Investigating the Hepatoprotective Properties of Mulberry Leaf Flavonoids against Oxidative Stress in HepG2 Cells
Abstract
1. Introduction
2. Results
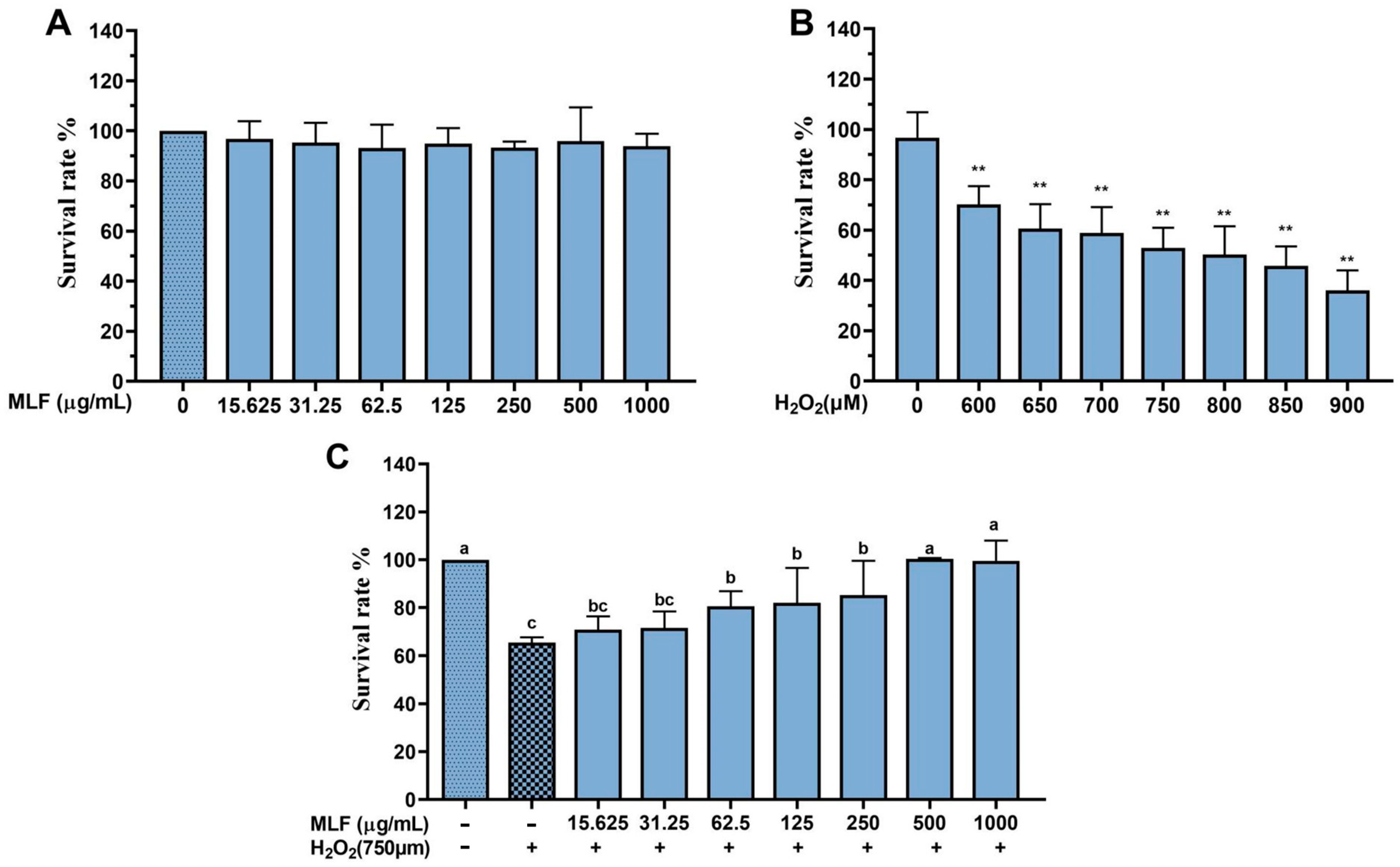
2.1. Effect of MLF on Survival of HepG2 Cells with H2O2–Mediated Oxidative Damage
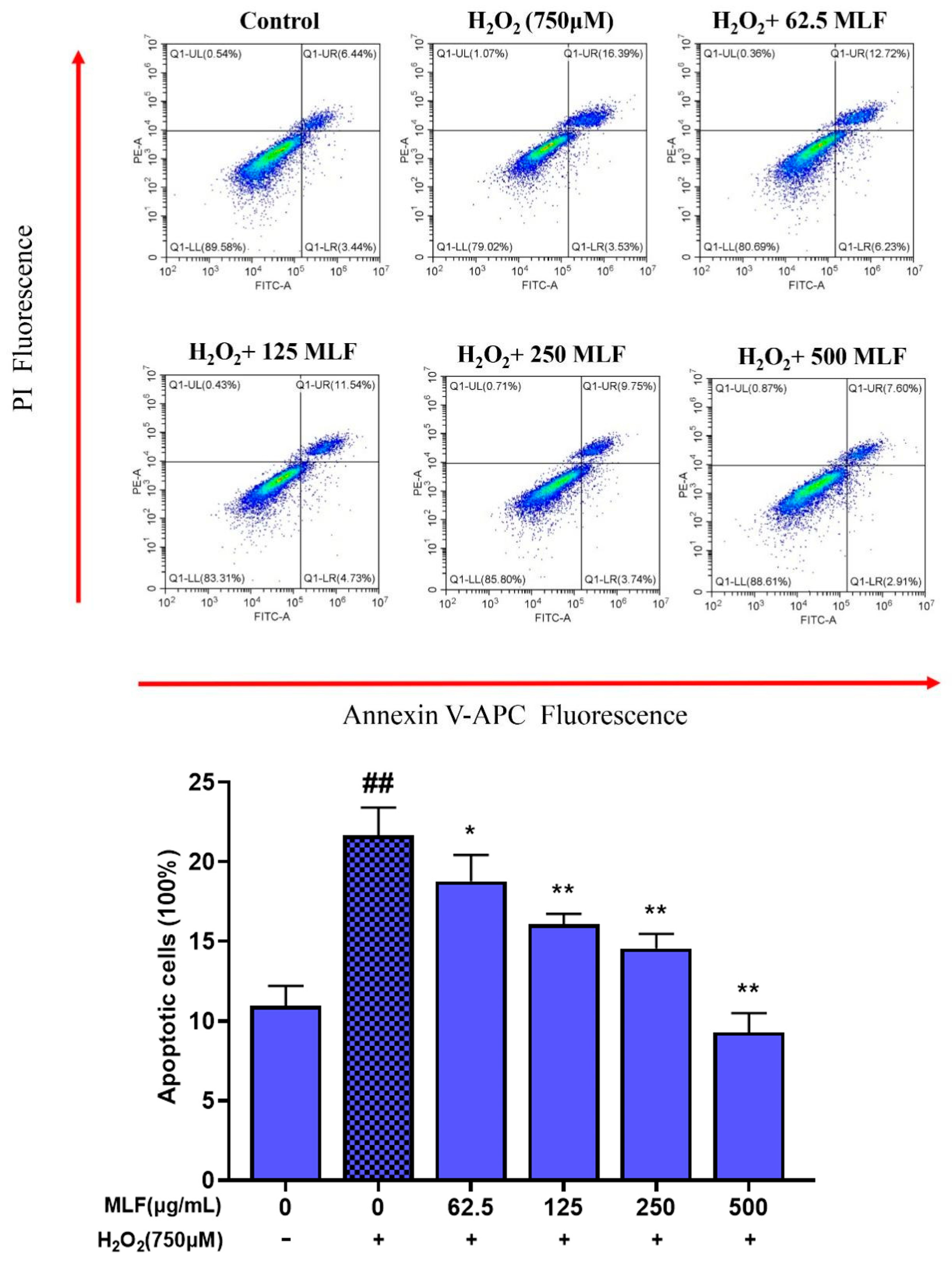
2.2. Effect of MLF on the Apoptosis Rate of HepG2 Cells with H2O2–Mediated Oxidative Damage
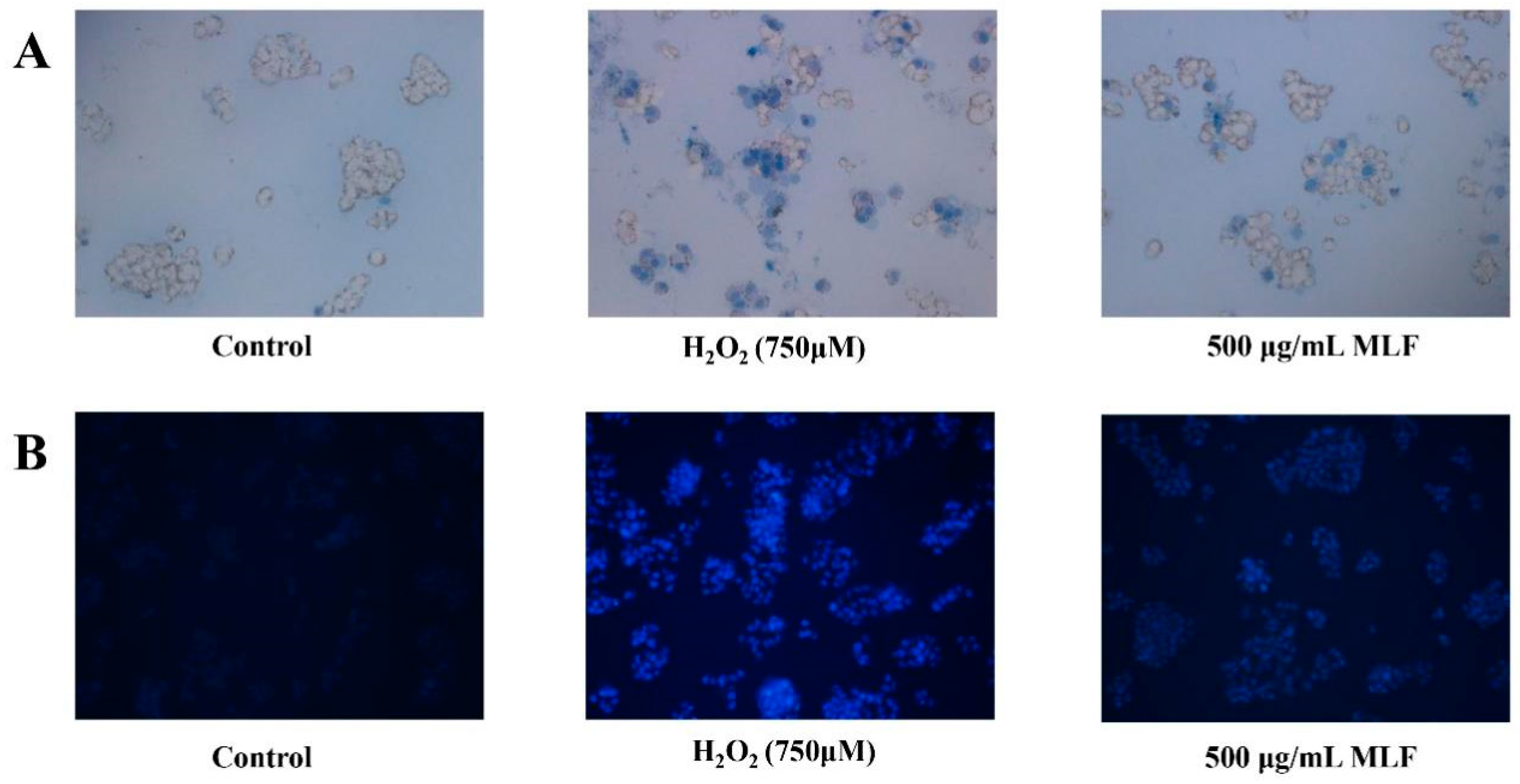
2.3. Analysis of Hoechstand Trypan Blue Staining
2.4. Effects of MLF Pretreatment on ROS Level in HepG2 Cells
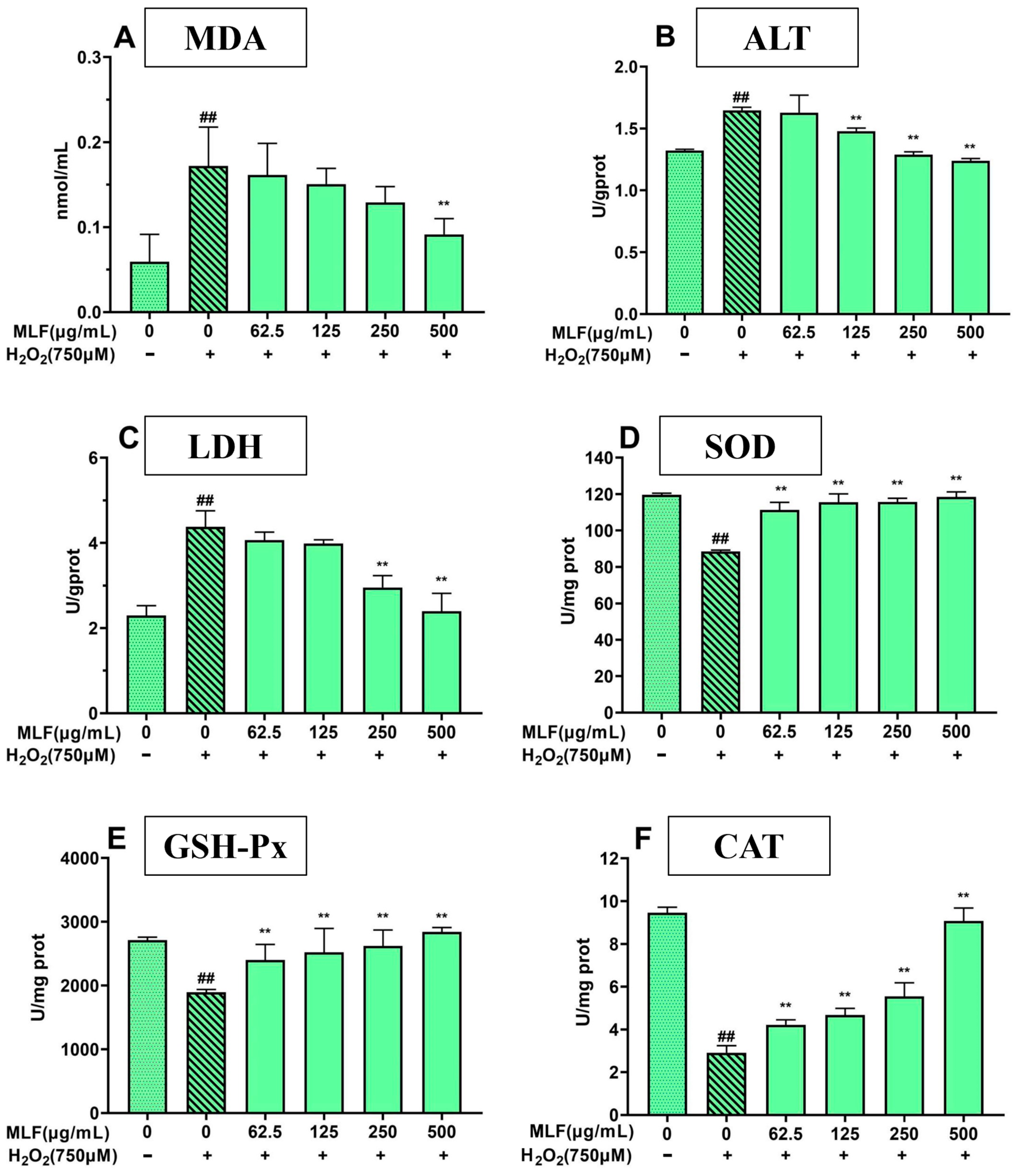
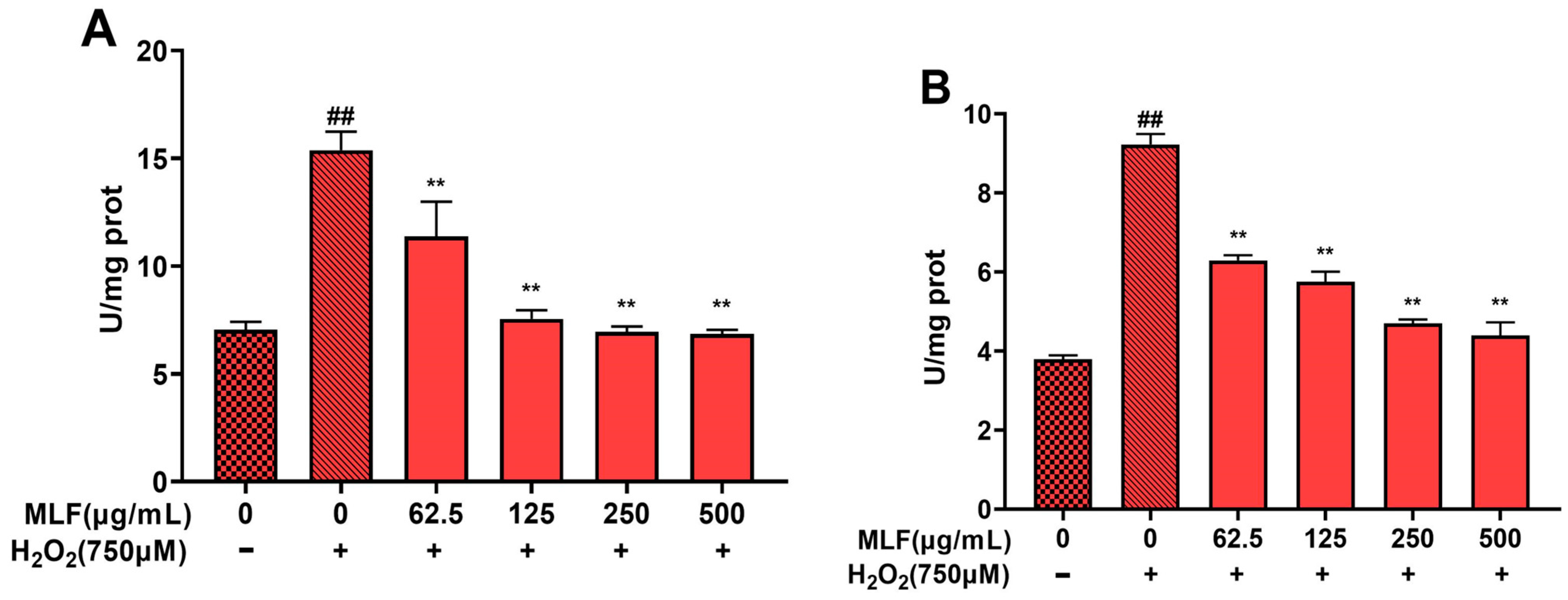
2.5. Effect of MLF Pretreatment on Biochemical Parameters in HepG2 Cells
2.6. Effect of MLF Pretreatment on Mitochondrial Membrane Potential Levels in HepG2 Cells
2.7. Results of Apoptosis-Related Protein Assay in HepG2 Cells
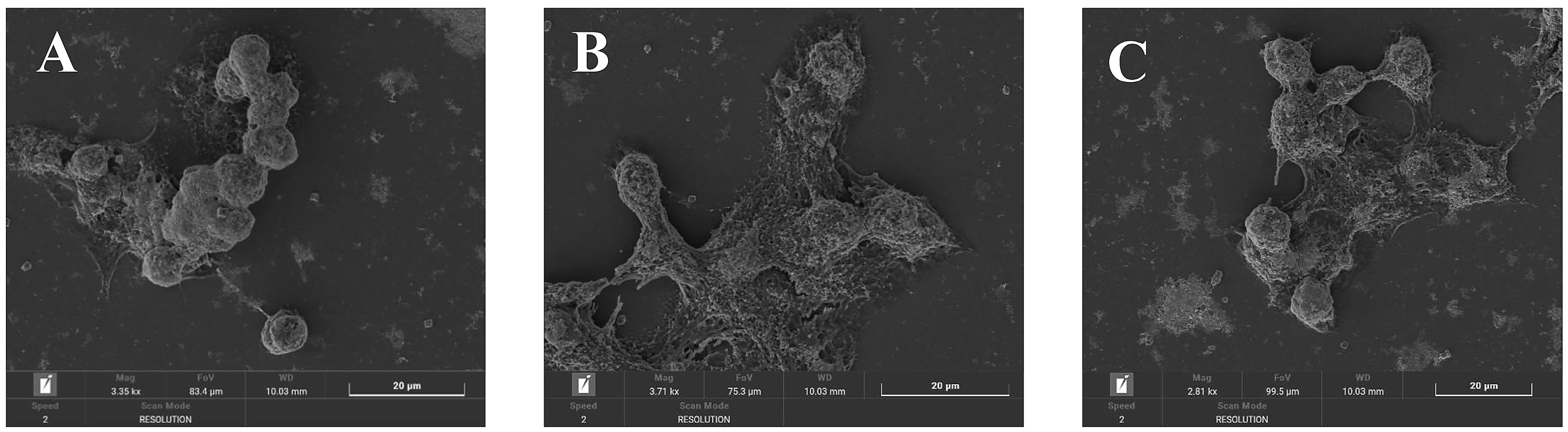
2.8. Observation of Cell Morphology
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Extraction and Purification of MLF
3.3. Cell Culture and Treatment
3.4. Cell Viability Assay
3.5. Influence of MLF on the Survival Rate of H2O2–Mediated Oxidative Injured HepG2 Cells
3.6. Determination of Apoptosis Rate of HepG2 Cells
3.7. Hoechst 33342 and Trypan Blue Staining Test
3.8. Determination of Intracellular ROS Level
