Adsorption and Aggregation Behaviors of Oleyl Alcohol-Based Extended Surfactant and Its Mixtures
Abstract
:1. Introduction
2. Results and Discussion
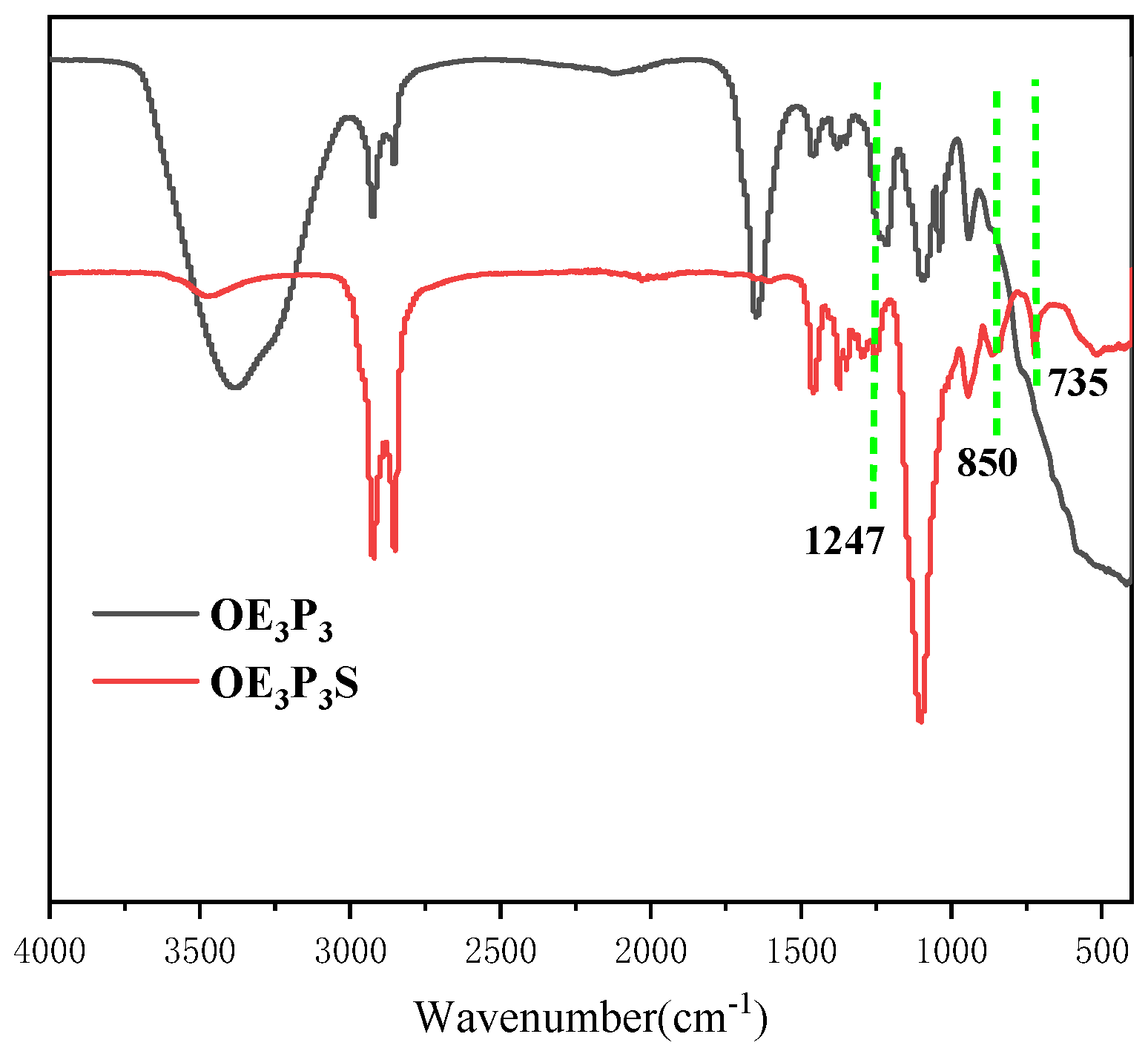
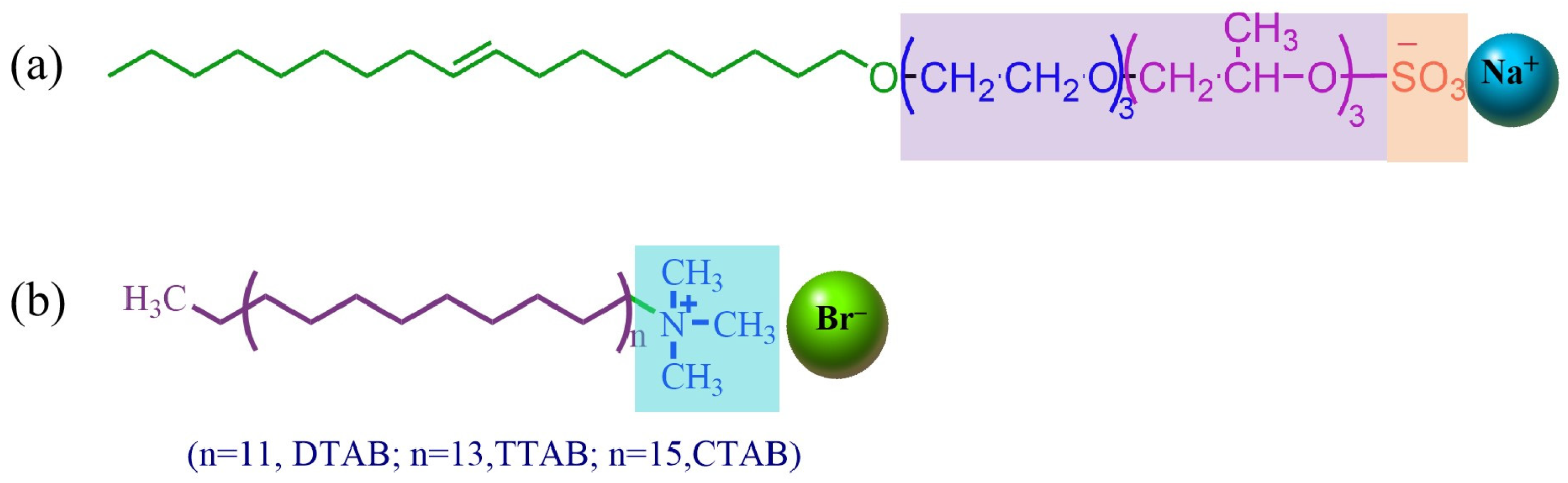
2.1. Structural Characterization
2.2. Krafft Point
2.3. Adsorption Properties
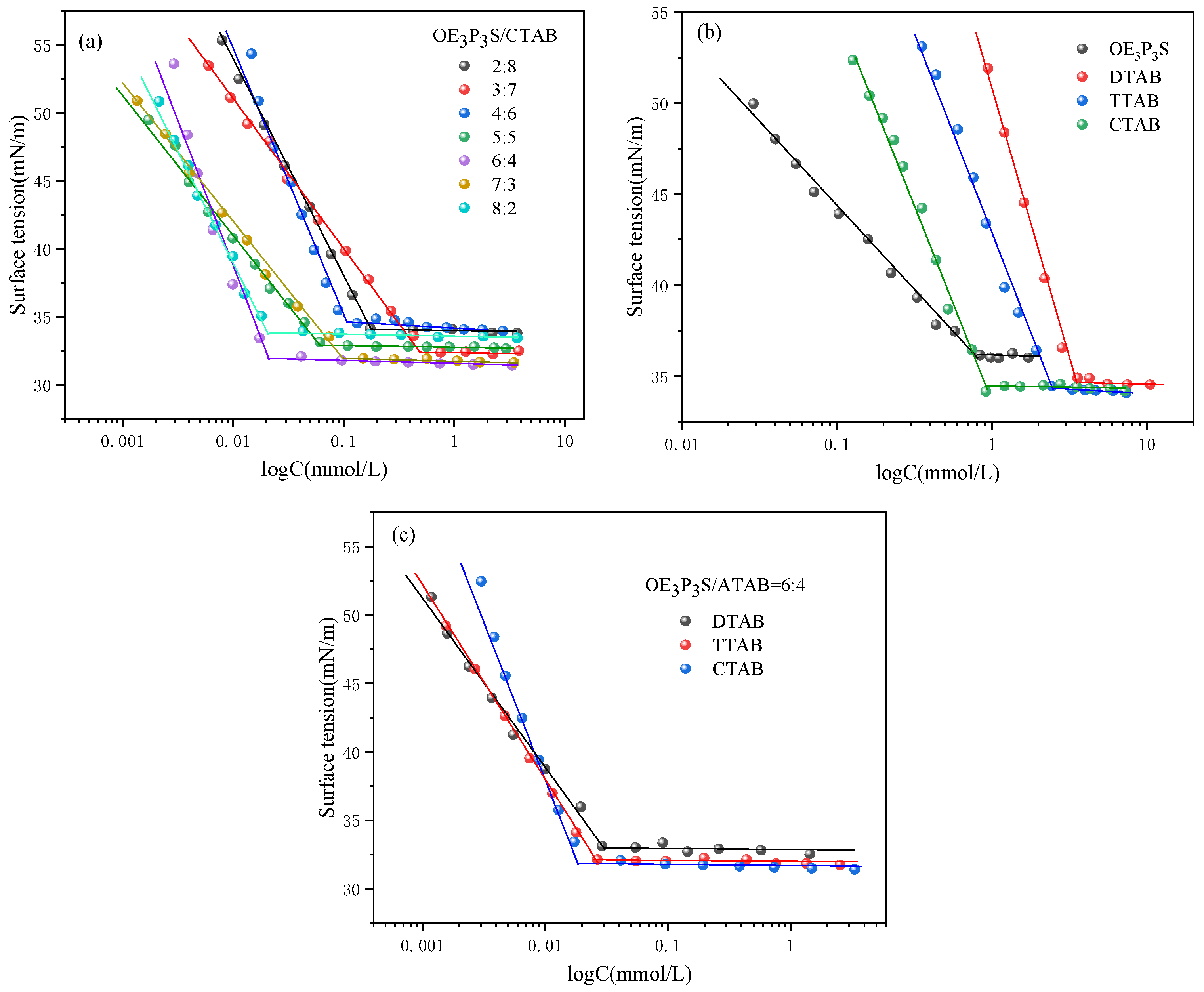
2.3.1. Equilibrium Surface Tension
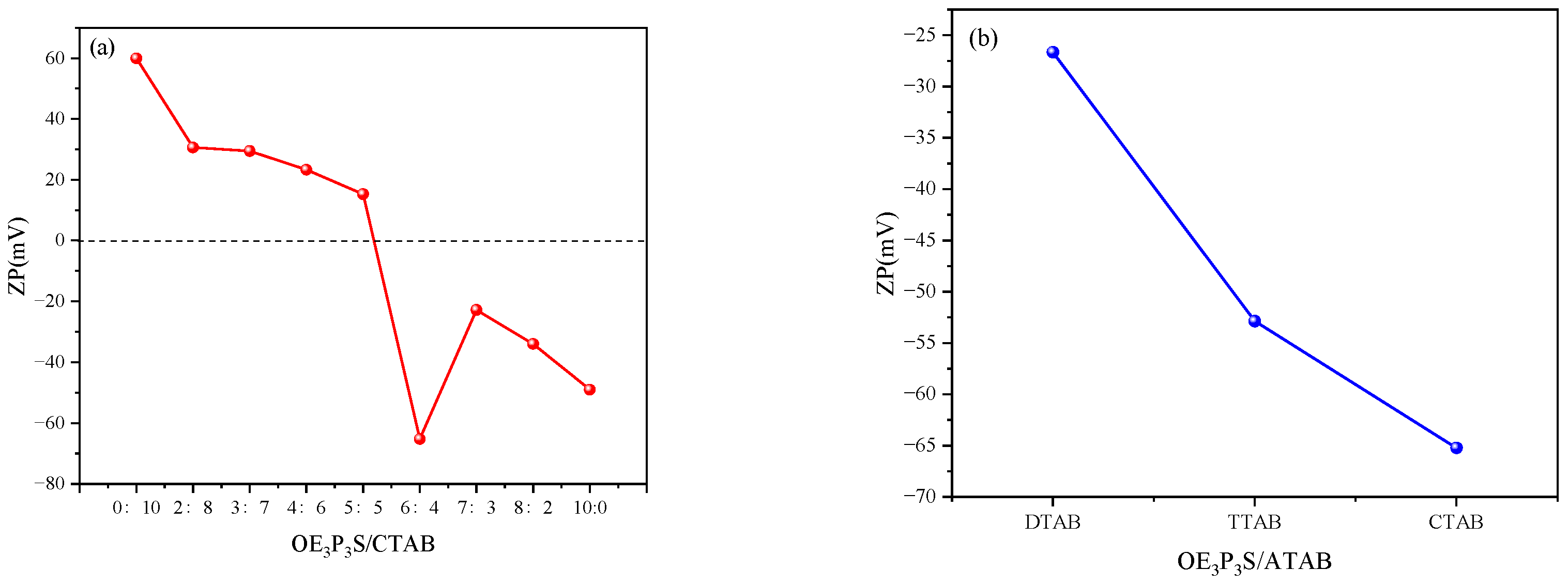
2.3.2. Zeta Potential Measurements
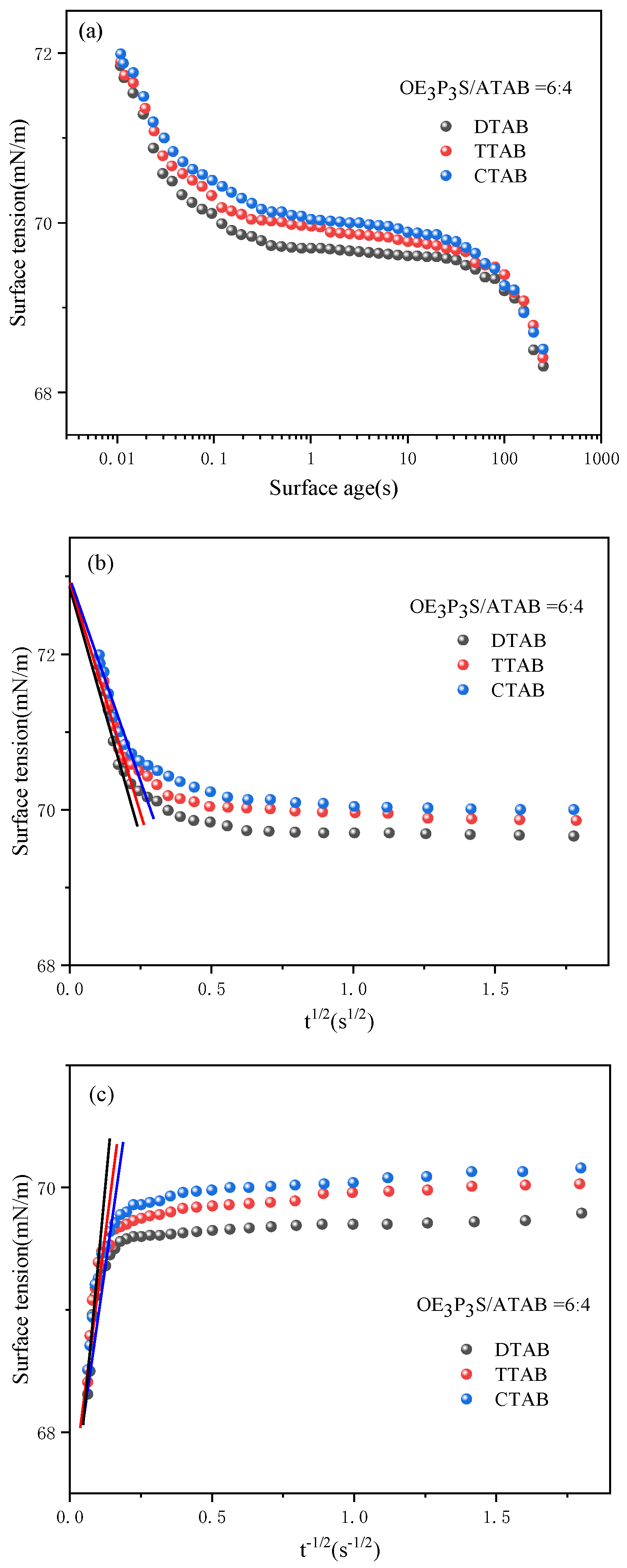
2.3.3. Dynamic Surface Tension
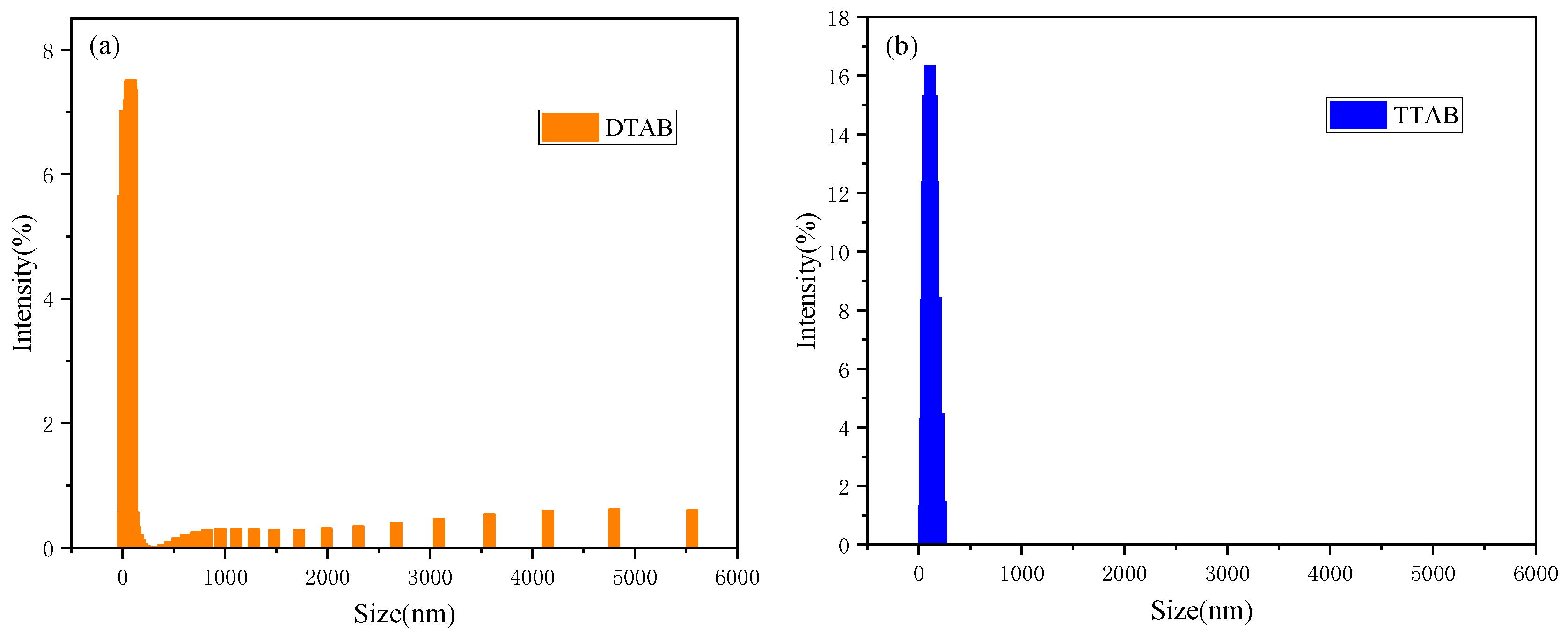
2.4. Aggregation Behaviors
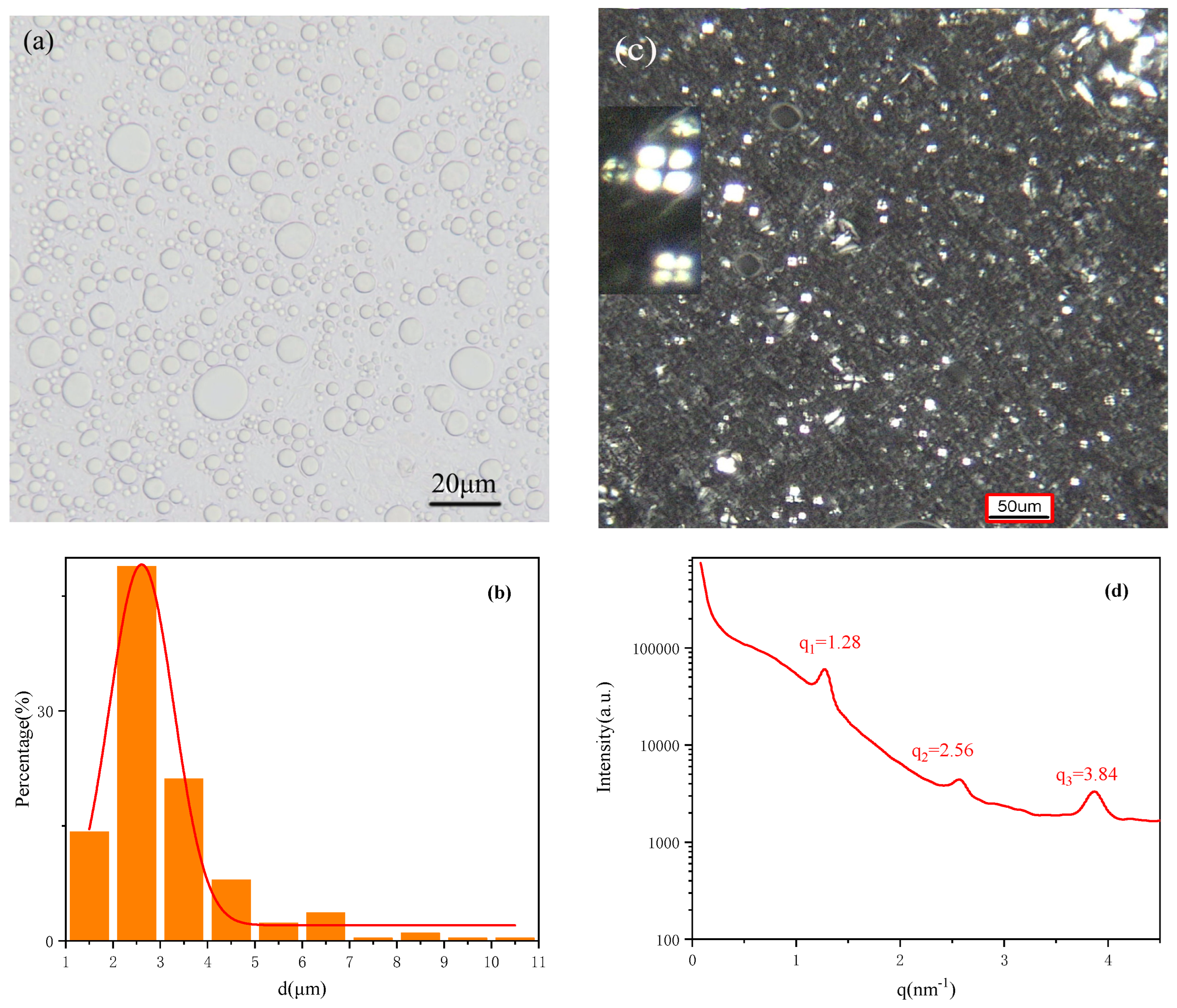
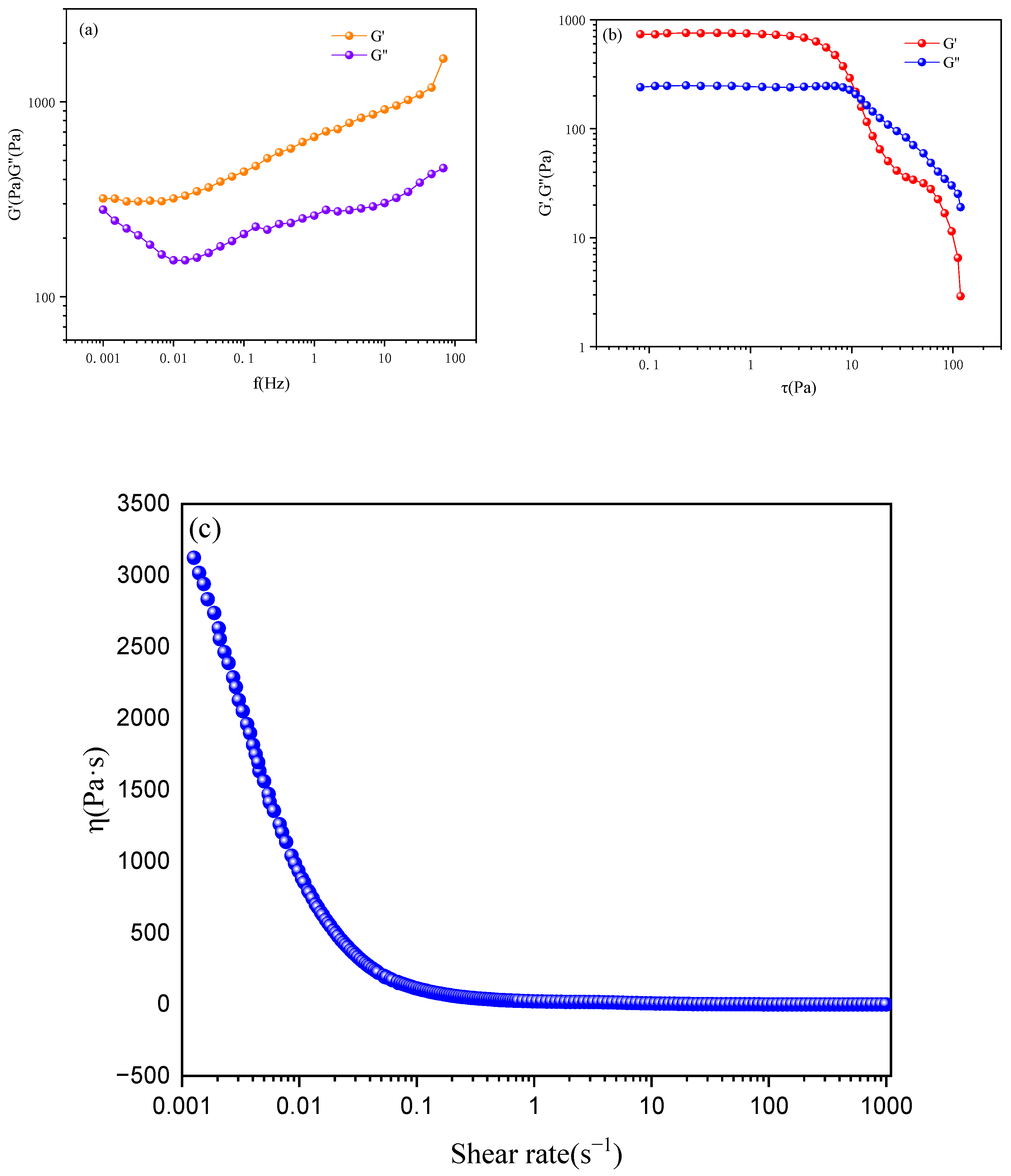
2.5. Liquid Crystal Emulsion
3. Materials and Methods
3.1. Materials
3.2. Sample Synthesis and Identification
3.3. Krafft Point (KT)
3.4. Measurements
3.5. Preparation of Emulsion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.G.; Pan, Y.; Xu, Z.C.; Zhang, L.; Zhang, L.; Yang, S.C. Ultralow interfacial tension achieved by extended anionic surfactants with a short hydrophobic chain. J. Mol. Liq. 2024, 400, 124514–124520. [Google Scholar] [CrossRef]
- Wang, Z.S.; Zhou, Z.H.; Han, L.; Chen, X.; He, H.; Zhang, Q.; Xu, Z.C.; Gong, Q.T.; Zhang, L.; Ma, G.Y.; et al. The mechanism for lowering interfacial tension by Extended surfactant containing ethylene oxide and propylene oxide groups. J. Mol. Liq. 2022, 359, 119364–119373. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, Y.; Tian, F.; Liu, H.; Lu, X.; Yi, X.; Wang, Z. Performance of extended surfactant and its mixture with betaine surfactant for enhanced oil recovery in sandstone reservoirs with low permeability. J. Mol. Liq. 2023, 391, 123228–123242. [Google Scholar] [CrossRef]
