In Vitro Study of Tumor-Homing Peptide-Modified Magnetic Nanoparticles for Magnetic Hyperthermia
Abstract
:1. Introduction
2. Results and Discussion
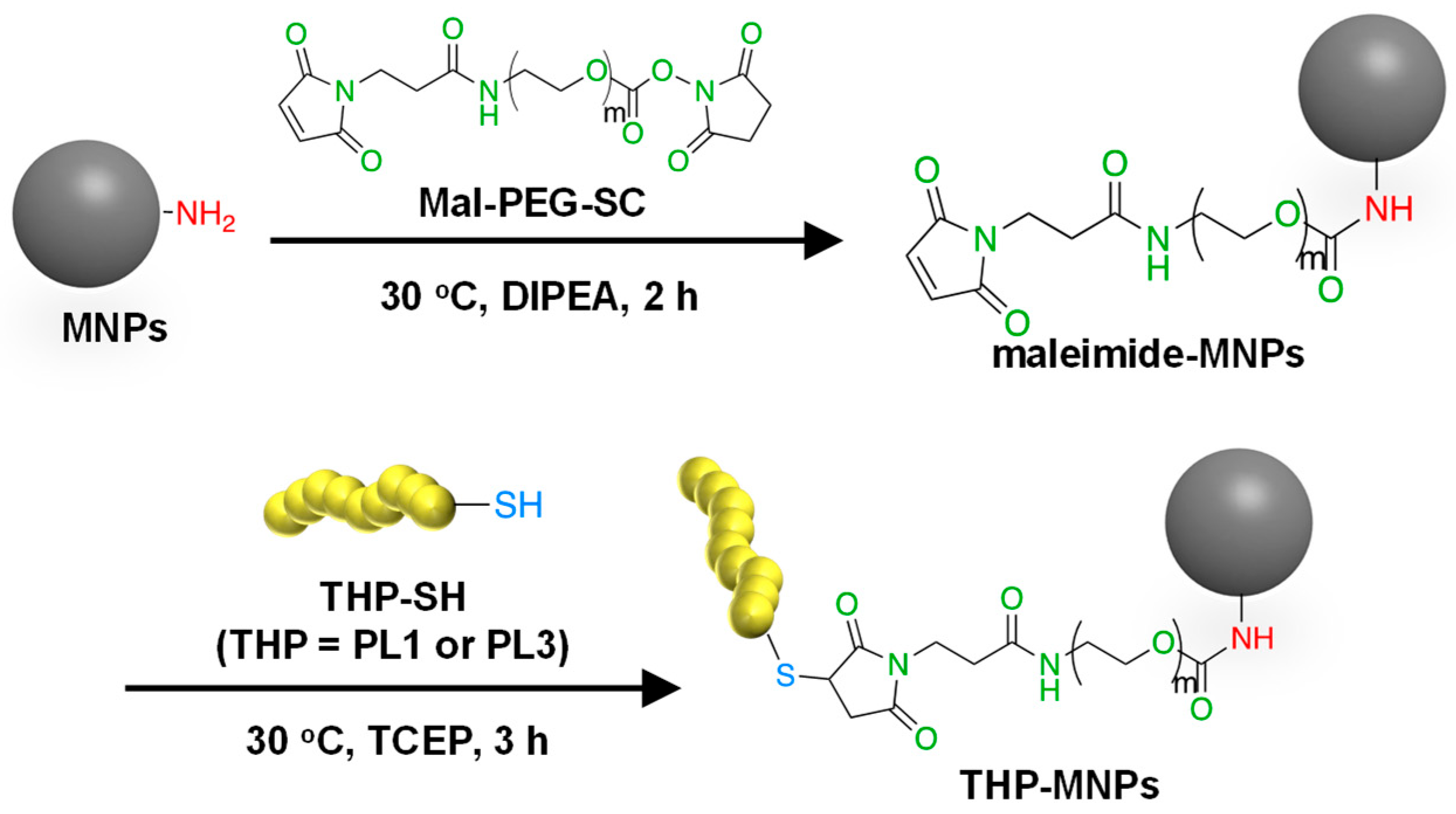
2.1. Syntheses of THP-MNPs
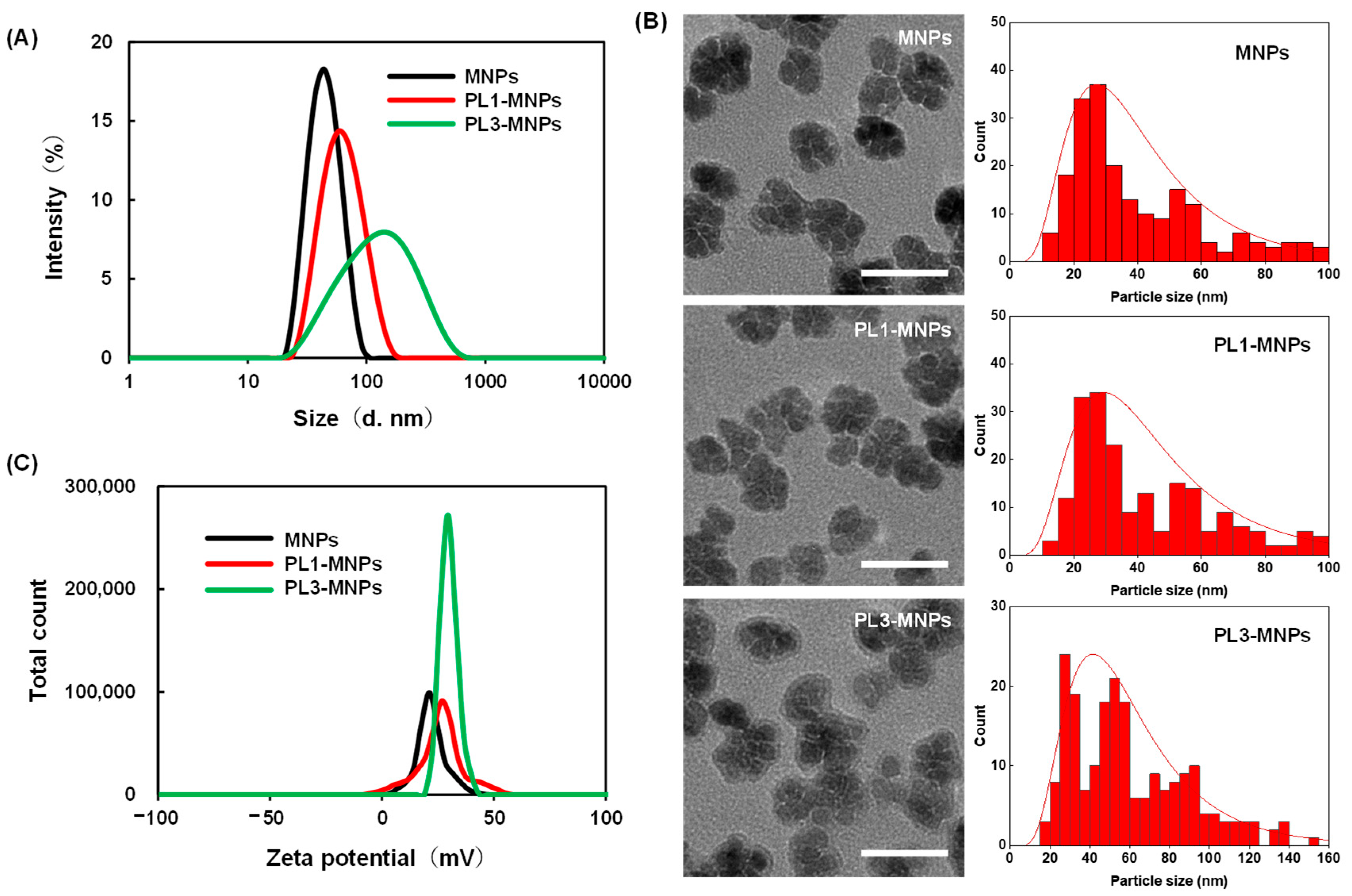
2.2. The Characterization of the THP-MNPs
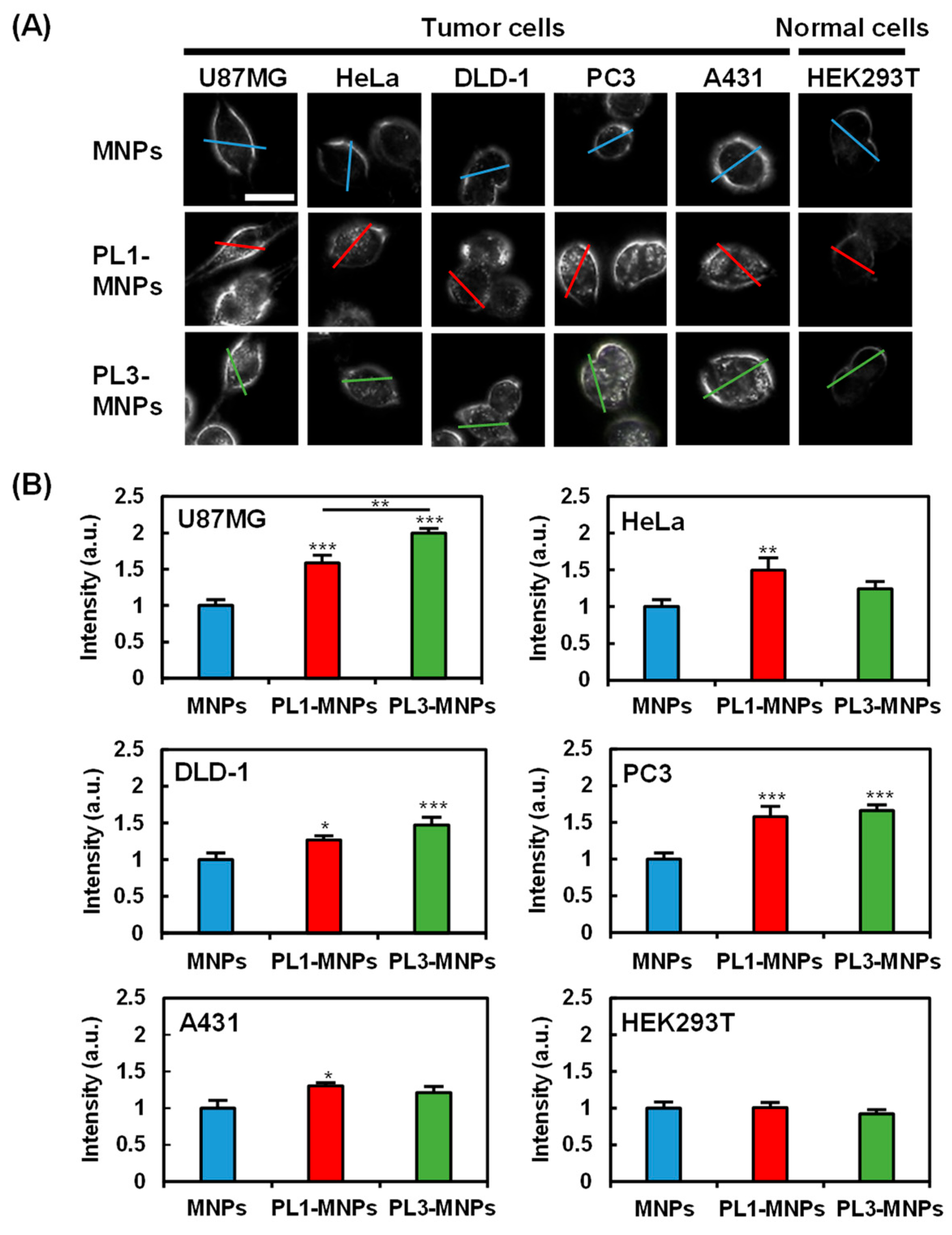
2.3. Cell Specificity of THP-Modified MNPs
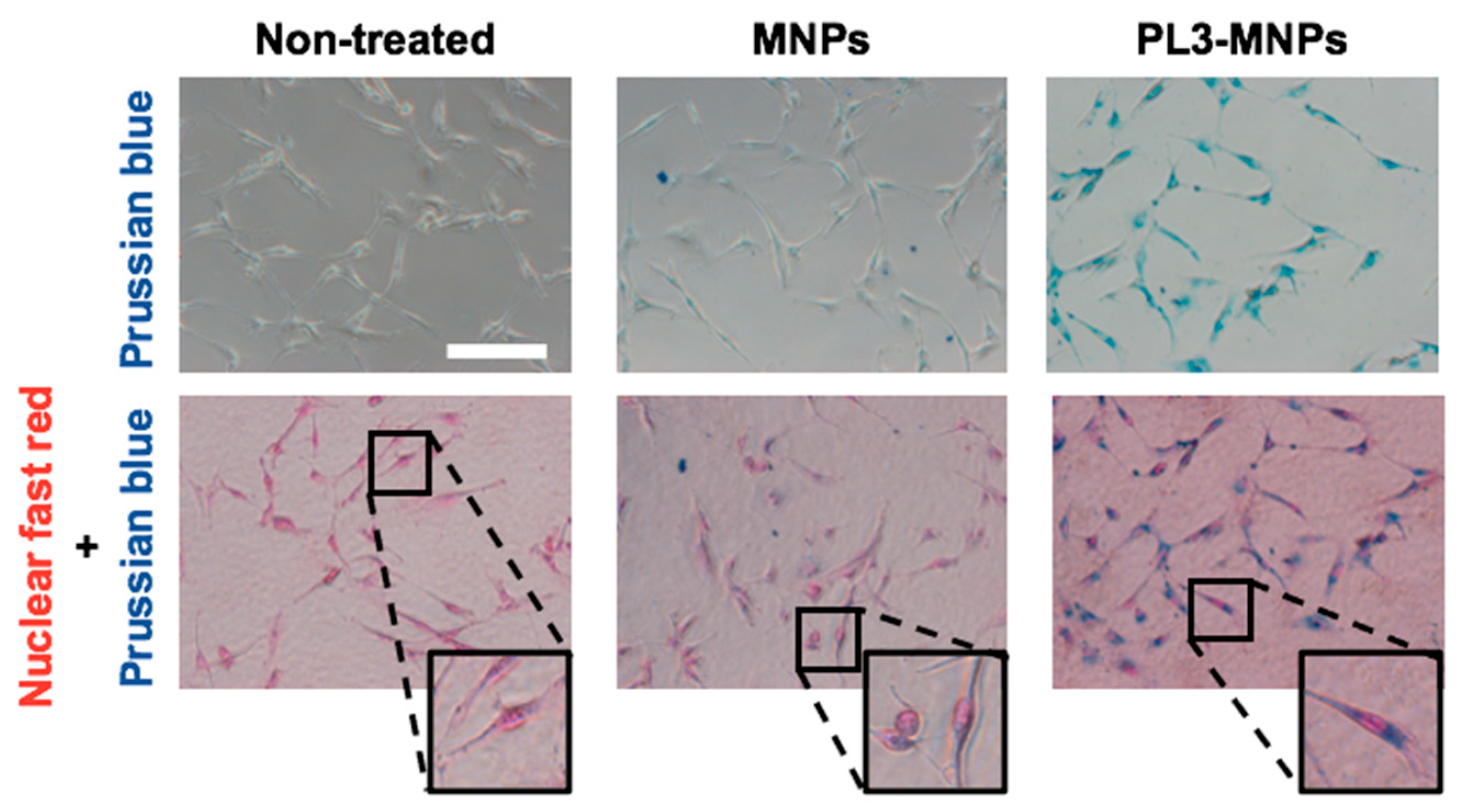
2.4. Intracellular Iron Uptake
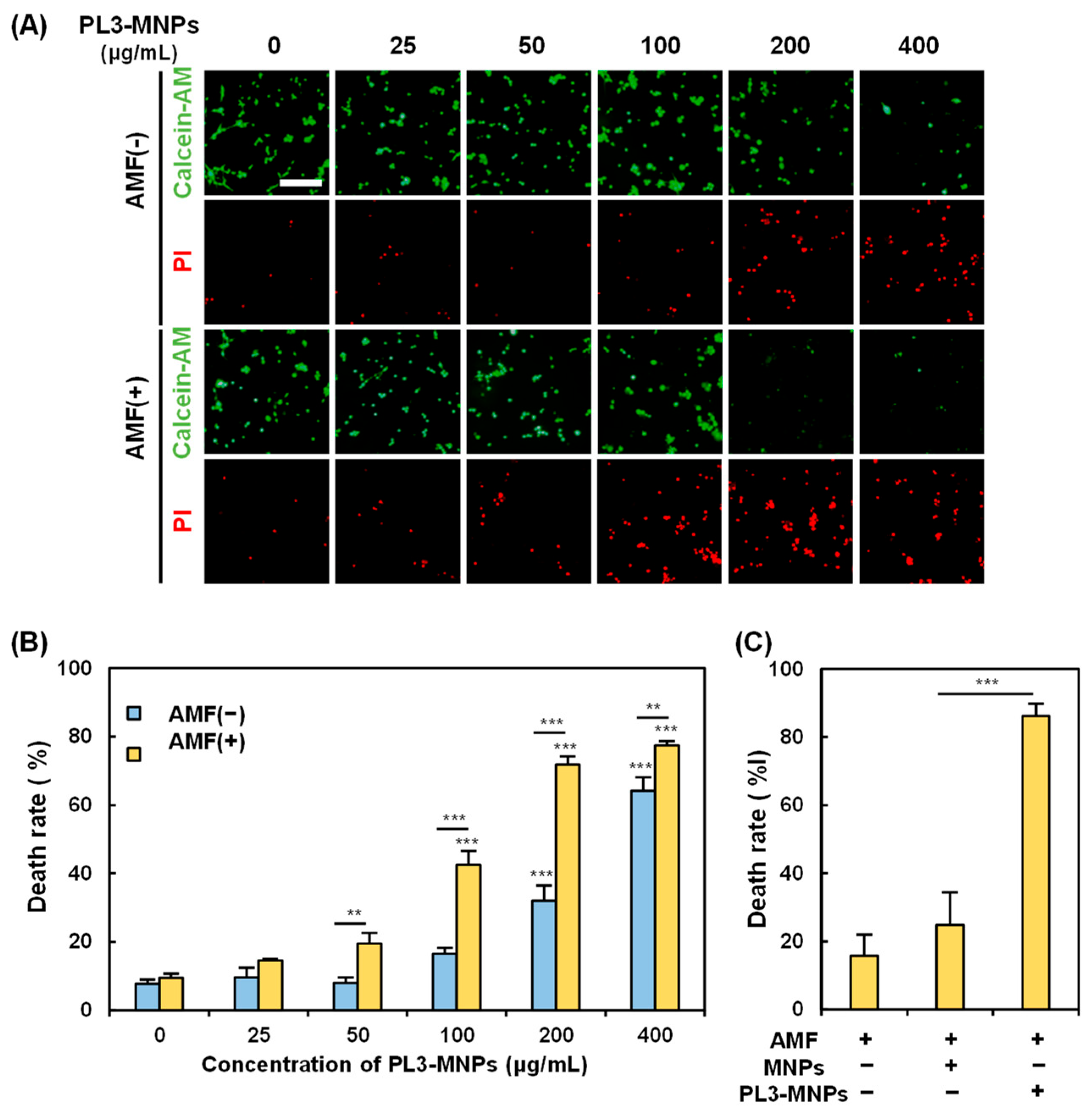
2.5. In Vitro Hyperthermia
2.6. Ferroptosis with PL3-MNPs
3. Materials and Methods
3.1. Synthesis of THP-Modified MNPs
3.2. Characterization
3.3. Cell Culture
3.4. Evaluation of Cell Specificity of THP-Modified MNPs
3.5. Intracellular Iron Uptake
3.6. In Vitro Hyperthermia Assessment
3.7. Detection of Ferroptosis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Li, Q.; Wang, C.; Hao, Y.; Yang, N.; Chen, M.; Ji, J.; Feng, L.; Liu, Z. Rational Design of Biomaterials to Potentiate Cancer Thermal Therapy. Chem. Rev. 2023, 123, 7326–7378. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R. Tissue Physiology and the Response to Heat. Int. J. Hyperth. 2006, 22, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dudar, T.E.; Jain, R.K. Differential Response of Normal and Tumor Microcirculation to Hyperthermia. Cancer Res. 1984, 44, 605–612. [Google Scholar] [PubMed]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18, 1534735419876345. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.M.; Courteau, A.; Bellaye, P.-S.; Kohli, E.; Oudot, A.; Doulain, P.-E.; Petitot, C.; Walker, P.-M.; Decréau, R.; Collin, B. Superparamagnetic Iron Oxide Nanoparticles for Immunotherapy of Cancers through Macrophages and Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in Hyperthermia Cancer Therapy: From Fundamental Principles to Advanced Applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Shasha, C.; Krishnan, K.M. Nonequilibrium Dynamics of Magnetic Nanoparticles with Applications in Biomedicine. Adv. Mater. 2021, 33, e1904131. [Google Scholar] [CrossRef] [PubMed]
- Beković, M.; Ban, I.; Drofenik, M.; Stergar, J. Magnetic Nanoparticles as Mediators for Magnetic Hyperthermia Therapy Applications: A Status Review. Appl. Sci. 2023, 13, 9548. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic Nanoparticles and Clusters for Magnetic Hyperthermia: Optimizing Their Heat Performance and Developing Combinatorial Therapies to Tackle Cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Kawai, N.; Gonda, M.; Iida, K.; Etani, T.; Kobayashi, D.; Naiki, T.; Naiki-Ito, A.; Ando, R.; Yamaguchi, S.; et al. Role of HIKESHI on Hyperthermia for Castration-Resistant Prostate Cancer and Application of a Novel Magnetic Nanoparticle with Carbon Nanohorn for Magnetic Hyperthermia. Pharmaceutics 2023, 15, 626. [Google Scholar] [CrossRef] [PubMed]