3.9. Determination of Biochemical Parameters in HepG2 Cells
3.10. Determination of Mitochondrial Membrane Potential in HepG2 Cells
3.11. Determination of Apoptosis Proteins Activities in HepG2 Cells
3.12. Scanning Electron Microscope (SEM) Observation
3.13. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.M.; Bong, H.Y.; Jeong, H.I.; Kim, Y.K.; Kim, J.Y.; Kwon, O. Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats. Nutr. Res. Pract. 2009, 3, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, J.; Naik, P.R. Evaluation of hypoglycemic effect of Morus alba in an animal model. Indian J. Pharmacol. 2008, 40, 15–18. [Google Scholar] [PubMed]
- Wilson, R.D.; Islam, M.S. Effects of white mulberry (Morus alba) leaf tea investigated in a type 2 diabetes model of rats. Acta Pol. Pharm. 2015, 72, 153–160. [Google Scholar] [PubMed]
- Park, E.; Lee, S.M.; eun Lee, J.; Kim, J.H. Anti-inflammatory activity of mulberry leaf extract through inhibition of NF-κB. J. Funct. Foods 2013, 5, 178–186. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Chen, W.; Ma, J.; Gong, F.; Xi, H.; Zhan, Q.; Li, X.; Wei, F.; Wu, H.; Lai, F. Two novel polysaccharides from the torus of Saussurea laniceps protect against AAPH-induced oxidative damage in human erythrocytes. Carbohydr. Polym. 