Non-Glycosylated SARS-CoV-2 Omicron BA.5 Receptor Binding Domain (RBD) with a Native-like Conformation Induces a Robust Immune Response with Potent Neutralization in a Mouse Model
Abstract
1. Introduction
2. Results
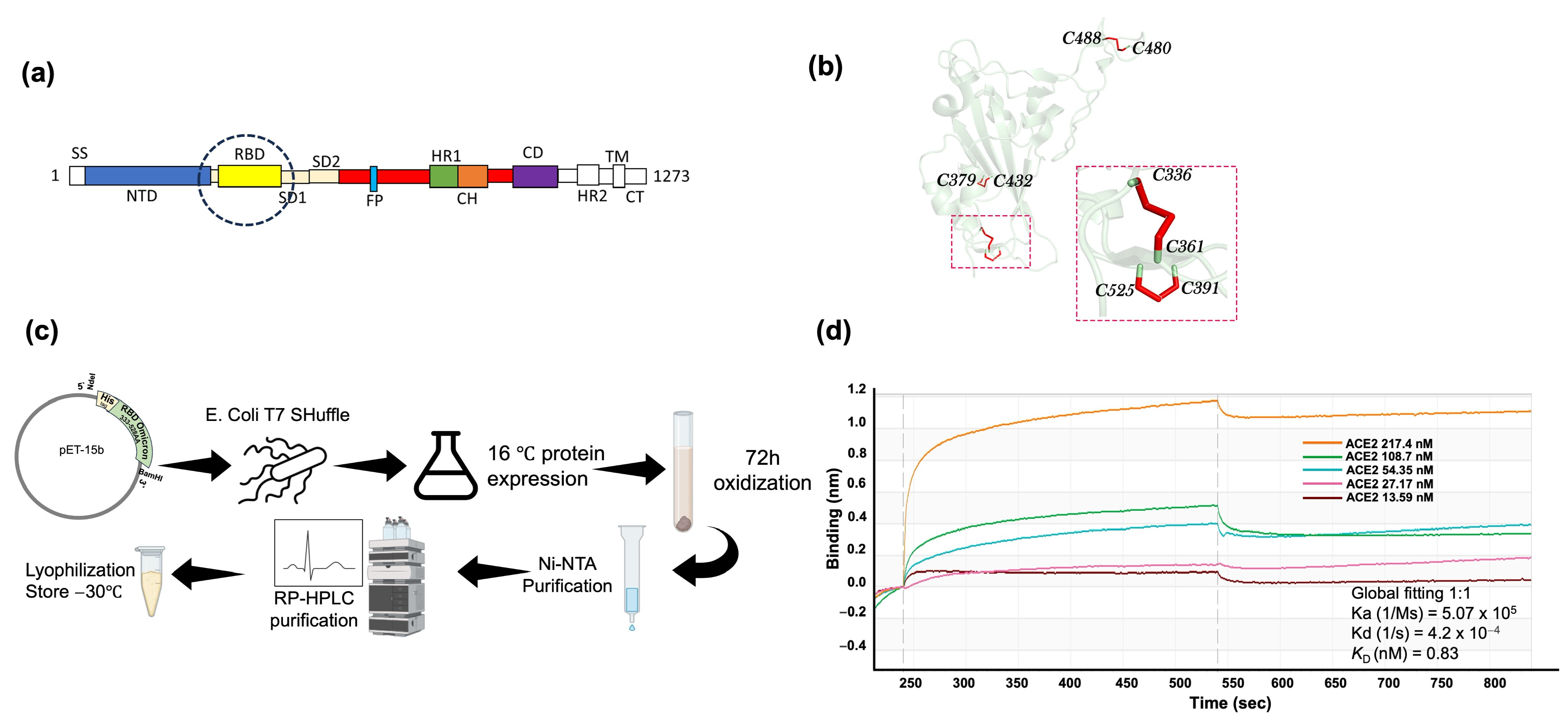
2.1. SARS-CoV-2 Omicron BA.5 RBD Expression and Purification in E. coli and Biochemical Characterization
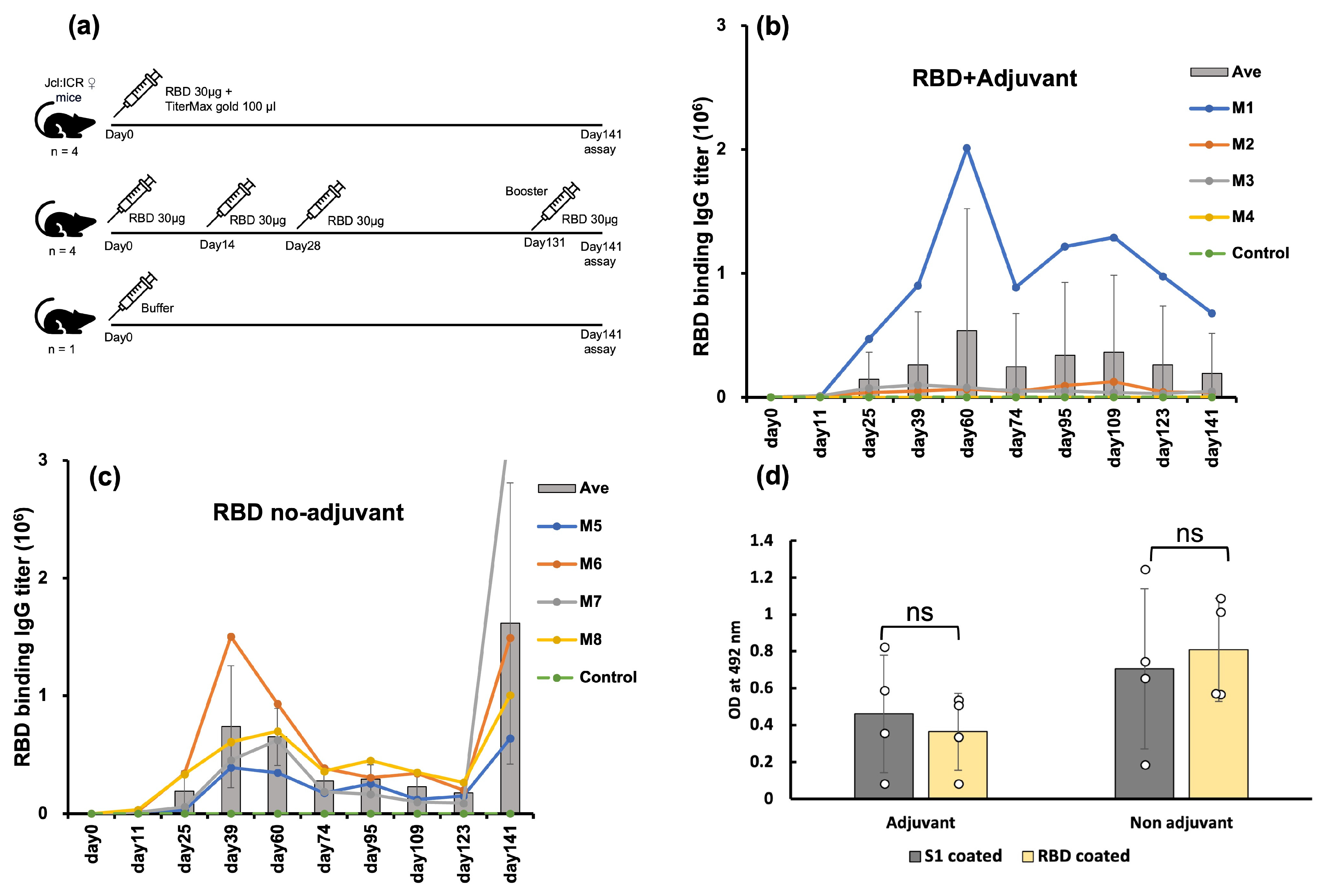
2.2. E. coli-Expressed RBD Induced a High Level of Anti-RBD Antisera in a Mouse Model
2.3. E. coli-Expressed RBD Elicited Antisera Recognized the Mammalian-Produced S1
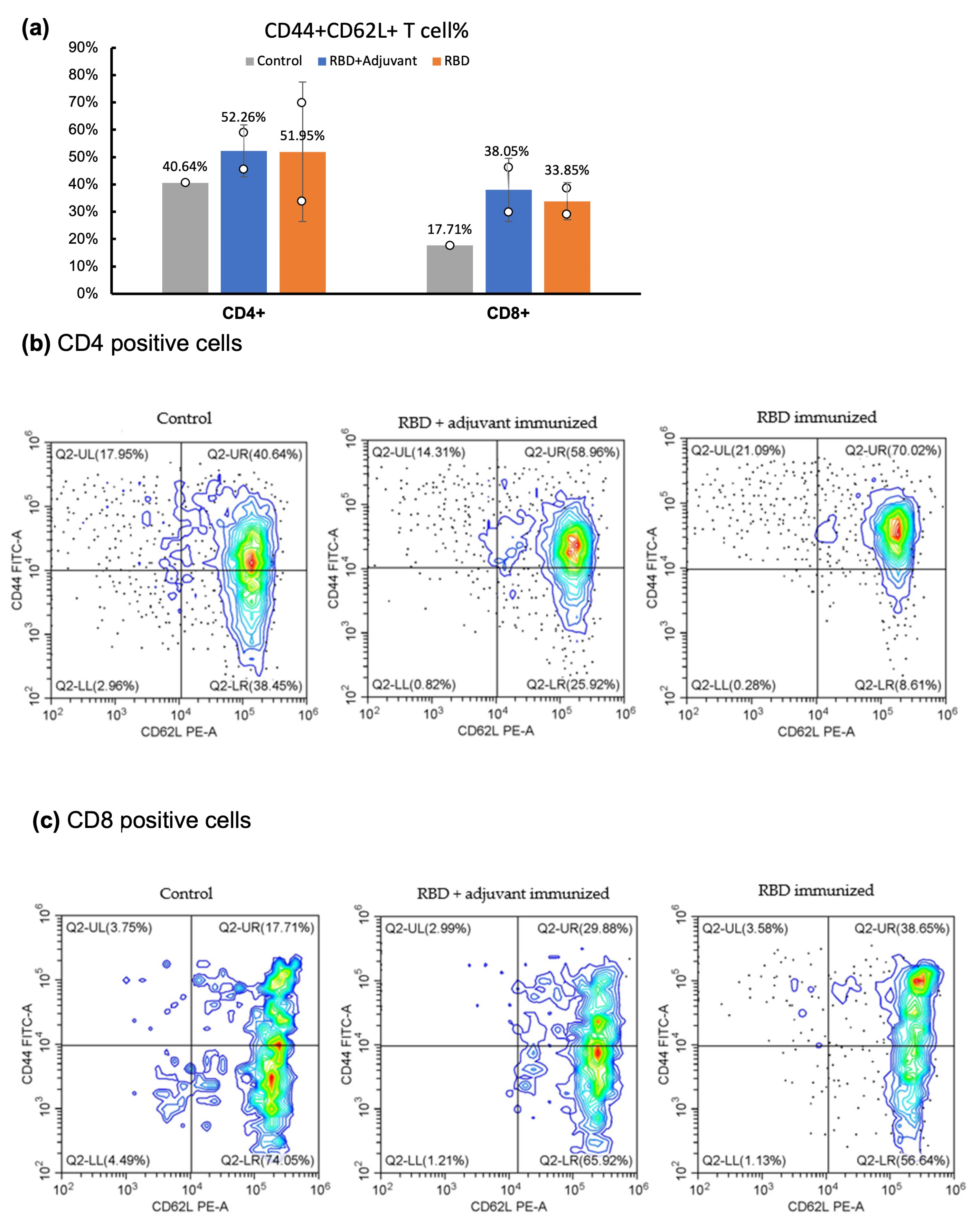
2.4. CD Marker Analysis through Flow Cytometry
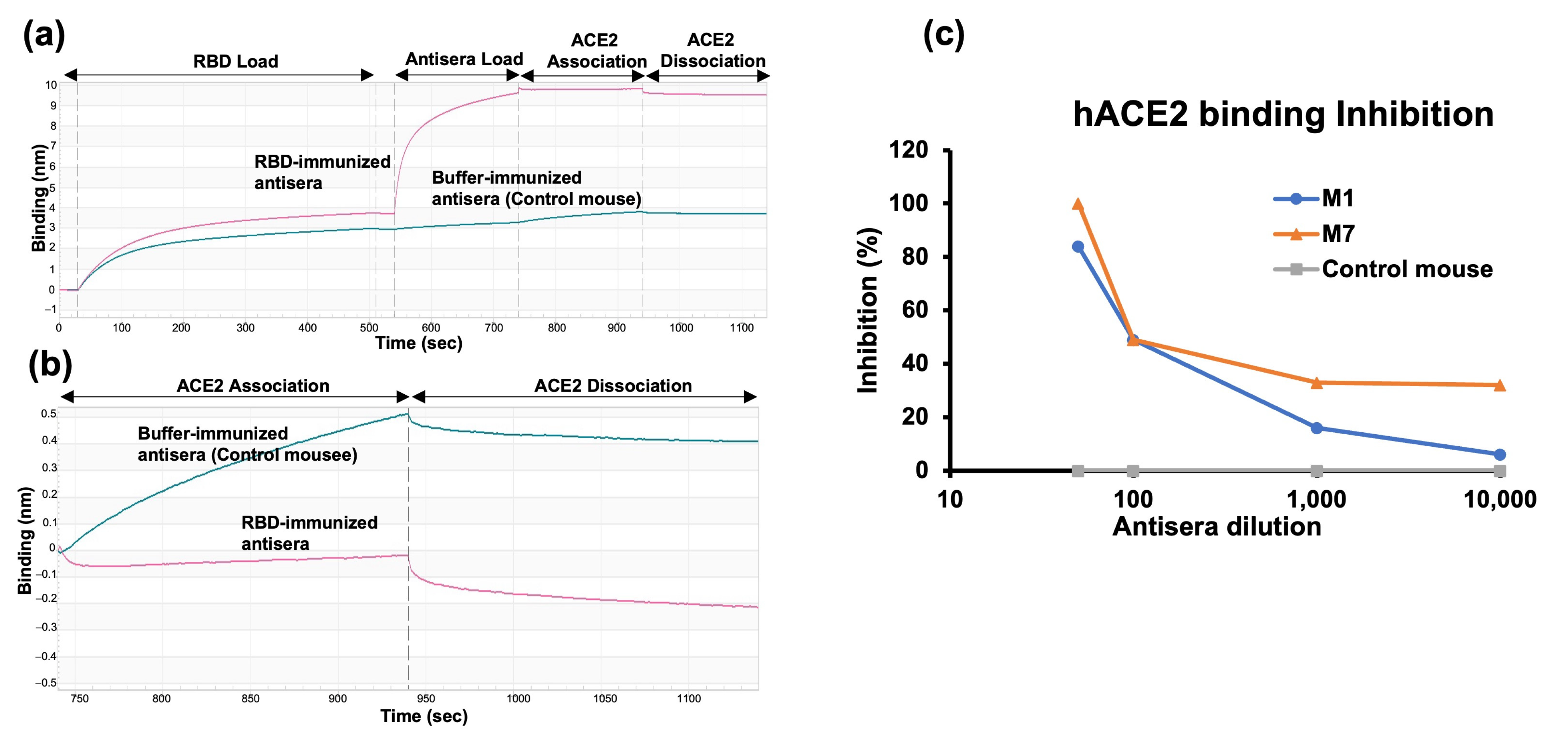
2.5. RBD-hACE2 Binding Inhibition Assay
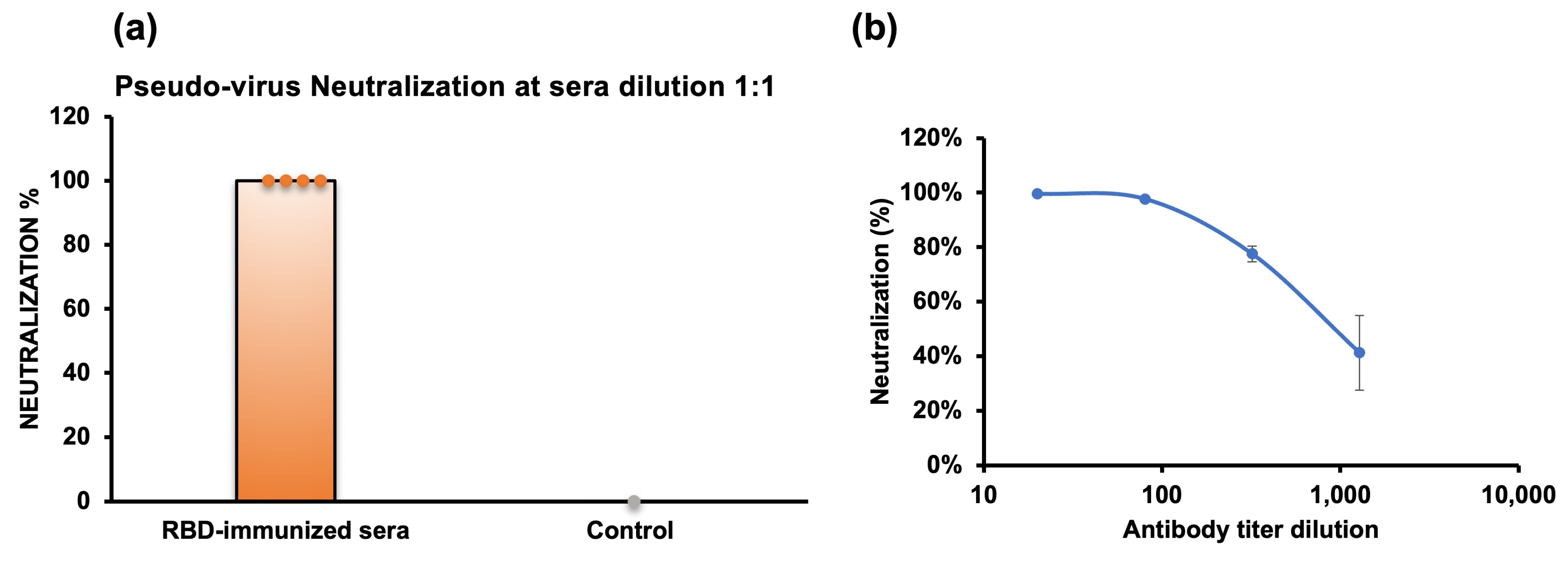
2.6. Pseudovirus Neutralization
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Binding Activity by Bio-Layer Interferometry
4.3. Mice Immunization
4.4. Anti-RBD IgG Titer by ELISA
4.5. Cell Surface CD Marker Analysis
4.6. ACE2 Binding Inhibition Assay
4.7. Pseudovirus Neutralization Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, Q.; Shi, K.; Ye, G.; Zhang, W.; Aihara, H.; Li, F. Structural Basis for Human Receptor Recognition by SARS-CoV-2 Omicron Variant BA.1. J. Virol. 2022, 96, e0024922. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Alhumaid, S.; AlMusa, Z.; Fatimawali; Kusumawaty, D.; Alynbiawi, A.; Alshukairi, A.N.; Rabaan, A.A. Update on the Omicron Sub-Variants BA.4 and BA.5. Rev. Med. Virol. 