Conversion of Phosphogypsum into Porous Calcium Silicate Hydrate for the Removal and Recycling of Pb(II) and Cd(II) from Wastewater
Abstract
1. Introduction
2. Results and Discussion
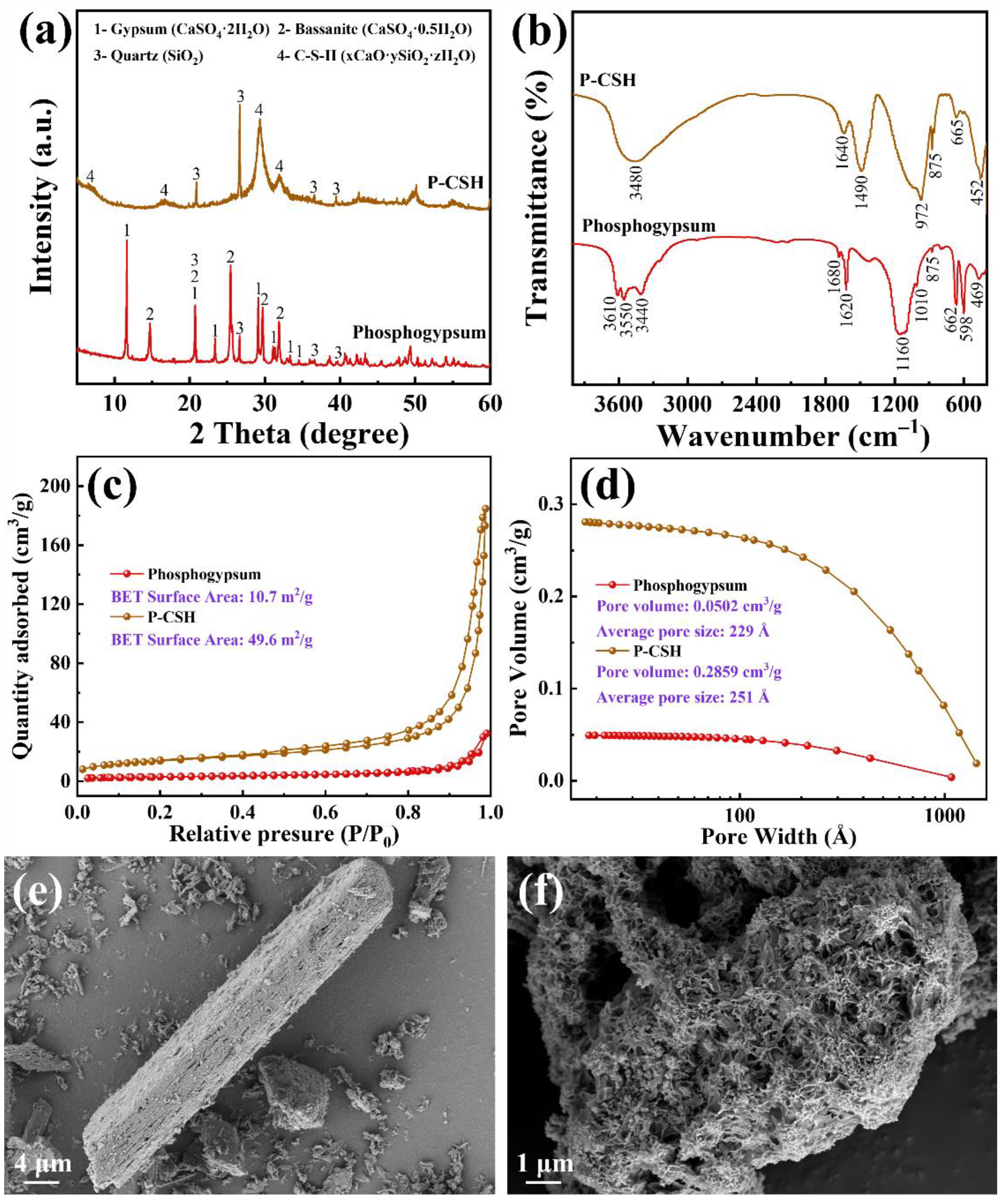
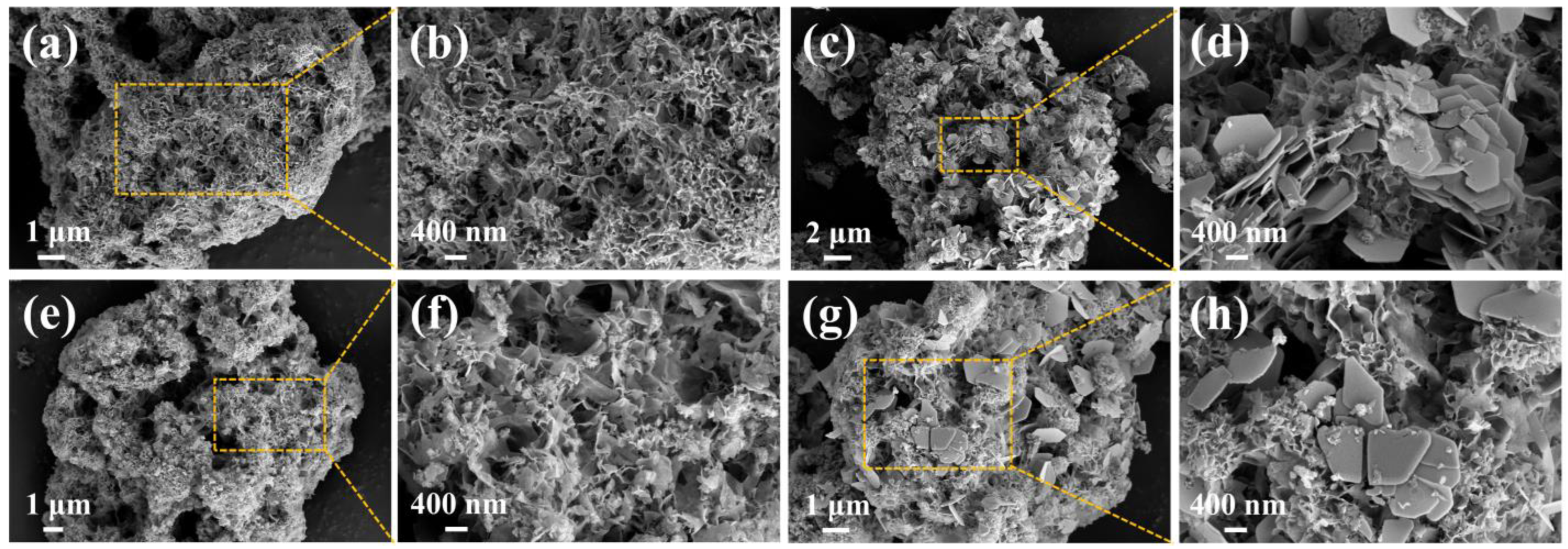
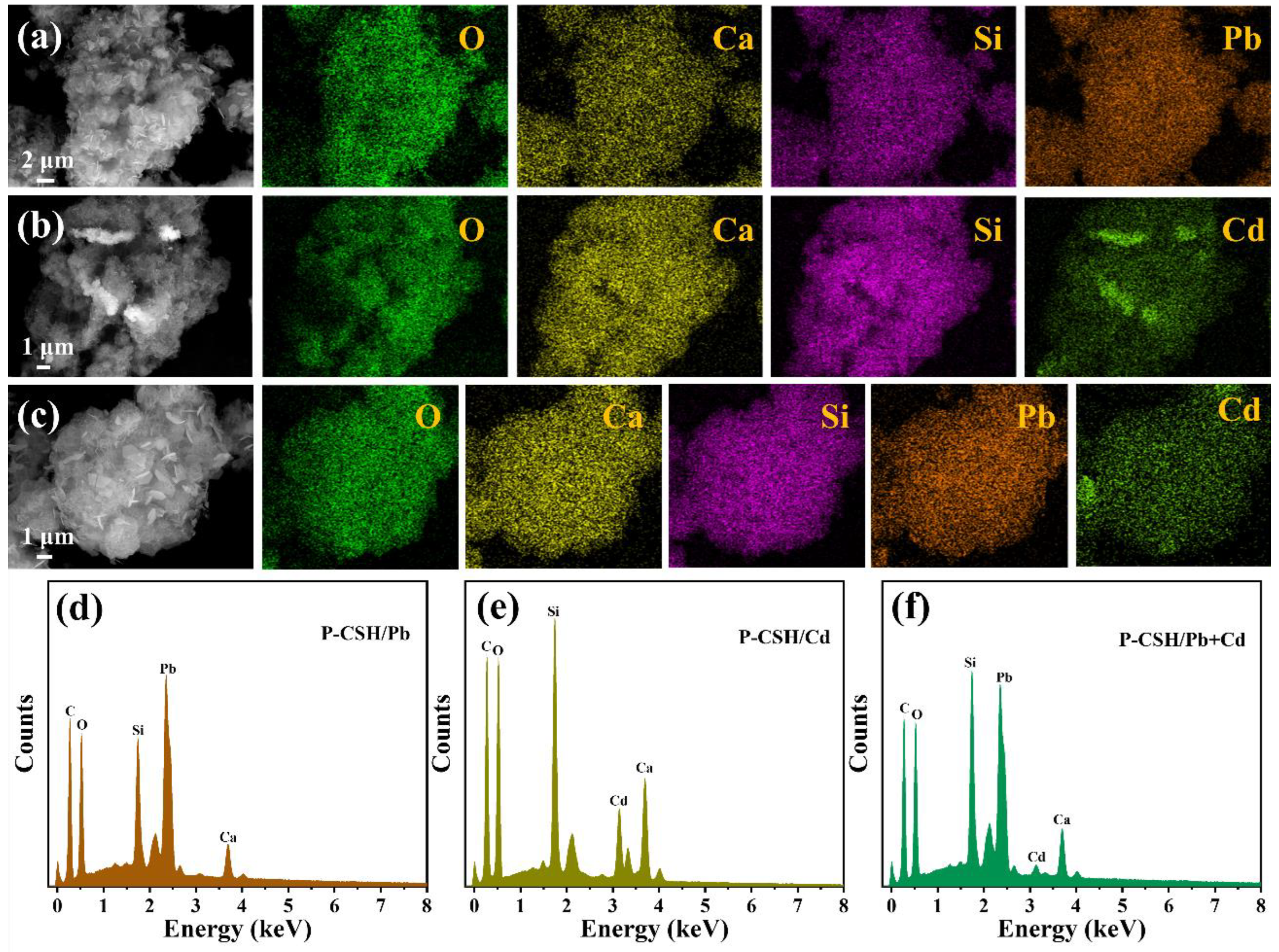
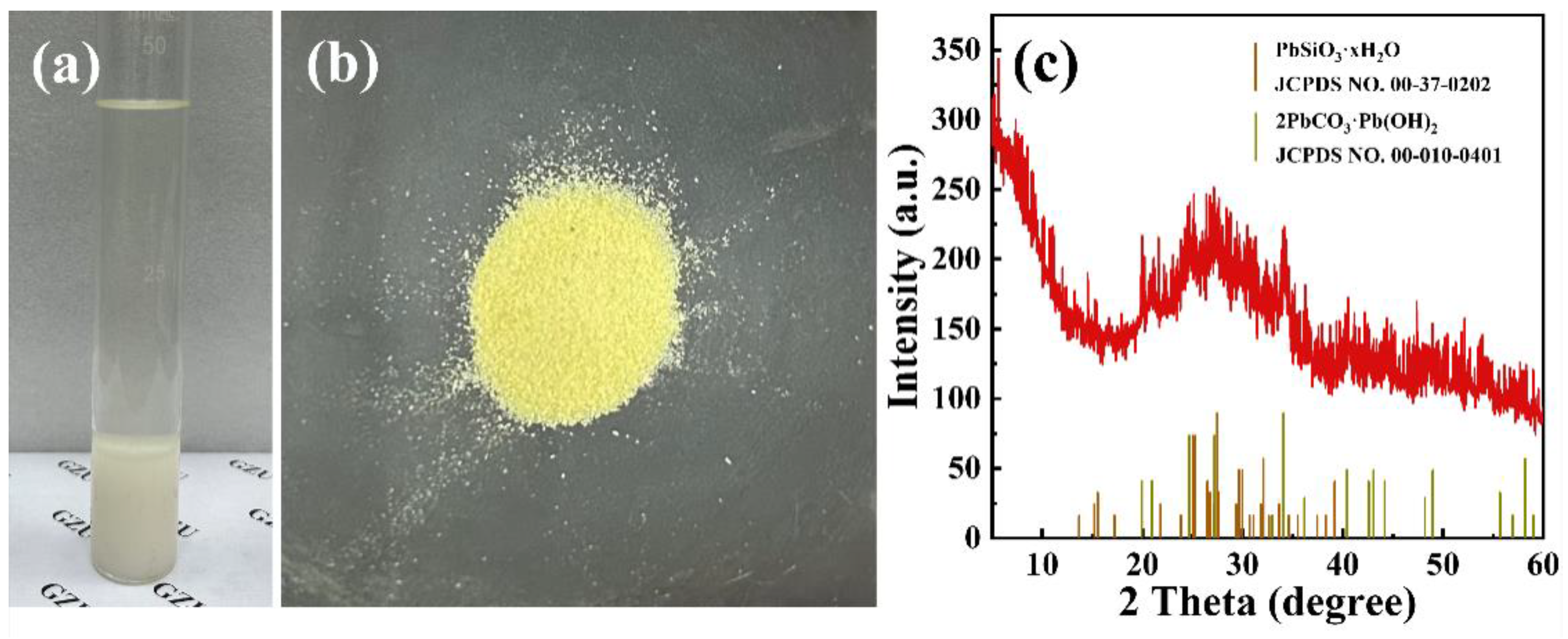
2.1. Characterizations of Phosphogypsum and P-CSH
2.2. Adsorption Performance of Pb(II) and Cd(II)
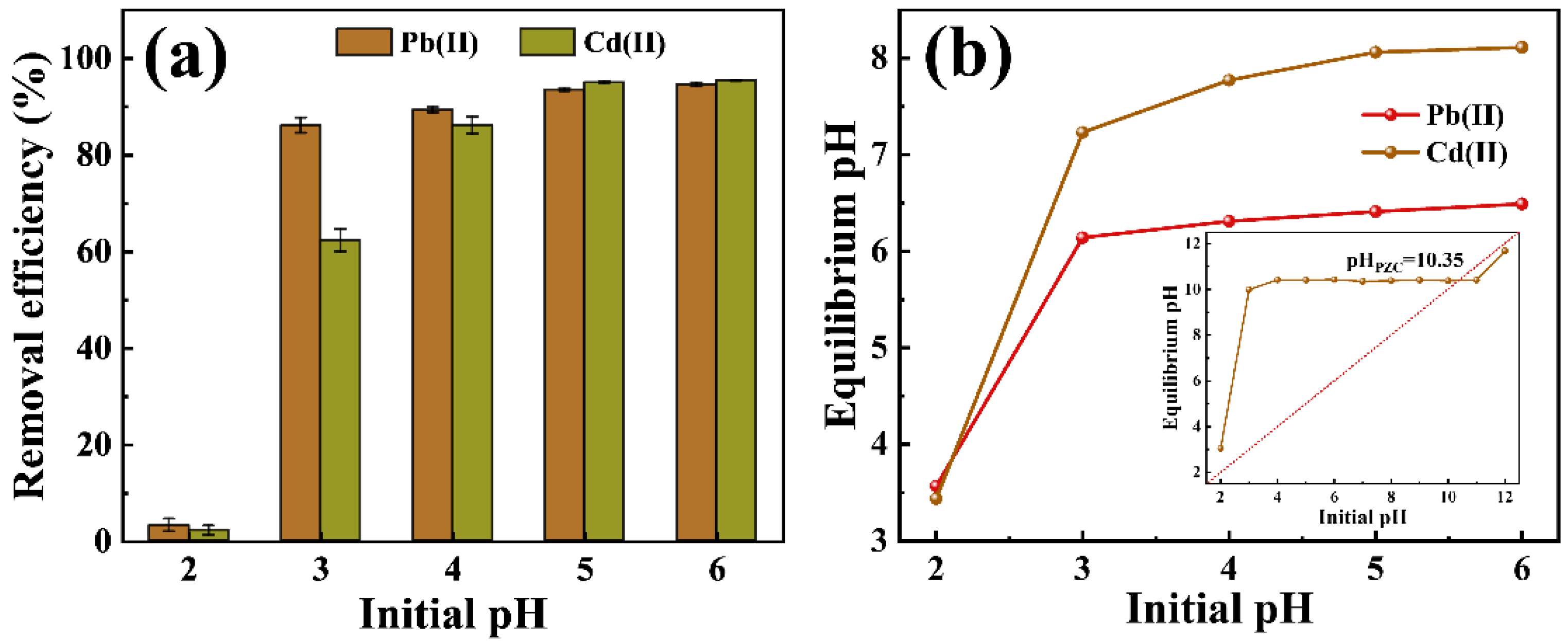
2.2.1. Effect of Initial pH
2.2.2. Adsorption Kinetics
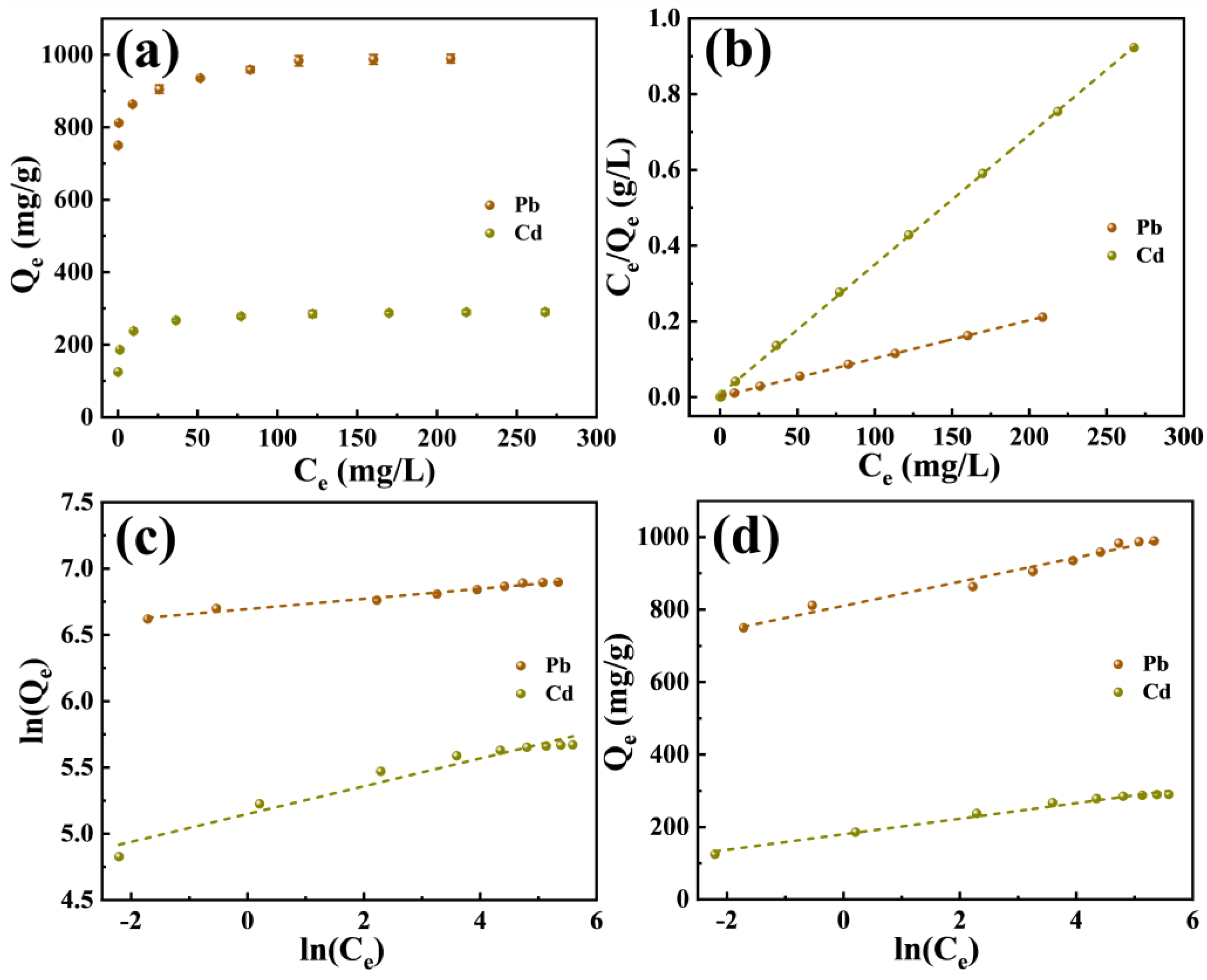
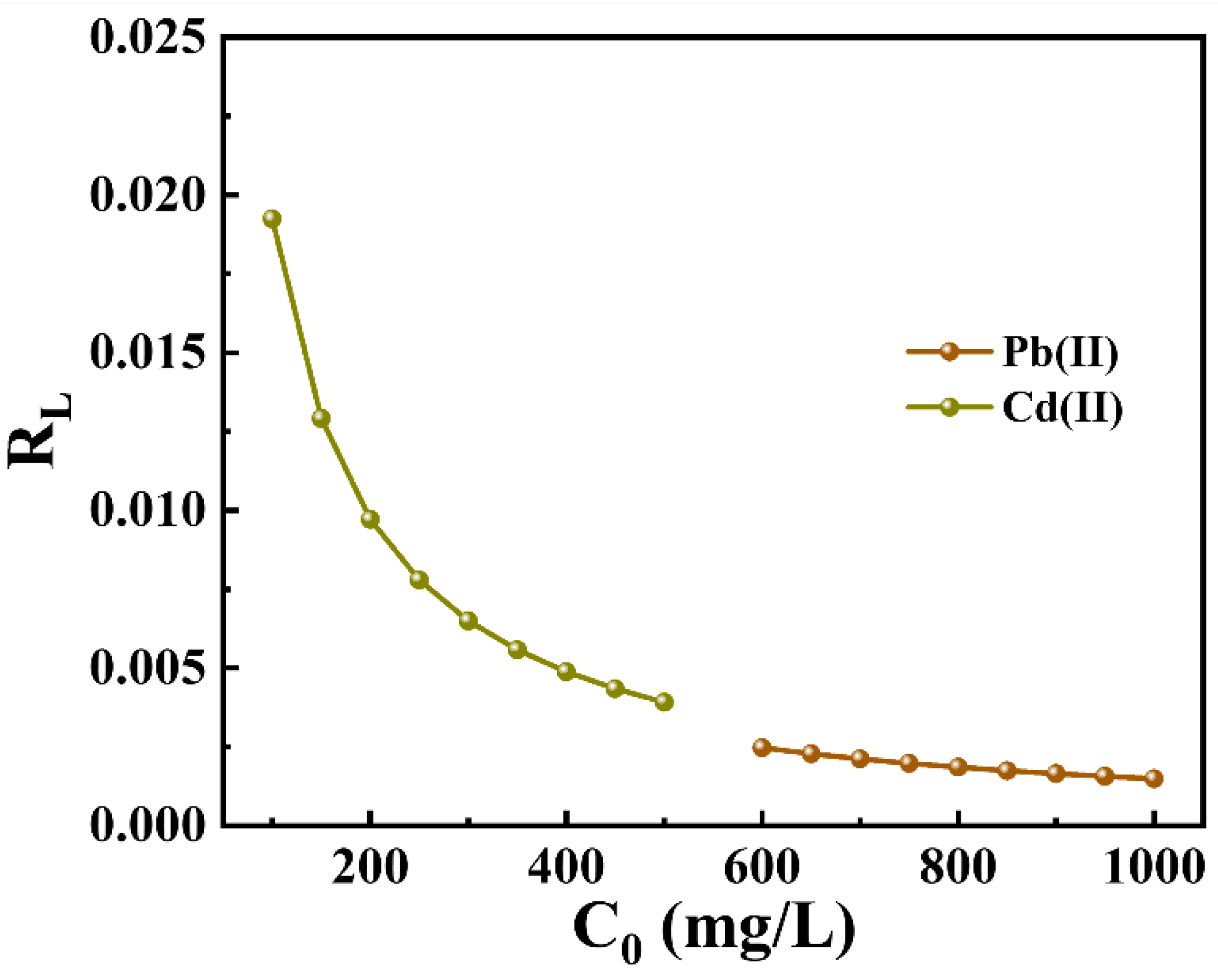
2.2.3. Adsorption Isotherms
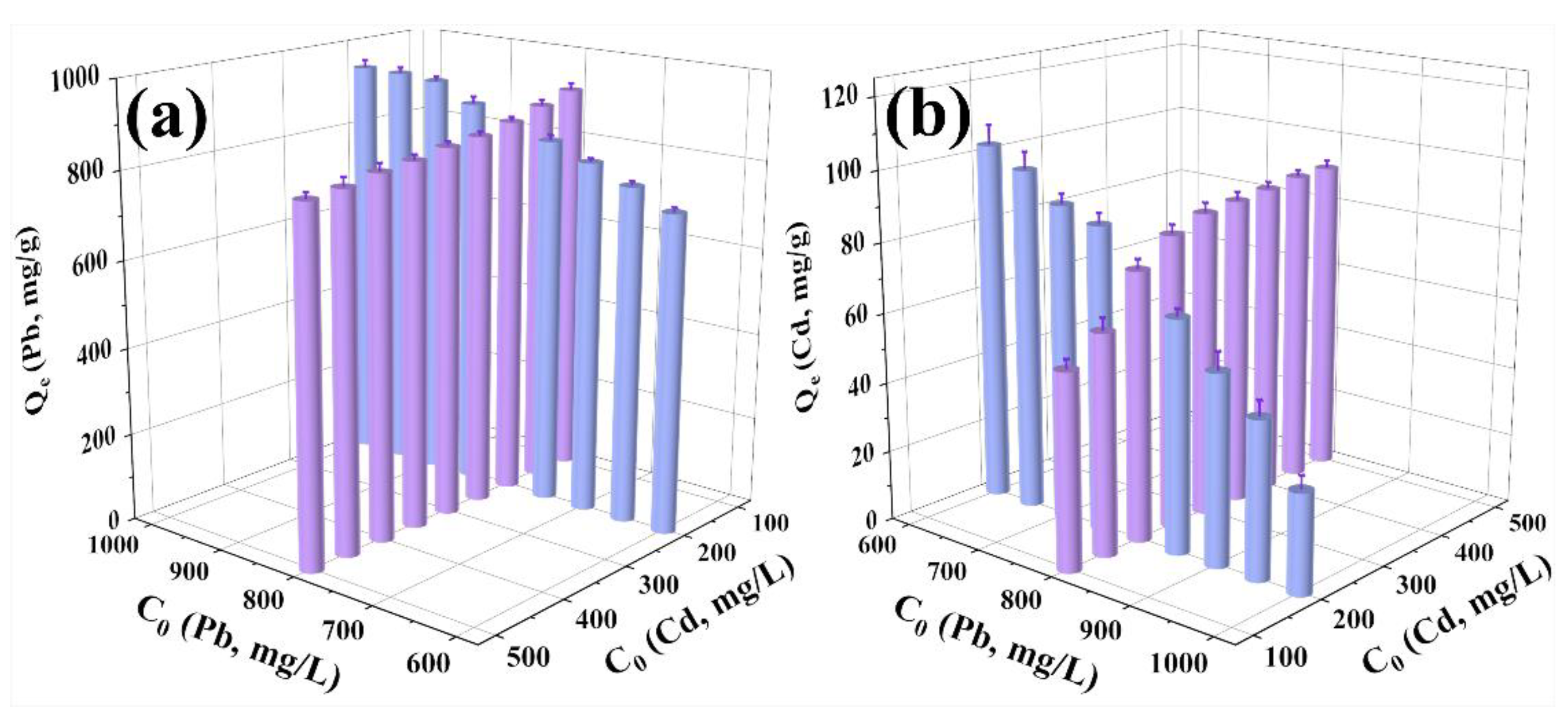
2.2.4. Competitive or Cooperative Adsorption
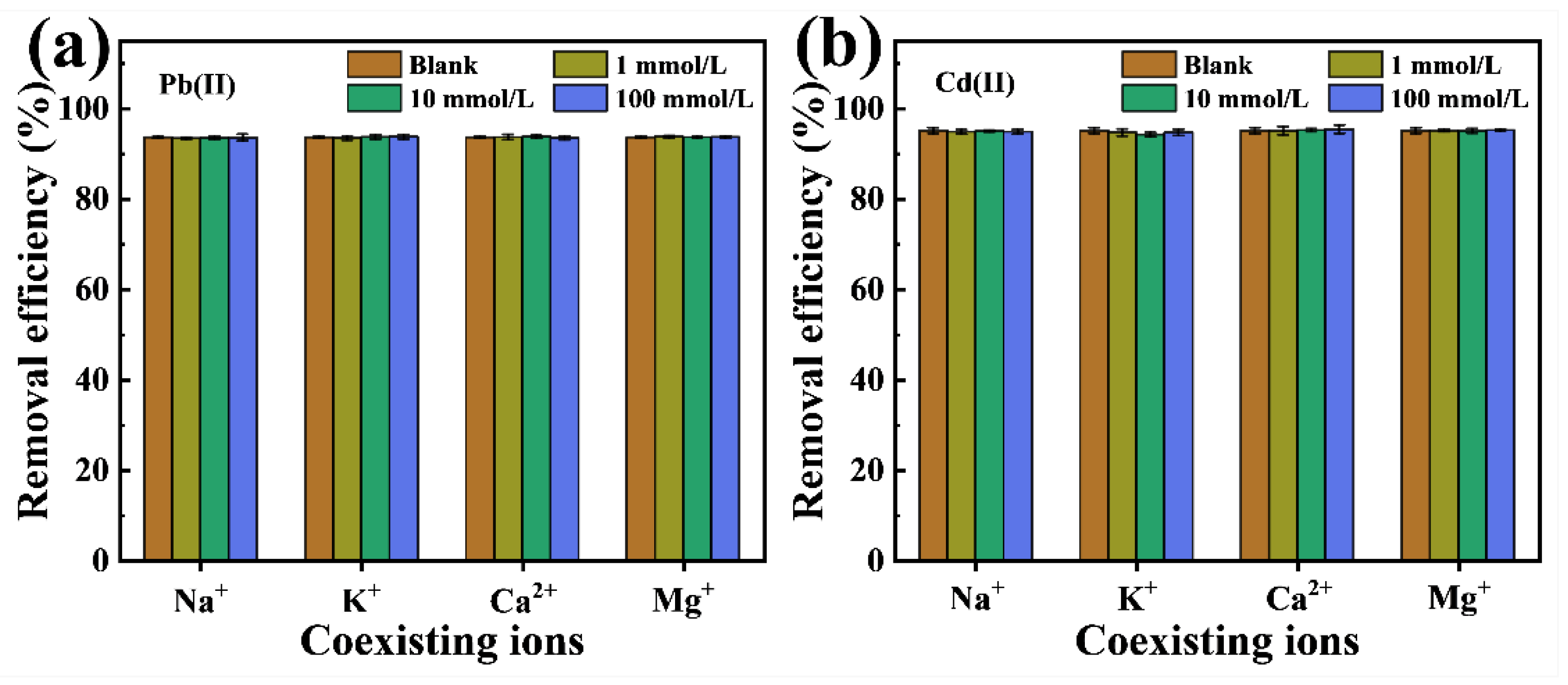
2.2.5. Effect of Coexisting Ions
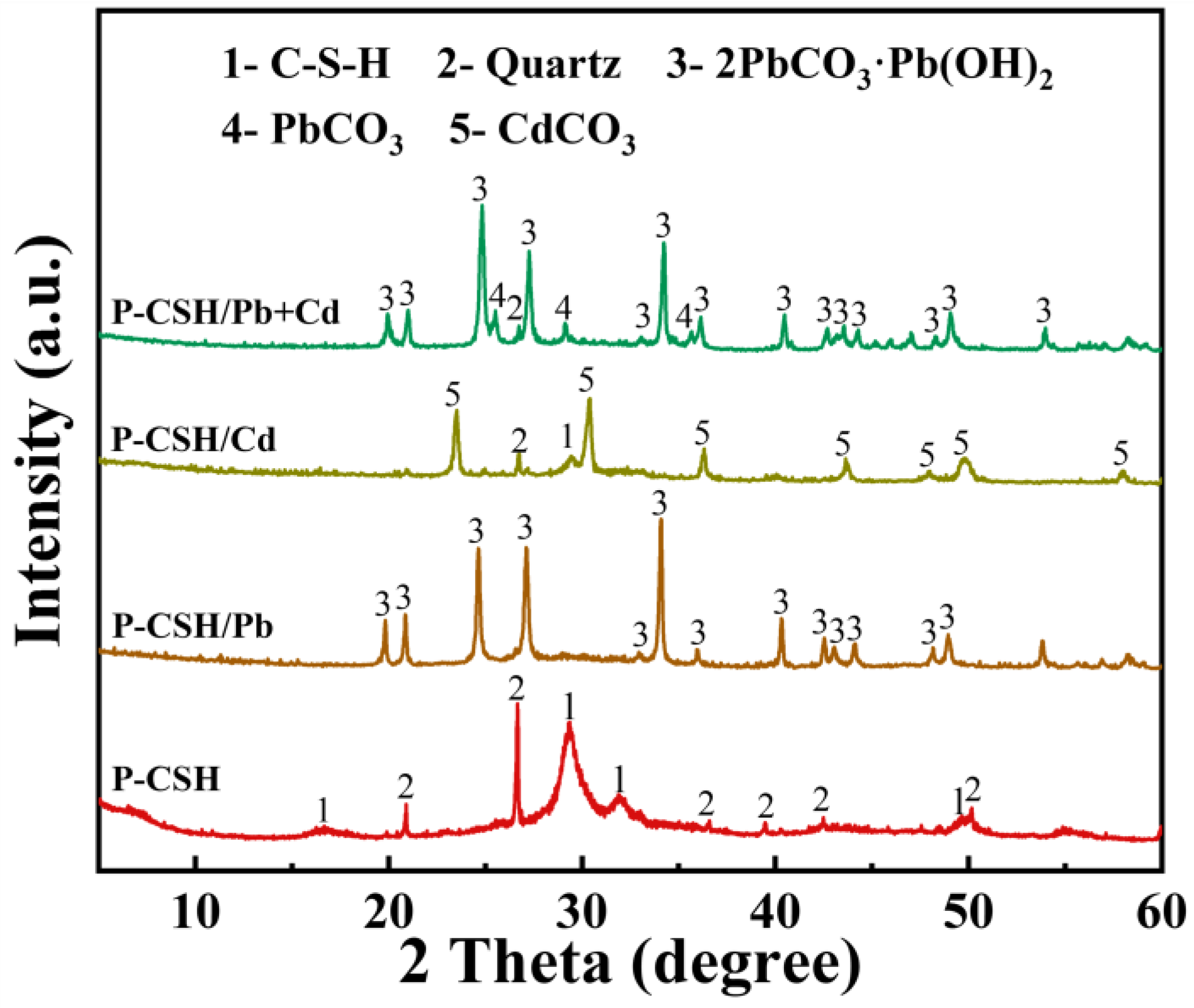
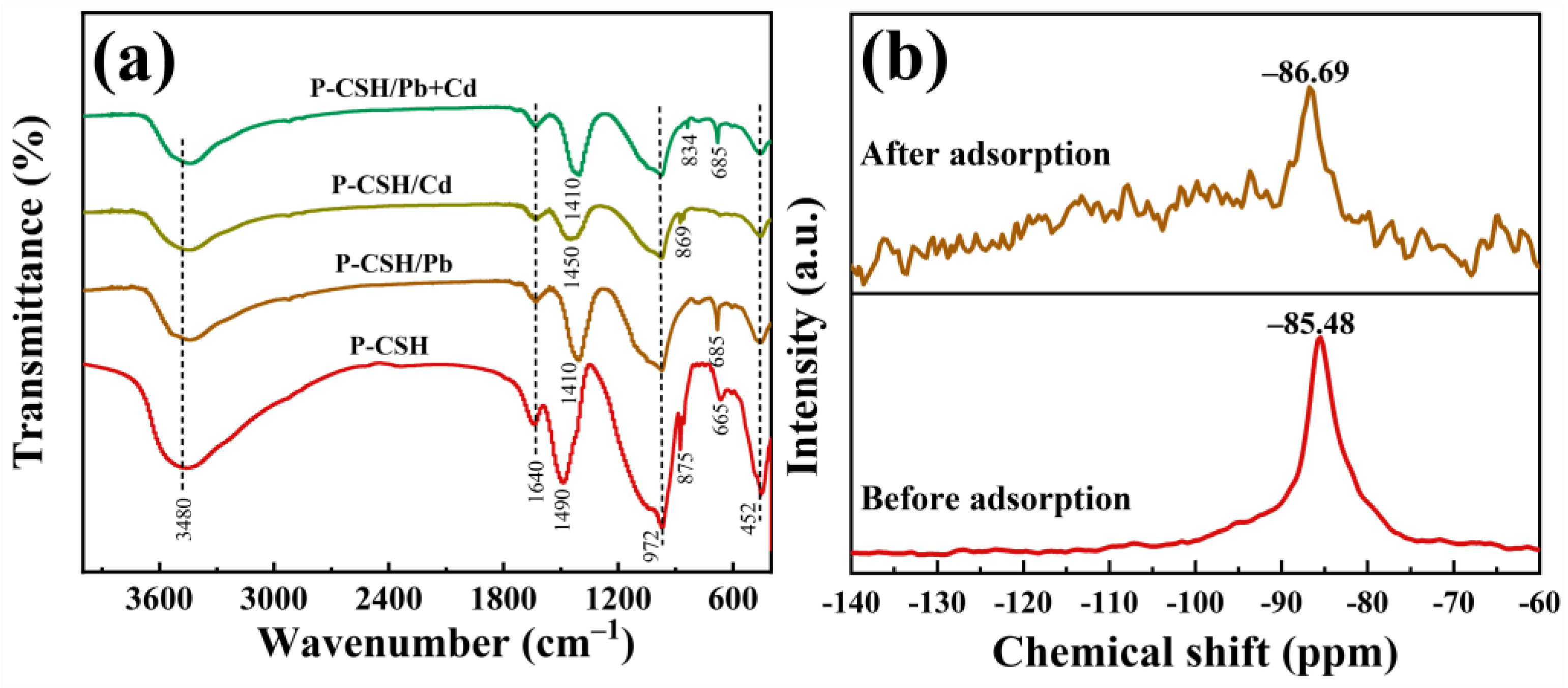
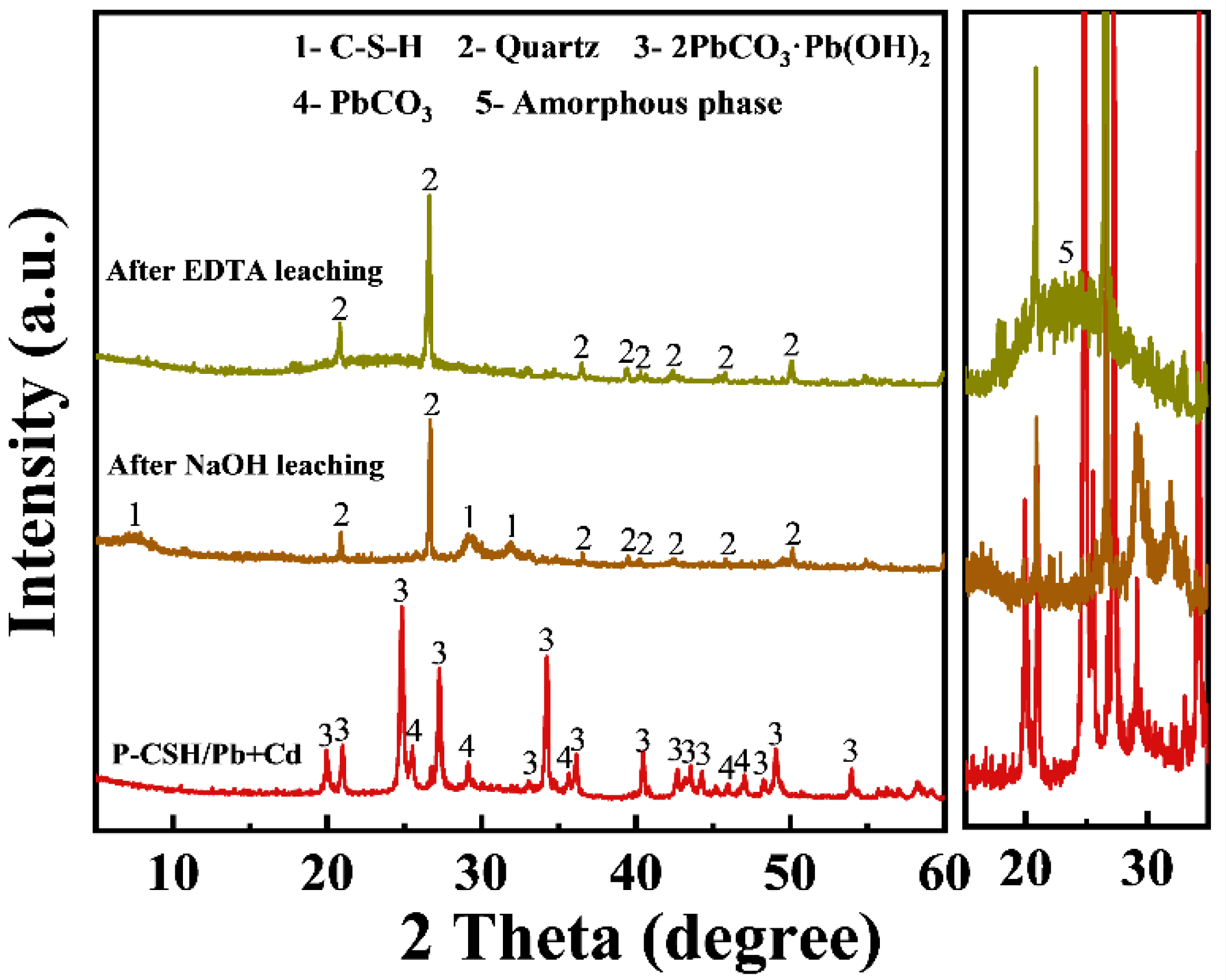
2.3. Adsorption Mechanism
2.4. Stepwise Desorption and Recovery of Pb(II) and Cd(II)
3. Materials and Methods
3.1. Materials
3.2. Phosphogypsum Conversion to P-CSH
3.3. Batch Adsorption Experiment
3.4. Stepwise Desorption and Recovery of Pb(II) and Cd(II)
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, M.; Yan, T.; Shen, J.; Zhang, J.; Zhang, D. Selective Capacitive Removal of Heavy Metal Ions from Wastewater over Lewis Base Sites of S-Doped Fe-N-C Cathodes via an Electro-Adsorption Process. Environ. Sci. Technol. 2021, 55, 7665–7673. [Google Scholar] [CrossRef] [PubMed]
- Ismail, U.M.; Vohra, M.S.; Onaizi, S.A. Adsorptive removal of heavy metals from aqueous solutions: Progress of adsorbents development and their effectiveness. Environ. Res. 2024, 251, 118562. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, H.; Huang, T.; Zhang, Y.; Lu, Y.; Tan, J.; Chen, R. Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue. Molecules 2021, 26, 2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, L.; Hu, M.; Gao, L.; Lian, B. The competitive and selective adsorption of heavy metals by struvite in the Pb(II)-Cd(II)-Zn(II) composite system and its environmental significance. Water Res. 2024, 250, 121087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Xu, L.; Wu, D.; Li, Q.; Ai, Y.; Liu, W.; Li, D.; Zhou, Y.; Zhang, B.; et al. EDTA functionalized Mg/Al hydroxides modified biochar for Pb(II) and Cd (II) removal: Adsorption performance and mechanism. Sep. Purif. Technol. 2024, 335, 126199. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Meng, N.; Song, S.; Zhu, Q.; Li, D.; Gong, L.; Ding, Y.; Zhang, R.; Shi, X. Simultaneously-efficient electro-sorption of Pb(II), Cu(II) and Cd(II) by Cu2+ modified superactive carbons. Sep. Purif. Technol. 2024, 338, 126604. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; Zhang, Y.D.; Zhang, J.-Q.; Zeng, Q.-P.; Zhang, Z.-L.; Tian, D.; Li, C.; Peng, C.-L.; Ye, K.; et al. Vertical migration behavior simulation and prediction of Pb and Cd in co-contaminated soil around Pb-Zn smelting slag site. J. Hazard. Mater. 2024, 469, 133990. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, P.; Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E. Zinc recovery from purified electric arc furnace dust leach liquors by chemical precipitation. J. Environ. Chem. Eng. 2017, 5, 3550–3559. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W.; Yang, Q.; Chen, W. Chemical coagulation process for the removal of heavy metals from water: A review. Desalination Water Treat. 2016, 57, 1733–1748. [Google Scholar] [CrossRef]
- Oden, M.K.; Sari-Erkan, H. Treatment of metal plating wastewater using iron electrode by electrocoagulation process: Optimization and process performance. Process Saf. Environ. Prot. 2018, 119, 207–217. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpaa, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Yang, Y.; Han, T.; Wang, J. Ultrafast and highly efficient Cd(II) and Pb(II) removal by magnetic adsorbents derived from gypsum and corncob: Performances and mechanisms. Ecotoxicol. Environ. Saf. 2024, 275, 116265. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, Q.; Tian, C.; Li, D.; Li, H.; Qin, G.; Hu, K.; Zhang, Q. Preparation of Biomass Carbon Composites MgO@ZnO@BC and Its Adsorption and Removal of Cu(II) and Pb(II) in Wastewater. Molecules 2023, 28, 6982. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, A.K.; Kumar, P.S.; Hoang, T.K.A.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, Y.; Liu, Y.; Shi, Y.; Lin, Q.; Cai, M.; Jia, Z.; Yu, C.; Shang, A.; Fei, Y.; et al. Cobalt/Iron Bimetallic Biochar Composites for Lead(II) Adsorption: Mechanism and Remediation Performance. Molecules 2024, 29, 1595. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Takaizumi, M.; Kumagai, S.; Saito, Y.; Yoshioka, T. Adsorption of various metals by carboxymethyl-β-cyclodextrin-modified Zn-Al layered double hydroxides. Appl. Clay Sci. 2020, 187, 105479. [Google Scholar] [CrossRef]
- Wang, K.; Gu, J.; Yin, N. Efficient Removal of Pb(II) and Cd(II) Using NH2-Functionalized Zr-MOFs via Rapid Microwave-Promoted Synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, M.; Han, Y.; Luo, X.; Li, C.; Tang, W.; Yue, T.; Li, Z. Heavy metal ions’ poisoning behavior-inspired etched UiO-66/CTS aerogel for Pb(II) and Cd(II) removal from aqueous and apple juice. J. Hazard. Mater. 2021, 401, 123318. [Google Scholar] [CrossRef]
- Wang, C.P.; Li, F.Z.; Zhou, M.K.; Chen, Y.; Chen, X. Effect of cement-MSWI fly ash hydration on the stabilisation/solidification of Pb and Cd. Mater. Res. Innov. 2015, 19, 1161–1166. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Peng, S.; Zhou, C. Synthesis of Calcium Silicate Hydrate from Coal Gangue for Cr(VI) and Cu(II) Removal from Aqueous Solution. Molecules 2021, 26, 6192. [Google Scholar] [CrossRef]
- Le, Q.T.N.; Vivas, E.L.; Cho, K. Calcium oxalate/calcium silicate hydrate (Ca-Ox/C-S-H) from blast furnace slag for the highly efficient removal of Pb2+ and Cd2+ from water. J. Environ. Chem. Eng. 2021, 9, 106287. [Google Scholar] [CrossRef]