2018, 200, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, Z.; Wu, X.; Mao, D.; Feng, C.; Zhang, J.; Chen, G. Comprehensive study of the interaction between Puerariae Radix flavonoids and DNA: From theoretical simulation to structural analysis to functional analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 231, 118109. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhu, Y.-W.; Jiang, Y.-W.; Li, H.-K.; Liu, Z.-M.; Wang, W.; Shan, C.-H.; Fu, Y.-J. Improvement of flavonoid aglycone and biological activity of mulberry leaves by solid-state fermentation. Ind. Crop. Prod. 2020, 148, 112287. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wan, Y.; Xu, J.Y.; Wu, G.H.; Li, L.; Yao, X.H. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr. Polym. 2016, 137, 473–479. [Google Scholar] [CrossRef]
- Ju, W.-T.; Kwon, O.-C.; Kim, H.-B.; Sung, G.-B.; Kim, H.-W.; Kim, Y.-S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef]
- Ahmad, A.; Alkharfy, K.M.; Wani, T.A.; Raish, M. Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015, 72, 990–997. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of Reactive Oxygen Species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What role do they play in physical activity and health? Am. J. Clin. Nutr. 2000, 72, 637S–646S. [Google Scholar] [CrossRef]
- Hu, Y.M.; Lu, S.Z.; Li, Y.S.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z.C. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373 Pt B, 131539. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Bakken, H.E. Preventive effect of Nile tilapia hydrolysate against oxidative damage of HepG2 cells and DNA mediated by H2O2 and AAPH. J. Food Sci. Technol. 2015, 52, 6194–6205. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; Homem de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.Y.; Lin, K.H.; Chiu, C.C.; Yang, Y.Y.; Huang, M.Y.; Yang, C.M. Inhibitive effects of mulberry leaf-related extracts on cell adhesion and inflammatory response in human aortic endothelial cells. Evid. Based Complement. Alternat Med. 2013, 2013, 267217. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, R.; Huo, W.; Dong, H.; Chang, Z.; Xia, X. Cytotoxicity, genotoxicity, oxidative stress, and apoptosis in HepG2 cells induced by the imidazole ionic liquid 1-dodecyl-3-methylimidazolium chloride. Environ. Toxicol. 2020, 35, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Zheng, Q.; Tan, W.; Feng, X.; Feng, K.; Zhong, W.; Liao, C.; Liu, Y.; Li, S.; Hu, W. Protective Effect of Flavonoids from Mulberry Leaf on AAPH-Induced Oxidative Damage in Sheep Erythrocytes. Molecules 2022, 27, 7625. [Google Scholar] [CrossRef] [PubMed]