- Mehak; Thummer, R.P.; Pandey, L.M. Surface Modified Iron-Oxide Based Engineered Nanomaterials for Hyperthermia Therapy of Cancer Cells. Biotechnol. Genet. Eng. Rev. 2023, 1–47. [Google Scholar] [CrossRef]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment-The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef] [PubMed]
- Widder, K.J.; Senyei, A.E.; Ranney, D.F. Magnetically Responsive Microspheres and Other Carriers for the Biophysical Targeting of Antitumor Agents. Adv. Pharmacol. 1979, 16, 213–271. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.M.; Yu, X.; Winter, P.M.; Abendschein, D.R.; Karukstis, K.K.; Scott, M.J.; Chinen, L.K.; Fuhrhop, R.W.; Scherrer, D.E.; Wickline, S.A. Targeted Antiproliferative Drug Delivery to Vascular Smooth Muscle Cells with a Magnetic Resonance Imaging Nanoparticle Contrast Agent: Implications for Rational Therapy of Restenosis. Circulation 2002, 106, 2842–2847. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Weissleder, R.; Lee, A.S.; Wittenberg, J.; Brady, T.J. Receptor Imaging: Application to MR Imaging of Liver Cancer. Radiology 1990, 177, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Wadghiri, Y.Z.; Sigurdsson, E.M.; Sadowski, M.; Elliott, J.I.; Li, Y.; Scholtzova, H.; Tang, C.Y.; Aguinaldo, G.; Pappolla, M.; Duff, K.; et al. Detection of Alzheimer’s Amyloid in Transgenic Mice Using Magnetic Resonance Microimaging. Magn. Reson. Med. 2003, 50, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of Magnetic Iron Oxide Nanoparticles for Medical Applications. J. Nanobiotechnology 2022, 20, 305. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological Applications of Magnetic Nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Weiss, L. The Role of the Sinus Wall in the Passage of Erythrocytes through the Spleen. Blood 1973, 41, 529–537. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J. V Renal Clearance of Nanoparticles. Nat. Biotechnol. 2009, 25, 1165–1170. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, P.; Porkka, K.; Hoffman, J.A.; Ruoslahti, E. A Tumor-Homing Peptide with a Targeting Specificity Related to Lymphatic Vessels. Nat. Med. 2002, 8, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.S.; Gunassekaran, G.R.; Lee, S.M.; Yoon, J.W.; Lee, Y.K.; Lee, B. Peptides as Multifunctional Players in Cancer Therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.M.; Meenach, S.A.; Anderson, K.W.; Hilt, J.Z. Synthesis and Characterization of CREKA-Conjugated Iron Oxide Nanoparticles for Hyperthermia Applications. Acta Biomater. 2014, 10, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.R.; Bhojani, M.S.; McConville, P.; Moody, J.; Moffat, B.A.; Hall, D.E.; Kim, G.; Koo, Y.-E.L.; Woolliscroft, M.J.; Sugai, J.V.; et al. Vascular Targeted Nanoparticles for Imaging and Treatment of Brain Tumors. Clin. Cancer Res. 2006, 12, 6677–6686. [Google Scholar] [CrossRef] [PubMed]
