Combining the Elicitor Up-Regulated Production of Unusual Linear Diterpene-Derived Variants for an In-Depth Assessment of the Application Value and Risk of the Medicinal and Edible Basidiomycete Schizophyllum commune
Abstract
:1. Introduction
2. Results and Discussion
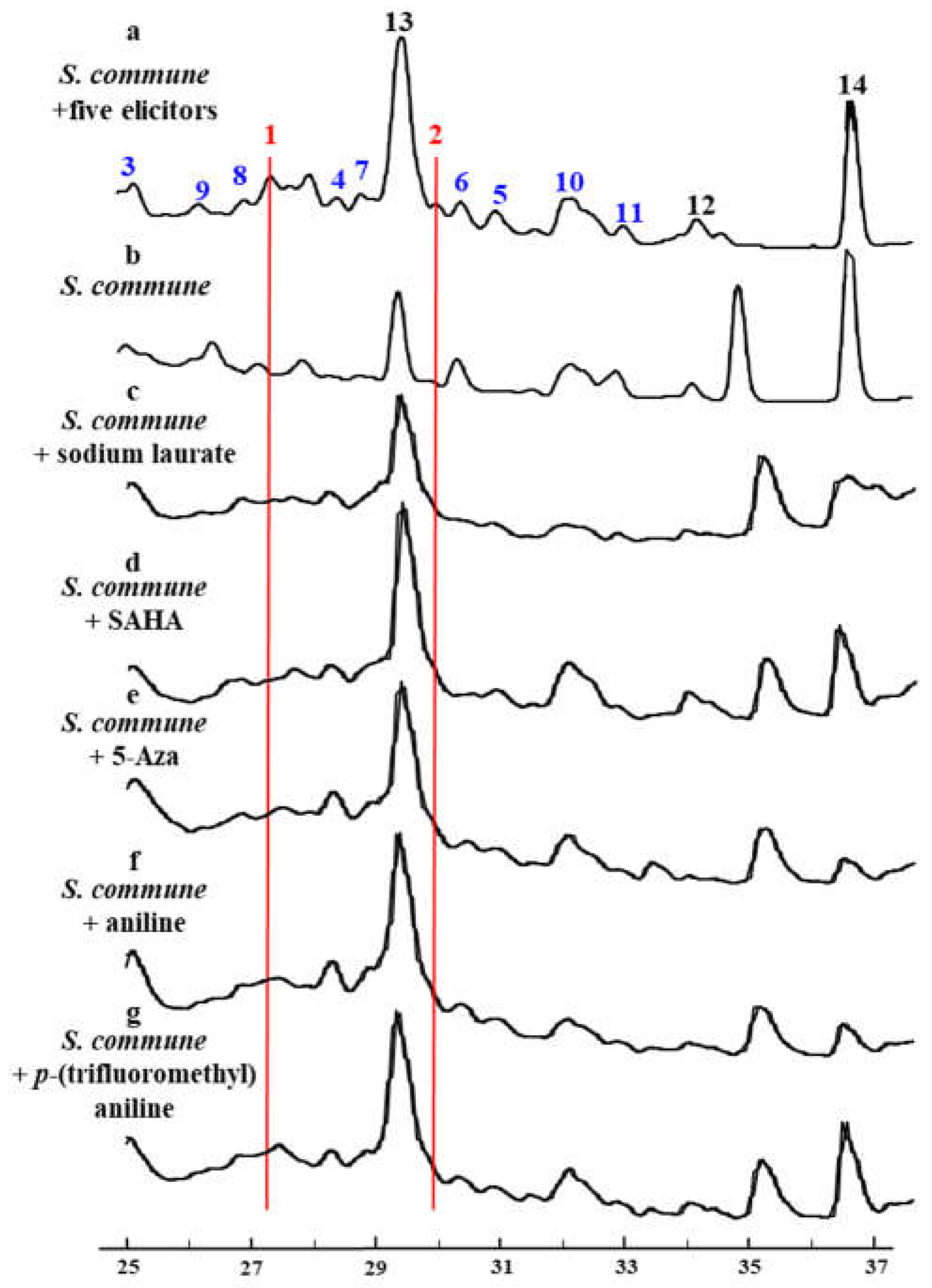
2.1. Selection and Screening
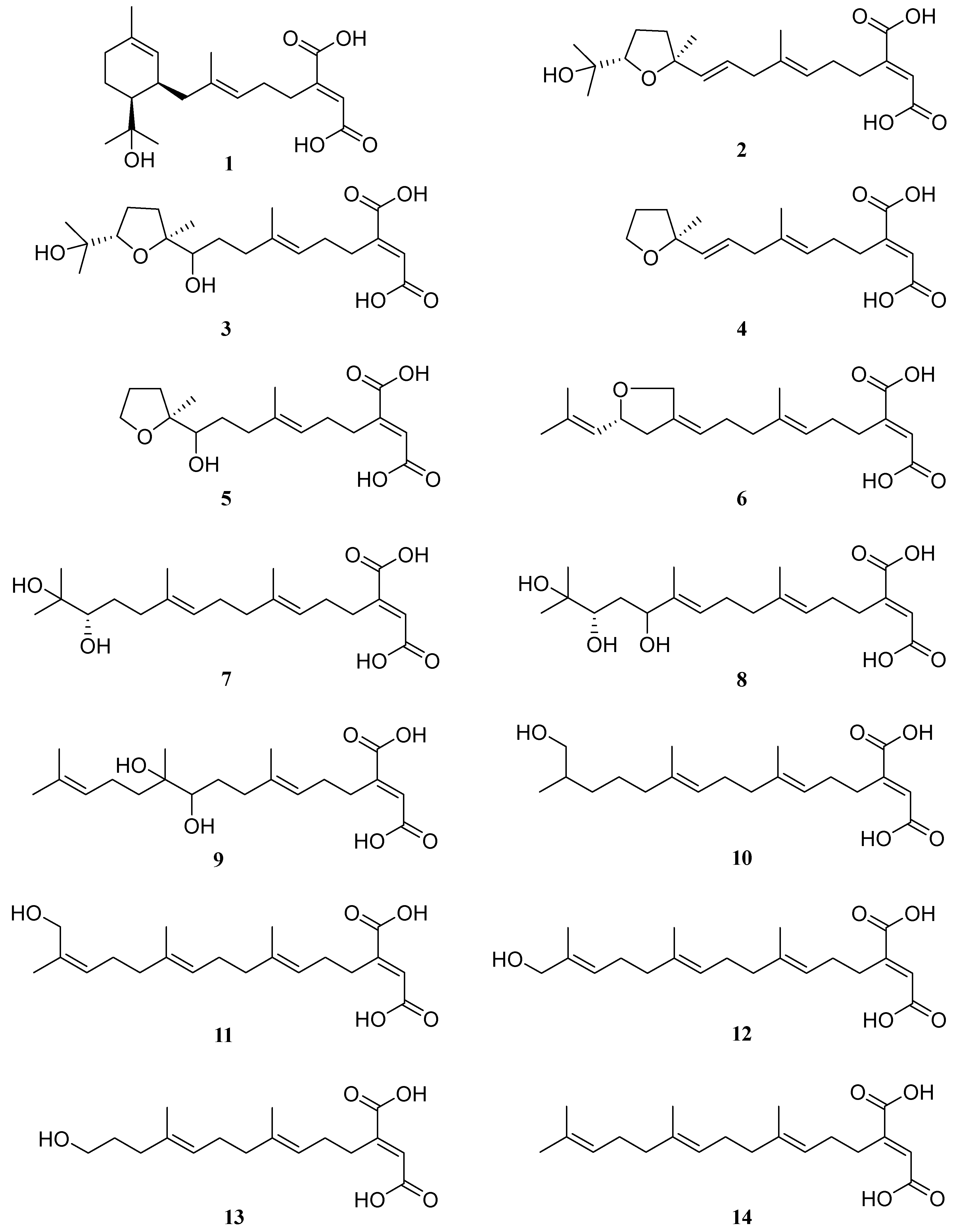
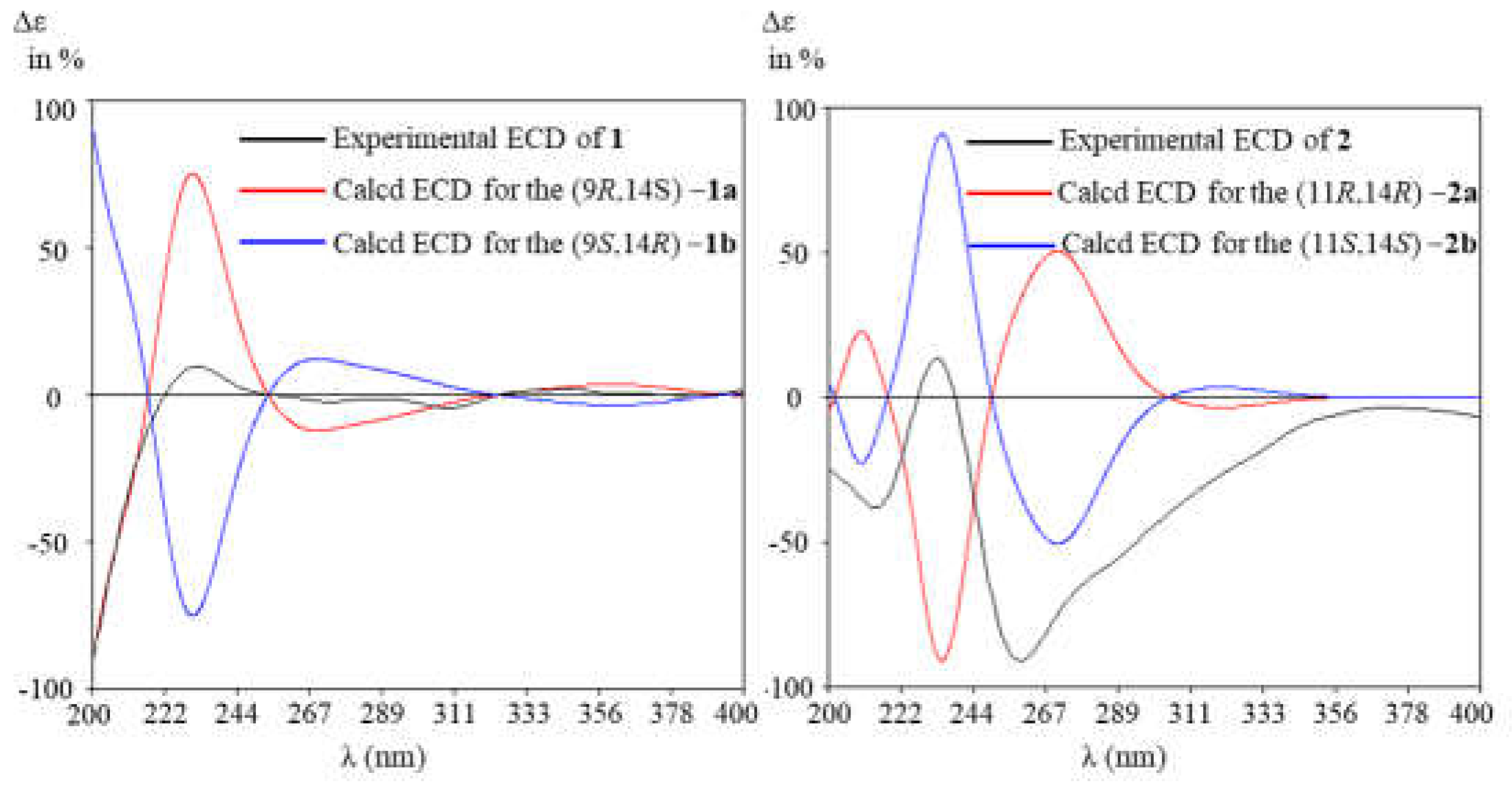
2.2. Structural Elucidation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungi Materials
3.3. The Screening of Elicitors
3.4. Fermentation and Extraction
3.5. Nuclear Magnetic Resonance (NMR) Calculation Assay
3.6. Electric Circular Dichroism (ECD) Calculation Assay
3.7. Regarding the Optical Rotation (OR) Calculation Assay
3.8. Vibrational Circular Dichroism (VCD) Calculation Assay
3.9. Assay of Antimicrobial Activity
3.10. Hydroxyl Radical Scavenging Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Characterization and Anti-Inflammatory Potential of an Exopolysaccharide from Submerged Mycelial Culture of Schizophyllum commune. Front. Pharmacol. 2017, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Basso, V.; Schiavenin, C.; Mendonça, S.; de Siqueira, F.G.; Salvador, M.; Camassola, M. Chemical features and antioxidant profile by Schizophyllum commune produced on different agroindustrial wastes and byproducts of biodiesel production. Food. Chem. 2020, 329, 127089. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Yang, Y.; Bian, Z.; Xu, B. Molecular weight and helix conformation determine intestinal anti-inflammatory effects of exopolysaccharide from Schizophyllum commune. Carbohyd. Polym. 2017, 172, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Frydenvang, K.; Liu, H.; Zhai, L.; Chen, M.; Olsen, C.E.; Christensen, S.B. Iminolactones from Schizophyllum commune. J. Nat. Prod. 2015, 78, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- National Medical Products Administration. 2023. Available online: https://www.nmpa.gov.cn (accessed on 27 September 2023).