2023, 33, e2391. [Google Scholar] [CrossRef]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron Lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody Escape of SARS-CoV-2 Omicron BA.4 and BA.5 from Vaccine and BA.1 Serum. Cell 2022, 185, 2422–2433.e13. [Google Scholar] [CrossRef]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.Y.; et al. Neutralizing Immunity in Vaccine Breakthrough Infections from the SARS-CoV-2 Omicron and Delta Variants. Cell 2022, 185, 1539. [Google Scholar] [CrossRef]
- Erabi, G.; Faridzadeh, A.; Parvin, A.; Deravi, N.; Rahmanian, M.; Fathi, M.; Aleebrahim-Dehkordi, E.; Rezaei, N. SARS-CoV-2 Omicron (BA.4, BA.5) Variant: Lessons Learned from a New Variant during the COVID-19 Pandemic. Health Sci. Rep. 2024, 7, e1873. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. New Engl. J. Med. 2022, 387, 468–470. [Google Scholar] [CrossRef]
- Shafqat, A.; Omer, M.H.; Ahmad, O.; Niaz, M.; Abdulkader, H.S.; Shafqat, S.; Mushtaq, A.H.; Shaik, A.; Elshaer, A.N.; Kashir, J.; et al. SARS-CoV-2 Epitopes Inform Future Vaccination Strategies. Front. Immunol. 2022, 13, 1041185. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, X.; Gu, Y.; Jiang, J.; Yang, Z.; Lv, Q.; Guo, D.; Yang, Y.; Lu, H.; Yuan, J. Distinct and Relatively Mild Clinical Characteristics of SARS-CoV-2 BA.5 Infections against BA.2. Signal Transduct. Target. Ther. 2023, 8, 1041185. [Google Scholar] [CrossRef]
- Goller, K.V.; Ziemann, J.; Kohler, C.; Becker, K.; Hübner, N.O. Clinical Manifestations of Infections with the Omicron Sub-Lineages BA.1, BA.2, and BA.5: A Retrospective Follow-Up Analysis of Public Health Data from Mecklenburg-Western Pomerania, Germany. Viruses 2024, 16, 454. [Google Scholar] [CrossRef]