- Qi, G.; Lei, X.; Li, L.; Yuan, C.; Sun, Y.; Chen, J.; Chen, J.; Wang, Y.; Hao, J. Preparation and evaluation of a mesoporous calcium-silicate material (MCSM) from coal fly ash for removal of Co(II) from wastewater. Chem. Eng. J. 2015, 279, 777–787. [Google Scholar] [CrossRef]
- Fang, D.; Huang, L.; Fang, Z.; Zhang, Q.; Shen, Q.; Li, Y.; Xu, X.; Ji, F. Evaluation of porous calcium silicate hydrate derived from carbide slag for removing phosphate from wastewater. Chem. Eng. J. 2018, 354, 1–11. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bai, J.; Chang, I.S.; Wu, J. A systematic review of phosphogypsum recycling industry based on the survey data in China—Applications, drivers, obstacles, and solutions. Environ. Impact Assess. Rev. 2024, 105, 107405. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitras, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Lu, W.; Su, Y.; Li, Y.; Gao, C.; He, X. Synthesis of α-hemihydrate gypsum from cleaner phosphogypsum. J. Clean. Prod. 2018, 195, 396–405. [Google Scholar] [CrossRef]
- Tokpayev, R.; Khavaza, T.; Ibraimov, Z.; Kishibayev, K.; Atchabarova, A.; Abdimomyn, S.; Abduakhytova, D.; Nauryzbayev, M. Phosphogypsum conversion under conditions of SC-CO2. J. CO2 Util. 2022, 63, 102120. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Wang, T.; Fang, M.; Li, Y. Preparation of calcium carbonate nanoparticles from waste carbide slag based on CO2 mineralization. J. Clean. Prod. 2022, 363, 132463. [Google Scholar] [CrossRef]
- Qi, F.; Zhu, G.; Zhang, Y.; Hou, X.; Li, S.; Yang, C.; Zhang, J.; Li, H. Eco-utilization of silicon-rich lye: Synthesis of amorphous calcium silicate hydrate and its application for recovering heavy metals. Sep. Purif. Technol. 2022, 282, 120092. [Google Scholar] [CrossRef]
- Hammas, I.; Horchani-Naifer, K.; Ferid, M. Solubility study and valorization of phosphogypsum salt solution. Int. J. Miner. Process. 2013, 123, 87–93. [Google Scholar] [CrossRef]