- Hazli, U.H.A.M.; Hwong, C.S.; Abdul-Aziz, A.; Mat-Junit, S.; Leong, K.H.; Kong, K.W. Effects of Alternanthera sessilis Red leaf extracts on hydrogen peroxide-induced oxidative stress in HepG2 cells and identification of phytochemicals using HPLC-QToF-MS/MS. S. Afr. J. Bot. 2022, 151, 440–450. [Google Scholar] [CrossRef]
- Silva, S.V.E.; Gallia, M.C.; Luz, J.R.D.D.; Rezende, A.A.; Bongiovanni, G.A.; Araujo-Silva, G.; Almeida, M.D.G. Antioxidant Effect of Coenzyme Q10 in the Prevention of Oxidative Stress in Arsenic-Treated CHO-K1 Cells and Possible Participation of Zinc as a Pro-Oxidant Agent. Nutrients 2022, 14, 3265. [Google Scholar] [CrossRef] [PubMed]
- Senes-Lopes, T.F.; Luz, J.R.D.D.; Guterres, Z.D.R.; Barbosa, E.A.; Batista, D.; Galdino, O.A.; Ururahy, M.A.G.; Gomes Dos Santos, E.C.; López, J.A.; Araujo-Silva, G.; et al. Pseudobombax parvifolium Hydroalcoholic Bark Extract: Chemical Characterisation and Cytotoxic, Mutagenic, and Preclinical Aspects Associated with a Protective Effect on Oxidative Stress. Metabolites 2023, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- da Luz, J.R.D.; López, J.A.; Ferreira, M.P.; de Sousa, R.M.; e Silva, S.V.; Almeida, M.d.G.; Araujo-Silva, G. In Vitro Antithrombotic, Antitumor and Antiangiogenic Activities of Green Tea Polyphenols and Its Main Constituent Epigallocatechin-3-gallate. Processes 2022, 11, 76. [Google Scholar] [CrossRef]
