The Preparation of g-C3N4/ZnIn2S4 Nano-Heterojunctions and Their Enhanced Efficient Photocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD) Analysis
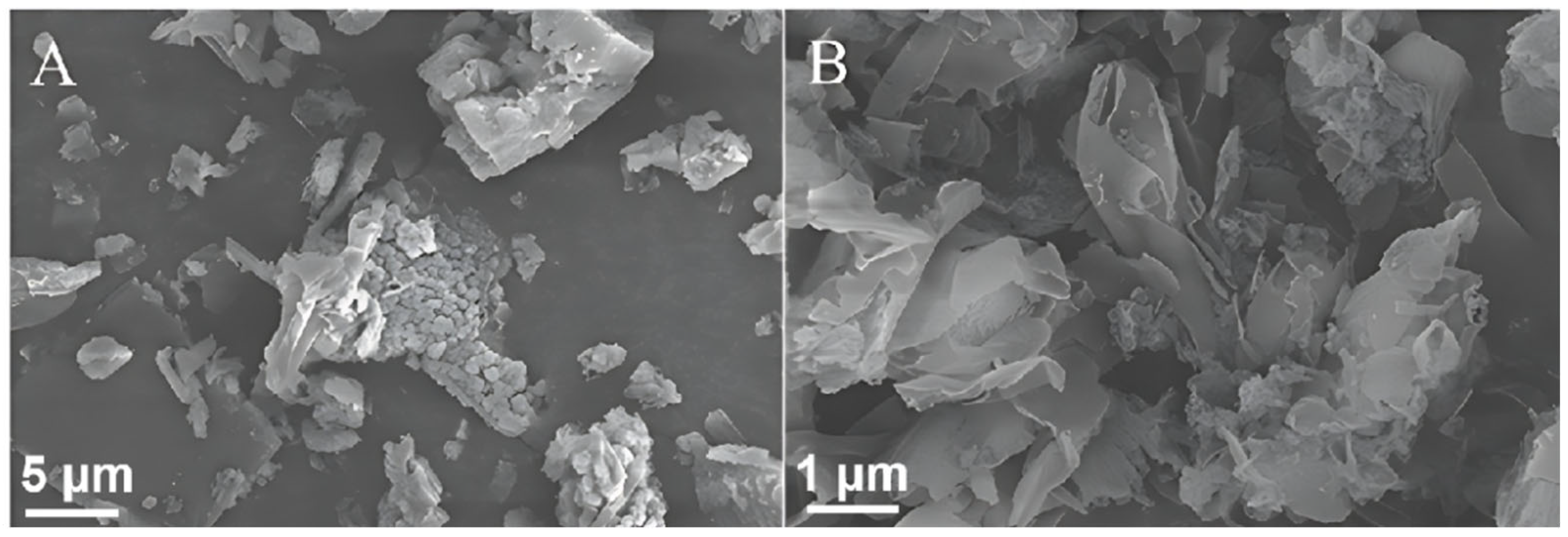
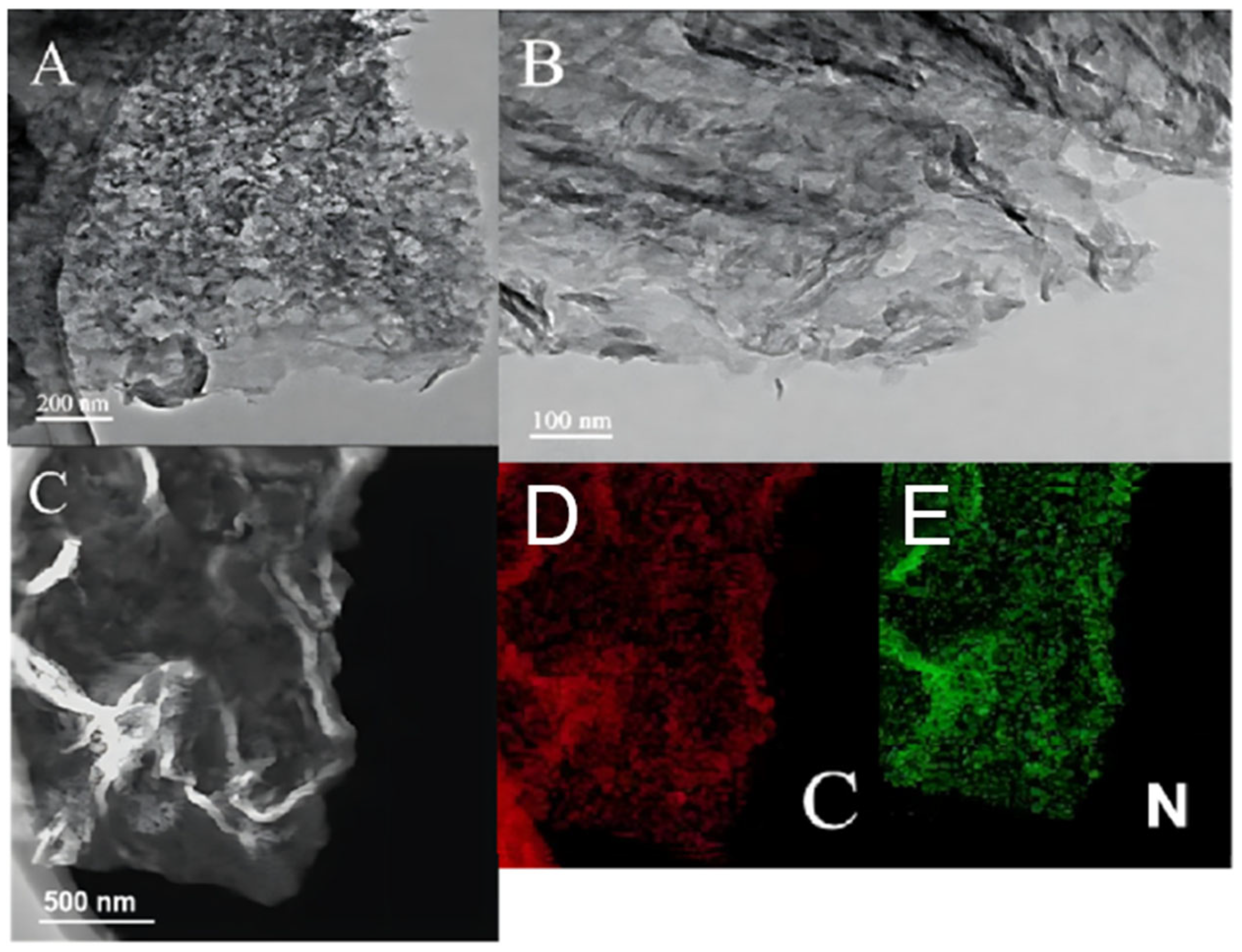
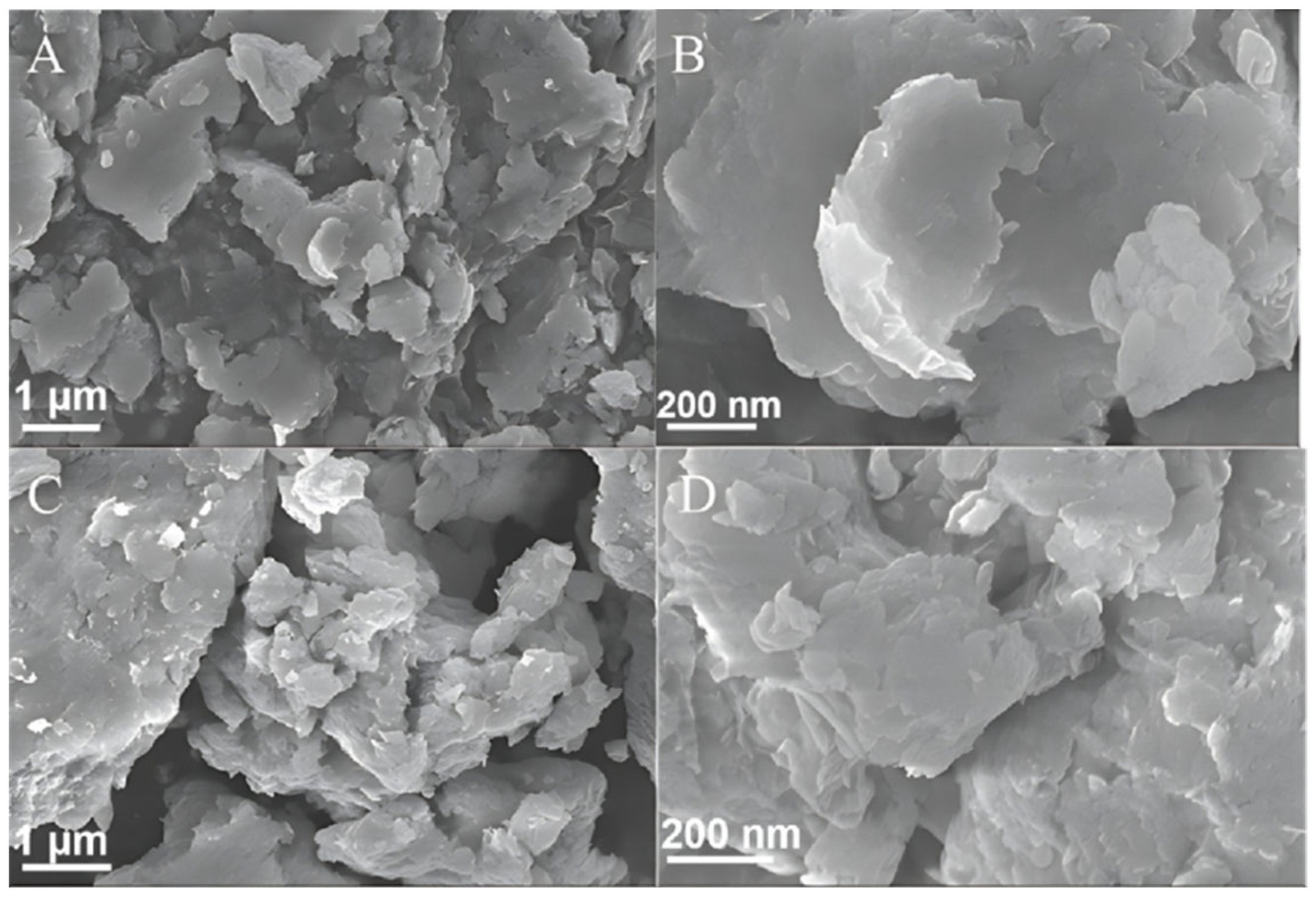
2.2. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) Analyses
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
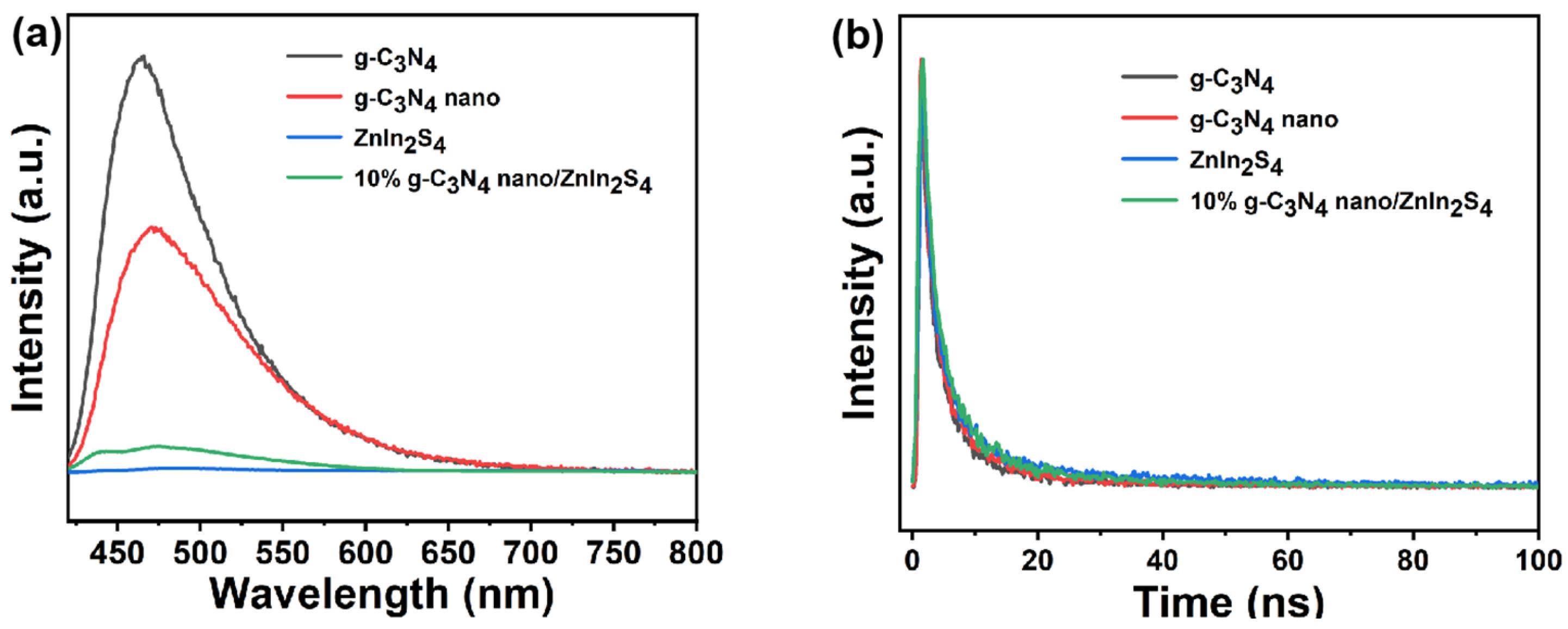
2.4. Photoluminescence (PL) Analysis
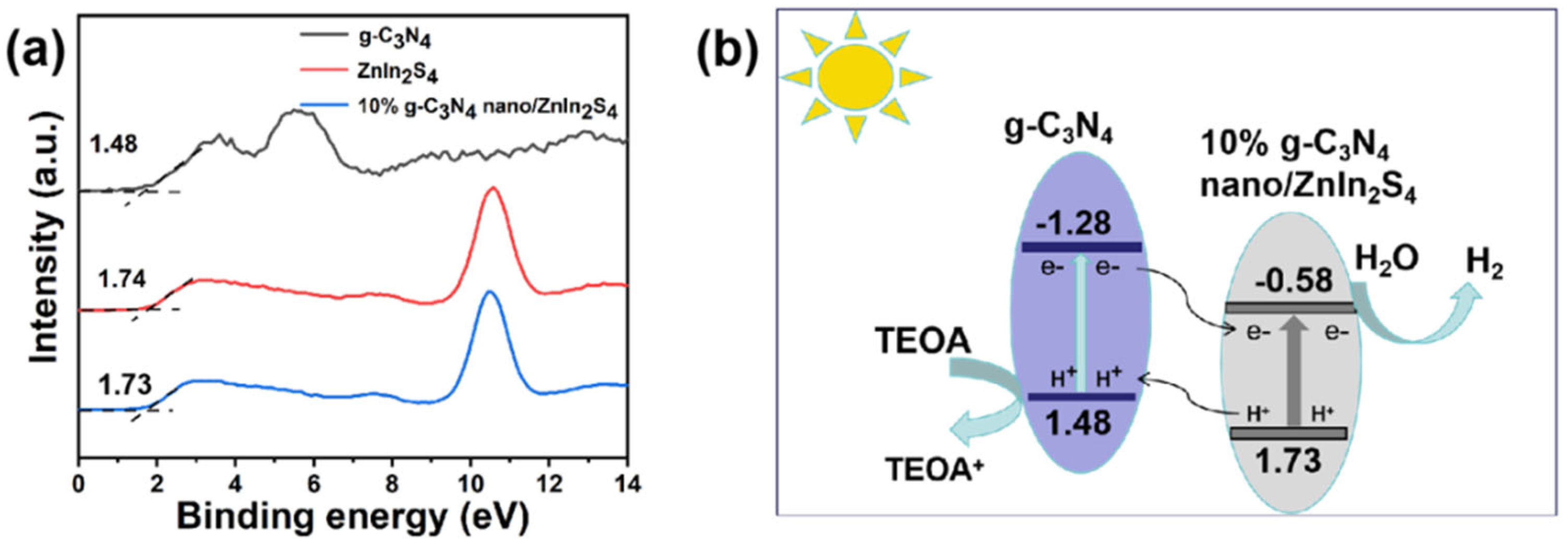
2.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.6. UV–Visible Diffuse Reflectance Spectroscopy
2.7. N2 Adsorption–Desorption Isotherm
2.8. Photocatalytic Activity Studies
2.9. Stability Analysis of 10% g-C3N4/ZnIn2S4 Nano-Heterojunction
2.10. Mechanism Analysis
3. Experimental Section
3.1. Preparation of Photocatalysts
3.1.1. Preparation of g-C3N4 Nanoflakes
3.1.2. Preparation of ZnIn2S4 Material
3.1.3. Preparation of 10% g-C3N4/ZnIn2S4 Nano-Heterojunction Composites
3.2. Material Characterization
3.3. Photocatalytic Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; Mao, L.; Yang, P.; Zheng, H.; Fujitsuka, M.; Zhang, J.; Majima, T. Ultrathin ZnIn2S4 nanosheets with active (110) facet exposure and efficient charge separation for cocatalyst free photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 265, 118616. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, M.; Lv, X.; Liu, Y.; Shi, C.; Dai, Y.; Wang, A.; Majima, T. Insight into iron group transition metal phosphides (Fe2P, Co2P, Ni2P) for improving photocatalytic hydrogen generation. Appl. Catal. B Environ. 2019, 246, 330–336. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Yan, Y.; Liu, C.; Hu, X.; Liu, E.; Fan, J. A novel S-scheme 1D/2D Bi2S3/g-C3N4 heterojunctions with enhanced H2 evolution activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125598. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Y.; Lin, Q.; Zhang, L.; Zhang, X.; Wang, H. Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis. Energy Environ. Sci. 2021, 14, 2954–3009. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhao, P.; Zhang, L.; Dai, B.; Xu, J.; Huang, H.; He, Y.; Leung, D.Y. Novel Z-scheme Ag-C3N4/SnS2 plasmonic heterojunction photocatalyst for degradation of tetracycline and H2 production. Chem. Eng. J. 2021, 405, 126555. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Ultrathin ZnIn2S4 nanosheets anchored on Ti3C2TX MXene for photocatalytic H2 evolution. Angew. Chem. 2020, 132, 11383–11388. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Ge, H.; Li, M.; Li, Y.; Duan, T.; He, R.; Zhu, W. Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J. Hazard. Mater. 2020, 384, 121383. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, S.; Wang, D.; Zhu, C.; Shi, W.; Tao, H.; Wang, X.; Yang, F. Boosting carrier separation over ultrathin g-C3N4 by f-ionic intercalation for improved photocatalytic activity. Appl. Surf. Sci. 2024, 644, 158808. [Google Scholar] [CrossRef]
- Jang, D.; Lee, S.; Kwon, N.H.; Kim, T.; Park, S.; Jang, K.Y.; Yoon, E.; Choi, S.; Han, J.; Lee, T.W.; et al. Preparation of carbon nitride nanotubes with P-doping and their photocatalytic properties for hydrogen evolution. Carbon 2023, 208, 290–302. [Google Scholar] [CrossRef]
- Gorai, D.K.; Kuila, S.K.; Oraon, A.; Kumar, A.; Suthar, M.; Mitra, R.; Biswas, K.; Roy, P.; Ahmad, M.I.; Kundu, T.K. A facile and green synthesis of Mn and P functionalized graphitic carbon nitride nanosheets for spintronics devices and enhanced photocatalytic performance under visible-light. J. Colloid Interface Sci. 2023, 644, 397–414. [Google Scholar] [CrossRef]
- Guo, S.; Tang, Y.; Xie, Y.; Tian, C.; Feng, Q.; Zhou, W.; Jiang, B. P-doped tubular g-C3N4 with surface carbon defects: Universal synthesis and enhanced visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Lin, P.; Shen, J.; Yu, X.; Liu, Q.; Li, D.; Tang, H. Construction of Ti3C2 MXene/O-doped g-C3N4 2D-2D Schottky-junction for enhanced photocatalytic hydrogen evolution. Ceram. Int. 2019, 45, 24656–24663. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B Environ. 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. A facile fabrication of Br-modified g-C3N4/rGO composite catalyst for enhanced visible photocatalytic activity towards the degradation of harmful dyes. Mater. Res. Bull. 2020, 130, 110870. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Lin, Q.; Chen, Y.; Liao, X.; Yu, H.; Yu, C. Three-dimensional P-doped porous g-C3N4 nanosheets as an efficient metal-free photocatalyst for visible-light photocatalytic degradation of Rhodamine B model pollutant. J. Taiwan Inst. Chem. Eng. 2020, 114, 249–262. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, X.; Zeng, Q.; Yan, T.; Ji, P.; Wu, T.; Wei, D.; Du, B. Facile synthesis of hierarchical ZnIn2S4/CdIn2S4 microspheres with enhanced visible light driven photocatalytic activity. Appl. Surf. Sci. 2017, 407, 328–336. [Google Scholar] [CrossRef]
- Xu, S.; Dai, J.; Yang, J.; You, J.; Hao, J. Facile synthesis of novel CaIn2S4/ZnIn2S4 composites with efficient performance for photocatalytic reduction of Cr(VI) under simulated sunlight irradiation. Nanomaterials 2018, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Tian, F.; Bao, S.; Sun, C.; Yang, W.; Yu, Y. MnIn2S4 