Nutritional, Bioactive, and Volatile Characteristics of Two Types of Sorbus domestica Undervalued Fruit from Northeast of Iberian Peninsula, Spain
Abstract
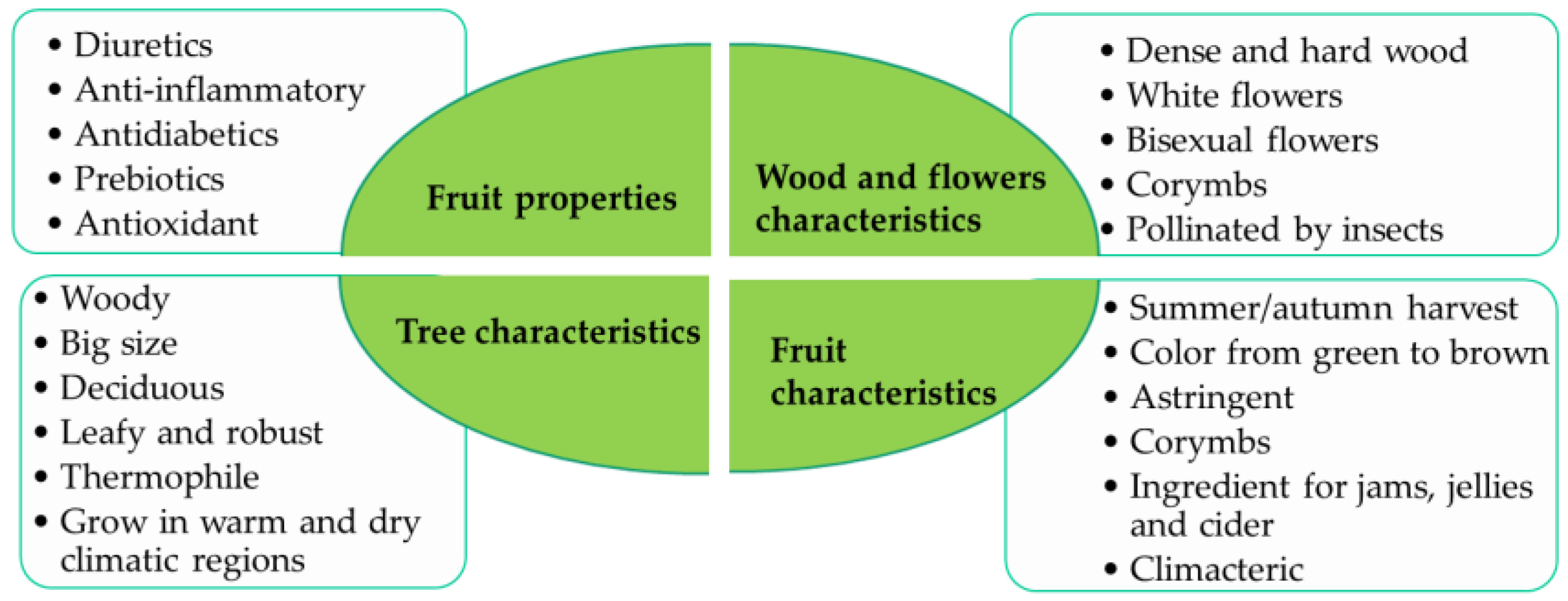
1. Introduction
2. Results
2.1. Individual Weights, Proximal and Mineral Composition of S. domestica Fruits
2.2. pH, Soluble Solids Content, Total Acidity, Total Sugars, and Glucose Composition of S. domestica Fruits
2.3. Total Phenolic Content and Antioxidant Activity by DPPH Assay of S. domestica Fruits
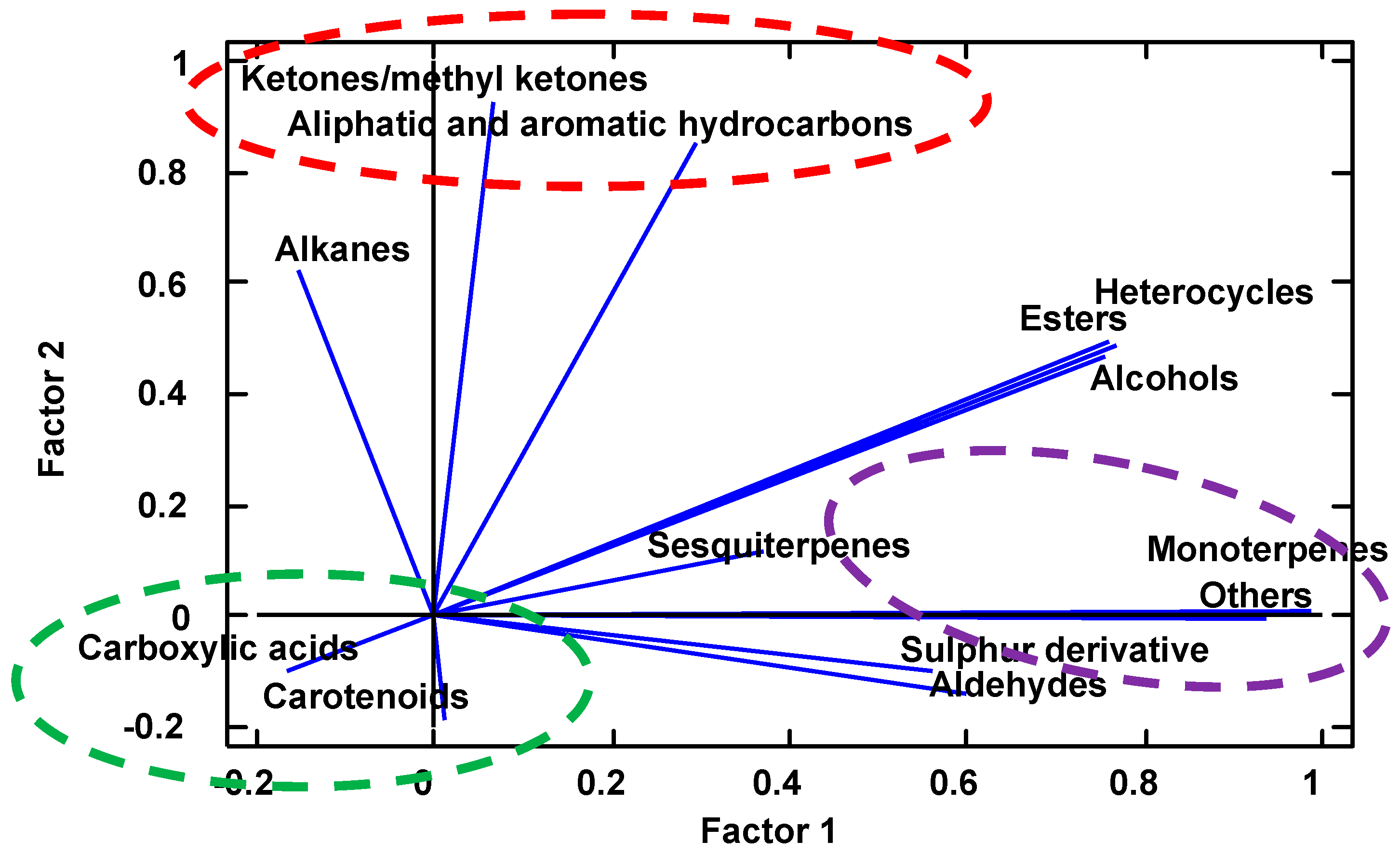
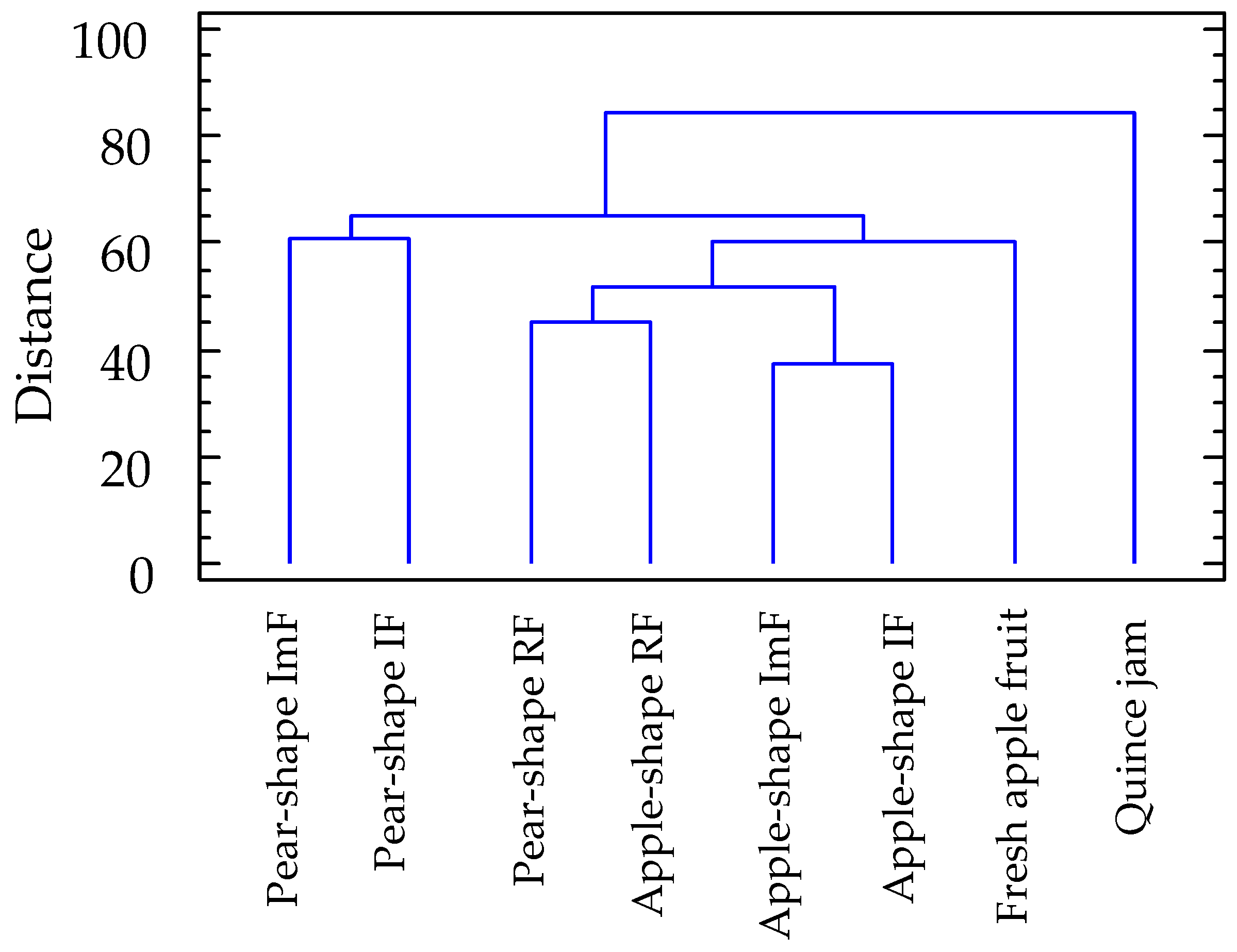
2.4. Volatiles Profile of S. domestica Fruits
3. Discussion
4. Materials and Methods
4.1. Vegetal Material and Sample Preparation
4.2. Proximal and Mineral Composition
4.3. Minor Analytical Determinations: pH, Soluble Solids Content and Total Acidity
4.4. Sugars Analytical Determinations: Total Sugars and Glucose
4.5. Bioactive Compounds Analytical Determinations: Total Phenolic Content and Antioxidant Activity by DPPH Assay
4.6. Analysis of Volatiles Profile
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreira, M.R.; Ponce, A.G. Bioactive compounds in functional fruits and vegetables: A strategy for promoting wellness and preventing diseases. Stud. Nat. Prod. Chem. 2024, 82, 315–345. [Google Scholar] [CrossRef]
- Bridgewater, P.; Rotherham, I.D. A critical perspective on the concept of biocultural diversity and its emerging role in nature and heritage conservation. People Nat. 2019, 1, 291–304. [Google Scholar] [CrossRef]
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Pilling, D.; Bélanger, J.; Hoffmann, I. Declining biodiversity for food and agriculture needs urgent global action. Nat. Food 2020, 1, 144–147. [Google Scholar] [CrossRef]
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, e261. [Google Scholar] [CrossRef]
- Melchior, I.C.; Newig, J. Governing transitions towards sustainable agriculture—Taking stock of an emerging field of research. Sustainability 2021, 13, 528. [Google Scholar] [CrossRef]
- Lucas, E.; Guo, M.; Guillén-Gosálbez, G. Low-carbon diets can reduce global ecological and health costs. Nat. Food 2023, 4, 394–406. [Google Scholar] [CrossRef]
- James-Martin, G.; Baird, D.L.; Hendrie, G.A.; Bogard, J.; Anastasiou, K.; Brooker, P.G.; Wiggins, B.; Williams, G.; Herrero, M.; Lawrence, M.; et al. Environmental sustainability in national food-based dietary guidelines: A global review. Lancet Planet. Health 2022, 6, e977–e986. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Ulian, T.; Diazgranados, M.; Pironon, S.; Padulosi, S.; Liu, U.; Davies, L.; Howes, M.J.R.; Borrell, J.S.; Ondo, I.; Pérez-Escobar, O.A.; et al. Unlocking plant resources to support food security and promote sustainable agriculture. Plants People Planet 2020, 2, 421–445. [Google Scholar] [CrossRef]
- Singh, V.; Shukla, S.; Singh, A. The principal factors responsible for biodiversity loss. Open J. Plant Sci. 2021, 6, 011–014. [Google Scholar]
- Katoch. Underutilized Crops: An Overview. In Ricebean: Exploiting the Nutritional Potential of an Underutilized Legume; Katoch, A., Ed.; Springer: Singapore, 2020; pp. 1–27. [Google Scholar] [CrossRef]