- Batista, D.; Romáryo Duarte da Luz, J.; Evellyn Silva Do Nascimento, T.; Felipe de Senes-Lopes, T.; Araújo Galdino, O.; Victor ESilva, S.; Ferreira, M.P.; dos Santos Azevedo, M.A.; Brandão-Neto, J.; Araujo-Silva, G.; et al. Licania rigida leaf extract: Protective effect on oxidative stress, associated with cytotoxic, mutagenic and preclinical aspects. J. Toxicol. Environ. Health Part A 2021, 85, 276–290. [Google Scholar] [CrossRef]
- Vukmirović, S.; Ilić, V.; Tadić, V.; Čapo, I.; Pavlović, N.; Tomas, A.; Kusturica, M.P.; Tomić, N.; Maksimović, S.; Stilinović, N. Comprehensive Analysis of Antioxidant and Hepatoprotective Properties of Morus nigra L. Antioxidants 2023, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Malfa, G.A.; Santangelo, R.; Bianchi, S.; Pappalardo, F.; Taviano, M.F.; Miceli, N.; Di Giacomo, C.; Tomasello, B. Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells. Molecules 2023, 28, 2475. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef]


| Number | Compound | Formula (M) | Calculated Conc. (ng/mL) |
|---|---|---|---|
| 1 | Rutoside | C27H30O16 | 125,000 |
| 2 | Hyperoside | C21H20O12 | 68,300 |
| 3 | Catechin | C15H14O6 | 163 |