- Gao, J.M. New biologically active metabolites from Chinese higher fungi. Curr. Org. Chem. 2006, 10, 849–871. [Google Scholar]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, C.C.; Yin, X.; Wei, J.; Gao, J.M.; Striatoids, A.-F. Cyathane Diterpenoids with Neurotrophic Activity from Cultures of the Fungus Cyathus striatus. J. Nat. Prod. 2015, 78, 783–788. [Google Scholar] [CrossRef]
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef]
- Kogen, H.; Tago, K.; Kaneko, S.; Hamano, K.; Onodera, K.; Haruyama, H.; Minagawa, K.; Kinoshita, T.; Ishikawa, T.; Tanimoto, T.; et al. Schizostatin, a novel squalene synthase inhibitor produced by the mushroom, Schizophyllum commune. II. Structure elucidation and total synthesis. J. Antibiot. 1996, 49, 624–630. [Google Scholar] [CrossRef]
- Chunyu, W.X.; Ding, Z.G.; Zhao, J.Y.; Wang, Y.X.; Han, X.L.; Li, M.G.; Wen, M.L. Two new diketopiperazines from the tin mine tailings-derived fungus Schizophyllum commune YIM DT 10058. Nat. Prod. Res. 2017, 31, 1566–1572. [Google Scholar] [CrossRef]
- Chen, G.G.; Zhu, Q.F.; Long, X.M.; Lu, Q.; Li, K.Y.; Chen, Q.; Zhou, M.; Liao, G.-S.; Xu, G.B. Antibacterial activities of the chemical constituents of Schizophyllum commune MST7-3 collected from coal area. Nat. Prod. Res. 2022, 36, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Yang, X.Q.; Sun, J.; Wang, T.; Cui, H.R.; Yang, Y.B. New Metabolites, Antifeedant, Insecticidal Activities, and Reciprocal Relationship Between Insect and Fungus from Endophyte Schizophyllum commune. Chem. Biodivers. 