- He, H.J.; Xiao, H.Y.; Zhou, Z.H.; Zhang, Q.; Gao, M.; Chen, X.F.; Zhang, L.; Zhang, L. Effect of oxyethylene and oxypropylene groups on the interfacial structure and property of Extended surfactants: Molecular simulation and experimental study. J. Mol. Liq. 2023, 382, 121944–121954. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.Y.; Fang, Y.; Liu, H.H.; Xia, Y.M. Comparative study of conventional/ethoxylated/Extended n-alkylsulfate surfactants. Langmuir 2019, 35, 3116–3125. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, Q.; Zhu, Y.; Zhou, Z.; Zhang, F.; Zhang, L.; Yan, F.; Wang, M.; Zhang, L. Adsorption of extended surfactants at the water-PTFE interface: The effect of PO number. J. Mol. Liq. 2022, 348, 118465–118473. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.Y.; Fang, Y.; Xia, Y.M. Cooperative effects of polypropylene oxide spacers and alkyl chains on dynamic amphipathicity of extended surfactants. J. Mol. Liq. 2020, 311, 113276–113285. [Google Scholar] [CrossRef]
- Ren, J.; Xiao, H.Y.; Cao, X.L.; Yuan, F.Q.; Pan, B.L.; Ma, B.D.; Zhang, L.; Zhang, L. Molecular dynamics simulation study on interfacial behaviors of betaines and Extended surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131323–131332. [Google Scholar] [CrossRef]
- Wang, G.; Liu, L.; He, D.; Lu, R.; Xie, Y.; Lai, L. Cationic-anionic surfactant mixtures based on gemini surfactant as a candidate for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132297–132305. [Google Scholar] [CrossRef]
- Li, S.J.; Lai, L.; Mei, P.; Li, Y.; Cheng, L.; Ren, Z.H.; Liu, Y. Equilibrium and dynamic surface properties of cationic/anionic surfactant mixtures based on bisquaternary ammonium salt. J. Mol. Liq. 2018, 254, 248–254. [Google Scholar] [CrossRef]
- Zhou, H.T.; Lin, S.S.; Xing, H.; Gao, A.T.; Xiao, J.X. The role of nascn on the equimolar cationic–anionic surfactant mixtures: Evidence from nmr, surface tension and dynamic light scattering. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 16–22. [Google Scholar] [CrossRef]
