution. Ultrason. Sonochem. 2015, 22, 198–204. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Kumar, P.; Headley, J.; Peru, K.; Bailey, J.; Dalai, A. Removal of Dicyclohexyl Acetic Acid from Aqueous Solution Using Ultrasound, Ozone and Their Combination. J. Environ. Sci. Health Part A 2014, 49, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-Y.; Moed, N.M.; Ku, Y.; Lee, H.-Y. Ultrasonic Regeneration Studies on Activated Carbon Loaded with Isopropyl Alcohol. Appl. Sci. 2020, 10, 7596. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Q.; Wei, R.; Ma, J.; Wang, Y. Mechanism and Dynamic Study of Reactive Red X-3B Dye Degradation by Ultrasonic-Assisted Ozone Oxidation Process. Ultrason. Sonochem. 2017, 38, 681–692. [Google Scholar] [CrossRef]
- Yargeau, V.; Danylo, F. Removal and Transformation Products of Ibuprofen Obtained during Ozone- and Ultrasound-Based Oxidative Treatment. Water Sci. Technol. 2015, 72, 491–500. [Google Scholar] [CrossRef]
- Condón-Abanto, S.; Arroyo, C.; Álvarez, I.; Condón, S.; Lyng, J.G. Application of Ultrasound in Combination with Heat and Pressure for the Inactivation of Spore Forming Bacteria Isolated from Edible Crab (Cancer Pagurus). Int. J. Food Microbiol. 2016, 223, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, S.; Yang, Y.; Yao, B.; Tang, Y.; Luo, L.; Zhi, D.; Wan, Z.; Wang, L.; Zhou, Y. A Review on Percarbonate-Based Advanced Oxidation Processes for Remediation of Organic Compounds in Water. Environ. Res. 2021, 200, 111371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced Oxidation Processes for Water Purification Using Percarbonate: Insights into Oxidation Mechanisms, Challenges, and Enhancing Strategies. J. Hazard. Mater. 2023, 442, 130014. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M. Sonochemical Degradation of Rhodamine B in Aqueous Phase: Effects of Additives. Chem. Eng. J. 2010, 158, 550–557. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Nejumal, K.K.; Satayev, M.I.; Rayaroth, M.P.; Arun, P.; Dineep, D.; Aravind, U.K.; Azimov, A.M.; Aravindakumar, C.T. Degradation Studies of Bisphenol S by Ultrasound Activated Persulfate in Aqueous Medium. Ultrason. Sonochem. 2023, 101, 106700. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Guo, W.; Yi, C.; Leng, Y.; Ma, Z. Degradation of Amoxicillin in Aqueous Solution Using Sulphate Radicals under Ultrasound Irradiation. Ultrason. Sonochem. 2012, 19, 469–474. [Google Scholar] [CrossRef]
- Lin, M.; Ning, X.; An, T.; Zhang, J.; Chen, C.; Ke, Y.; Wang, Y.; Zhang, Y.; Sun, J.; Liu, J. Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Textile Dyeing Sludge with Ultrasound and Fenton Processes: Effect of System Parameters and Synergistic Effect Study. J. Hazard. Mater. 2016, 307, 7–16. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Chen, L.; Wang, S.; Zhang, D. Ozonation Combined with Ultrasound for the Degradation of Tetracycline in a Rectangular Air-Lift Reactor. Sep. Purif. Technol. 2012, 84, 138–146. [Google Scholar] [CrossRef]
- Waldemer, R.H.; Tratnyek, P.G.; Johnson, R.L.; Nurmi, J.T. Oxidation of Chlorinated Ethenes by Heat-Activated Persulfate: Kinetics and Products. Environ. Sci. Technol. 2007, 41, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, X.; Cong, Q.; Li, Q.; Wang, X.; Zhong, S.; Deng, H.; Yan, B. Research Progress of Ozone/Peroxymonosulfate Advanced Oxidation Technology for Degrading Antibiotics in Drinking Water and Wastewater Effluent: A Review. Molecules 2024, 29, 1170. [Google Scholar] [CrossRef]
