Synthesis and Inclusion Properties of a β-Cyclodextrin Heptaphosphoramidate
Abstract
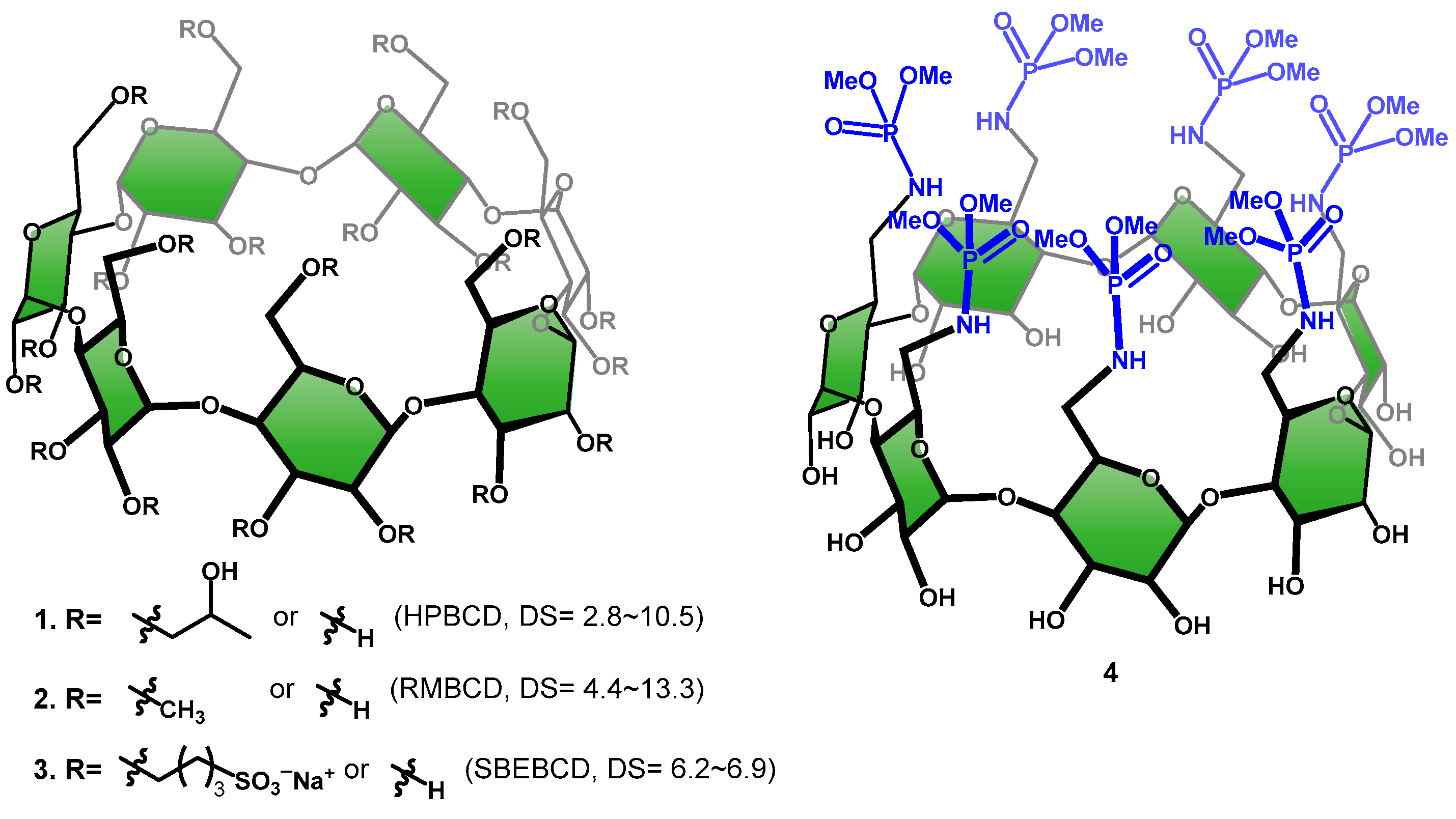
:1. Introduction
2. Results and Discussion
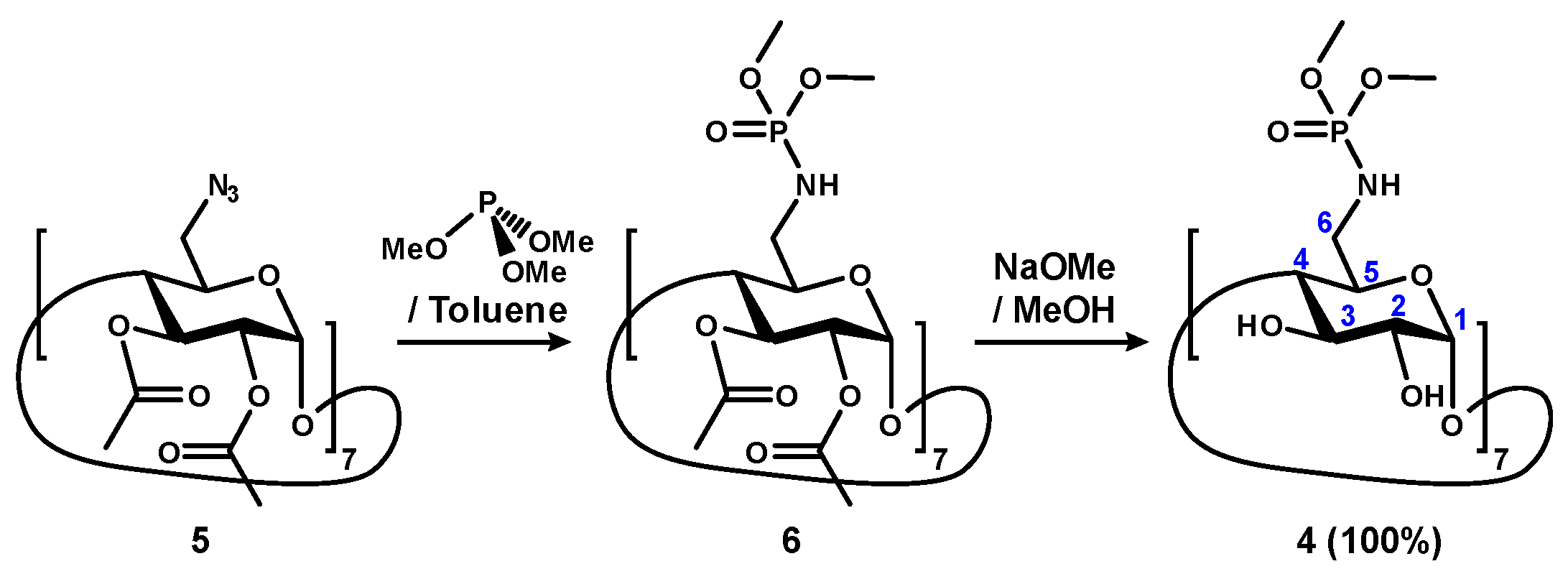
2.1. Synthesis and Characterization
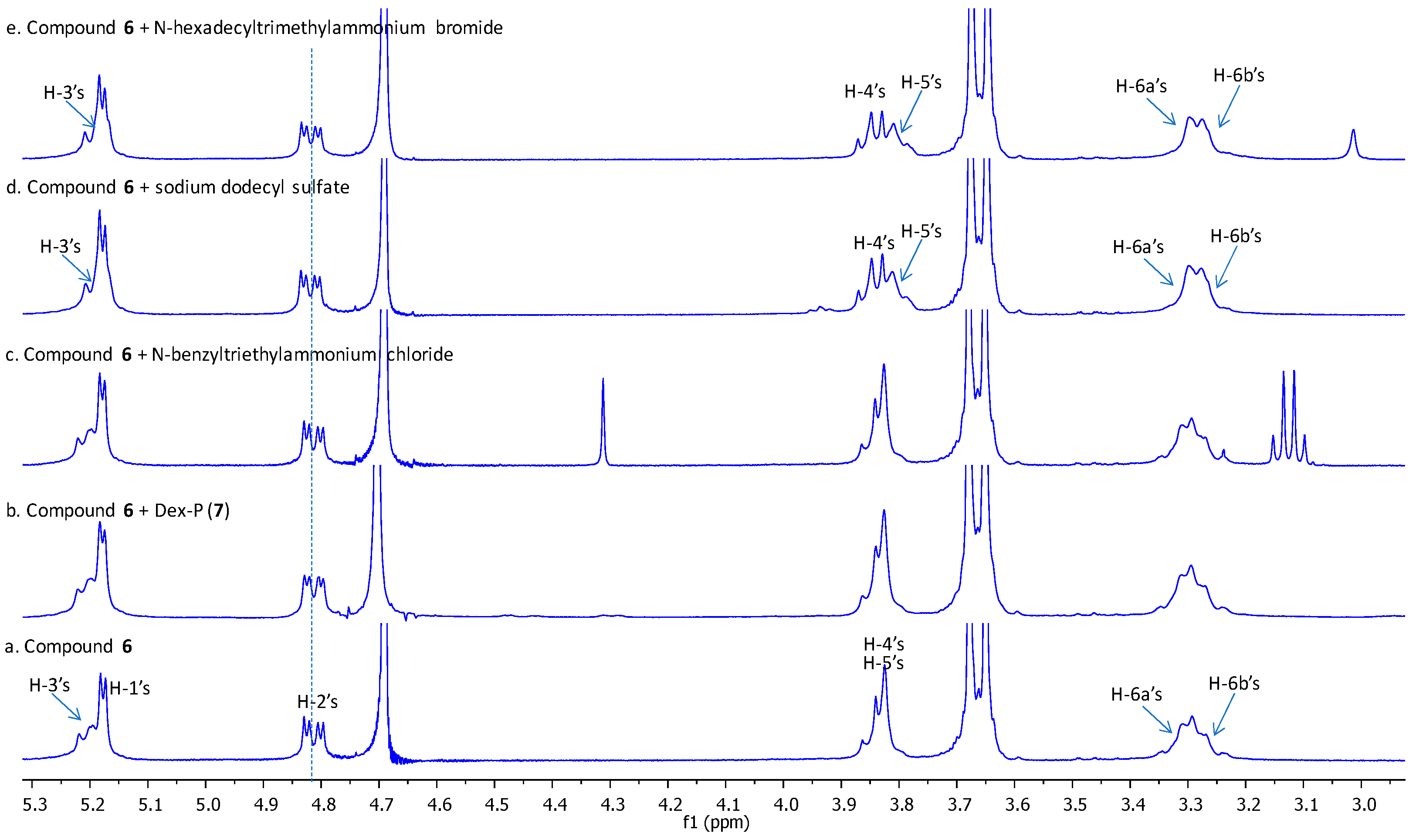
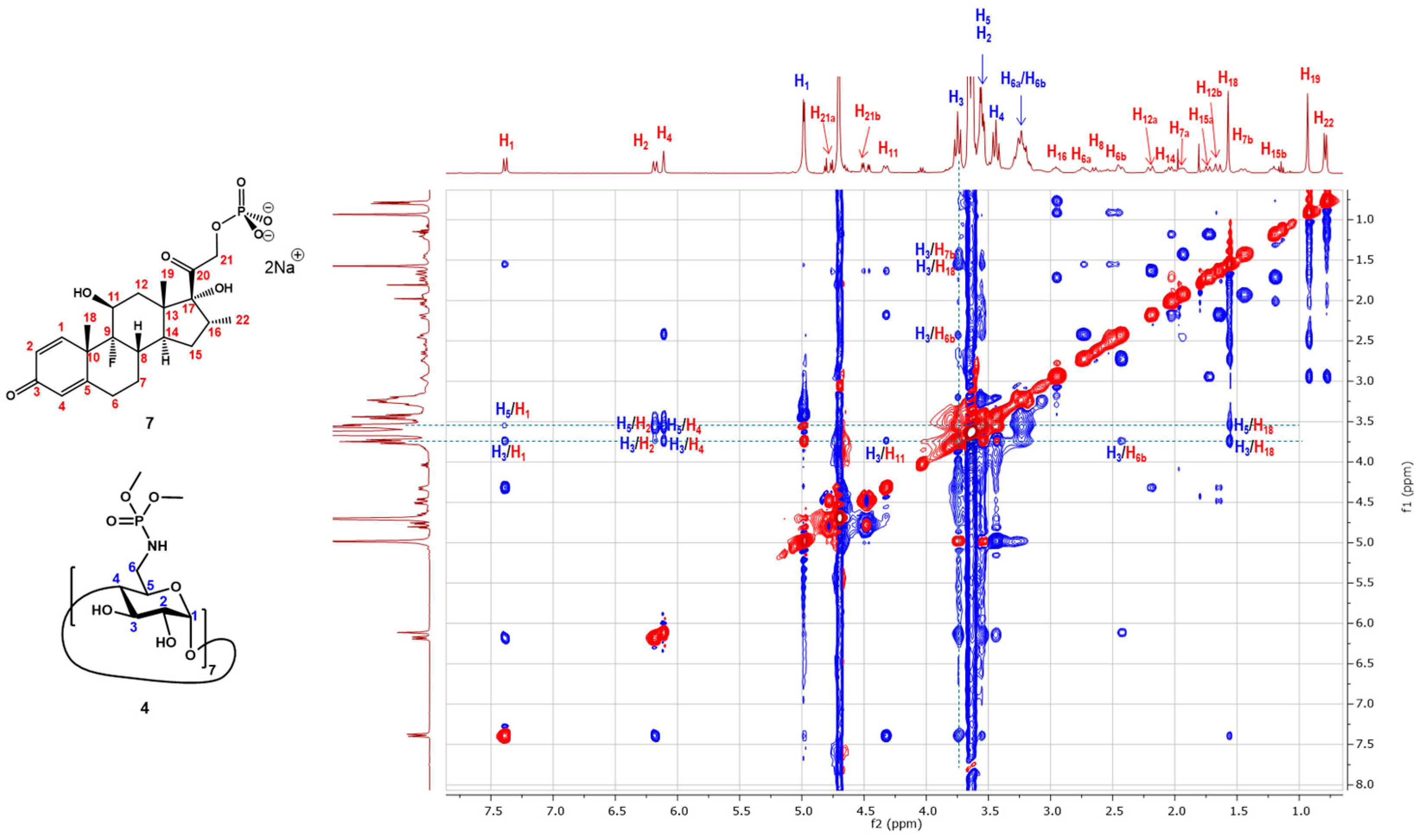
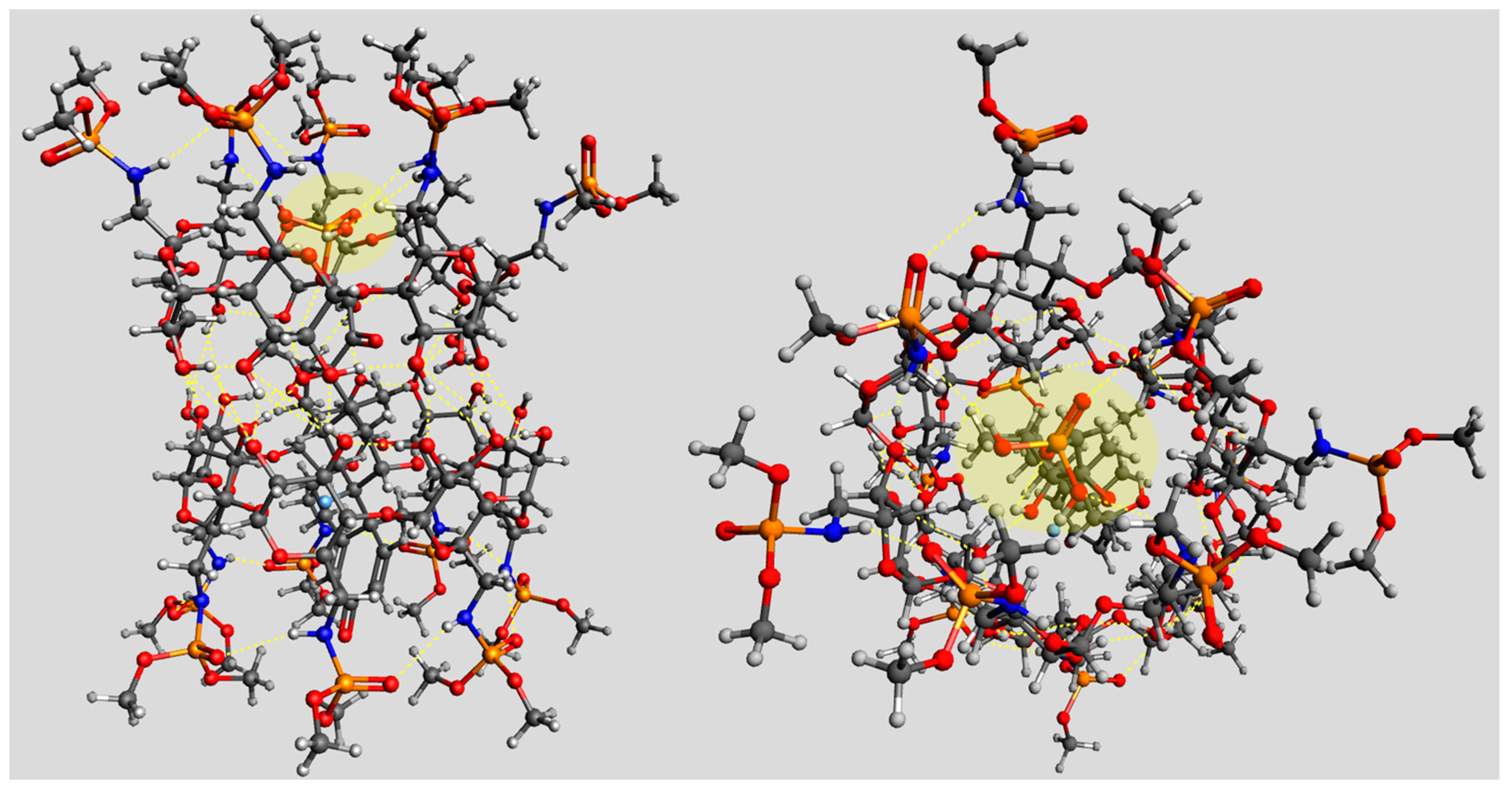
2.2. Inclusion Studies
3. Conclusions
4. Materials and Methods
4.1. Chemical Synthesis
4.2. Isothermal Calorimetry
4.3. Molecular Model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, L.; Zhou, L.; Sun, X.-Q.; Lin, C.; Wang, L. Dynamic Hydrogels Mediated by Macrocyclic Host–Guest Interactions. J. Mater. Chem. B 2019, 7, 1526–1540. [Google Scholar] [CrossRef]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The Aqueous Supramolecular Chemistry of Cucurbit[n]Urils, Pillar[n]Arenes and Deep-Cavity Cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef]
- Niedzwiecka, A.; Achebe, N.; Ling, C.-C. Glycoclusters and Glycodendrimers. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 263–345. ISBN 978-0-12-822244-7. [Google Scholar]