nanosheets growing on rods-like β-MnO2 via covalent bonds as high-performance photocatalyst for boosting Cr (VI) photocatalytic reduction under visible light irradiation: Behavior and mechanism study. J. Colloid Interface Sci. 2022, 625, 264–277. [Google Scholar] [CrossRef]
- Zhang, M.; Arif, M.; Hua, Y.; Qiu, B.; Mao, Y.; Liu, X. Direct Z-scheme α-MnO2@MnIn2S4 hierarchical photocatalysts with atomically defined junctions for improved photocatalytic activities. Nanoscale Adv. 2021, 3, 812–822. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Wang, X.; Lou, X.W.D. Formation of hierarchical Co9S8@ZnIn2S4 heterostructured cages as an efficient photocatalyst for hydrogen evolution. J. Am. Chem. Soc. 2018, 140, 15145–15148. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cao, R.; Sun, P.; Yin, J.; Zhang, S.; Xu, X. Constructing electrostatic self-assembled 2D/2D ultra-thin ZnIn2S4/protonated g-C3N4 heterojunctions for excellent photocatalytic performance under visible light. Appl. Catal. B Environ. 2019, 256, 117862. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Ismail, A.F.; Kiong, T.S. g-C3N4/La2O3 nanocomposite as a photo-electrocatalyst in solar water splitting. Surf. Interfaces 2024, 44, 103639. [Google Scholar] [CrossRef]