- Sun, S.; Ren, Y.; Guo, F.; Zhou, Y.; Cui, M.; Ma, J.; Han, Z.; Khim, J. Comparison of Effects of Multiple Oxidants with an Ultrasonic System under Unified System Conditions for Bisphenol A Degradation. Chemosphere 2023, 329, 138526. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yi, P.; Wang, X.; Gao, H.; Zhang, H. Degradation of Acid Orange 7 by an Ultrasound/ZnO-GAC/Persulfate Process. Sep. Purif. Technol. 2018, 194, 181–187. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Li, X.; Zhou, Z.; Wei, B. Ultrasonic–Thermal Regeneration of Spent Powdered Activated Carbon. Sustainability 2023, 15, 9060. [Google Scholar] [CrossRef]
- Ghanbari, F.; Khatebasreh, M.; Mahdavianpour, M.; Lin, K.-Y.A. Oxidative Removal of Benzotriazole Using Peroxymonosulfate/Ozone/Ultrasound: Synergy, Optimization, Degradation Intermediates and Utilizing for Real Wastewater. Chemosphere 2020, 244, 125326. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; El-taliawy, H.; Durán, A.; Caro, G.; Bester, K. Sono-Activated Persulfate Oxidation of Diclofenac: Degradation, Kinetics, Pathway and Contribution of the Different Radicals Involved. J. Hazard. Mater. 2018, 357, 457–465. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, H.; Xian, T.; Li, R.S.; Zhang, H.M.; Wang, X.X. Sonocatalytic Degradation of RhB over LuFeO3 Particles under Ultrasonic Irradiation. J. Hazard. Mater. 2015, 289, 149–157. [Google Scholar] [CrossRef]
- Lu, X.; Qiu, W.; Peng, J.; Xu, H.; Wang, D.; Cao, Y.; Zhang, W.; Ma, J. A Review on Additives-Assisted Ultrasound for Organic Pollutants Degradation. J. Hazard. Mater. 2021, 403, 123915. [Google Scholar] [CrossRef]
- Ekande, O.S.; Johnson, I.; Nagasai, K.; Kumar, M. Single and Multi-Antibiotics Removal via Peroxymonosulfate Activation Using Molybdenum Disulfide (MoS2): Central Composite Design and Degradation Pathway. Chemosphere 2023, 338, 139554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, X.; Hao, J.; Wang, Z.; Huo, B.; Qi, J.; Wang, Y.; Meng, F. Sustainable Self-Powered Degradation of Antibiotics Using Fe3O4@MoS2/PVDF Modified Pipe with Superior Piezoelectric Activity: Mechanism Insight, Toxicity Assessment and Energy Consumption. Appl. Catal. B Environ. 2023, 331, 122655. [Google Scholar] [CrossRef]
- Du, J.; Tang, S.; Ling, H.; Zheng, H.; Xiao, G.; Luo, L.; Bao, J. Insights into Periodate Oxidation of Bisphenol A Mediated by Manganese. Chem. Eng. J. 2019, 369, 1034–1039. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chen, M.-J.; Huang, C.-P.; Kuo, J.; Lo, S.-L. Efficient Sonochemical Degradation of Perfluorooctanoic Acid Using Periodate. Ultrason. Sonochem. 2016, 31, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Saadi, S.; Vahid, B.; Joo, S.W.; Min, B.-K. Sonocatalytic Degradation of Acid Blue 92 Using Sonochemically Prepared Samarium Doped Zinc Oxide Nanostructures. Ultrason. Sonochem. 2016, 29, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Moradian, F.; Ramavandi, B.; Jaafarzadeh, N.; Kouhgardi, E. Activation of Periodate Using Ultrasonic Waves and UV Radiation for Landfill Leachate Treatment: In Review. Environ. Sci. Pollut. Res. 2022, 29, 90338–90350. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Soltani, R.D.C.; Karimi, A.; Joo, S.W. Sonocatalytic Degradation of a Textile Dye over Gd-Doped ZnO Nanoparticles Synthesized through Sonochemical Process. Ultrason. Sonochem. 2015, 23, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sponza, D.T.; Oztekin, R. Dephenolization, Dearomatization and Detoxification of Olive Mill Wastewater with Sonication Combined with Additives and Radical Scavengers. Ultrason. Sonochem. 2014, 21, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Andaluri, G.; Rokhina, E.V.; Suri, R.P.S. Evaluation of Relative Importance of Ultrasound Reactor Parameters for the Removal of Estrogen Hormones in Water. Ultrason. Sonochem. 2012, 19, 953–958. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Merouani, S. Improvement of Sonochemical Degradation of Brilliant Blue R in Water Using Periodate Ions: Implication of Iodine Radicals in the Oxidation Process. Ultrason. Sonochem. 2017, 37, 344–350. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Computer Simulation of Chemical Reactions Occurring in Collapsing Acoustical Bubble: Dependence of Free Radicals Production on Operational Conditions. Res. Chem. Intermed. 2015, 41, 881–897. [Google Scholar] [CrossRef]
- Weng, C.-H.; Tsai, K.-L. Ultrasound and Heat Enhanced Persulfate Oxidation Activated with Fe0 Aggregate for the Decolorization of C.I. Direct Red 23. Ultrason. Sonochem. 2016, 29, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sukhatskiy, Y.; Sozanskyi, M.; Shepida, M.; Znak, Z.; Gogate, P.R. Decolorization of an Aqueous Solution of Methylene Blue Using a Combination of Ultrasound and Peroxate Process. Sep. Purif. Technol. 2022, 288, 120651. [Google Scholar] [CrossRef]
- Eslami, A.; Asadi, A.; Meserghani, M.; Bahrami, H. Optimization of Sonochemical Degradation of Amoxicillin by Sulfate Radicals in Aqueous Solution Using Response Surface Methodology (RSM). J. Mol. Liq. 2016, 222, 739–744. [Google Scholar] [CrossRef]
- Darvishi Cheshmeh Soltani, R.; Safari, M. Periodate-Assisted Pulsed Sonocatalysis of Real Textile Wastewater in the Presence of MgO Nanoparticles: Response Surface Methodological Optimization. Ultrason. Sonochem. 2016, 32, 181–190. [Google Scholar] [CrossRef]
- Seid-Mohammadi, A.; Asgari, G.; Shokoohi, R.; Baziar, M.; Mirzaei, N.; Adabi, S.; Partoei, K. Degradation of Phenol Using US/Periodate/nZVI System from Aqueous Solutions. Glob. NEST J. 2019, 21, 360–367. [Google Scholar]
- Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-Assisted Heterogeneous Fenton-like Degradation of Tetracycline over a Magnetite Catalyst. J. Hazard. Mater. 2016, 302, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rahdar, A.; Igwegbe, C.A.; Mortazavi-Derazkola, S.; Banach, A.M.; Rahdar, S.; Singh, A.K.; Rodriguez-Couto, S.; Kyzas, G.Z. Praseodymium-Doped Cadmium Tungstate (CdWO4) Nanoparticles for Dye Degradation with Sonocatalytic Process. Polyhedron 2020, 190, 114792. [Google Scholar] [CrossRef]