2022, 19, e202200130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.; Zou, G.; Wang, G.; Kang, W.; Yuan, J.; She, Z. Cytotoxic Bromine- and Iodine-Containing Cytochalasins Produced by the Mangrove Endophytic Fungus Phomopsis sp. QYM-13 Using the OSMAC Approach. J. Nat. Prod. 2022, 85, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.Q.; Tang, H.Y.; Li, X.J.; Zhang, L.; Xiao, J.; Gao, Q.Y.; Zhang, A.L.; Gao, J.M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain-many compounds method. J. Agric. Food. Chem. 2013, 61, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.-Y.; Ji, C.-h.; Lee, D.; Shim, S.H.; Joo, H.-S.; Kang, H.S. Acidonemycins A–C, Glycosylated Angucyclines with Antivirulence Activity Produced by the Acidic Culture of Streptomyces indonesiensis. J. Nat. Prod. 2023, 86, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhou, H.; Zhang, P.; Wang, X.; Li, W.; Zhang, W.; Liu, X.; Liu, H.-W.; Keller, N.P.; An, Z.; et al. Polyketide Production of Pestaloficiols and Macrodiolide Ficiolides Revealed by Manipulations of Epigenetic Regulators in an Endophytic Fungus. Org. Lett. 2016, 18, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Zhang, Q.; Gao, Y.Q.; Zhang, A.L.; Gao, J.M. Miniolins A–C, novel isomeric furanones induced by epigenetic manipulation of Penicillium minioluteum. RSC. Advances. 2015, 5, 2185–2190. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhong, Y.; Yu, Y.; Shi, D.F.; Huang, H.Y.; Tang, X.L.; Wang, Y.H.; Chen, G.D.; Zhang, H.P.; Liu, C.L.; et al. 4-Hydroxy Pyridones from Heterologous Expression and Cultivation of the Native Host. J. Nat. Prod. 2020, 83, 3338–3346. [Google Scholar] [CrossRef]
- Lee, S.R.; Seyedsayamdost, M.R. Induction of Diverse Cryptic Fungal Metabolites by Steroids and Channel Blockers. Angew Chem. Int. Ed. Engl. 2022, 61, e202204519. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Merten, C.; Firsova, D.; Fearnhead, H.; Tasdemir, D. Oxygenated Acyclic Diterpenes with Anticancer Activity from the Irish Brown Seaweed Bifurcaria bifurcata. Mar. Drugs 2020, 18, 581. [Google Scholar] [CrossRef]