- Wang, S.; Xu, Z.; Wang, T.; Xiao, T.; Hu, X.-Y.; Shen, Y.-Z.; Wang, L. Warm/Cool-Tone Switchable Thermochromic Material for Smart Windows by Orthogonally Integrating Properties of Pillar[6]Arene and Ferrocene. Nat. Commun. 2018, 9, 1737. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zhou, L.; Xu, L.; Zhong, W.; Zhao, W.; Sun, X.-Q.; Elmes, R.B.P. Dynamic Materials Fabricated from Water Soluble Pillar[n]Arenes Bearing Triethylene Oxide Groups. Chin. Chem. Lett. 2019, 30, 271–276. [Google Scholar] [CrossRef]
- Shimizu, S.; Kito, K.; Sasaki, Y.; Hirai, C. Water-Soluble Calixarenes as New Inverse Phase-Transfer Catalysts.Nucleophilic Substitution of Alkyl and Arylalkyl Halides in Aqueousmedia. Chem. Commun. 1997, 1629–1630. [Google Scholar] [CrossRef]
- Ryu, E.-H.; Zhao, Y. Efficient Synthesis of Water-Soluble Calixarenes Using Click Chemistry. Org. Lett. 2005, 7, 1035–1037. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The Utility of Cyclodextrins for Enhancing Oral Bioavailability. J. Control. Release 2007, 123, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for Selective Modifications of Cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. “Back to the Future”: A New Look at Hydroxypropyl Beta-Cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E.; Derendorf, H.; Bodor, N. 2-Hydroxypropyl-β-Cyclodextrin: Properties and Usage in Pharmaceutical Formulations. PZ Wiss. 1991, 136, 5–10. [Google Scholar]
