Assessment of the Novel Anti-Seizure Potential of Validamycin A Using Zebrafish Epilepsy Model
Abstract
:1. Introduction
2. Results
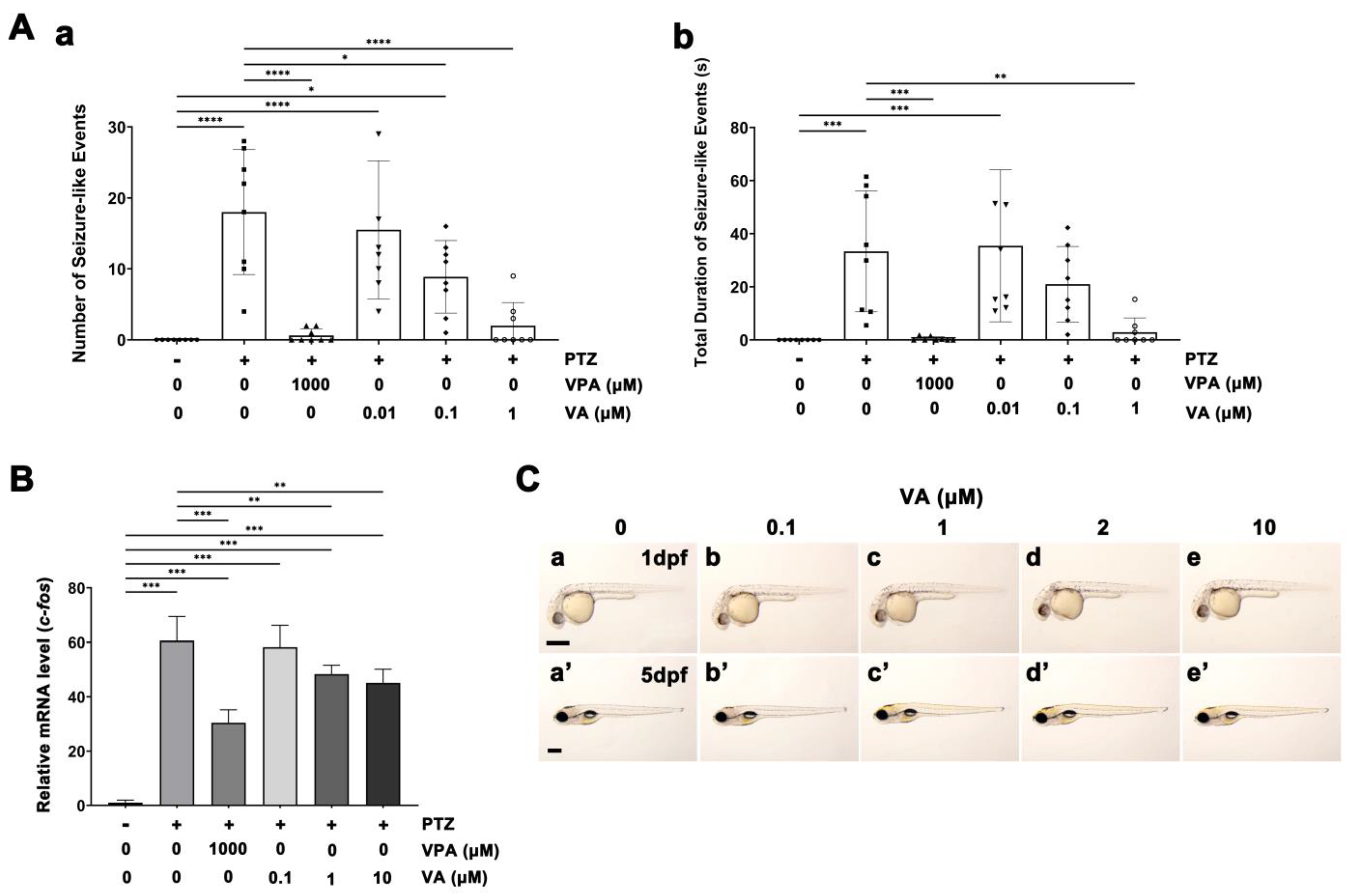
2.1. Assessment of the Anti-Seizure Efficacy of VA Using Zebrafish Larval Epilepsy Model
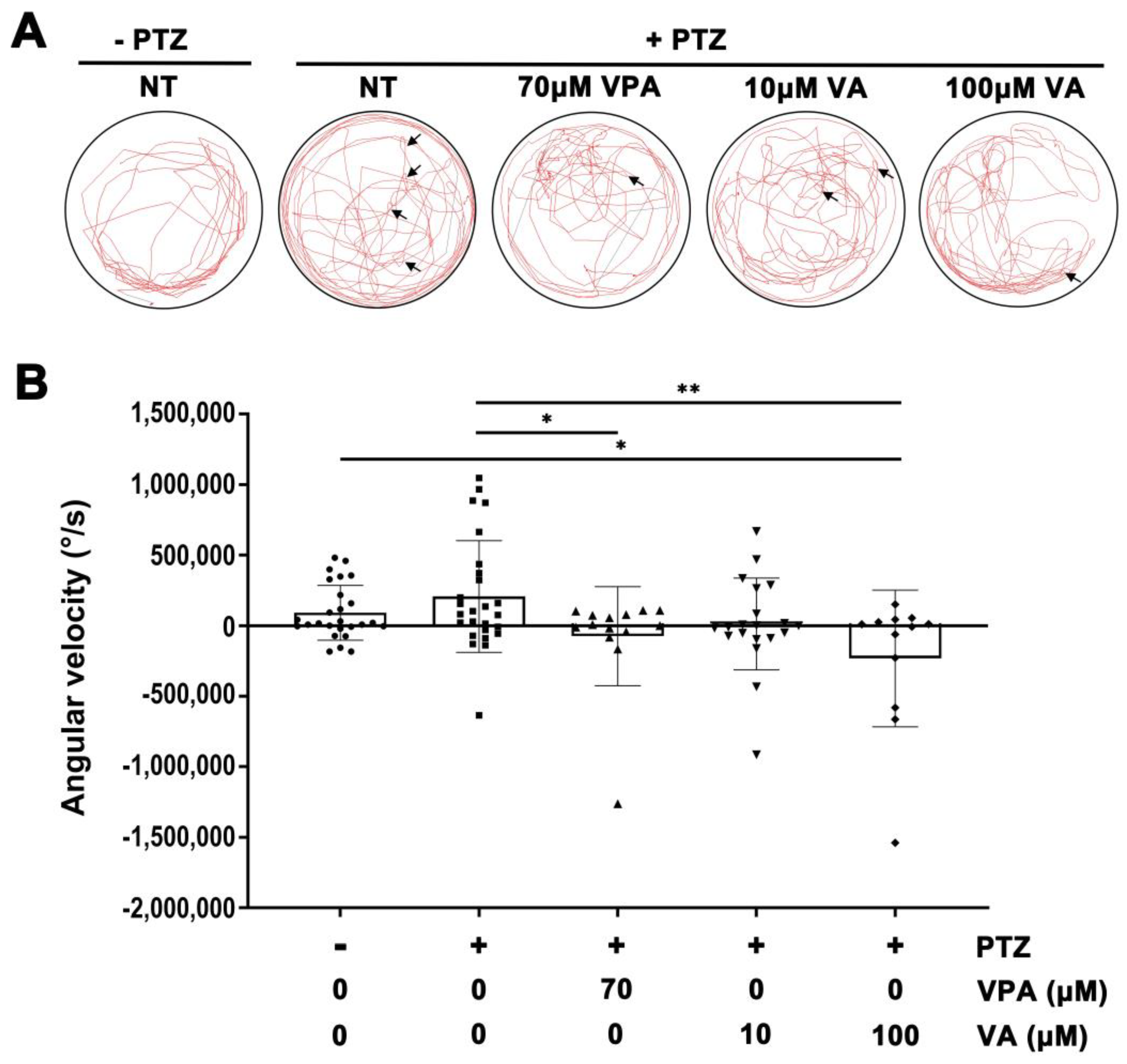
2.2. Assessment of Anti-Seizure Efficacy of VA in the Adult Brain Using a Zebrafish Epilepsy Model
2.3. Assessment of the Efficacy of VA via Angular Velocity Analysis of Adult Zebrafish Movement
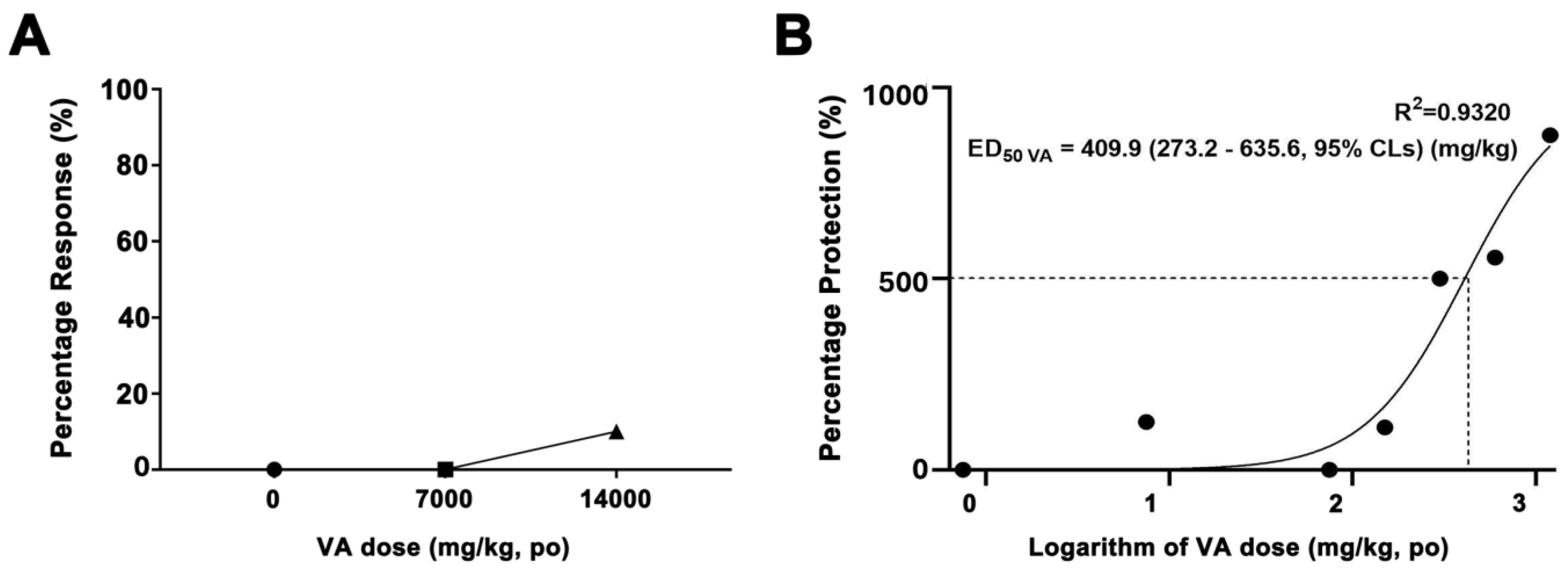
2.4. Calculation of the Therapeutic Dosage of VA
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance
4.2. Electroencephalographic Evaluation of Brain Wave Signals in Zebrafish Larvae
4.3. VA Treatment and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.4. VA Treatment and Bright-Field Imaging of Live Zebrafish Embryos
4.5. Recording of EEG Signals in Adult Zebrafish Brain
4.6. Assessment of Angular Velocity of Adult Zebrafish Movement
4.7. Measurement of the Therapeutic Dosage of VA
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bian, C.; Duan, Y.; Wang, J.; Xiu, Q.; Wang, J.; Hou, Y.; Song, X.; Zhou, M. Validamycin A induces broad-spectrum resistance involving salicylic acid and jasmonic acid/ethylene signaling pathways. Mol. Plant Microbe Interact. 2020, 33, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Guirao-Abad, J.P.; Sanchez-Fresneda, R.; Valentin, E.; Martinez-Esparza, M.; Arguelles, J.C. Analysis of validamycin as a potential antifungal compound against Candida albicans. Int. Microbiol. 