- Ling, T.; Jiang, Z.; Yang, X.; Li, P.; Li, J.; Ren, X.; Guo, C. Synthesis, surface properties, aggregation behavior of oleyl ether sulfates and their application to liquid crystal emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128655–128665. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, Z.H.; Shangguan, Y.N.; Han, L.; Wang, L.L.; Fan, W.; Zhang, Q.; Zhang, L.; Ma, G.Y.; Zhang, L. Effect of bivalent cations on the interfacial tensions of Extended anionic surfactant solutions. J. Mol. Liq. 2022, 349, 118162–118169. [Google Scholar] [CrossRef]
- Chabba, S.; Kumar, S.; Aswal, V.K.; Kang, T.S.; Mahajan, R.K. Interfacial and aggregation behavior of aqueous mixtures of imidazolium based surface active ionic liquids and anionic surfactant sodium dodecylbenzenesulfonate. Colloids Surf. A Physicochem. Eng. Asp. 2015, 472, 9–20. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, B. Principles of Surfactant Action; China Light Industry Press: Beijing, China, 2003; pp. 140–180. [Google Scholar]
- Dudek, M.; Olsen, A.O.J.; Øye, G. The influence of divalent cations on the dynamic surface tension of sodium dodecylbenzenesulfonate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132007–132017. [Google Scholar] [CrossRef]
- Sohrabi, B.; Gharibi, H.; Tajik, B.; Javadian, S.; Hashemianzadeh, M. Molecular interactions of cationic and anionic surfactants in mixed monolayers and aggregates. J. Phys. Chem. B 2008, 112, 14869–14876. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. A simple thermodynamic analysis of the stability of ionic/nonionic mixed micelles. J. Colloid Interface Sci. 1995, 172, 98–105. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Synthesis, characterization, and physicochemical properties of a series of quaternary gemini surfactants with different spacer lengths. Colloid Polym. Sci. 2017, 295, 2261–2277. [Google Scholar] [CrossRef]