- Agemy, L.; Friedmann-Morvinski, D.; Kotamraju, V.R.; Roth, L.; Sugahara, K.N.; Girard, O.M.; Mattrey, R.F.; Verma, I.M.; Ruoslahti, E. Targeted Nanoparticle Enhanced Proapoptotic Peptide as Potential Therapy for Glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17450–17455. [Google Scholar] [CrossRef]
- Al Faruque, H.; Choi, E.-S.; Kim, J.-H.; Kim, E. Enhanced Effect of Autologous EVs Delivering Paclitaxel in Pancreatic Cancer. J. Control. Release 2022, 347, 330–346. [Google Scholar] [CrossRef]
- Lingasamy, P.; Tobi, A.; Haugas, M.; Hunt, H.; Paiste, P.; Asser, T.; Rätsep, T.; Kotamraju, V.R.; Bjerkvig, R.; Teesalu, T. Bi-Specific Tenascin-C and Fibronectin Targeted Peptide for Solid Tumor Delivery. Biomaterials 2019, 219, 119373. [Google Scholar] [CrossRef]
- Lingasamy, P.; Tobi, A.; Kurm, K.; Kopanchuk, S.; Sudakov, A.; Salumäe, M.; Rätsep, T.; Asser, T.; Bjerkvig, R.; Teesalu, T. Tumor-Penetrating Peptide for Systemic Targeting of Tenascin-C. Sci. Rep. 2020, 10, 5809. [Google Scholar] [CrossRef] [PubMed]
- Leins, A.; Riva, P.; Lindstedt, R.; Davidoff, M.S.; Mehraein, P.; Weis, S. Expression of Tenascin-C in Various Human Brain Tumors and Its Relevance for Survival in Patients with Astrocytoma. Cancer 2003, 98, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Saw, P.E.; Lee, I.-H.; Yu, M.K.; Kim, M.; Lee, K.; Kim, Y.-C.; Jeong, Y.Y.; Jon, S. Fibronectin Extra Domain B-Specific Aptide Conjugated Nanoparticles for Targeted Cancer Imaging. J. Control. Release 2012, 163, 111–118. [Google Scholar] [CrossRef]