- Knez, M.; Ranić, M.; Gurinović, M. Underutilized plants increase biodiversity, improve food and nutrition security, reduce malnutrition, and enhance human health and well-being. Let’s put them back on the plate! Nutr. Rev. 2023, 82, 1111–1124. [Google Scholar] [CrossRef]
- Chandra, M.S.; Naresh, R.K.; Thenua, O.V.S.; Singh, R.; Geethanjali, D. Improving resource conservation, productivity and profitability of neglected and underutilized crops in the breadbasket of India: A review. Pharma Innov. 2020, 70, 19–31. [Google Scholar]
- Moskalets, V.; Hulko, B.; Rozhko, I.; Moroz, V.; Ivankiv, M. Morpho-physiological characteristics of plants and biochemical parameters of rowan berries, common rowan, and domestic rowan grown in the conditions of the Northern Forest-Steppe of Ukraine. Sci. Horiz. 2023, 26, 78–92. [Google Scholar] [CrossRef]
- Cáceres Escudero, Y. Sorbus domestica L. (Rosaceae) en Extremadura (España). Acta Bot. Malacit. 2020, 45, 173–178. [Google Scholar] [CrossRef]
- de Rueda Salgueiro, J.O.; de Azagra Paredes, A.M.; Nieto, A.Á. The genus Sorbus in Spain. For. Syst. 2006, 15, S166–S186. [Google Scholar] [CrossRef]
- del Río, J.; de Azagra Paredes, A.M.; de Rueda, J.A.O. Ecología del paisaje del género “Sorbus” L. en la península Ibérica y Baleares. Ecología 2009, 22, 25–44. [Google Scholar]
- García-Fayos, P.; Gulias, J.; Martínez, J.; Marzo, A.; Melero, J.P.; Traveset, A.; Veintimilla, P.; Verd, M.; Cerd, V.; Gasque, M.; et al. Bases Ecológicas para la Recolección, Almacenamiento y Germinación de Semillas de Especies de uso Forestal de la Comunidad Valenciana; Banc de Llavors Forestals: Valencia, Spain, 2001; p. 91. Available online: https://www.uv.es/patricio/docs/bases-ecologicas_Garcia_Fayos_2001.pdf (accessed on 8 July 2024).
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer International Publishing: Gewerbestr, Switzerland, 2016. [Google Scholar]
- Rotach, P. EUFORGEN. Technical Guidelines for Genetic Conservation and Use for Service Tree (Sorbus domestica); International Plant Genetic Resources Institute: Rome, Italy, 2003. [Google Scholar]
- Enescu, C.M.; de Rigo, D.; Durrant, T.H.; Caudullo, G. Sorbus domestica in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Galabova, M.; Stoyanov, N.; Mitev, P. Primary studies of the composition of distillate beverages produced from Sorbus domestica fruits. BIO Web Conf. 2022, 45, 01012. [Google Scholar] [CrossRef]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. Antioxidant activities of various extracts and fractions of Sorbus domestica fruits at different maturity stages. Food Chem. 2006, 98, 599–608. [Google Scholar] [CrossRef]
- Ognyanov, M.; Denev, P.; Petkova, N.; Petkova, Z.; Stoyanova, M.; Zhelev, P.; Matev, G.; Teneva, D.; Georgiev, Y. Nutrient constituents, bioactive phytochemicals, and antioxidant properties of service tree (Sorbus domestica L.) fruits. Plants 2022, 11, 1832. [Google Scholar] [CrossRef]
- Brindza, J.; Cerve náková, J.; Tóth, D.; Bíro, D.; Sajbidor, J. Unutilized potential of true service tree (Sorbus domestica L.). ISHS Acta Hortic. 2009, 806, 717–726. [Google Scholar] [CrossRef]
- Majić, B.; Šola, I.; Likić, S.; Cindrić, I.J.; Rusak, G. Characterisation of Sorbus domestica L. bark, fruits and seeds: Nutrient composition and antioxidant activity. Food Technol. Biotechnol. 2015, 53, 463–471. [Google Scholar] [CrossRef]