- Nakakubo, S.; Kishida, N.; Okuda, K.; Kamada, K.; Iwama, M.; Suzuki, M.; Yokota, I.; Ito, Y.M.; Nasuhara, Y.; Boucher, R.C.; et al. Associations of COVID-19 Symptoms with Omicron Subvariants BA.2 and BA.5, Host Status, and Clinical Outcomes in Japan: A Registry-Based Observational Study. Lancet Infect. Dis. 2023, 23, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Nwagwu, C.S.; Ugwu, C.N.; Ogbonna, J.D.N.; Onugwu, A.L.; Agbo, C.P.; Echezona, A.C.; Ezeibe, E.N.; Uzondu, S.; Kenechukwu, F.C.; Akpa, P.A.; et al. Recent and Advanced Nano-Technological Strategies for COVID-19 Vaccine Development. In Methods in Microbiology; Elsevier: Amsterdam, Netherlands, 2022; Volume 50. [Google Scholar]
- Abinaya, R.V.; Viswanathan, P. Biotechnology-Based Therapeutics. In Translational Biotechnology: A Journey from Laboratory to Clinics; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Chavda, V.P.; Ghali, E.N.H.K.; Balar, P.C.; Chauhan, S.C.; Tiwari, N.; Shukla, S.; Athalye, M.; Patravale, V.; Apostolopoulos, V.; Yallapu, M.M. Protein Subunit Vaccines: Promising Frontiers against COVID-19. J. Control. Release 2024, 366, 761–782. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the Receptor-Binding Domain (RBD) of 2019 Novel Coronavirus: Implication for Development of RBD Protein as a Viral Attachment Inhibitor and Vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 1727–11734. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, W.; Xia, S.; Gu, C.; Wang, X.; Wang, Q.; Zhou, J.; Wu, Y.; Cai, X.; Qu, D.; et al. RBD-Fc-Based COVID-19 Vaccine Candidate Induces Highly Potent SARS-CoV-2 Neutralizing Antibody Response. Signal Transduct. Target. Ther. 2020, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Sun, Q. Antibodies and Vaccines Target RBD of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 671633. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Chen, R.H.; Hamdoun, S.; Coghi, P.; Ng, J.P.L.; Zhang, D.W.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. Corilagin Prevents SARS-CoV-2 Infection by Targeting RBD-ACE2 Binding. Phytomedicine 2021, 87, 153591. [Google Scholar] [CrossRef] [PubMed]
- Tubiana, J.; Xiang, Y.; Fan, L.; Wolfson, H.J.; Chen, K.; Schneidman-Duhovny, D.; Shi, Y. Reduced B Cell Antigenicity of Omicron Lowers Host Serologic Response. Cell Rep. 2022, 41, 111512. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, X.; Yang, J.; Lei, H.; Hong, W.; Song, X.; Yang, L.; Li, J.; Wang, W.; Shen, G.; et al. Spike Protein of SARS-CoV-2 Omicron (B.1.1.529) Variant Have a Reduced Ability to Induce the Immune Response. Signal Transduct. Target. Ther. 2022, 7, 119. [Google Scholar] [CrossRef]
- Chen, R. Bacterial Expression Systems for Recombinant Protein Production: E. Coli and Beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia Coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Wongnak, R.; Brindha, S.; Yoshizue, T.; Onchaiya, S.; Mizutani, K.; Kuroda, Y.E. Coli Production of a Multi-Disulfide Bonded SARS-CoV-2 Omicron BA.5 RBD Exhibiting Native-like Biochemical and Biophysical Properties. Biophys. Physicobiol. 2023, 20, e200036. [Google Scholar] [CrossRef] [PubMed]