- Pitha, J.; Milecki, J.; Fales, H.; Pannell, L.; Uekama, K. Hydroxypropyl-β-Cyclodextrin: Preparation and Characterization; Effects on Solubility of Drugs. Int. J. Pharm. 1986, 29, 73–82. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, C.; Mao, J.; Yu, Y. A Facile and Practical Approach to Randomly Methylated β-Cyclodextrin. J. Chem. Technol. Biotechnol. 2010, 85, 248–251. [Google Scholar] [CrossRef]
- Bucur, S.; Niculaua, M.; Ciobanu, C.I.; Lungu, N.C.; Mangalagiu, I. A Simple Synthesis Route for Selectively Methylated β-Cyclodextrin Using a Copper Complex Sandwich Protecting Strategy. Molecules 2021, 26, 5669. [Google Scholar] [CrossRef]
- Luna, E.A.; Bornancini, E.R.N.; Thompson, D.O.; Rajewski, R.A.; Stella, V.J. Fractionation and Characterization of 4-Sulfobutyl Ether Derivatives of Cyclomaltoheptaose (β-Cyclodextrin). Carbohydr. Res. 1997, 299, 103–110. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kothawade, R.V.; Markad, A.R.; Pardeshi, S.R.; Kulkarni, A.D.; Chaudhari, P.J.; Longhi, M.R.; Dhas, N.; Naik, J.B.; Surana, S.J.; et al. Sulfobutylether-β-Cyclodextrin: A Functional Biopolymer for Drug Delivery Applications. Carbohydr. Polym. 2023, 301, 120347. [Google Scholar] [CrossRef] [PubMed]