- Mrkonjić, Z.; Nađpal, J.; Beara, I.; Šibul, F.; Knežević, P.; Lesjak, M.; Mimica-Dukić, N. Fresh fruits and jam of Sorbus domestica L. and Sorbus intermedia (Ehrh.) Pers.: Phenolic profiles, antioxidant action and antimicrobial activity. Bot. Serb. 2019, 43, 187–196. [Google Scholar] [CrossRef]
- Piagnania, M.C.; Debellinia, C.; LoScalzob, R. Phyllometry and carpometry, chemical and functional characterization of fruits of Sorbus domestica L. (service tree) selections. J. Berry Res. 2012, 2, 7–22. [Google Scholar] [CrossRef]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. LC-DAD-MS (ESI+) analysis of the phenolic content of Sorbus domestica fruits in relation to their maturity stage. Food Chem. 2008, 106, 1234–1245. [Google Scholar] [CrossRef]
- Termentzi, A.; Zervou, M.; Kokkalou, E. Isolation and structure elucidation of novel phenolic constituents from Sorbus domestica fruits. Food Chem. 2009, 116, 371–381. [Google Scholar] [CrossRef]
- Zeiner, M.; Cindrić, I.J.; Majić, B.; Stingeder, G. Study of the accumulation of toxic and essential ultra-trace elements in fruits of Sorbus domestica L. Int. J. Environ. Res. Public Health 2017, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Dereli, F.T.G.; Taştan, H.; Sobarzo-Sánchez, E.; Khan, H. Effect of Sorbus domestica and its active constituents in an experimental model of colitis rats induced by acetic acid. J. Ethnopharmacol. 2020, 251, 112521. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; De Pascual-Teresa, S.; De Ancos, B.; Cano, M.P. Nutritional Quality of Fruits. In Handbook of Fruits and Fruit Processing; Sinha, N.K., Sidhu, J.S., Barta, J., Wu, J.S.B., Cano, M.P., Eds.; Wiley & Sons, Ltd.: Ames, IA, USA, 2012; pp. 73–84. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Marcio, F.R.; Resende, M.F., Jr. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Sun, B.; Rahman, T.; Chen, F. Tracking volatile flavor changes during two years of aging of Chinese vinegar by HS-SPME-GC-MS and GC-O. J. Food Compos. Anal. 2022, 106, 104295. [Google Scholar] [CrossRef]
- Paganova, V. Ecology and distribution of service tree Sorbus domestica (L.) in Slovakia. Ekológia 2008, 27, 152–167. [Google Scholar]
- Paganová, V. Sorbus domestica L. in urban context and in landscape. In Proceedings of the International Conference of the Service Tree-Tree for New Europe, Tvarozná Lhota, Morava, Czech Republic, 20–21 September 2015; pp. 18–21. [Google Scholar]
- Špíšek, Z.; Uherková, A.; Svitok, M.; Vašut, R.J. Sorbus domestica L. at its northern Pannonian distribution limits: Distribution of individuals, fruit shapes and dendrometric characteristics. Dendrobiology 2018, 80, 37–47. [Google Scholar] [CrossRef]
- Brus, R.; Ballian, D.; Bogunić, F.; Bobinac, M.; IdžOjtić, M. Leaflet morphometric variation of service tree (Sorbus domestica L.) in the Balkan Peninsula. Plant Biosyst. 2011, 145, 278–285. [Google Scholar] [CrossRef]
- Špíšek, Z.; Otto, L.G.; Vašut, R.J. Genotypic variability of Sorbus domestica in Central Europe revealed by the SSR markers. Plant Biosyst. 2022, 156, 938–946. [Google Scholar] [CrossRef]
- Butorac, L.; Rošin, J.; Ninčević Runjić, T.; Runjić, M.; Tadić, J.; Čagalj, M.; Limić, I.; Dulčić, Ž.; Jelić, G.; Radunić, M. Morphological variability of Sorbus domestica L. fruits and seeds in the Mediterranean part of Croatia. Acta Hortic. 2022, 1384, 505–512. [Google Scholar] [CrossRef]
