Effect of Calcination Atmosphere on the Performance of Cu/Al2O3 Catalyst for the Selective Hydrogenation of Furfural to Furfuryl Alcohol
Abstract
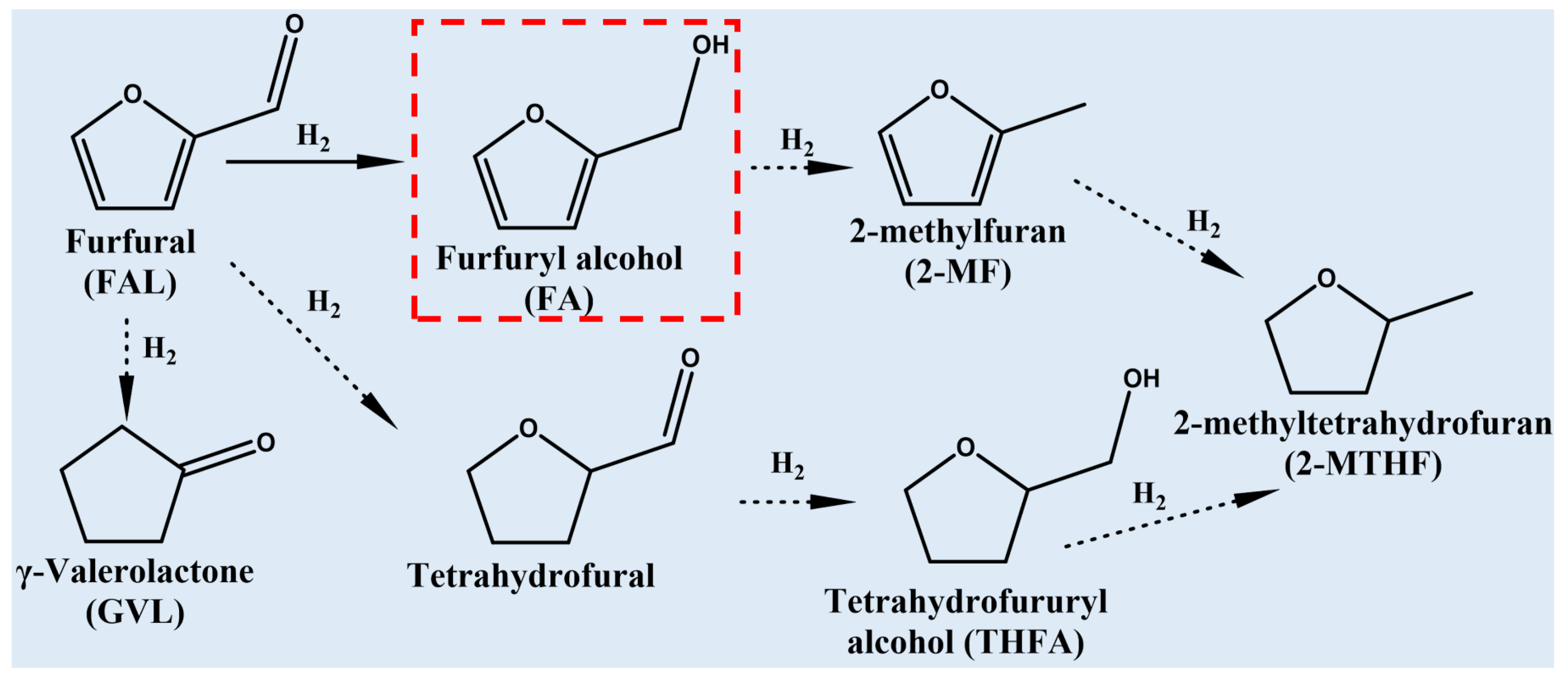
:1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalyst Evaluation
3. Results and Discussion
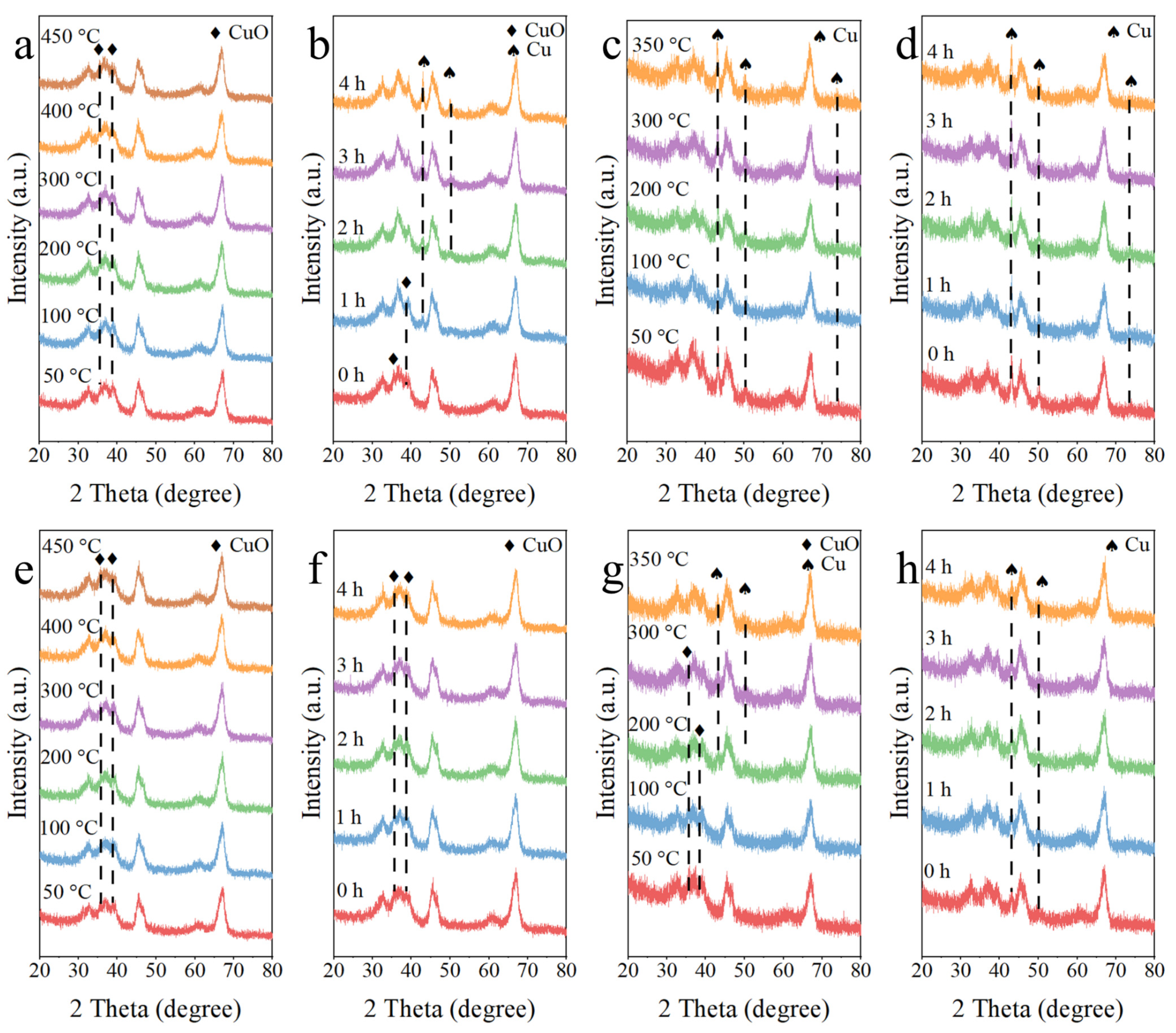