- Du, C.; Cai, D.; Qin, M.; Zheng, P.; Hao, Z.; Yin, T.; Zhao, J.; Shen, W. Thermodynamics of mixed surfactant solutions of n,n′-bis(dimethyldodecyl)-1,2-ethanediammoniumdibromide with 1-dodecyl-3-methylimidazolium bromide. J. Phys. Chem. B 2014, 118, 1168–1179. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C. Synergistic interactions in the mixed micelles of anionic amino acid surfactant with cationic gemini surfactant. J. Chem. Thermodyn. 2021, 162, 106568–106579. [Google Scholar] [CrossRef]
- Amalendu, P.; Renu, P. Self-aggregation behaviour of cationic surfactant tetradecyltrimethylammonium bromide and bi-amphiphilic surface active ionic liquid 3-methyl-1-pentylimidazolium dodecylsulfate in aqueous solution. J. Mol. Liq. 2020, 304, 112803–112812. [Google Scholar]
- Gao, Y.; Yang, X. Equilibrium and dynamic surface properties of sulfosuccinate surfactants. J. Surfactants Deterg. 2014, 17, 1117–1123. [Google Scholar] [CrossRef]
- Manousakis, M.; Avranas, A. Dynamic surface tension studies of mixtures of hydroxypropylmethylcellulose with the double chain cationic surfactants didodecyldimethylammonium bromide and ditetradecyldimethylammonium bromide. J. Colloid Interface Sci. 2013, 402, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Fainerman, V.B.; Makievski, A.V.; Krägel, J.; Grigoriev, D.O.; Kazakov, V.N.; Sinyachenko, O.V. Dynamics of protein and mixed protein/surfactant adsorption layers at the water/fluid interface. Adv. Colloid Interface Sci. 2000, 86, 39–82. [Google Scholar] [CrossRef]
- Nahringbauer, I. Dynamic surface tension of aqueous polymer solutions, i: Ethyl(hydroxyethyl)cellulose (bermocoll cst-103). J. Colloid Interface Sci. 1995, 176, 318–328. [Google Scholar] [CrossRef]
- Avranas, A.; Tasopoulos, V. Aqueous solutions of sodium deoxycholate and hydroxypropylmethylcellulose: Dynamic surface tension measurements. J. Colloid Interface Sci. 2000, 221, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.E.; Sánchez, C.C.; Pilosof, A.M.R.; Rodríguez Patino, J.M. Dynamics of adsorption of hydroxypropyl methylcellulose at the air–water interface. Food Hydrocoll. 