- Sottile, F.; Del Signore, M.B.; Giuggioli, N.R.; Peano, C. The potential of the Sorb (Sorbus domestica L.) as a minor fruit species in the Mediterranean areas: Description and quality traits of underutilized accessions. Prog. Nutr. 2017, 19, 41–48. [Google Scholar] [CrossRef]
- Pardo-De-Santayana, M.; Tardío, J.; Morales, R. The gathering and consumption of wild edible plants in the Campoo (Cantabria, Spain). Int. J. Food Sci. Nutr. 2005, 56, 529–542. [Google Scholar] [CrossRef]
- Camarero, J.J.; Campelo, F.; Sánchez-Sancho, J.A.; Santana, J.C. Mediterranean service trees respond less to drought than oaks. For. Ecol. Manag. 2023, 541, 121070. [Google Scholar] [CrossRef]
- Armbruster, G.F.J.; Lucek, K.; Willi, Y. Cryptic population structure at the northern range margin of the service tree Sorbus domestica. PeerJ 2022, 10, e14397. [Google Scholar] [CrossRef]
- Schmucker, J.; Skovsgaard, J.P.; Uhl, E.; Pretzsch, H. Crown structure, growth, and drought tolerance of true service tree (Sorbus domestica L.) in forests and urban environments. Urban For. Urban Green. 2024, 91, 128161. [Google Scholar] [CrossRef]
- Hasbal, G.; Ozden, T.Y.; Sen, M.; Yanardag, R.; Can, A. In vitro investigation of Sorbus domestica as an enzyme inhibitor. Istanbul J. Pharm. 2020, 50, 28–32. [Google Scholar]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M. Sorbus domestica L. leaf extracts as functional products: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Miletić, R.; Paunović, M.S. Research into service tree (Sorbus domestica L.) population in eastern Serbia. Genetika 2012, 44, 483–490. [Google Scholar] [CrossRef]
- Tas, A.; Gundogdu, M.; Ercisli, S.; Orman, E.; Celik, K.; Marc, R.A.; Buckova, M.; Adamkova, B.A.; Mlcek, J. Fruit quality characteristics of service tree (Sorbus domestica L.) genotypes. ACS Omega 2023, 8, 19862–19873. [Google Scholar] [CrossRef]
- Miko, M.; Gažo, J. Morphological and biological characteristics of fruits and seed of the service tree (Sorbus domestica L.). J. Fruit Ornam. Plant Res. 2004, 12, 139–146. [Google Scholar]
- Sulusoglu, M. Distribution of service tree in Kocaeli-Marmara area: Phenological, morphological and chemical properties. J. Appl. Biol. Sci. 2019, 8, 35–41. [Google Scholar]
- Petkova, N.T.; Ognyanov, M.H.; Vrancheva, R.Z.; Zhelev, P. Phytochemical, nutritional and antioxidant characteristics of whitebeam (Sorbus aria) fruits. Acta Sci. Pol. Technol. Aliment. 2020, 19, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, E.; Yildiz, M.; Gul, E.N. The effect of ripening periods on physical, chemical and mechanical properties of service tree (Sorbus domestica L.) fruits. Agric. Eng. Int. CIGR J. 2015, 17, 259–266. [Google Scholar]
- Poljak, I.; Vahčić, N.; Liber, Z.; Tumpa, K.; Pintar, V.; Zegnal, I.; Vidaković, A.; Valković, B.; Kajba, D.; Idžojtić, M. Morphological and chemical diversity and antioxidant capacity of the service tree (Sorbus domestica L.) fruits from two eco-geographical regions. Plants 2021, 10, 1691. [Google Scholar] [CrossRef]
- USDA: U.S. Department of Agriculture. National Nutrient Database for Standard Reference. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/ (accessed on 28 July 2024).