- Li, J.; Peng, X.; Zheng, J.; Mao, M.; Sun, X.; Wang, J.; Li, X.; Chai, X.; Lin, Z.; Liu, W. Simultaneous removal of phosphorus and organic contaminants from phosphogypsum using hydrothermal method for gypsum resource regeneration. J. Environ. Chem. Eng. 2022, 10, 108441. [Google Scholar] [CrossRef]
- Han, M.; Shen, X.; Shao, H.; Liu, Y.; Han, Q.; Zhai, Y. Facile one-pot hydrothermal synthesis of reticulated porous tobermorite for fast phosphorus recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131349. [Google Scholar] [CrossRef]
- Shen, X.; Feng, P.; Zhang, Q.; Lu, J.; Liu, X.; Ma, Y.; Jin, P.; Wang, W.; Ran, Q.; Hong, J. Toward the formation mechanism of synthetic calcium silicate hydrate (C-S-H)—pH and kinetic considerations. Cem. Concr. Res. 2023, 172, 107248. [Google Scholar] [CrossRef]
- Qi, F.; Zhu, G.; Zhang, Y.; Li, H.; Li, S.; Yang, C.; Zhang, J. Eco-friendly recycling of silicon—Rich lye: Synthesis of hierarchically structured calcium silicate hydrate and its application for phosphorus removal. Sci. Total Environ. 2022, 848, 157431. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Li, T.; Yang, G.; Tang, A.; Ling, Y. Adsorption efficiency, thermodynamics, and kinetics of amino-functionalized mesoporous calcium silicate for the removal of heavy metal ions. Desalination Water Treat. 2018, 107, 165–181. [Google Scholar] [CrossRef]
- Shang, Z.; Wang, T.; Ye, Q.; Wu, P.; Wu, J.; Sun, L.; Zhu, N. Immobilization of Pb2+ and Cd2+ on the novel calcium/magnesium silicate and their transformation in the presence of phosphate. Desalination 2023, 568, 117007. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, J.; Shen, J.; Yan, P.; Liu, S.; Kang, J.; Bi, L.; Wang, B.; Zhao, S.; Chen, Z. Preparation of P-doped biochar and its high-efficient removal of sulfamethoxazole from water: Adsorption mechanism, fixed-bed column and DFT study. Chem. Eng. J. 2023, 468, 143748. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Salinas, A.; Copello, G.J.; Díaz, L.E. Point of zero charge as a factor to control biofilm formation of Pseudomonas aeruginosa in sol-gel derivatized aluminum alloy plates. Surf. Coat. Technol. 2014, 254, 145–150. [Google Scholar] [CrossRef]