- Rajavaram, R.; Vattikuti, S.P.; Shim, J.; Liu, X.; Hoai, N.T.; Dang, N.N. Enriched photocatalytic and photoelectrochemical activities of a 2D/0D gC3N4/CeO2 nanostructure. Nanoscale Adv. 2023, 5, 6489–6500. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Zeng, G.; Huang, D.; Xiao, R.; Zhang, C.; Zhou, C.; Xiong, W.; Wang, W.; Cheng, M.; et al. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal. B Environ. 2019, 258, 117956. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Nitrogen doped g-C3N4 with the extremely narrow band gap for excellent photocatalytic activities under visible light. Appl. Catal. B Environ. 2021, 281, 119474. [Google Scholar] [CrossRef]
- Tan, S.; Xing, Z.; Zhang, J.; Li, Z.; Wu, X.; Cui, J.; Kuang, J.; Zhu, Q.; Zhou, W. Ti3+-TiO2/g-C3N4 mesostructured nanosheets heterojunctions as efficient visible-light-driven photocatalysts. J. Catal. 2018, 357, 90–99. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, J.; Jiang, C.; Cheng, B.; Yu, J. First principle investigation of halogen-doped monolayer g-C3N4 photocatalyst. Appl. Catal. B Environ. 2017, 207, 27–34. [Google Scholar] [CrossRef]
- Lee, S.; Shin, E.Y.; Jang, D.; Choi, S.; Park, H.; Kim, J.; Park, S. Production of mesoporous carbon nitrides and their photocatalytic properties for degradation of organic pollutants. Bull. Korean Chem. Soc. 2022, 43, 1124–1129. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xiao, M.; Yu, S.; Wu, H.; Wang, Y.; Sun, J.; Chen, G.; Li, C. Insight into the activity and stability of Rh x P Nano-species supported on g-C3N4 for photocatalytic H2 production. ACS Catal. 2019, 10, 458–462. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, K.; Wang, L.; Bai, B.; Liu, H.; Wang, Q. Aminated flower-like ZnIn2S4 coupled with benzoic acid modified g-C3N4 nanosheets via covalent bonds for ameliorated photocatalytic hydrogen generation. Appl. Catal. B Environ. 2020, 268, 118462. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Tian, W.; Meng, A.; Yang, L. CoNi bimetal cocatalyst modifying a hierarchical ZnIn2S4 nanosheet-based microsphere noble-metal-free photocatalyst for efficient visible-light-driven photocatalytic hydrogen production. ACS Sustain. Chem. Eng. 2019, 7, 20190–20201. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Yang, P.; Luo, Z.; Yuan, R.; Asiri, A.M.; Wakeel, M.; Wang, X. Layered heterostructures of ultrathin polymeric carbon nitride and ZnIn2S4 nanosheets for photocatalytic CO2 reduction. Chemistry 2018, 24, 18529–18534. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal sulfide photocatalysts for hydrogen generation: A review of recent advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]