2013, 16, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Plabutong, N.; Ekronarongchai, S.; Niwetbowornchai, N.; Edwards, S.W.; Virakul, S.; Chiewchengchol, D.; Thammahong, A. The inhibitory effect of validamycin A on aspergillus flavus. Int. J. Microbiol. 2020, 2020, 3972415. [Google Scholar] [CrossRef]
- Asano, N.; Tanaka, K.; Kameda, Y.; Matsui, K. All eight possible mono-beta-D-glucosides of validoxylamine A. II. Biological activities. Jpn. J. Antibiot. 1991, 44, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Ji, S.; Si, Y.X.; Yang, J.M.; Qian, G.Y.; Lee, J.; Yin, S.J. The effect of validamycin A on tyrosinase: Inhibition kinetics and computational simulation. Int. J. Biol. Macromol. 2013, 55, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Canzian, J.; Muller, T.E.; Franscescon, F.; Michelotti, P.; Fontana, B.D.; Costa, F.V.; Rosemberg, D.B. Modeling psychiatric comorbid symptoms of epileptic seizures in zebrafish. J. Psychiatr. Res. 2019, 119, 14–22. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.K.; Gloss, D.; Devinsky, O. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav. 2017, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K.; Langlois, M.; Martins, T.; van der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the moment: Zebrafish epilepsy models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.D.; Westfall, T.A.; Das, T.; Dawson, D.V.; Slusarski, D.C. High-throughput behavioral assay to investigate seizure sensitivity in zebrafish implicates ZFHX3 in epilepsy. J. Neurogenet. 2018, 32, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Park, E.; Baker, A.; Reid, A.Y. Age Bias in Zebrafish Models of Epilepsy: What can we learn from old fish? Front. Cell Dev. Biol. 2020, 8, 573303. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A. Antiseizure medication discovery: Recent and future paradigm shifts. Epilepsia Open 2022, 7 (Suppl. S1), S133–S141. [Google Scholar] [CrossRef]
- Loscher, W.; Klein, P. The pharmacology and clinical efficacy of antiseizure medications: From bromide salts to cenobamate and beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, K.J.; Jang, J.W.; Lee, S.I.; Kim, S. An EEG system to detect brain signals from multiple adult zebrafish. Biosens. Bioelectron. 2020, 164, 112315. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, D.L.; Sive, H. Noninvasive Multielectrode array for brain and spinal cord local field potential recordings from live zebrafish larvae. Zebrafish 2020, 17, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.N.; Lee, K.B.; Butterworth, W.; Park, S.K.; Kim, J.Y.; Kim, S. Zebrafish EEG predicts the efficacy of antiepileptic drugs. Front. Pharmacol. 2022, 13, 1055424. [Google Scholar] [CrossRef]
- Perucca, P.; Scheffer, I.E.; Kiley, M. The management of epilepsy in children and adults. Med. J. Aust. 2018, 208, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.; Kwon, S.H.; Eum, J.; Raslan, A.A.; Kim, K.N.; Hwang, B.J.; Kee, Y. 3D light-sheet assay assessing novel valproate-associated cardiotoxicity and folic acid relief in zebrafish embryogenesis. Chemosphere 2019, 227, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Eum, J.; Hwang, B.J.; Kee, Y. Differential neuroprotective effects of N-acetylcysteine against dithianon toxicity in