- Wang, M.; Ye, H.; Zheng, X.; Chen, S.; Xing, H.; Tao, X.; Dang, Z.; Lu, G. Adsorption behaviors and mechanisms of simultaneous cadmium and fluoride removal on waste bovine bone from aqueous solution. J. Environ. Chem. Eng. 2023, 11, 109035. [Google Scholar] [CrossRef]
- Yan, Y.; Dong, X.; Sun, X.; Sun, X.; Li, J.; Shen, J.; Han, W.; Liu, X.; Wang, L. Conversion of waste FGD gypsum into hydroxyapatite for removal of Pb2+ and Cd2+ from wastewater. J. Colloid Interface Sci. 2014, 429, 68–76. [Google Scholar] [CrossRef]
- Shao, N.; Tang, S.; Liu, Z.; Li, L.; Yan, F.; Liu, F.; Li, S.; Zhang, Z. Hierarchically Structured Calcium Silicate Hydrate-Based Nanocomposites Derived from Steel Slag for Highly Efficient Heavy Metal Removal from Wastewater. ACS Sustain. Chem. Eng. 2018, 6, 14926–14935. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.-H.; Meng, L.-H.; Li, Y.; Sun, M.-Y.; Wang, C.-C.; Wang, P.; Li, H.-Y. Mining Ag+ ions from wastewater with Bio-MOF-1: From adsorption to high value-added application. Sep. Purif. Technol. 2024, 341, 126928. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, D.; Yang, Q.; Liu, X.; Gholami, Z.; Vakili, M.; Zhou, S.; Wang, W. Triazole and methylthio modified covalent organic frameworks for enhancing Hg(II) adsorption from water. Mater. Today Commun. 2024, 38, 107976. [Google Scholar] [CrossRef]
- Monárrez-Cordero, B.E.; Amézaga-Madrid, P.; Fuentes-Cobas, L.; Montero-Cabrera, M.E.; Miki-Yoshida, M. High and fast adsorption efficiency of simultaneous As+3, As+5 and F− by Al-doped magnetite synthesized via AACVD. J. Alloys Compd. 2017, 718, 414–424. [Google Scholar] [CrossRef]
- Czech, B.; Hojamberdiev, M.; Bogusz, A. Impact of thermal treatment of calcium silicate-rich slag on the removal of cadmium from aqueous solution. J. Clean. Prod. 2018, 200, 369–379. [Google Scholar] [CrossRef]