- Carnemolla, B.; Castellani, P.; Ponassi, M.; Borsi, L.; Urbini, S.; Nicolo, G.; Dorcaratto, A.; Viale, G.; Winter, G.; Neri, D.; et al. Identification of a Glioblastoma-Associated Tenascin-C Isoform by a High Affinity Recombinant Antibody. Am. J. Pathol. 1999, 154, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Bergé, M.; Allanic, D.; Bonnin, P.; de Montrion, C.; Richard, J.; Suc, M.; Boivin, J.-F.; Contrerès, J.-O.; Lockhart, B.P.; Pocard, M.; et al. Neuropilin-1 Is Upregulated in Hepatocellular Carcinoma and Contributes to Tumour Growth and Vascular Remodelling. J. Hepatol. 2011, 55, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Lingasamy, P.; Põšnograjeva, K.; Kopanchuk, S.; Tobi, A.; Rinken, A.; General, I.J.; Asciutto, E.K.; Teesalu, T. PL1 Peptide Engages Acidic Surfaces on Tumor-Associated Fibronectin and Tenascin Isoforms to Trigger Cellular Uptake. Pharmaceutics 2021, 13, 1998. [Google Scholar] [CrossRef] [PubMed]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, O.; Sajjamark, K.; Franke, J.; Wei, H.; Behrends, A.; Münkel, C.; Grüttner, C.; Levan, P.; von Elverfeldt, D.; Graeser, M.; et al. In Situ Theranostic Platform Combining Highly Localized Magnetic Fluid Hyperthermia, Magnetic