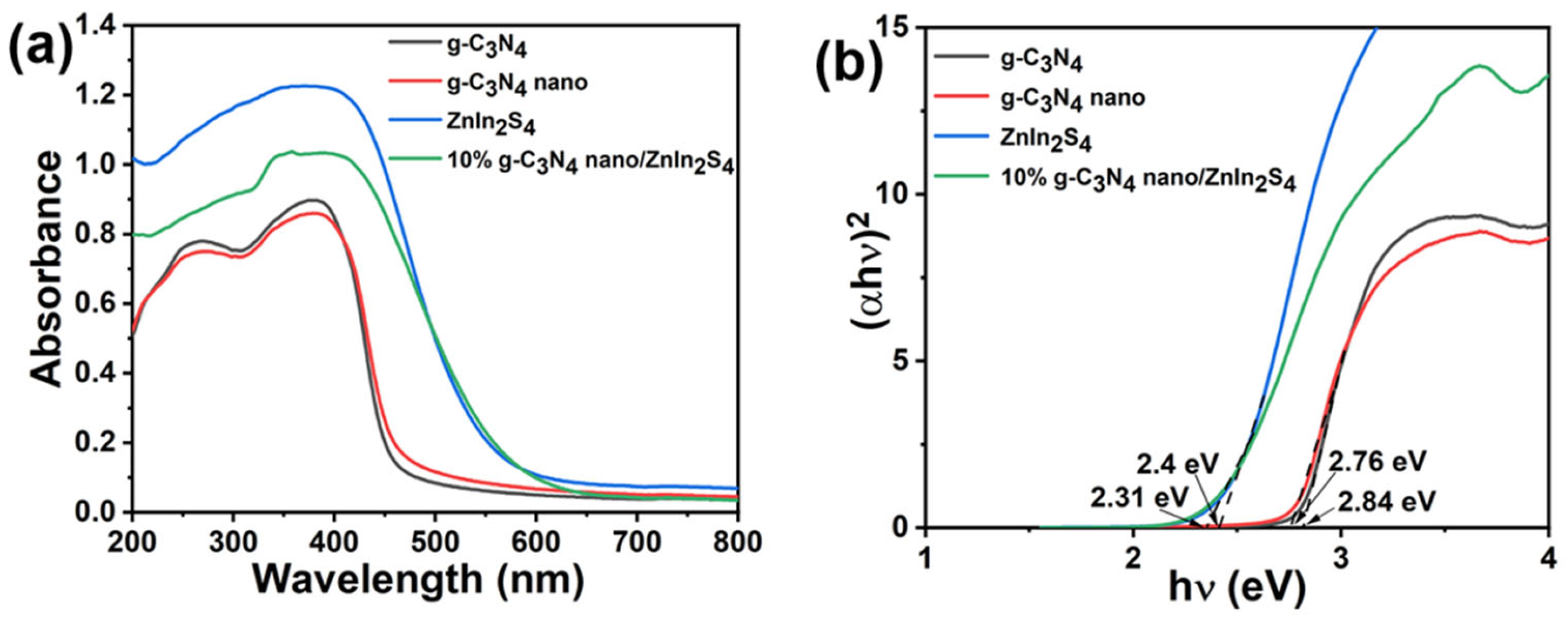
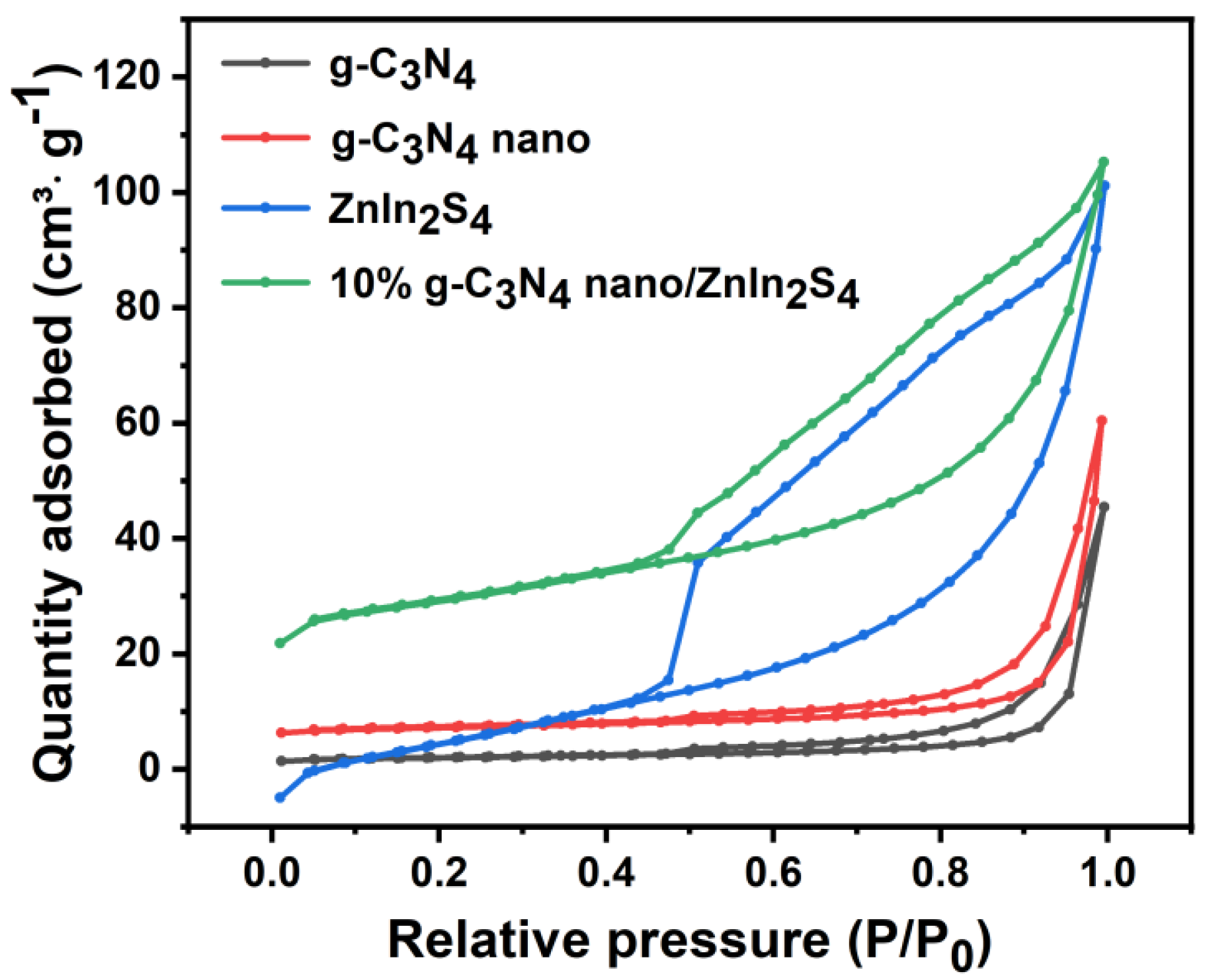


| Sample | SBET (m2·g−1) | VTotal (m3·g−1) | DAverage (nm) |
|---|---|---|---|
| g-C3N4 | 6.9 | 0.07 | 20.05 |
| g-C3N4 nano | 9.36 | 0.09 | 19.1 |
| Znln2S4 | 65.85 | 0.18 | 10.56 |
| 10% g-C3N4 nano/Znln2S4 | 90.57 | 0.24 | 9.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, Y.; Wang, S.; Xiao, X. The Preparation of g-C3N4/ZnIn2S4 Nano-Heterojunctions and Their Enhanced Efficient Photocatalytic Hydrogen Production. Molecules 2024, 29, 2571. https://doi.org/10.3390/molecules29112571
Li H, Wang Y, Wang S, Xiao X. The Preparation of g-C3N4/ZnIn2S4 Nano-Heterojunctions and Their Enhanced Efficient Photocatalytic Hydrogen Production. Molecules. 2024; 29(11):2571. https://doi.org/10.3390/molecules29112571
Chicago/Turabian StyleLi, Hubing, Yaoting Wang, Song Wang, and Xin Xiao. 2024. "The Preparation of g-C3N4/ZnIn2S4 Nano-Heterojunctions and Their Enhanced Efficient Photocatalytic Hydrogen Production" Molecules 29, no. 11: 2571. https://doi.org/10.3390/molecules29112571
APA StyleLi, H., Wang, Y., Wang, S., & Xiao, X. (2024). The Preparation of g-C3N4/ZnIn2S4 Nano-Heterojunctions and Their Enhanced Efficient Photocatalytic Hydrogen Production. Molecules, 29(11), 2571. https://doi.org/10.3390/molecules29112571