- Brindha, S.; Yoshizue, T.; Wongnak, R.; Takemae, H.; Oba, M.; Mizutani, T.; Kuroda, Y. An Escherichia Coli Expressed Multi-Disulfide Bonded SARS-CoV-2 RBD Shows Native-like Biophysical Properties and Elicits Neutralizing Antisera in a Mouse Model. Int. J. Mol. Sci. 2022, 23, 15744. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. SHuffle, a Novel Escherichia Coli Protein Expression Strain Capable of Correctly Folding Disulfide Bonded Proteins in Its Cytoplasm. Microb. Cell Fact. 2012, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Vasina, J.A.; Baneyx, F. Expression of Aggregation-Prone Recombinant Proteins at Low Temperatures: A Comparative Study of the Escherichia Coli CspA and Tac Promoter Systems. Protein Expr. Purif. 1997, 9, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, R.; Lilie, H. In Vitro Folding of Inclusion Body Proteins. FASEB J. 1996, 10, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Guo, Y.; Yeh, A.Y.; Liu, M.; Yu, J.; Sheng, Z.; Huang, Y.; Liu, L.; et al. Antigenic Characterization of the SARS-CoV-2 Omicron Subvariant BA.2.75. Cell Host Microbe 2022, 30, 1512. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jin, S.; Gilmartin, L.; Toth, I.; Hussein, W.M.; Stephenson, R.J. Advances in Infectious Disease Vaccine Adjuvants. Vaccines 2022, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Roberts, A.D.; Ely, K.H.; Woodland, D.L. Differential Contributions of Central and Effector Memory T Cells to Recall Responses. J. Exp. Med. 2005, 202, 123–133. [Google Scholar] [CrossRef]