2008, 22, 387–402. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S. Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface. Adv. Colloid Interface Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S.; Heenan, R.K. Dynamic surface tensions and micelle structures of dichained phosphatidylcholine surfactant solutions. Langmuir 1998, 14, 5719–5724. [Google Scholar] [CrossRef]
- Jafarlou, A.; Javadi, A.; Miller, R.; Eckert, K. Dynamics of adsorption of CTAB-Silica nanoparticle complexes: New experiments and modeling approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127448–127456. [Google Scholar] [CrossRef]
- Jiang, R.; Ma, Y.; Zhao, J. Adsorption dynamics of binary mixture of gemini surfactant and opposite-charged conventional surfactant in aqueous solution. J. Colloid Interface Sci. 2006, 297, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.B.; Zhu, Y.; Zhu, B.Y.; Li, R.K.; Fu, H.L. Spontaneous vesicle formation in aqueous mixtures of cationic surfactants and partially hydrolyzed polyacrylamide. J. Colloid Interface Sci. 2001, 236, 201–207. [Google Scholar] [CrossRef]
- Terescenco, D.; Picard, C.; Clemenceau, F.; Grisel, M.; Savary, G. Influence of the emollient structure on the properties of cosmetic emulsion containing lamellar liquid crystals. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 10–19. [Google Scholar] [CrossRef]
- Friberg, S.E. Micelies, microemulsions, liquid crystals, and the structure of stratum corneum lipids. J. Soc. Cosmet. Chem. 1990, 41, 155–171. [Google Scholar]
- Savić, S.; Savić, M.; Tamburić, S.; Vuleta, G.; Vesić, S.; Müller-Goymann, C.C. An alkylpolyglucoside surfactant as a prospective pharmaceutical excipient for topical formulations: The influence of oil polarity on the colloidal structure and hydrocortisone in vitro/in vivo permeation. Eur. J. Pharm. Sci. 2007, 30, 441–450. [Google Scholar] [CrossRef]