- Puskás, I.; Erzsebet, V.; Tuza, K.; Szemán, J.; Fenyvesi, É.; Sohajda, T.; Szente, L. Sulfobutylether-Cyclodextrins: Structure, Degree of Substitution and Functional Performance; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 293–320. [Google Scholar]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-Cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef]
- Fenyvesi, É.; Szemán, J.; Csabai, K.; Malanga, M.; Szente, L. Methyl-Beta-Cyclodextrins: The Role of Number and Types of Substituents in Solubilizing Power. J. Pharm. Sci. 2014, 103, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Zia, V.; Rajewski, R.A.; Stella, V.J. Thermodynamics of Binding of Neutral Molecules to Sulfobutyl Ether β-Cyclodextrins (SBE-β-CDs): The Effect of Total Degree of Substitution. Pharm. Res. 2000, 17, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Bragagni, M.; Maestrelli, F.; Mura, P. Physico-Chemical Characterization in Solution and in the Solid State of Clonazepam Complexes with Native and Chemically-Modified Cyclodextrins. J. Pharm. Biomed. Anal. 2014, 89, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Itumoh, E.J.; Data, S.; Leitao, E.M. Opening up the Toolbox: Synthesis and Mechanisms of Phosphoramidates. Molecules 2020, 25, 3684. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, C.; Pathirana, R.N.; Balzarini, J.; De Clercq, E. Intracellular Delivery of Bioactive AZT Nucleotides by Aryl Phosphate Derivatives of AZT. J. Med. Chem. 1993, 36, 1048–1052. [Google Scholar] [CrossRef]