- Yun, B.S.; Lee, I.K.; Woo, E.E.; Kim, Y.J.; Lee, J.Y.; Wo, S.K.; Jin, K.S.; Seon, K.J.; Ja, S.S. Antimicrobial Composition Including Extract of Schizophyllum commune Strain Culture and Preparing Method Thereof. KR Patent 20190050037-A, 1 August 2018. [Google Scholar]
- Tanimoto, T.; Tsujita, Y.; Hamano, K.; Haruyama, H.; Kinoshita, T.; Hosoya, T.; Kaneko, S.; Tago, K.; Kogen, H. Schizostatin, a potent squalene synthase inhibitor from Schizophyllum commune: Isolation, structure elucidation, and total synthesis. Tetrahedron. Lett. 1995, 36, 6301–6304. [Google Scholar] [CrossRef]
- Anjum, K.; Huang, X.; Zhou, L.; Zhu, T.; Che, Q.; Zhang, G.; Li, D. New cyclic dipeptide discovered from deep-sea derived Aspergillus sp. HDN20-1401. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Xing, L.; Sun, C.; Liang, S.; Liu, T.; Zhang, X.; Zhu, T.; Pfeifer, B.A.; Che, Q.; Zhang, G.; et al. Monacycliones G-K and ent-Gephyromycin A, Angucycline Derivatives from the Marine-Derived Streptomyces sp. HDN15129. J. Nat. Prod. 2020, 83, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Mándi, A.; Kurtán, T. Applications of OR/ECD/VCD to the structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 889–918. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Merten, C.; Kaiser, M.; Tasdemir, D. Bifurcatriol, a New Antiprotozoal Acyclic Diterpene from the Brown Alga Bifurcaria bifurcata. Mar. Drugs 2017, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Alishir, A.; Zhang, S.; Zhang, Y.; Choi, S.; Pang, C.; Bae, H.Y.; Jung, W.H.; Kim, K.H. Identification of Obscurolide-Type Metabolites and Antifungal Metabolites from the Termite-Associated Streptomyces neopeptinius BYF101. J. Nat. Prod. 2023, 86, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, J. A comparative study of caffeic acid and a novel caffeic acid conjugate SMND-309 on antioxidant properties in vitro. Lwt. Food. Sci. Technol. 