- Duncke, A.C.P.; Marinho, T.O.; Barbato, C.N.; Freitas, G.B.; de Oliveira, M.C.K.; Nele, M. Liquid crystal observations in emulsion fractions from brazilian crude oils by polarized light microscopy. Energy Fuels 2016, 30, 3815–3820. [Google Scholar] [CrossRef]
- Capaccio, A.; Caserta, S.; Guido, S.; Rusciano, G.; Sasso, A. Dissolution of a surfactant-water lamellar phase investigated by combining time-lapse polarized light microscopy and confocal raman spectroscopy. J. Colloid Interface Sci. 2020, 561, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Lee, J.H.; Kim, E.M.; Heo, G.S.; Kim, D.H.; Oh, J.Y.; Liu, Y.; Seo, D.-S. Surface modification of a poly(ethylene-co-vinyl acetate) layer by ion beam irradiation for the uniform alignment of liquid crystals. J. Mol. Liq. 2021, 339, 116700–116706. [Google Scholar] [CrossRef]
- Cao, B.; Guo, H.Y.; Hao, X.L.; Wu, Z.H.; Wu, F.G.; Yu, Z.W. Transition mechanism from nonlamellar to well-ordered lamellar phases: Is the lamellar liquid-crystal phase a must? J. Phys. Chem. Lett. 2021, 12, 4484–4489. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Xia, C.; Zhao, L.; Yin, Y.; Zhang, Y.; Pan, W.; Xin, X. Photoluminescent lyotropic liquid crystals formed by tyloxapol and n-dodecyl tetraethylene monoether. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 343–350. [Google Scholar] [CrossRef]
- Savic, S.; Vuleta, G.; Daniels, R.; Müller-Goymann, C.C. Colloidal microstructure of binary systems and model creams stabilized with an alkylpolyglucoside non-ionic emulsifier. Colloid Polym. Sci. 2005, 283, 439–451. [Google Scholar] [CrossRef]
- Ma, W.; Yang, B.; Zhang, H.; Sun, D. From emulsion to gel: Self-assembly behavior of ionic liquid surfactants in the water–oil interface. J. Mol. Liq. 2023, 390, 123133–123142. [Google Scholar] [CrossRef]
- Marlow, J.B.; Pottage, M.J.; McCoy, T.M.; de Campo, L.; Sokolova, A.; Bell, T.D.M.; Tabor, R.F. Structural and rheological changes of lamellar liquid crystals as a result of compositional changes and added silica nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 16592–16603. [Google Scholar] [CrossRef] [PubMed]