- Yingjie, S.; Zedong, S.; Yaping, J.; Xinhua, Z.; Xiaoan, L.; Fujun, L. Effects of preharvest regulation of ethylene on carbohydrate metabolism of apple (Malus domestica Borkh cv. Starkrimson) fruit at harvest and during storage. Sci. Hortic. 2021, 276, 109748. [Google Scholar] [CrossRef]
- Steinnes, E. Soil and Human Health. In Sustaining Soil Productivity in Response to Global Climate Change: Science, Policy, and Ethics; Sauer, T., Norman, J., Sivakumar, M., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 214–239. [Google Scholar] [CrossRef]
- Ekholm, P.; Reinivuo, H.; Mattila, P.; Pakkala, H.; Koponen, J.; Happonen, A.; Hellström, J.; Ovaskainen, M. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food Compost. Anal. 2007, 20, 487–495. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Kartal, T.; Gür, E. Üvez (Sorbus domestica L.) meyvesi pomolojik özelliklerinin belirlenmesi. ÇOMÜ LJAR 2020, 1, 24–30. [Google Scholar]
- Öz Atasever, Ö.; Kepenek, G.; Gercekcioglu, R. Performances of genotypes selected from Tokat natural service tree (Sorbus domestica.) population (Selection II). Acta Hortic. 2020, 1282, 351–356. [Google Scholar] [CrossRef]
- Shao, X.; Tu, K.; Tu, S.; Tu, J. A combination of heat treatment and chitosan coating delays ripening and reduces decay in “gala” apple fruit. J. Food Qual. 2012, 35, 83–92. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2020, 140, 109854. [Google Scholar] [CrossRef]
- Bennett, A.B.; Labavitch, J.M. Ethylene and ripening-regulated expression and function of fruit cell wall modifying proteins. Plant Sci. 2008, 175, 130–136. [Google Scholar] [CrossRef]
- Jiang, C.C.; Fang, Z.Z.; Zhou, D.R.; Pan, S.L.; Ye, X.F. Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv.‘Furongli’(Prunus salicina Lindl). J. Sci. Food Agric. 2019, 99, 1010–1019. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The sorbus spp.—Underutilised plants for foods and nutraceuticals: Review on polyphenolic phytochemicals and antioxidant potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Kültür, S. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Labuda, R.; Krivánek, L.; Tančinová, D.; Mátéová, S.; Hrubcová, S. Mycological survey of ripped service tree fruits (Sorbus domestica L.) with an emphasis on toxinogenic fungi. Int. J. Food Microbiol. 2005, 99, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewska, M.A. Seasonal variation in phenylpropanoid biosynthesis and in vitro antioxidant activity of Sorbus domestica leaves: Harvesting time optimisation for medicinal application. Ind. Crops Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Razola-Díaz, M.D.C.; Aznar-Ramos, M.J.; Benítez, G.; Gómez-Caravaca, A.M.; Verardo, V. Exploring the potential of phenolic and antioxidant compounds in new Rosaceae fruits. J. Sci. Food Agric. 2024, 104, 3705–3718. [Google Scholar] [CrossRef]
- Ceylan, B.; Yeşiloğlu, Y. Investigation of antioxidant activity of Sorbus domestica L. extracts and determination of phenolic contents by LC-MS/MS. Rev. Roum. Chim. 2022, 67, 343–351. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudone, L.; Gaivelyte, K.; Viškelis, P.; Janulis, V. Phenolic and antioxidant profiles of rowan (Sorbus L.) fruits. Nat. Prod. Res. 2014, 28, 1231–1240. [Google Scholar] [CrossRef]
- Vyviurska, O.; Pysarevska, S.; Jánošková, N.; Špánik, I. Comprehensive two-dimensional gas chromatographic analysis of volatile organic compounds in distillate of fermented Sorbus domestica fruit. Open Chem. 2015, 13, 96–104. [Google Scholar] [CrossRef]
- Maoz, I.; Lewinsohn, E.; Gonda, I. Amino acids metabolism as a source for aroma volatiles biosynthesis. Curr. Opin. Plant Biol. 2022, 67, 102221. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Petersen, M.A.; Bredie, W.L. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Techakanon, C.; Thitithanakul, S. Impact of the ripening stage of wax apples on chemical profiles of juice and cider. ACS Omega 2018, 3, 6710–6718. [Google Scholar] [CrossRef]
- Maeda, T.; Kikuma, S.; Araki, T.; Ikeda, G.; Takeya, K.; Sagara, Y. The effects of mixing stage and fermentation time on the quantity of flavor compounds and sensory intensity of flavor in white bread. Food Sci. Technol. Res. 2009, 15, 117–126. [Google Scholar] [CrossRef][Green Version]
- Kyoui, D.; Saito, Y.; Takahashi, A.; Tanaka, G.; Yoshida, R.; Maegaki, Y.; Kawarai, T.; Ogihara, H.; Suzuki, C. Antibacterial activity of hexanol vapor in vitro and on the surface of vegetables. Foods 2023, 12, 3097. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qin, D.; Huang, G.; Jiang, X.; Fang, K.; Wang, Q.; Ni, E.; Li, B.; Pan, C.; Li, H.; et al. Identification and characterization of the key volatile flavor compounds in black teas from distinct regions worldwide. J. Food Sci. 2022, 87, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Peppard, T.L.; Halsey, S.A. The occurrence of two geometrical isomers of 2, 4, 5-trimethyl-1, 3-dioxolane in beer. J. Inst. Brew. 1982, 88, 309–312. [Google Scholar] [CrossRef]