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-Activated Persulfate Oxidation of Bisphenol A: Kinetics, Pathways and the Controversial Role of Temperature. Chem. Eng. J. 2015, 280, 623–633. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical Degradation of Coomassie Brilliant Blue: Effect of Frequency, Power Density, pH and Various Additives. Chemosphere 2015, 119, 848–855. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Singlet-Oxygen Generation in Alkaline Periodate Solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, S.; Raghavan, N.; Kadarkarai, G.; Kim, S.-J.; Venugopal, G. Graphene-Oxide (GO)–Fe3+ Hybrid Nanosheets with Effective Sonocatalytic Degradation of Reactive Red 120 and Study of Their Kinetics Mechanism. Ultrason. Sonochem. 2015, 24, 123–131. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic Degradation of Antibiotic Sulfadiazine in a Heterogeneous Ultrasound-Enhanced Fe0/Persulfate Fenton-like System. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Anju, S.G.; Yesodharan, S.; Yesodharan, E.P. Zinc Oxide Mediated Sonophotocatalytic Degradation of Phenol in Water. Chem. Eng. J. 2012, 189–190, 84–93. [Google Scholar] [CrossRef]
- Saien, J.; Fallah Vahed Bazkiaei, M. Homogenous UV/Periodate Process in Treatment of p-Nitrophenol Aqueous Solutions under Mild Operating Conditions. Environ. Technol. 2018, 39, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical Decomposition of Perfluorooctanoic Acid in Aqueous Periodate with VUV and UV Light Irradiation. J. Hazard. Mater. 2010, 179, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Moumeni, O.; Hamdaoui, O. Intensification of Sonochemical Degradation of Malachite Green by Bromide Ions. Ultrason. Sonochem. 2012, 19, 404–409. [Google Scholar] [CrossRef]
- Deng, J.; Fang, Y.; Hou, C.; Zhang, Y.; Li, M.; Han, J.; Du, W.; Tang, C.; Hu, X. Ultrasonic Assisted Activation of Persulfate for the Treatment of Spent Porous Biochar: Degradation of Adsorbed PFOA and Adsorbent Regeneration. J. Environ. Chem. Eng. 2023, 11, 111146. [Google Scholar] [CrossRef]
- Yang, L.; Xue, J.; He, L.; Wu, L.; Ma, Y.; Chen, H.; Li, H.; Peng, P.; Zhang, Z. Review on Ultrasound Assisted Persulfate Degradation of Organic Contaminants in Wastewater: Influences, Mechanisms and Prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Chen, W.-S.; Huang, C.-P. Mineralization of Dinitrotoluenes in Aqueous Solution by Sono-Activated Persulfate Enhanced with Electrolytes. Ultrason. Sonochem. 2019, 51, 129–137. [Google Scholar] [CrossRef]
- Golash, N.; Gogate, P.R. Degradation of Dichlorvos Containing Wastewaters Using Sonochemical Reactors. Ultrason. Sonochem. 2012, 19, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Prakash, L.V.; Gopinath, A.; Gandhimathi, R.; Velmathi, S.; Ramesh, S.T.; Nidheesh, P.V. Ultrasound Aided Heterogeneous Fenton Degradation of Acid Blue 15 over Green Synthesized Magnetite Nanoparticles. Sep. Purif. Technol. 2021, 266, 118230. [Google Scholar] [CrossRef]
- Mishra, K.P.; Gogate, P.R. Intensification of Degradation of Aqueous Solutions of Rhodamine B Using Sonochemical Reactors at Operating Capacity of 7L. J. Environ. Manag. 2011, 92, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, N.; Deng, Y.; Lin, T.F.; Ma, Y.; Li, L.; Sui, M. Degradation of Bisphenol-A Using Ultrasonic Irradiation Assisted by Low-Concentration Hydrogen Peroxide. J. Environ. Sci. 2011, 23, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Degradation of Pharmaceuticals by Ultrasound-Based Advanced Oxidation Process. Environ. Chem. Lett. 2016, 14, 259–290. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Mashayekhi, M.; Naderi, M.; Boczkaj, G.; Jorfi, S.; Safari, M. Sonocatalytic Degradation of Tetracycline Antibiotic Using Zinc Oxide Nanostructures Loaded on Nano-Cellulose from Waste Straw as Nanosonocatalyst. Ultrason. Sonochem. 2019, 55, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sukhatskiy, Y.; Shepida, M.; Sozanskyi, M.; Znak, Z.; Gogate, P.R. Periodate-Based Advanced Oxidation Processes for Wastewater Treatment: A Review. Sep. Purif. Technol. 2023, 304, 122305. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Ma, Y.; Wu, L.; Zheng, L.; Wang, J.; Chen, Y.; Li, Y.; Zhang, Z. Periodate-Based Oxidation Focusing on Activation, Multivariate-Controlled Performance and Mechanisms for Water Treatment and Purification. Sep. Purif. Technol. 2022, 289, 120746. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Martín, I.S.; García, S. Ultrasound-Assisted Homogeneous Photocatalytic Degradation of Reactive Blue 4 in Aqueous Solution. Appl. Catal. B: Environ. 2014, 152–153, 59–67. [Google Scholar] [CrossRef]
| Technologies | Advantages | Disadvantages | References |
|---|---|---|---|
| US/PI oxidation method | Efficient degradation of pollutants; rapid reaction kinetics; outstanding environmental compatibility; wide applicability; straightforward operation. | Substantial energy consumption; significant equipment expenditure. | [22] |
| Photochemical catalytic oxidation method | Lighting is sustainable and eco-friendly; exerts minimal impact on water quality; characterized by low operating costs; boasts a long service life | Optimization is required for its integration with other advanced technologies such as UV/H2O2 and UV/PDS; the UV radiation from built-in light tubes poses health risks to humans. | [23] |
| Electrochemical oxidation process | The electrolysis device is streamlined, user-friendly, and offers straightforward control, with an anode that efficiently oxidizes pollutants. Altering the anode material enables the targeted degradation of various organic compounds. | The energy consumption is excessively high, the reactor exhibits suboptimal efficiency, and the associated equipment costs are considerable. | [24] |
| Ozone technology | Fast oxidation speed and high oxidation efficiency. | The equipment configuration is intricate and characterized by high energy demands accompanied by significant financial outlays. | [9] |
| Contaminant | Degradation Products | Key Mechanism | End Products | References |
|---|---|---|---|---|
| Perfluorooctanoic acid |
Perfluoroolefins. 1 h-perfluoroalkanes | Cleavage of C–C and C–F bonds in perfluoroanions; ultrasonic pyrolysis occurring at or near the bubble interface; oxidation of PFOA via electron transfer mediated by IO3• radicals. | CO, CO2, HF, etc. | [34] |
| Acid Blue 92 |
1,2-Benzenedicarboxylic acid 1-Hydroxycyclohexane-1-carboxylic acid Aniline; acetic acid; formamide |
Dyes undergo degradation through the cleavage of N≡N, C-C, C-S, and C-N bonds. Hydroxyl and amino groups attached to the benzene ring undergo oxidation. The aromatic ring undergoes opening, leading to the formation of the final product. | CO2, H2O, etc. | [35] |
| landfill leachate |
Cyclohexylsiloxane Cyclotridecane Phenol | IO3•, IO4• and • OH radicals oxidize litter permeability, forming intermediates that are finally broken down into CO2 and H2O. | CO2, H2O, etc. | [36] |