- Zeng, G.; Si, M.; Dong, C.; Liao, Q.; He, F.; Johnson, V.E.; Arinzechi, C.; Yang, W.; Yang, Z. Adsorption behavior of lead, cadmium, and arsenic on manganese-modified biochar: Competition and promotion. Environ. Geochem. Health 2024, 46, 86. [Google Scholar] [CrossRef]
- Nassar, N.N. Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Guérin, T.; Oustrière, N.; Bulteel, D.; Betrancourt, D.; Ghinet, A.; Malladi, S.; Kaleo-Bioh, J.G.; Blanc-Brude, A.; Pappoe, A.; Waterlot, C. Removal of heavy metals from contaminated water using industrial wastes containing calcium and magnesium. J. Clean. Prod. 2022, 337, 130472. [Google Scholar] [CrossRef]
- Guo, H.; Hu, S.; Wang, Z.; Li, Y.; Guo, X.; He, Z.; Wang, W.; Feng, J.; Yang, K.; Zheng, H. Synthesis of a Magnetic Carnation-like Hydroxyapatite/Basic Calcium Carbonate Nanocomposite and Its Adsorption Behaviors for Lead Ions in Water. Molecules 2022, 27, 5565. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, Z.; Du, Y.; Lyu, Q.; Yang, Z.; Liu, Y.; Yan, Z. Microbiologically induced calcite precipitation technology for mineralizing lead and cadmium in landfill leachate. J. Environ. Manag. 2021, 296, 113199. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Hegazey, R.M.; AlReshaidan, S.; Naglah, A.M. Facile hydrothermal synthesis of calcium silicate nanostructures for removal of Hg(II) and Cd(II) ions from aqueous media. Int. J. Environ. Anal. Chem. 2023, 103, 6687–6703. [Google Scholar] [CrossRef]
- Ba, H.; Li, J.; Ni, W.; Li, Y.; Ju, Y.; Zhao, B.; Wen, G.; Hitch, M. Effect of calcium to silicon ratio on the microstructure of hydrated calcium silicate gels prepared under medium alkalinity. Constr. Build. Mater. 2023, 379, 131240. [Google Scholar] [CrossRef]
- Generalic, E. Solubility Product Constants. EniG. Periodic Table of the Elements. 2024. Available online: https://www.periodni.com/solubility_product_constants.html (accessed on 26 May 2024).