- Nistor, O.V.; Mocanu, G.D.; Andronoiu, D.G.; Barbu, V.V.; Ceclu, L. A complex characterization of pumpkin and quince purees obtained by a combination of Freezing and conventional cooking. Foods 2022, 11, 2038. [Google Scholar] [CrossRef]
- Simonato, B.; Lorenzini, M.; Zapparoli, G. Effects of post-harvest fungal infection of apples on chemical characteristics of cider. LWT 2021, 138, 110620. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus× domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef]
- Rossouw, M.; Geldenhuys, G.G.; Marini, F.; Manley, M. Multivariate statistical visualisation and modelling of GC–MS data for sensory quality prediction of flavoured cider as influenced by storage time and temperature. Microchem. J. 2023, 195, 109393. [Google Scholar] [CrossRef]
- Wu, X.; Bi, J.; Fauconnier, M.L. Characteristic volatiles and cultivar classification in 35 apple varieties: A case study of two harvest years. Foods 2022, 11, 690. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemist (AOAC International): Washington, DC, USA, 2005. [Google Scholar]
- Ma, X.; Luo, H.; Zhang, F.; Gao, F. Study on the influence of region of interest on the detection of total sugar content in apple using hyperspectral imaging technology. Food Sci. Technol. 2022, 42, e87922. [Google Scholar] [CrossRef]
- Ukeda, H.; Fujita, Y.; Ohira, M.; Sawamura, M. Immobilized enzyme-based microtiter plate assay for glucose in foods. J. Agric. Food Chem. 1996, 44, 3858–3863. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compost. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rufino, M.S.; Alves, R.E.; Fernandes, F.A.; Brito, E.S. Free radical scavenging behavior of ten exotic tropical fruits extracts. Food Res. Int. 2011, 44, 2072–2075. [Google Scholar] [CrossRef]
- Moreno, E.; Fita, A.; González-Mas, M.C.; Rodríguez-Burruezo, A. HS-SPME study of the volatile fraction of Capsicum accessions and hybrids in different parts of the fruit. Sci Hortic. 2012, 135, 87–97. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative analysis of the volatile fraction of fruit juice from different Citrus species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef]


| Parameter | S. domestica Apple-Shaped Type | S. domestica Pear-Shaped Type | p-Value | ||
|---|---|---|---|---|---|
| Mean Value ± SD | CV (%) | Mean Value ± SD | CV (%) | ||
| Weight (g) | 7.61 ± 0.39 | 5.15 | 4.42 ± 0.54 | 12.23 | 0.0012 * |
| Moisture (%) | 65.46 ± 0.31 | 0.47 | 65.63 ± 1.19 | 1.81 | 0.8189 |
| Dry matter (%) | 34.54 ± 0.31 | 0.90 | 34.37 ± 1.19 | 3.46 | 0.8189 |
| Ash (%) | 0.51 ± 0.04 | 7.07 | 0.60 ± 0.06 | 9.53 | 0.0897 |
| Fiber (%) | 4.91 ± 1.51 | 30.79 | 2.52 ± 0.11 | 4.29 | 0.0525 |
| Fat (%) | 0.67 ± 0.07 | 10.45 | 0.43 ± 0.09 | 21.44 | 0.0244 * |
| Protein (%) | 0.54 ± 0.06 | 10.51 | 0.68 ± 0.12 | 18.13 | 0.1484 |
| Carbohydrates (%) | 27.91 ± 1.08 | 3.88 | 30.13 ± 1.31 | 4.33 | 0.0858 |
| Total energy (kcal 100 g−1 fw) | 129.65 ± 2.21 | 1.71 | 132.20 ± 4.06 | 3.07 | 0.3933 |
| Na (mg 100 g−1 fw) | 7.35 ± 0.14 | 1.95 | 6.85 ± 2.21 | 10.53 | 0.3100 |
| K (mg 100 g−1 fw) | 185.56 ± 7.75 | 4.18 | 221.19 ± 7.76 | 3.51 | 0.0049 * |
| P (mg 100 g−1 fw) | 18.16 ± 4.10 | 22.59 | 17.19 ± 4.69 | 27.27 | 0.8006 |
| Mg (mg 100 g−1 fw) | 9.81 ± 0.09 | 0.92 | 11.12 ± 0.46 | 4.11 | 0.0082 * |
| Ca (mg 100 g−1 fw) | 44.59 ± 1.84 | 4.13 | 43.93 ± 3.58 | 8.14 | 0.7894 |
| Mn (mg 100 g−1 fw) | 0.12 ± 0.10 | 85.09 | 0.09 ± 0.01 | 15.25 | 0.5740 |
| Fe (mg 100 g−1 fw) | 1.42 ± 0.23 | 16.45 | 0.47 ± 0.10 | 20.68 | 0.0029 * |
| Cu (mg 100 g−1 fw) | 0.08 ± 0.01 | 11.52 | 0.06 ± 0.00 | 5.72 | 0.0373 * |
| Zn (mg 100 g−1 fw) | 0.11 ± 0.01 | 12.49 | 0.06 ± 0.01 | 13.87 | 0.0069 * |
| Parameter | S. domestica Apple-Shaped Type | S. domestica Pear-Shaped Type | p-Value | ||
|---|---|---|---|---|---|
| Mean Value ± SD | CV (%) | Mean Value ± SD | CV (%) | ||
| pH | 3.56 ± 0.03 | 0.81 | 3.98 ± 0.01 | 0.25 | 0.0000 * |
| Soluble solids content (°Brix) | 16.57 ± 0.48 | 2.90 | 16.29 ± 10.70 | 1.81 | 0.7994 |
| Total acidity (g malic acid 100 g−1 fw) | 1.17 ± 0.03 | 2.33 | 0.79 ± 0.01 | 1.38 | 0.0000 * |
| Total sugars (g·100 g−1 fw) | 14.19 ± 0.55 | 3.88 | 11.42 ± 0.49 | 4.33 | 0.0029 * |
| Glucose (g·100 g−1 fw) | 6.42 ± 0.25 | 3.88 | 5.41 ± 0.17 | 3.18 | 0.0044 * |
| Parameter | S. domestica Apple-Shaped Type | S. domestica Pear-Shaped Type | p-Value | ||
|---|---|---|---|---|---|
| Mean Value ± SD | CV (%) | Mean Value ± SD | CV (%) | ||
| Total phenolics (mg ECA 100 g−1 fw) | 93.67 ± 19.99 | 21.34 | 107.40 ± 7.32 | 6.81 | 0.3265 |
| Antioxidant activity by DPPH assay (µmol TE·g−1 fw). | 283.67 ± 66.16 | 23.32 | 170.58 ± 42.08 | 24.67 | 0.0669 |