2012, 46, 239–244. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food. Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Zou, Y. Genome mining of fungal globin-like enzymes for catalyzing the synthesis of linear terpenes. Chin. J. Nat. Med. 2022, 20, 795–800. [Google Scholar] [CrossRef]



| 1 | 2 | 3 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|
| Position | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) |
| 2 | 6.72, s | 6.72, s | 6.72, s | 6.71, s | 6.71, s | 6.72, s | 6.71, s | 6.72, s | 6.71, s |
| 4 | 2.79, t (7.6) | 2.80, t (7.6) | 2.79, t (7.5) | 2.79, t (7.6) | 2.78, t (7.7) | 2.79, t (7.6) | 2.78, t (7.6) | 2.78, t (7.6) | 2.78, t (7.6) |
| 5 | 2.19, q, (7.6) | 2.20, q, (7.5) | 2.19, m | 2.18, m | 2.17, q, (7.6) | 2.18, m | 2.18, m | 2.17, q, (7.6) | 2.17, m |
| 6 | 5.11, t, (7.5) | 5.18, t, 7.4 | 5.21, m | 5.17, m | 5.14, m | 5.18, t, (7.4) | 5.22, t, (7.4) | 5.15, m | 5.12, m |
| 8 | 1.77, m, 2.43, m a | 2.66, d, (6.6) | 2.02, m, 2.21, m | 2.01, m a | 1.97, m a, 2.08, m a | 2.13, m a, 2.02, m a | 1.98, m, 2.24, m | 1.97, m a | 1.97, m a |
| 9 | 2.43, m a | 5.55, m | 1.34, m, 1.71, m | 2.01, m a | 1.97, m a, 2.08, m a | 2.13, m a, 2.02, m a | 1.37, m, 1.73, m | 2.07, m, 1.97, m a | 2.07, q, (7.5) |
| 10 | 5.33, m | 5.49, m | 3.39, m | 5.30, m | 5.17, m | 5.43, t, (6.6) | 3.26, m | 5.11, m | 5.16, m |
| 12 | 1.99, m | 1.87, m a, 1.68, m | 1.59, m, 2.04, m | 2.59, m, 2.20, m | 2.22, m, 1.99, m | 4.22, t, (7.0) | 1.45, m, 1.52, m | 2.08, q, (7.3) | 1.97, m a |
| 13 | 1.55, m, 1.75, m | 1.87, m a | 1.82, m | 4.57, m | 1.33, m, 1.70, m | 1.78, m, 1.54, m | 2.07, m | 1.46, m, 1.37, m a | 2.14, m, |