- Zlatev, I.; Giraut, A.; Morvan, F.; Herdewijn, P.; Vasseur, J.-J. δ-Di-Carboxybutyl Phosphoramidate of 2′-Deoxycytidine-5′-Monophosphate as Substrate for DNA Polymerization by HIV-1 Reverse Transcriptase. Bioorg. Med. Chem. 2009, 17, 7008–7014. [Google Scholar] [CrossRef] [PubMed]
- Derudas, M.; Carta, D.; Brancale, A.; Vanpouille, C.; Lisco, A.; Margolis, L.; Balzarini, J.; McGuigan, C. The Application of Phosphoramidate Protide Technology to Acyclovir Confers Anti-HIV Inhibition. J. Med. Chem. 2009, 52, 5520–5530. [Google Scholar] [CrossRef]
- Bondada, L.; Detorio, M.; Bassit, L.; Tao, S.; Montero, C.M.; Singletary, T.M.; Zhang, H.; Zhou, L.; Cho, J.-H.; Coats, S.J.; et al. Adenosine Dioxolane Nucleoside Phosphoramidates As Antiviral Agents for Human Immunodeficiency and Hepatitis B Viruses. ACS Med. Chem. Lett. 2013, 4, 747–751. [Google Scholar] [CrossRef]
- Modolo, L.V.; da-Silva, C.J.; Brandão, D.S.; Chaves, I.S. A Minireview on What We Have Learned about Urease Inhibitors of Agricultural Interest since Mid-2000s. J. Adv. Res. 2018, 13, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.J.; Sanmartín, C.; Font, M.; Palop, J.A.; San Francisco, S.; Urrutia, O.; Houdusse, F.; García-Mina, J.M. Design, Synthesis, and Biological Evaluation of Phosphoramide Derivatives as Urease Inhibitors. J. Agric. Food Chem. 2008, 56, 3721–3731. [Google Scholar] [CrossRef]
- Kang, J.; Zettel, V.H.; Ward, N.I. The Organophosphate Pesticides. J. Nutr. Environ. Med. 1995, 5, 325–339. [Google Scholar] [CrossRef]
- Neely, W.B.; Whitney, W.K. Statistical Analysis of Insectiscidal Activity in a Series of Phosphoramidates. J. Agric. Food Chem. 1968, 16, 571–573. [Google Scholar] [CrossRef]
- Neisius, M.; Liang, S.; Mispreuve, H.; Gaan, S. Phosphoramidate-Containing Flame-Retardant Flexible Polyurethane Foams. Ind. Eng. Chem. Res. 2013, 52, 9752–9762. [Google Scholar] [CrossRef]
- Nguyen, T.-M.; Chang, S.; Condon, B.; Slopek, R.; Graves, E.; Yoshioka-Tarver, M. Structural Effect of Phosphoramidate Derivatives on the Thermal and Flame Retardant Behaviors of Treated Cotton Cellulose. Ind. Eng. Chem. Res. 2013, 52, 4715–4724. [Google Scholar] [CrossRef]
- Che, A.; Duong, J.; Ling, C.-C. Synthesis of β-Cyclodextrin-Based per-6-Phosphoramidates. Tetrahedron 2023, 149, 133723. [Google Scholar] [CrossRef]