| 4 | Myricitrin | C21H20O12 | 44,500 |
| 5 | Isoquercitrin | C21H20O12 | 43.1 |
| 6 | Kaempferol 3-rutinoside | C27H30O15 | 15,700 |
| 7 | Paeoniflorin | C23H28O11 | 3570 |
| 8 | Epicatechin | C15H14O6 | 64.2 |
| 9 | Taxifolin | C15H12O7 | 518 |
| 10 | Quercetin | C15H10O7 | 886 |
| 11 | Luteolin | C15H10O6 | 95.6 |
| 12 | Morin | C15H10O7 | 698 |
| 13 | Astragalin | C21H20O11 | 16,700 |
| 14 | Quercitrin | C21H20O11 | 18,600 |
| 15 | Isorhamnetin-3-O-glucoside | C22H22O12 | 3.65 |
| 16 | Curculigoside | C22H26O11 | 23.0 |
| 17 | Hesperidin | C28H34O15 | 180,000 |
| 18 | Cynaroside | C21H20O11 | 16,200 |
| 19 | Vitexin | C21H20O10 | 4990 |
| 20 | Kaempferol | C15H10O6 | 209 |
| 21 | Guaiaverin | C20H18O11 | 12,300 |
| 22 | Licochalcone-A | C21H22O4 | 15.2 |
| 23 | Ginsenoside Rg2 | C42H72O13 | 116 |
| 24 | Ginsenoside C-K | C36H62O8 | 211 |
| 25 | Ginsenoside Rc | C53H90O22 | 230 |
| 26 | Ginsenoside Rg1 | C42H72O14 | 1150 |
| 27 | Ginsenoside Rf | C42H72O14 | 4.54 |
| 28 | Ginsenoside F1 | C36H62O9 | 3140 |
| 29 | Ginsenoside Rb2 | C53H90O22 | 110 |
| 30 | Ginsenoside Rd | C48H82O18 | 71.2 |
| 31 | Ginsenoside Re | C48H82O18 | 284 |
| 32 | Hesperetin | C16H14O6 | 1190 |
| 33 | Polydatin | C20H22O8 | 318 |
| 34 | Cyanidin 3-O-glucoside | C21H21ClO11 | 64,800 |