| 14 | 1.58 (m) | 3.80 (t, 6.7) | 3.75, dd (9.7, 5.9) | 5.20 (m) | 3.22, m | 3.28 (m) | 5.11, m | 1.05, m, 1.37, m a | 5.26, m |
| 15 | 1.55, m | ||||||||
| 16 | 1.25, s | 1.15, s | 1.17, s | 1.70, s | 1.15, s | 1.15, s | 1.62, s | 3.30, m, 3.41, m | 4.05, m |
| 17 | 1.23, s | 1.17, s | 1.14, s | 1.73, s | 1.12, s | 1.13, s | 1.67, s | 0.89, d, (6.7) | 1.75, q, (1.3) |
| 18 | 1.61, s | 1.30, s | 1.13, s | 4.38, m, 4.22, m | 1.62, s | 1.61, s | 1.10, s | 1.59, s | 1.59, s |
| 19 | 1.63, s | 1.57, s | 1.61, s | 1.59, s | 1.60, s | 1.61, s | 1.61, s | 1.59, s | 1.60, s |
| 1 | 2 | 3 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|
| Position | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type |
| 1-COOH | 170.1, C | 170.1, C | 170.2, C | 170.2 a, C | 170.2 a, C | 170.3, C | 170.3, C | 170.2, C | 170.3, C |
| 2 | 128.3, CH | 128.4, CH | 128.4, CH | 128.6, CH | 128.4, CH | 128.4, CH | 128.4, CH | 128.3, CH | 128.6, CH |
| 3 | 148.6, C | 148.4, C | 148.4, C | 148.3 a, C | 148.5, C | 148.5, C | 148.4, C | 148.5, C | 148.5, C |
| 4 | 28.7, CH2 | 28.6, CH2 | 28.8, CH2 | 28.8, CH2 | 28.8, CH2 | 28.7, CH2 | 28.8, CH2 | 28.8, CH2 | 28.8, CH2 |
| 5 | 28.6, CH2 | 28.6, CH2 | 28.6, CH2 | 28.6, CH2 | 28.7, CH2 | 28.6, CH2 | 28.7, CH2 | 28.7, CH2 | 28.7, CH2 |
| 6 | 125.6, CH | 125.0, CH | 124.6, CH | 124.9, CH | 124.5, CH | 124.5, CH | 124.5, CH | 125.4, CH | 125.7, CH |
| 7 | 135.9, C | 136.3, C | 137.2, C | 136.7, C | 137.2, C | 137.6, C | 137.4, C | 137.2, C | 137.2, C |
| 8 | 42.5, CH2 | 43.4, CH2 | 37.5, CH2 | 40.3, CH2 | 40.8, CH2 | 40.4, CH2 | 38.0, CH2 | 41.0, CH2 | 40.8, CH2 |
| 9 | 35.5, CH | 126.8, CH | 30.9, CH2 | 28.9, CH2 | 27.6, CH2 | 27.2, CH2 | 30.4, CH2 | 27.6, CH2 | 27.6, CH2 |