| Compounds * and Odor Descriptor | S. domestica Apple-Shaped Type | S. domestica Pear-Shaped Type | Apple Fruit | Quince Jam | ||||
|---|---|---|---|---|---|---|---|---|
| ImF | IF | RF | ImF | IF | RF | |||
| Esters | ||||||||
| Bytil acetate (sweet, fruity) | 1.14 ± 1.07 | 0.33 ± 0.11 | 0.25 ± 0.05 | 0.12 ± 0.00 | 0.12 ± 0.02 | 0.39 ± 0.18 | 1.59 ± 0.61 | - |
| Methyl hexanoate (pineapple) | 0.21 ± 0.02 | - | - | 0.33 ± 0.06 | 0.29 ± 0.04 | - | 0.14 ± 0.10 | - |
| Hexyl acetate (fruity) | 0.47 ± 0.12 | 0.82 ± 0.26 | 1.68 ± 0.30 | 0.47 ± 0.13 | 0.50 ± 0.06 | 0.87 ± 0.39 | 71.68 ± 5.71 | - |
| Methyl octanoate (fruity, floral) | 0.13 ± 0.09 | 0.18 ± 0.13 | 0.22 ± 0.16 | 0.19 ± 0.09 | 0.20 ± 0.02 | - | - | - |
| Ethyl octanoate (fruity, floral) | - | 0.13 ± 0.10 | 0.40 ± 0.17 | - | 0.11 ± 0.08 | 0.29 ± 0.04 | - | - |
| Methyl nonanoate (sweet, coconut-like) | - | 0.05 ± 0.04 | - | 0.07 ± 0.00 | 0.14 ± 0.01 | 0.12 ± 0.09 | - | - |
| Ethyl nonanoate (fruity brandy-like) | - | - | - | - | - | 0.28 ± 0.03 | - | - |
| Sesquiterpenes | ||||||||
| β-caryophyllene (spicy) | - | 0.31 ± 0.06 | - | - | - | - | - | - |
| α-farnesene (floral) | 4.13 ± 1.38 | 2.40 ± 0.48 | 2.84 ± 0.02 | 5.04 ± 0.41 | 1.39 ± 0.22 | 2.47 ± 0.11 | - | 0.64 ± 0.19 |
| δ-cadinene (woody) | 0.05 ± 0.04 | 0.04 ± 0.03 | - | - | - | - | 0.28 ± 0.02 | - |
| Sulfur derivative compounds | ||||||||
| Diallyl disulfide (garlic) | 0.28 ± 0.04 | 0.16 ± 0.12 | - | 0.81 ± 0.31 | 0.30 ± 0.08 | - | - | - |
| Monoterpenes | ||||||||
| Linalool (floral, fresh, slightly sweet) | 0.26 ± 0.19 | 0.35 ± 0.15 | - | 0.52 ± 0.22 | 0.35 ± 0.17 | - | - | - |
| Epoxy linalool (floral, fresh, slightly sweet) | 0.18 ± 0.14 | 0.10 ± 0.09 | - | - | 1.10 ± 0.06 | 1.35 ± 0.01 | - | - |
| β-citronellol (smell citrussy) | 0.14 ± 0.10 | 0.16 ± 0.12 | - | 0.24 ± 0.05 | 0.13 ± 0.01 | - | - | - |
| Alcohols | ||||||||
| 3-metyl-1-butanol (spicy) | - | 1.42 ± 1.05 | 3.18 ± 0.30 | 0.92 ± 0.09 | 1.68 ± 0.09 | 2.61 ± 0.25 | - | 13.25 ± 0.11 |
| 2-methyl-1-butanol (acidic, sharp, spicy) | - | 1.53 ± 1.14 | 4.03 ± 0.09 | 0.99 ± 0.10 | 1.51 ± 0.13 | 2.57 ± 0.87 | 8.60 ± 1.76 | 5.58 ± 0.04 |
| 1-pentanol (like apricot) | 1.39 ± 0.62 | 0.57 ± 0.13 | 0.55 ± 0.07 | 1.09 ± 0.02 | 1.03 ± 0.10 | 0.77 ± 0.18 | - | - |
| cis-3-hexenol (grass) | 0.22 ± 0.16 | 0.13 ± 0.10 | 0.05 ± 0.04 | 0.37 ± 0.08 | 0.27 ± 0.05 | - | - | - |
| 1-hexanol (green, fresh fruit) | 35.31 ± 2.00 | 28.13 ± 0.97 | 26.20 ± 0.90 | 25.38 ± 0.25 | 21.88 ± 0.29 | 18.94 ± 1.07 | 15.13 ± 3.74 | 1.09 ± 0.03 |
| 1-heptanol (green) | 0.38 ± 0.11 | 0.26 ± 0.10 | 0.56 ± 0.14 | 0.25 ± 0.01 | 0.38 ± 0.00 | 0.45 ± 0.04 | - | - |
| 6-metil-5-hepten-2-ol (fruity, citrus-like) | 0.19 ± 0.14 | 0.21 ± 0.16 | - | 0.24 ± 0.03 | 0.15 ± 0.01 | - | - | - |
| 3-octanol (nutty, herbaceous) | 0.31 ± 0.23 | 0.35 ± 0.10 | - | 0.50 ± 0.02 | 0.24 ± 0.01 | - | - | - |
| Benzyl alcohol (floral) | - | 0.10 ± 0.07 | 0.50 ± 0.06 | - | 0.30 ± 0.01 | 1.99 ± 0.91 | - | 4.69 ± 0.11 |
| 1-octanol (rose, mushroom) | 3.38 ± 0.90 | 2.66 ± 0.60 | 3.65 ± 0.58 | 3.34 ± 0.19 | 1.92 ± 0.03 | 2.07 ± 0.02 | - | |
| Phenethyl alcohol (floral) | 1.30 ± 0.15 | 2.29 ± 1.70 | 15.17 ± 2.79 | 0.90 ± 0.04 | 2.61 ± 0.07 | 6.33 ± 1.73 | - | 13.94 ± 0.27 |
| 1,3-octanediol (green, fatty, mushroom) | 15.66 ± 2.58 | 11.24 ± 1.98 | 15.40 ± 0.59 | 4.35 ± 1.43 | 8.30 ± 0.41 | 11.46 ± 1.23 | - | - |
| Compounds * and Odor Descriptor | S. domestica Apple-Shaped Type | S. domestica Pear-Shaped Type | Apple Fruit | Quince Jam | ||||
|---|---|---|---|---|---|---|---|---|
| ImF | IF | RF | ImF | IF | RF | |||
| Aldehydes | ||||||||
| Hexanal (green grass) | 1.14 ± 0.31 | 0.97 ± 0.28 | 0.61 ± 0.08 | 8.07 ± 1.59 | 2.21 ± 0.29 | 1.16 ± 0.03 | 0.21 ± 0.16 | 2.83 ± 0.01 |
| 2-hexenal (green, fresh fruit) | 0.18 ± 0.14 | - | - | 1.64 ± 0.35 | 0.06 ± 0.04 | - | 1.07 ± 0.21 | - |
| Heptanal (strong fruity) | 0.11 ± 0.08 | 0.05 ± 0.03 | - | 0.09 ± 0.01 | 0.08 ± 0.00 | - | - | 0.62 ± 0.00 |
| cis-2-heptenal (green) | 0.36 ± 0.27 | 0.50 ± 0.37 | - | 0.16 ± 0.01 | 2.76 ± 1.99 | - | - | 19.89 ± 0.14 |
| Benzaldehyde (fruity, almond-like) | 1.78 ± 0.06 | 2.44 ± 0.38 | 1.11 ± 0.05 | 3.37 ± 0.58 | 2.94 ± 2.09 | 6.82 ± 0.58 | - | 3.79 ± 0.11 |
| Phenylacetaldehyde (green floral) | 8.67 ± 1.02 | 8.38 ± 0.86 | 1.10 ± 0.04 | 8.65 ± 0.92 | 12.61 ± 0.50 | 8.55 ± 0.23 | - | - |
| (E)-2-octenal (fatty, citric) | 0.74 ± 0.19 | 0.77 ± 0.10 | 0.77 ± 0.04 | 0.40 ± 0.01 | 5.56 ± 0.03 | 0.61 ± 0.02 | - | 14.12 ± 0.98 |
| Nonanal (fatty, citric) | 2.86 ± 0.05 | 2.23 ± 0.18 | 2.23 ± 0.58 | 3.83 ± 0.81 | 5.21 ± 0.22 | 4.72 ± 0.16 | - | 5.66 ± 0.73 |
| Decanal (citric) | 0.24 ± 0.03 | 0.17 ± 0.03 | 0.16 ± 0.00 | 0.20 ± 0.01 | 0.08 ± 0.06 | - | - | |
| Ketones/methyl ketones | ||||||||
| 2-heptanone (fruity, banana-like) | 0.27 ± 0.20 | - | 0.90 ± 0.26 | - | 0.10 ± 0.07 | 0.21 ± 0.05 | - | - |
| Acetophenone (sweet, floral, citric) | 0.04 ± 0.03 | - | 0.15 ± 0.03 | - | - | - | - | - |