- van Faassen, H.; Saldanha, M.; Gilbertson, D.; Dudani, R.; Krishnan, L.; Sad, S. Reducing the Stimulation of CD8+ T Cells during Infection with Intracellular Bacteria Promotes Differentiation Primarily into a Central (CD62LhighCD44high) Subset. J. Immunol. 2005, 174, 5341–5350. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest, I. Quest GraphTM IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 10 April 2024).
- Wu, N.; Kobayashi, N.; Tsuda, K.; Unzai, S.; Saotome, T.; Kuroda, Y.; Yamazaki, T. Solution Structure of Gaussia Luciferase with Five Disulfide Bonds and Identification of a Putative Coelenterazine Binding Cavity by Heteronuclear NMR. Sci. Rep. 2020, 10, 20069. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, P.; Udgaonkar, J.B. How Cooperative Are Protein Folding and Unfolding Transitions? Protein Sci. 2016, 25, 1924–1941. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M. Differential Scanning Calorimetry as a Tool for Protein Folding and Stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kuwajima, K. The Molten Globule State as a Clue for Understanding the Folding and Cooperativity of Globular-protein Structure. Proteins: Struct. Funct. Bioinform. 1989, 6, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Endo, S.; Nakamura, H. How a Novel Scientific Concept Was Coined the “Molten Globule State”. Biomolecules 2020, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Kissel, T.; Derksen, V.F.A.M.; Bentlage, A.E.H.; Koeleman, C.; Hafkenscheid, L.; van der Woude, D.; Wuhrer, M.; Vidarsson, G.; Toes, R.E.M. N-Linked Fc Glycosylation Is Not Required for IgG-B-Cell Receptor Function in a GC-Derived B-Cell Line. Nat. Commun. 2024, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A. For Many but Not for All: How the Conformational Flexibility of the Peptide/MHCII Complex Shapes Epitope Selection. Immunol. Res. 2013, 56, 85–95. [Google Scholar] [CrossRef] [PubMed]