| 10 | 127.4, CH | 138.1, CH | 77.1, CH | 121.3, CH | 125.6, CH | 128.2, CH | 78.2, CH | 124.4, CH | 124.4, CH |
| 11 | 133.6, C | 84.2, C | 86.8, C | 139.7, C | 136.1, C | 137.0, C | 75.5, C | 136.1, C | 135.7, C |
| 12 | 32.9, CH2 | 38.5, CH2 | 34.7, CH2 | 40.2, CH2 | 37.9, CH2 | 78.0, CH | 39.4, CH2 | 40.8, CH2 | 41.0, CH2 |
| 13 | 20.5, CH2 | 27.5, CH2 | 27.8, CH2 | 77.5, CH | 30.9, CH2 | 36.9, CH2 | 23.0, CH2 | 26.5, CH | 27.2, CH2 |
| 14 | 49.5, CH | 86.6, CH | 88.1, CH | 125.9, CH | 79.1, CH | 78.0, CH | 126.1, CH | 33.9, CH2 | 128.6, CH |
| 15 | 73.4, C | 72.7, C | 72.3, C | 138.0, C | 73.8, C | 73.4, C | 132.0, C | 36.8, CH | 135.7, C |
| 16 | 29.2, CH3 | 25.7, CH3 | 26.4, CH3 | 18.3, CH3 | 25.6, CH3 | 25.6, CH3 | 17.7, CH3 | 68.5, CH2 | 61.4, CH2 |
| 17 | 27.7, CH3 | 25.8, CH3 | 25.1, CH3 | 25.9, CH3 | 25.0, CH3 | 24.9, CH3 | 25.9, CH3 | 17.1, CH3 | 21.5, CH3 |
| 18 | 23.7, CH3 | 27.4, CH3 | 23.0, CH3 | 69.0, CH2 | 16.2, CH3 | 11.0, CH3 | 21.9, CH3 | 16.1, CH3 | 16.1, CH3 |
| 19 | 16.4, CH3 | 16.1, CH3 | 16.0, CH3 | 16.0, CH3 | 16.1, CH3 | 16.1, CH3 | 16.1, CH3 | 16.0, CH3 | 16.1, CH3 |
| 20-COOH | 169.0, C | 169.0, C | 169.1, C | 169.2 a, C | 169.0 a, C | 169.2, C | 169.2, C | 169.0, C | 169.2, C |
| 4 | 5 | |||
|---|---|---|---|---|
| Position | δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) |
| 1-COOH | 170.0, C | 170.2, C | ||
| 2 | 6.72, s | 128.4, CH | 6.71, s | 128.4, CH |
| 3 | 148.5, C | 148.4, C | ||
| 4 | 2.80, t, (7.5) | 28.6, CH2 | 2.78, t, (7.6) | 28.6, CH2 |
| 5 | 2.20, q, (7.5) | 28.6, CH2 | 2.19, m a | 28.8, CH2 |
| 6 | 5.18, m | 125.1, CH | 5.21, m | 124.6, CH |
| 7 | 136.2, C | 137.2, C | ||
| 8 | 2.65, d, (6.4) | 43.4, CH2 | 2.21, m a, 2.00, m a | 37.6, CH2 |
| 9 | 5.54, m | 127.3, CH | 1.68, m, 1.34, m | 31.1, CH2 |
| 10 | 5.50, m | 137.8, CH | 3.3, m | 77.0, CH |
| 11 | 83.9, C | 86.8, C | ||