| acid orange 7 |
1,3-Biphenyl Acetic acid 2-propionic acid Methyl ethyl Cyclohexene Ethylbenzene Trimethyl | Cleavage of the C–N bond in heterocyclic compounds; extraction of hydrogen atoms. oxidation of methyl groups; nitro group hydrolysis on aromatic rings; oxidation of hydroxyl groups and phenolic compounds; internal ultrasound-assisted pyrolysis. |
NH3, SO2 CO2, H2O, etc. | [37] |
|
industrial wastewater (TAAs) | Trimethylaniline, aniline, o-toluidine, o-aminoaniline, xylene, ethylbenzene, and duran | Cleavage of azo groups, as well as methyl, ethyl, and C-H-O bonds; ultrasound-assisted internal pyrolysis; oxidation of hydroxyl and amino groups on benzene rings; oxidation and decarboxylation of methyl groups, accompanied by hydrogen extraction; hydrolysis of aromatic rings. | NH3, CO2, etc. | [38] |
| Analytes | Selected pH Range | Optimal pH | Proposal Reactions | References |
|---|---|---|---|---|
| Perfluorooctanoic acid | 3.9, 7, 10.1 | 3.9 | [34] | |
| Brilliant Blue R | 2, 5.2, 8 | 8 | [40] | |
| Bisphenol A | 6, 7, 8.5 | 8.5 | [24] | |
| Phenol | 3, 7, 11 | 3 | [46] | |
| Garbage permeate | 3, 5, 7, 9, 11 | 3 | [36] |
| Contaminant |
Ultrasound Properties |
PI Concentration |
US Removal Efficiency
(%) |
PI Removal Efficiency
(%) | US/PI Removal/TOC Efficiency (%) | References |
|---|---|---|---|---|---|---|
|
Perfluorooctanoic acid (170.1 μmol/L) |
P:120 W f:40 kHz | 45 mmol/L | 2 h/26.2 | 2 h/2 | 2 h/96.5/95.7 | [34] |
|
Brilliant Blue R (5 mg/L) |
P:80 W f:300 kHz | 10 mmol/L | 140 min/100 | 60 min/0 | 60 min/100/100 | [40] |
|
Acid Blue 92 Dye (10 mg/L) |
P:150 W f:36 kHz | 0.15 mol/L | 150 min/46 | none | 150 min/98/none | [35] |
| Tetracycline (50 mg/L) |
P:256 W f:37 kHz | 0.01 mol/L | 45 min/12.8 | none | 45 min/94.2/77.9 | [66] |
| Orange 7 Acid (50 mg/L) |
P:150 W/L f:36 kHz | 2 mmol/L | 105 min/27.5 | 60 min/0 | 90 min/96/none | [37] |
| Industrial wastewater (2880 mg/L) |
P:100 W f:37 kHz | 0.11 mol/L | 120 min/18.43 | none | 120 min/98/79 | [45] |
| Ramazol Black B (100 mg/L) |
P:500 W f:60 kHz | 10 mg/L | 100 min/34.7 | none | 100 min/98.7/none | [48] |
| Aromatic amine (1990 mg/L) |
P:500 W f:35 kHz | 16 mg/L | 150 min/79 | none | 150 min/99/96 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Wang, Z.; Jiang, Y.; Yang, S.; Deng, Q. Research Progress on the Degradation of Organic Pollutants in Wastewater via Ultrasound/Periodate Systems: A Review. Molecules 2024, 29, 2562. https://doi.org/10.3390/molecules29112562
Song T, Wang Z, Jiang Y, Yang S, Deng Q. Research Progress on the Degradation of Organic Pollutants in Wastewater via Ultrasound/Periodate Systems: A Review. Molecules. 2024; 29(11):2562. https://doi.org/10.3390/molecules29112562
Chicago/Turabian StyleSong, Tiehong, Zhe Wang, Yi Jiang, Shenggang Yang, and Qiyuan Deng. 2024. "Research Progress on the Degradation of Organic Pollutants in Wastewater via Ultrasound/Periodate Systems: A Review" Molecules 29, no. 11: 2562. https://doi.org/10.3390/molecules29112562
APA StyleSong, T., Wang, Z., Jiang, Y., Yang, S., & Deng, Q. (2024). Research Progress on the Degradation of Organic Pollutants in Wastewater via Ultrasound/Periodate Systems: A Review. Molecules, 29(11), 2562. https://doi.org/10.3390/molecules29112562