- Terescenco, D.; Savary, G.; Picard, C.; Clemenceau, F.; Merat, E.; Grisel, M. Influence of the emollient on emulsions containing lamellar liquid crystals: From molecular organization towards applicative properties. Colloids Surf. A Physicochem. Eng. Asp. 2018, 40, 565–574. [Google Scholar] [CrossRef]
- Niu, X.; Luo, D.; Chen, R.; Wang, F.; Sun, X.; Dai, H. Optical biosensor based on liquid crystal droplets for detection of cholic acid. Opt. Commun. 2016, 381, 286–291. [Google Scholar] [CrossRef]




 | |||
|---|---|---|---|
| δH (ppm) | Integral Areas of Protons | Predicted Proton Numbers | |
| a | 0.81~1.00 | 3.00 | 3 |
| b | 1.00~1.55 | 36.08 | 24 + 3n |
| e | 3.10~3.91 | 23.98 | 2 + 4m + 3n |
| Systems | cmcexp (mmol/L) | cmcideal (mmol/L) | γcmc (mN/m) | Amin (nm2/molecule) | Γmax (μmol/m2) | |
|---|---|---|---|---|---|---|
| OE3P3S/CTAB | 0:10 | 0.94 | 0.94 | 34.89 | 107.13 | 1.55 |
| 2:8 | 0.18 | 1.06 | 33.06 | 62.30 | 2.67 | |
| 3:7 | 0.50 | 1.00 | 33.02 | 91.76 | 1.81 | |
| 4:6 | 0.11 | 0.95 | 33.40 | 41.62 | 3.99 | |
| 5:5 | 0.065 | 0.90 | 33.03 | 99.62 | 1.67 | |
| 6:4 | 0.016 | 0.86 | 32.13 | 35.21 | 4.72 | |
| 7:3 | 0.088 | 0.82 | 32.19 | 91.11 | 1.82 | |
| 8:2 | 0.017 | 0.78 | 33.93 | 56.02 | 2.97 | |
| 10:0 | 0.72 | 0.72 | 36.16 | 203.26 | 0.82 | |
| OE3P3S/DTAB | 6:4 | 0.029 | 0.068 | 33.13 | 78.95 | 2.10 |
| OE3P3S/TTAB | 6:4 | 0.026 | 0.062 | 32.75 | 67.76 | 2.45 |
| Systems | X1s | X1m | βs | βm | |
|---|---|---|---|---|---|
| OE3P3S/CTAB | 2:8 | 0.452 | 0.453 | −7.446 | −7.378 |
| 3:7 | 0.475 | 0.465 | −5.215 | −2.805 | |
| 4:6 | 0.504 | 0.505 | −7.628 | −8.617 | |
| 5:5 | 0.514 | 0.518 | −13.368 | −10.505 | |
| 6:4 | 0.524 | 0.523 | −14.756 | −16.134 | |
| 7:3 | 0.546 | 0.558 | −12.470 | −9.619 | |
| 8:2 | 0.552 | 0.551 | −16.111 | −16.706 | |
| OE3P3S/DTAB | 6:4 | 0.556 | 0.550 | −17.945 | −15.403 |