| 12 | 1.72, m, 1.90, m a | 38.6, CH2 | 2.02, m a, 1.58, m | 34.5, CH2 |
| 13 | 1.92, m a | 26.5, CH2 | 1.93, m | 27.1, CH2 |
| 14 | 3.85, m | 68.4, CH2 | 3.84, m, 3.79, m | 69.0, CH2 |
| 15 | 1.29, s | 26.8, CH3 | 1.12, s | 22.3, CH3 |
| 16 | 1.56, s | 16.1, CH3 | 1.6, s | 16.1, CH3 |
| 17-COOH | 168.9 | 169.1 | ||
| Compounds | % Inhibition (50 µM) | Compounds and VC a | % Inhibition (50 µM) |
|---|---|---|---|
| 1 | 76.0 | 9 | 71.2 |
| 2 | 70.4 | 10 | 68.0 |
| 3 | 71.4 | 11 | 69.6 |
| 4 | 69.2 | 12 | 67.7 |
| 5 | 71.0 | 13 | 71.7 |
| 6 | 71.7 | 14 | 73.4 |
| 7 | 71.4 | VC a | 81.7 |
| 8 | 72.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, F.; Zhou, L.; Liu, H.; Gao, H.; Cui, G.; Niu, C.; Zhang, P.; Li, D.; Liu, S.; et al. Combining the Elicitor Up-Regulated Production of Unusual Linear Diterpene-Derived Variants for an In-Depth Assessment of the Application Value and Risk of the Medicinal and Edible Basidiomycete Schizophyllum commune. Molecules 2024, 29, 2608. https://doi.org/10.3390/molecules29112608
Wang Y, Cao F, Zhou L, Liu H, Gao H, Cui G, Niu C, Zhang P, Li D, Liu S, et al. Combining the Elicitor Up-Regulated Production of Unusual Linear Diterpene-Derived Variants for an In-Depth Assessment of the Application Value and Risk of the Medicinal and Edible Basidiomycete Schizophyllum commune. Molecules. 2024; 29(11):2608. https://doi.org/10.3390/molecules29112608
Chicago/Turabian StyleWang, Ying, Fei Cao, Luning Zhou, Hanwei Liu, Hua Gao, Ge Cui, Changshan Niu, Peng Zhang, Dehai Li, Songqi Liu, and et al. 2024. "Combining the Elicitor Up-Regulated Production of Unusual Linear Diterpene-Derived Variants for an In-Depth Assessment of the Application Value and Risk of the Medicinal and Edible Basidiomycete Schizophyllum commune" Molecules 29, no. 11: 2608. https://doi.org/10.3390/molecules29112608