Particle Imaging, and Thermometry in 3D. Theranostics 2024, 14, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H.; Matsumura, Y. EPR Effect Based Drug Design and Clinical Outlook for Enhanced Cancer Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 129–130. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kataoka, K. Preclinical and Clinical Studies of Anticancer Agent-Incorporating Polymer Micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Quan, X.; Li, J.; Huo, J.; Li, X.; Zhao, Z.; Li, S.; Wan, J.; Li, J.; Liu, S.; et al. Liposomes Embedded with PEGylated Iron Oxide Nanoparticles Enable Ferroptosis and Combination Therapy in Cancer. Natl. Sci. Rev. 2023, 10, nwac167. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.R.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Antonini, J.M.; Roberts, J.R.; Qian, Y.; Sisler, J.D.; Castranova, V.; Porter, D.W.; et al. The Fate of Inhaled Nanoparticles: Detection and Measurement by Enhanced Dark-Field Microscopy. Toxicol. Pathol. 2018, 46, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Körsten, S.; Chen, X.; Yang, G.; Lv, Y.; Liu, M.; Wehner, S.; Fischer, C.B. High-Resolution Particle Size and Shape Analysis of the First Samarium Nanoparticles Biosynthesized from Aqueous Solutions via Cyanobacteria Anabaena Cylindrica. NanoImpact 2022, 26, 100398. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ohtsuki, T. Inhibition of Hsf1 and Safb Granule Formation Enhances Apoptosis Induced by Heat Stress. Int. J. Mol. Sci. 2021, 22, 4982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Watanabe, K.; Koide, S.; Kitamatsu, M.; Ohtsuki, T. Minimization of Apoptosis-Inducing CPP-Bim Peptide. Bioorganic Med. Chem. Lett. 2021, 36, 127811. [Google Scholar] [CrossRef]
- Watanabe, K.; Nawachi, T.; Okutani, R.; Ohtsuki, T. Photocontrolled Apoptosis Induction Using Precursor MiR-664a and an RNA Carrier-Conjugated with Photosensitizer. Sci. Rep. 2021, 11, 14936. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Tsutsumiuchi, K.; Imai, R.; Miki, Y.; Kondo, A.; Nakagawa, H.; Watanabe, K.; Ohtsuki, T. In Vitro Study of Tumor-Homing Peptide-Modified Magnetic Nanoparticles for Magnetic Hyperthermia. Molecules 2024, 29, 2632. https://doi.org/10.3390/molecules29112632
Zhou S, Tsutsumiuchi K, Imai R, Miki Y, Kondo A, Nakagawa H, Watanabe K, Ohtsuki T. In Vitro Study of Tumor-Homing Peptide-Modified Magnetic Nanoparticles for Magnetic Hyperthermia. Molecules. 2024; 29(11):2632. https://doi.org/10.3390/molecules29112632
Chicago/Turabian StyleZhou, Shengli, Kaname Tsutsumiuchi, Ritsuko Imai, Yukiko Miki, Anna Kondo, Hiroshi Nakagawa, Kazunori Watanabe, and Takashi Ohtsuki. 2024. "In Vitro Study of Tumor-Homing Peptide-Modified Magnetic Nanoparticles for Magnetic Hyperthermia" Molecules 29, no. 11: 2632. https://doi.org/10.3390/molecules29112632