| 35 | Cyanidin 3-O-rutinoside | C27H31O15 | 39,600 |
| 36 | Isorhamnetin-3-O-glucoside | C22H22O12 | 191 |
| 37 | Naringenin | C15H12O5 | 341 |
| 38 | Apigenin 7-glucoside | C21H20O10 | 6680 |
| 39 | Cyanidin | C21H28O8 | 19,500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Feng, K.; Zhong, W.; Tan, W.; Rengaowa, S.; Hu, W. Investigating the Hepatoprotective Properties of Mulberry Leaf Flavonoids against Oxidative Stress in HepG2 Cells. Molecules 2024, 29, 2597. https://doi.org/10.3390/molecules29112597
Zheng Q, Feng K, Zhong W, Tan W, Rengaowa S, Hu W. Investigating the Hepatoprotective Properties of Mulberry Leaf Flavonoids against Oxidative Stress in HepG2 Cells. Molecules. 2024; 29(11):2597. https://doi.org/10.3390/molecules29112597
Chicago/Turabian StyleZheng, Qinhua, Ke Feng, Wenting Zhong, Weijian Tan, Sa Rengaowa, and Wenzhong Hu. 2024. "Investigating the Hepatoprotective Properties of Mulberry Leaf Flavonoids against Oxidative Stress in HepG2 Cells" Molecules 29, no. 11: 2597. https://doi.org/10.3390/molecules29112597
APA StyleZheng, Q., Feng, K., Zhong, W., Tan, W., Rengaowa, S., & Hu, W. (2024). Investigating the Hepatoprotective Properties of Mulberry Leaf Flavonoids against Oxidative Stress in HepG2 Cells. Molecules, 29(11), 2597. https://doi.org/10.3390/molecules29112597