| OE3P3S/TTAB | 6:4 | 0.545 | 0.543 | −17.285 | −15.393 |
| Systems | ΔGmic (KJ/mol) | ΔGads (KJ/mol) | ΔHmic (KJ/mol) | TΔSmic (KJ/mol) | |TΔSmic/ΔGmic| | |
|---|---|---|---|---|---|---|
| OE3P3S/CTAB | 2:8 | −6.24 | −20.47 | −4.53 | 1.71 | 0.27 |
| 3:7 | −3.44 | −24.43 | −1.73 | 1.71 | 0.49 | |
| 4:6 | −7.05 | −16.48 | −5.34 | 1.72 | 0.24 | |
| 5:5 | −8.21 | −30.99 | −6.50 | 1.72 | 0.21 | |
| 6:4 | −11.69 | −19.93 | −9.97 | 1.71 | 0.15 | |
| 7:3 | −7.58 | −28.87 | −5.88 | 1.70 | 0.22 | |
| 8:2 | −11.94 | −24.45 | −10.24 | 1.70 | 0.14 | |
| OE3P3S/DTAB | 6:4 | −11.15 | −29.16 | −9.45 | 1.71 | 0.15 |
| OE3P3S/TTAB | 6:4 | −11.17 | −26.78 | −9.46 | 1.71 | 0.15 |
| Systems | Ds (m2/s) | Dl (m2/s) | Dl/Ds |
|---|---|---|---|
| OE3P3S/DTAB | 4.35 × 10−11 | 1.24 × 10−14 | 0.00029 |
| OE3P3S/TTAB | 3.21 × 10−11 | 1.66 × 10−14 | 0.00052 |
| OE3P3S/CTAB | 2.60 × 10−11 | 1.95 × 10−13 | 0.0075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ren, P.; Wang, S.; Wang, J.; Sun, Z.; Sun, J.; Gu, W. Adsorption and Aggregation Behaviors of Oleyl Alcohol-Based Extended Surfactant and Its Mixtures. Molecules 2024, 29, 2570. https://doi.org/10.3390/molecules29112570
Li P, Ren P, Wang S, Wang J, Sun Z, Sun J, Gu W. Adsorption and Aggregation Behaviors of Oleyl Alcohol-Based Extended Surfactant and Its Mixtures. Molecules. 2024; 29(11):2570. https://doi.org/10.3390/molecules29112570
Chicago/Turabian StyleLi, Ping, Peiyu Ren, Shuoyu Wang, Jiangshan Wang, Zidan Sun, Jiayi Sun, and Weibo Gu. 2024. "Adsorption and Aggregation Behaviors of Oleyl Alcohol-Based Extended Surfactant and Its Mixtures" Molecules 29, no. 11: 2570. https://doi.org/10.3390/molecules29112570
APA StyleLi, P., Ren, P., Wang, S., Wang, J., Sun, Z., Sun, J., & Gu, W. (2024). Adsorption and Aggregation Behaviors of Oleyl Alcohol-Based Extended Surfactant and Its Mixtures. Molecules, 29(11), 2570. https://doi.org/10.3390/molecules29112570