- Chen, Y.; Ding, H.; Sun, S. Preparation and Characterization of ZnO Nanoparticles Supported on Amorphous SiO2. Nanomaterials 2017, 7, 217. [Google Scholar] [CrossRef]
- Fertani-Gmati, M.; Jemal, M. Thermochemical and kinetic investigations of amorphous silica dissolution in NaOH solutions. J. Therm. Anal. Calorim. 2016, 123, 757–765. [Google Scholar] [CrossRef]
- Choi, J.-H.; Seo, Y.-S.; Chae, B.-G. A study of the pressure solution and deformation of quartz crystals at high pH and under high stress. Nucl. Eng. Technol. 2013, 45, 53–60. [Google Scholar] [CrossRef]
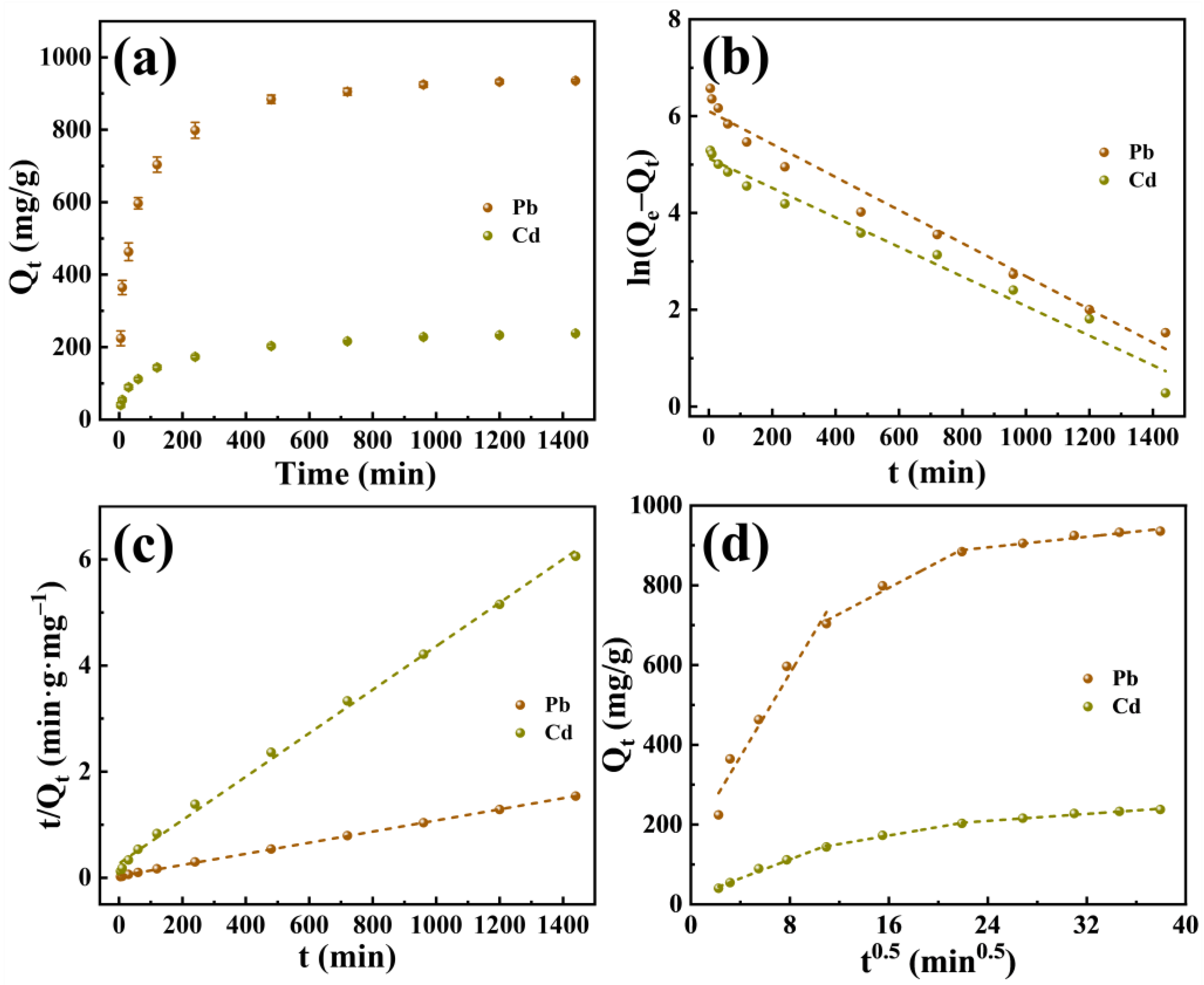

| Model | Parameter | Pb(II) | Cd(II) |
|---|---|---|---|
| Pseudo-first-order | k1 (min−1) | 0.003413 | 0.003052 |
| Qe (mg/g) | 448.2 | 168.6 | |
| R2 | 0.9715 | 0.9763 | |
| Pseudo-second-order | k2 (g/(mg·min)) | 3.27 × 10−5 | 6.24 × 10−5 |
| Qe (mg/g) | 954.2 | 244.1 | |
| R2 | 0.9996 | 0.9972 | |
| Intra-particle diffusion | C1 (mg/g) | 162.4 | 17.9 |
| ki1 (mg/(g·min0.5)) | 52.0802 | 11.8370 | |
| R2 | 0.9397 | 0.9846 | |
| C2 (mg/g) | 532.9 | 86.9 | |
| ki2 (mg/(g·min0.5)) | 16.2893 | 5.3534 | |
| R2 | 0.9678 | 0.9834 | |
| C3 (mg/g) | 815.6 | 156.8 | |
| ki3 (mg/(g·min0.5)) | 3.3091 | 2.1892 | |
| R2 | 0.9310 | 0.9676 |
| Model | Parameter | Pb(II) | Cd(II) |
|---|---|---|---|
| Langmuir | Qm (mg/g) | 993.1 | 291.5 |
| KL (L/mg) | 0.6735 | 0.5097 | |
| R2 | 0.9995 | 0.9994 | |
| Freundlich | KF(mg(1−1/n)·L1/n·g−1) | 808.3 | 172.2 |
| n | 26.14 | 9.54 | |
| R2 | 0.9776 | 0.9494 | |
| Temkin | KT (L/mg) | 3.60 × 1010 | 4366.8 |
| b | 75.57 | 117.33 | |
| R2 | 0.9734 | 0.9829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Chen, C.; Li, J.; Lan, Y.; Lin, X.; Chen, J. Conversion of Phosphogypsum into Porous Calcium Silicate Hydrate for the Removal and Recycling of Pb(II) and Cd(II) from Wastewater. Molecules 2024, 29, 2665. https://doi.org/10.3390/molecules29112665
Wang G, Chen C, Li J, Lan Y, Lin X, Chen J. Conversion of Phosphogypsum into Porous Calcium Silicate Hydrate for the Removal and Recycling of Pb(II) and Cd(II) from Wastewater. Molecules. 2024; 29(11):2665. https://doi.org/10.3390/molecules29112665
Chicago/Turabian StyleWang, Gangan, Chaoyi Chen, Junqi Li, Yuanpei Lan, Xin Lin, and Jiahang Chen. 2024. "Conversion of Phosphogypsum into Porous Calcium Silicate Hydrate for the Removal and Recycling of Pb(II) and Cd(II) from Wastewater" Molecules 29, no. 11: 2665. https://doi.org/10.3390/molecules29112665
APA StyleWang, G., Chen, C., Li, J., Lan, Y., Lin, X., & Chen, J. (2024). Conversion of Phosphogypsum into Porous Calcium Silicate Hydrate for the Removal and Recycling of Pb(II) and Cd(II) from Wastewater. Molecules, 29(11), 2665. https://doi.org/10.3390/molecules29112665