glutamatergic, dopaminergic, and GABAergic neurons: Assessment using zebrafish. Antioxidants 2023, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Wong, A.; Seguin, D.; Gerlai, R. Induction of social behavior in zebrafish: Live versus computer animated fish as stimuli. Zebrafish 2014, 11, 185–197. [Google Scholar] [CrossRef]
- Chitolina, R.; Gallas-Lopes, M.; Reis, C.G.; Benvenutti, R.; Stahlhofer-Buss, T.; Calcagnotto, M.E.; Herrmann, A.P.; Piato, A. chemically-induced epileptic seizures in zebrafish: A systematic review. Epilepsy Res. 2023, 197, 107236. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Banik, A.; Lee, K.-B.; Sim, S.M.; Kil, A.H.; Hwang, B.J.; Kee, Y. Assessment of the Novel Anti-Seizure Potential of Validamycin A Using Zebrafish Epilepsy Model. Molecules 2024, 29, 2572. https://doi.org/10.3390/molecules29112572
Lee E, Banik A, Lee K-B, Sim SM, Kil AH, Hwang BJ, Kee Y. Assessment of the Novel Anti-Seizure Potential of Validamycin A Using Zebrafish Epilepsy Model. Molecules. 2024; 29(11):2572. https://doi.org/10.3390/molecules29112572
Chicago/Turabian StyleLee, Eunhye, Amit Banik, Ki-Baek Lee, Seung Min Sim, Ah Hyun Kil, Byung Joon Hwang, and Yun Kee. 2024. "Assessment of the Novel Anti-Seizure Potential of Validamycin A Using Zebrafish Epilepsy Model" Molecules 29, no. 11: 2572. https://doi.org/10.3390/molecules29112572
APA StyleLee, E., Banik, A., Lee, K.-B., Sim, S. M., Kil, A. H., Hwang, B. J., & Kee, Y. (2024). Assessment of the Novel Anti-Seizure Potential of Validamycin A Using Zebrafish Epilepsy Model. Molecules, 29(11), 2572. https://doi.org/10.3390/molecules29112572