- Christoforides, E.; Papaioannou, A.; Bethanis, K. Crystal Structure of the Inclusion Complex of Cholesterol in β-Cyclodextrin and Molecular Dynamics Studies. Beilstein J. Org. Chem. 2018, 14, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.H.; Szeleszczuk, Ł.; Bethanis, K.; Christoforides, E.; Dudek, M.K.; Zielińska-Pisklak, M.; Pisklak, D.M. 17-β-Estradiol—β-Cyclodextrin Complex as Solid: Synthesis, Structural and Physicochemical Characterization. Molecules 2023, 28, 3747. [Google Scholar] [CrossRef]
- Lindner, K.; Saenger, W. Crystal and Molecular Structure of Cyclohepta-Amylose Dodecahydrate. Carbohydr. Res. 1982, 99, 103–115. [Google Scholar] [CrossRef]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool. Version 1.2.0n. Available online: http://avogadro.cc/ (accessed on 26 May 2024).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 09, Revision A.02, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian, Inc., Wallingford CT, 2016. Available online: https://gaussian.com/g09citation/ (accessed on 26 May 2024).



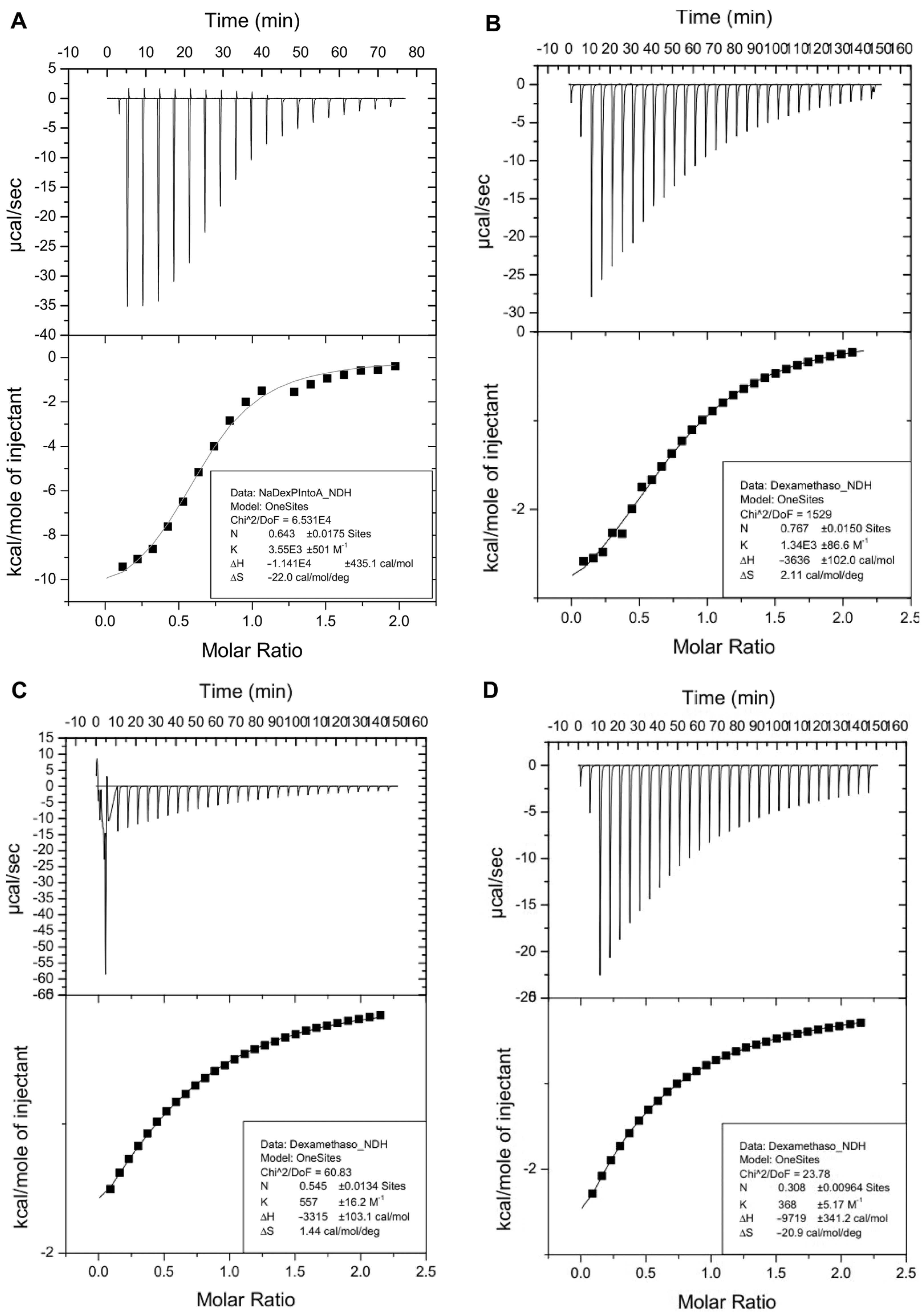

| 4 | HPBCD | SBEBCD | RMBCD | |
|---|---|---|---|---|
| Ka (M−1) | 3.6 × 103 | 1.3 × 103 | 3.7 × 102 | 5.6 × 102 |
| ΔH° (kcal/mol) | −11.4 | −3.6 | −9.7 | −3.3 |
| ΔS° (cal/(mol·K)) | −22.0 | 2.1 | −20.9 | 1.4 |
| ΔG° (kcal/mol) | −4.8 | −4.3 | −3.5 | −3.8 |