- DiPiazza, A.T.; Leist, S.R.; Abiona, O.M.; Moliva, J.I.; Werner, A.; Minai, M.; Nagata, B.M.; Bock, K.W.; Phung, E.; Schäfer, A.; et al. COVID-19 Vaccine MRNA-1273 Elicits a Protective Immune Profile in Mice That Is Not Associated with Vaccine-Enhanced Disease upon SARS-CoV-2 Challenge. Immunity 2021, 54, 1869. [Google Scholar] [CrossRef]
- Spencer, A.J.; McKay, P.F.; Belij-Rammerstorfer, S.; Ulaszewska, M.; Bissett, C.D.; Hu, K.; Samnuan, K.; Blakney, A.K.; Wright, D.; Sharpe, H.R.; et al. Heterologous Vaccination Regimens with Self-Amplifying RNA and Adenoviral COVID Vaccines Induce Robust Immune Responses in Mice. Nat. Commun. 2021, 12, 2893. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Wintjens, R.; Bifani, A.M.; Bifani, P. Impact of Glycan Cloud on the B-Cell Epitope Prediction of SARS-CoV-2 Spike Protein. NPJ Vaccines 2020, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Jan, J.-T.; Huang, Y.-J.; Chen, T.-H.; Wu, S.-C. Unmasking Stem-Specific Neutralizing Epitopes by Abolishing N-Linked Glycosylation Sites of Influenza Virus Hemagglutinin Proteins for Vaccine Design. J. Virol. 2016, 90, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Yoshizue, T.; Brindha, S.; Wongnak, R.; Takemae, H.; Oba, M.; Mizutani, T.; Kuroda, Y. Antisera Produced Using an E. Coli-Expressed SARS-CoV-2 RBD and Complemented with a Minimal Dose of Mammalian-Cell-Expressed S1 Subunit of the Spike Protein Exhibits Improved Neutralization. Int. J. Mol. Sci. 2023, 24, 10583. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongnak, R.; Brindha, S.; Oba, M.; Yoshizue, T.; Islam, M.D.; Islam, M.M.; Takemae, H.; Mizutani, T.; Kuroda, Y. Non-Glycosylated SARS-CoV-2 Omicron BA.5 Receptor Binding Domain (RBD) with a Native-like Conformation Induces a Robust Immune Response with Potent Neutralization in a Mouse Model. Molecules 2024, 29, 2676. https://doi.org/10.3390/molecules29112676
Wongnak R, Brindha S, Oba M, Yoshizue T, Islam MD, Islam MM, Takemae H, Mizutani T, Kuroda Y. Non-Glycosylated SARS-CoV-2 Omicron BA.5 Receptor Binding Domain (RBD) with a Native-like Conformation Induces a Robust Immune Response with Potent Neutralization in a Mouse Model. Molecules. 2024; 29(11):2676. https://doi.org/10.3390/molecules29112676
Chicago/Turabian StyleWongnak, Rawiwan, Subbaian Brindha, Mami Oba, Takahiro Yoshizue, Md. Din Islam, M. Monirul Islam, Hitoshi Takemae, Tetsuya Mizutani, and Yutaka Kuroda. 2024. "Non-Glycosylated SARS-CoV-2 Omicron BA.5 Receptor Binding Domain (RBD) with a Native-like Conformation Induces a Robust Immune Response with Potent Neutralization in a Mouse Model" Molecules 29, no. 11: 2676. https://doi.org/10.3390/molecules29112676
APA StyleWongnak, R., Brindha, S., Oba, M., Yoshizue, T., Islam, M. D., Islam, M. M., Takemae, H., Mizutani, T., & Kuroda, Y. (2024). Non-Glycosylated SARS-CoV-2 Omicron BA.5 Receptor Binding Domain (RBD) with a Native-like Conformation Induces a Robust Immune Response with Potent Neutralization in a Mouse Model. Molecules, 29(11), 2676. https://doi.org/10.3390/molecules29112676