| Carotenoids | ||||||||
| 6-metil-5-hepten-2-ona (fruity) | 0.27 ± 0.20 | 0.37 ± 0.28 | - | 1.81 ± 0.02 | 1.18 ± 0.27 | - | - | 6.83 ± 0.07 |
| Aliphatic and aromatic hydrocarbons | ||||||||
| β-methylnaphthalene (floral) | 0.66 ± 0.06 | 0.21 ± 0.18 | 0.62 ± 0.44 | 0.17 ± 0.01 | 0.16 ± 0.11 | - | - | - |
| α-methylnaphthalene (coal tar) | 0.20 ± 0.06 | 0.06 ± 0.04 | - | 0.08 ± 0.01 | - | - | - | - |
| Alkanes | ||||||||
| Tetradecane (gasoline-like) | 0.09 ± 0.07 | 0.08 ± 0.06 | 0.15 ± 0.00 | 0.07 ± 0.00 | - | - | - | - |
| Carboxylic acids | ||||||||
| Hexanoic acid (barnyard animals) | 0.46 ± 0.34 | 0.45 ± 0.34 | - | 0.23 ± 0.16 | - | - | - | - |
| Nonanoic acid (rancid) | - | - | 0.73 ± 0.10 | 0.63 ± 0.04 | 0.41 ± 0.04 | 0.43 ± 0.31 | 0.05 ± 0.04 | - |
| Lauric acid (bay, coconut) | 0.85 ± 0.48 | 0.62 ± 0.03 | 2.07 ± 0.29 | 0.72 ± 0.32 | 0.81 ± 0.12 | 2.20 ± 1.05 | 1.24 ± 0.38 | 7.08 ± 0.77 |
| Heterocycles | ||||||||
| γ-hexalactone (herbal) | - | 0.10 ± 0.08 | 0.13 ± 0.09 | 0.17 ± 0.02 | 0.14 ± 0.00 | 0.09 ± 0.06 | - | - |
| γ-nonalactone (creamy coconut) | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.09 ± 0.07 | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.14 ± 0.10 | - | - |
| γ-decalactone (fruity) | 0.66 ± 0.09 | 0.50 ± 0.07 | 0.68 ± 0.07 | 0.25 ± 0.02 | 0.29 ± 0.02 | 0.39 ± 0.04 | - | - |
| Others | ||||||||
| 2,4,5-trimethyl-1,3-dioxolane (nutty, phenolic) | 13.84 ± 0.67 | 25.50 ± 5.04 | 10.75 ± 1.91 | 22.41 ± 1.01 | 24.04 ± 0.57 | 20.13 ± 0.10 | - | - |
| 2-pentylfuran (fruity, green) | 0.21 ± 0.15 | 0.24 ± 0.10 | - | 0.17 ± 0.00 | 0.28 ± 0.03 | 0.20 ± 0.14 | - | - |
| Rose oxid (green, floral, rose) | 0.17 ± 0.12 | 0.17 ± 0.12 | - | 0.28 ± 0.03 | 0.19 ± 0.02 | 0.11 ± 0.08 | - | - |
| Camphor (fatty) | 0.92 ± 0.10 | 2.13 ± 0.14 | 3.27 ± 0.04 | 0.67 ± 0.11 | 0.72 ± 0.11 | 1.22 ± 0.20 | - | - |
| Chemical Family | S. domestica Apple-Shaped Type | S. domestica Pear-Shaped Type | Apple Fruit | Quince Jam | ||||
|---|---|---|---|---|---|---|---|---|
| ImF | IF | RF | ImF | IF | RF | |||
| Esters | 1.68 ± 0.86 | 2.22 ± 0.24 | 2.93 ± 0.66 | 1.19 ± 0.01 | 1.41 ± 0.15 | 1.96 ± 0.43 | 71.83 ± 5.02 | - |
| Sesquiterpenes | 3.65 ± 1.35 | 2.99 ± 0.51 | 3.04 ± 0.02 | 4.97 ± 0.41 | 1.39 ± 0.22 | 2.47 ± 0.11 | 0.29 ± 0.02 | 0.64 ± 0.19 |
| Sulfur derivative | 0.28 ± 0.04 | 0.16 ± 0.01 | - | 0.81 ± 0.03 | 0.30 ± 0.08 | - | - | - |
| Monoterpenes | 0.89 ± 0.23 | 0.94 ± 0.18 | - | 1.26 ± 0.54 | 1.58 ± 0.13 | 1.34 ± 0.01 | - | - |
| Alcohols | 57.52 ± 0.65 | 62.57 ± 2.78 | 74.25 ± 2.41 | 38.24 ± 0.79 | 40.18 ± 0.13 | 46.75 ± 1.81 | 25.32 ± 5.50 | 38.54 ± 0.29 |
| Aldehydes | 16.81 ± 1.97 | 19.26 ± 1.40 | 6.12 ± 0.79 | 26.36 ± 0.90 | 26.60 ± 0.55 | 21.92 ± 0.87 | 1.37 ± 0.11 | 46.90 ± 0.22 |
| Ketones/methyl-ketones | 0.55 ± 0.23 | - | 1.16 ± 0.29 | - | 0.16 ± 0.07 | 0.21 ± 0.05 | - | - |
| Carotenoids | 0.27 ± 0.20 | 0.37 ± 0.28 | - | 1.81 ± 0.02 | 1.18 ± 0.27 | - | - | 6.83 ± 0.07 |
| Aliphatic and aromatic hydrocarbons | 0.80 ± 0.12 | 0.36 ± 0.15 | 0.92 ± 0.44 | 0.26 ± 0.05 | 0.24 ± 0.11 | - | - | - |
| Alkenes | 0.09 ± 0.07 | 0.08 ± 0.06 | 0.15 ± 0.00 | 0.07 ± 0.00 | - | - | - | - |
| Carboxylic acids | 1.47 ± 0.25 | 1.71 ± 0.31 | 2.94 ± 0.38 | 1.73 ± 0.48 | 1.22 ± 0.08 | 2.95 ± 1.32 | 1.18 ± 0.42 | 7.09 ± 0.77 |
| Heterocycles | 0.77 ± 0.08 | 0.94 ± 0.02 | 1.10 ± 0.11 | 0.57 ± 0.06 | 0.58 ± 0.01 | 0.74 ± 0.13 | - | - |
| Others | 14.94 ± 0.31 | 7.67 ± 4.72 | 7.38 ± 0.88 | 23.29 ± 1.15 | 25.17 ± 0.67 | 21.66 ± 0.21 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raigón Jiménez, M.D.; García-Martínez, M.D.; Esteve Ciudad, P.; Fukalova Fukalova, T. Nutritional, Bioactive, and Volatile Characteristics of Two Types of Sorbus domestica Undervalued Fruit from Northeast of Iberian Peninsula, Spain. Molecules 2024, 29, 4321. https://doi.org/10.3390/molecules29184321
Raigón Jiménez MD, García-Martínez MD, Esteve Ciudad P, Fukalova Fukalova T. Nutritional, Bioactive, and Volatile Characteristics of Two Types of Sorbus domestica Undervalued Fruit from Northeast of Iberian Peninsula, Spain. Molecules. 2024; 29(18):4321. https://doi.org/10.3390/molecules29184321
Chicago/Turabian StyleRaigón Jiménez, María Dolores, María Dolores García-Martínez, Patricia Esteve Ciudad, and Tamara Fukalova Fukalova. 2024. "Nutritional, Bioactive, and Volatile Characteristics of Two Types of Sorbus domestica Undervalued Fruit from Northeast of Iberian Peninsula, Spain" Molecules 29, no. 18: 4321. https://doi.org/10.3390/molecules29184321
APA StyleRaigón Jiménez, M. D., García-Martínez, M. D., Esteve Ciudad, P., & Fukalova Fukalova, T. (2024). Nutritional, Bioactive, and Volatile Characteristics of Two Types of Sorbus domestica Undervalued Fruit from Northeast of Iberian Peninsula, Spain. Molecules, 29(18), 4321. https://doi.org/10.3390/molecules29184321