| 4 | HPBCD | SBEBCD | |
|---|---|---|---|
| Ka (M−1) | 1.2 × 103 | 4.0 × 103 | 1.9 × 103 |
| ΔH° (kcal/mol) | −11.2 | −1.7 | −1.7 |
| ΔS° (cal/mol·K) | −23.5 | 10.8 | 9.2 |
| ΔG° (kcal/mol) | −4.2 | −4.9 | −4.5 |
| 4 | HPBCD | SBEBCD | |
|---|---|---|---|
| Ka (M−1) | 4.6 × 103 | 1.6 × 103 | 1.2 × 103 |
| ΔH° (kcal/mol) | −13.0 | −3.4 | −3.4 |
| ΔS° (cal/(mol·K)) | −26.9 | 3.4 | 7.2 |
| ΔG° (kcal/mol) | −5.0 | −4.4 | −5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, A.; Espejo, J.; Ling, C.-C. Synthesis and Inclusion Properties of a β-Cyclodextrin Heptaphosphoramidate. Molecules 2024, 29, 2714. https://doi.org/10.3390/molecules29122714
Che A, Espejo J, Ling C-C. Synthesis and Inclusion Properties of a β-Cyclodextrin Heptaphosphoramidate. Molecules. 2024; 29(12):2714. https://doi.org/10.3390/molecules29122714
Chicago/Turabian StyleChe, Austin, Jayar Espejo, and Chang-Chun Ling. 2024. "Synthesis and Inclusion Properties of a β-Cyclodextrin Heptaphosphoramidate" Molecules 29, no. 12: 2714. https://doi.org/10.3390/molecules29122714
APA StyleChe, A., Espejo, J., & Ling, C.-C. (2024). Synthesis and Inclusion Properties of a β-Cyclodextrin Heptaphosphoramidate. Molecules, 29(12), 2714. https://doi.org/10.3390/molecules29122714