3.1. Structure and Morphology
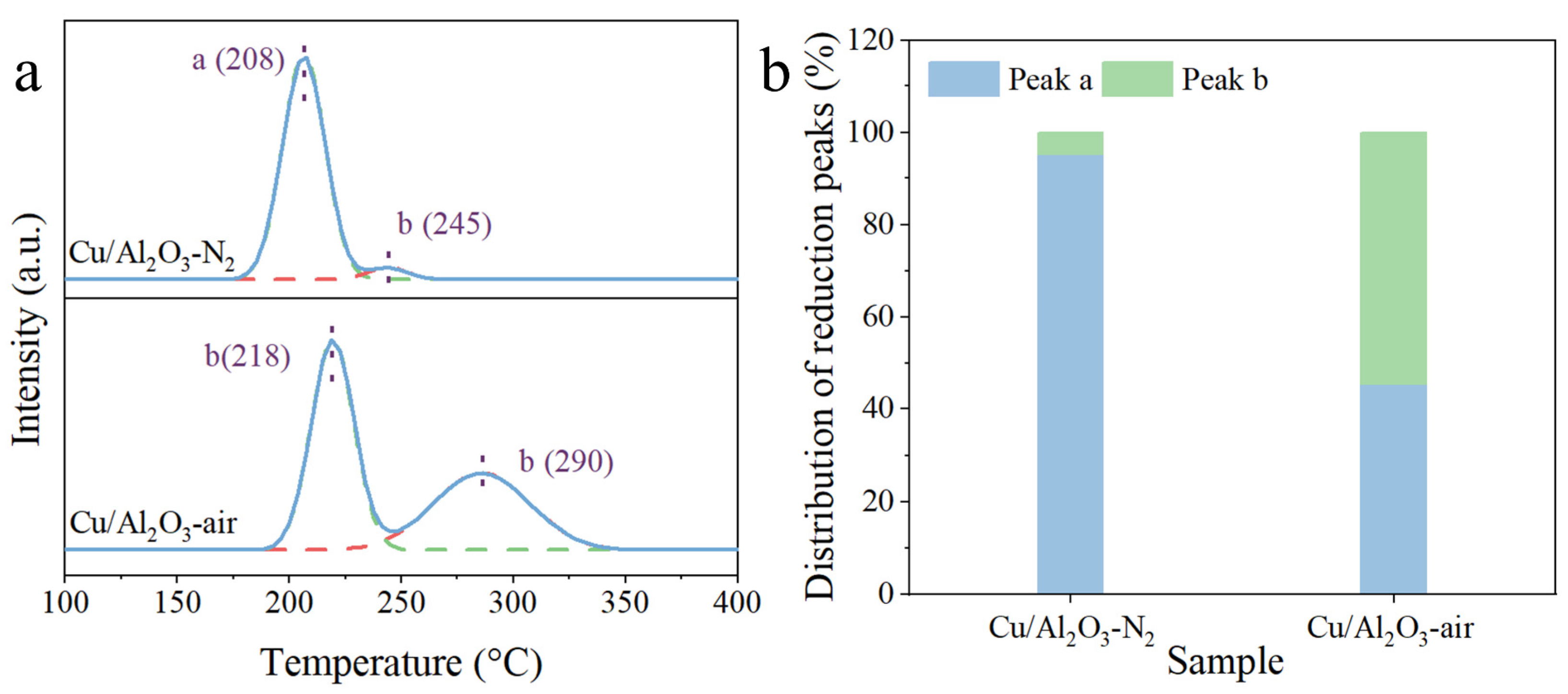
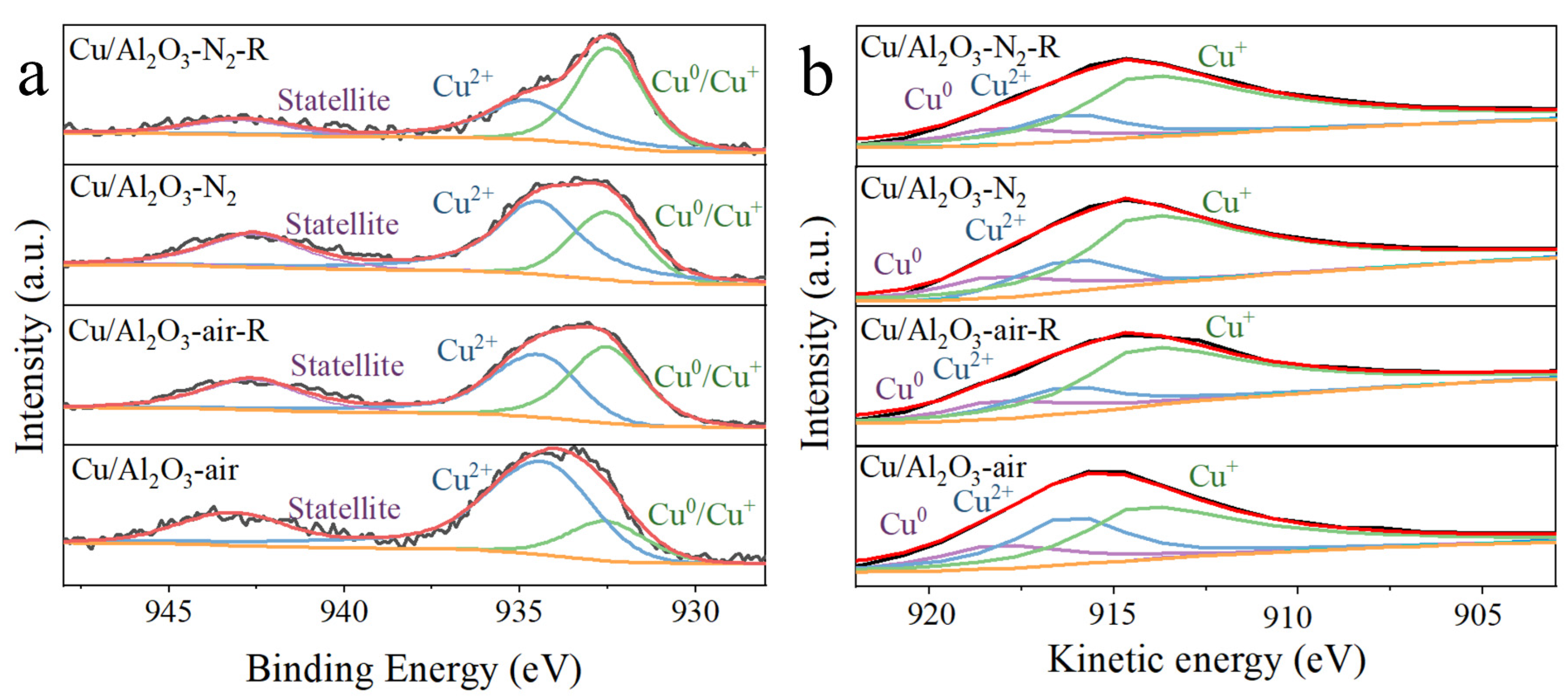
3.2. Surface Chemical State
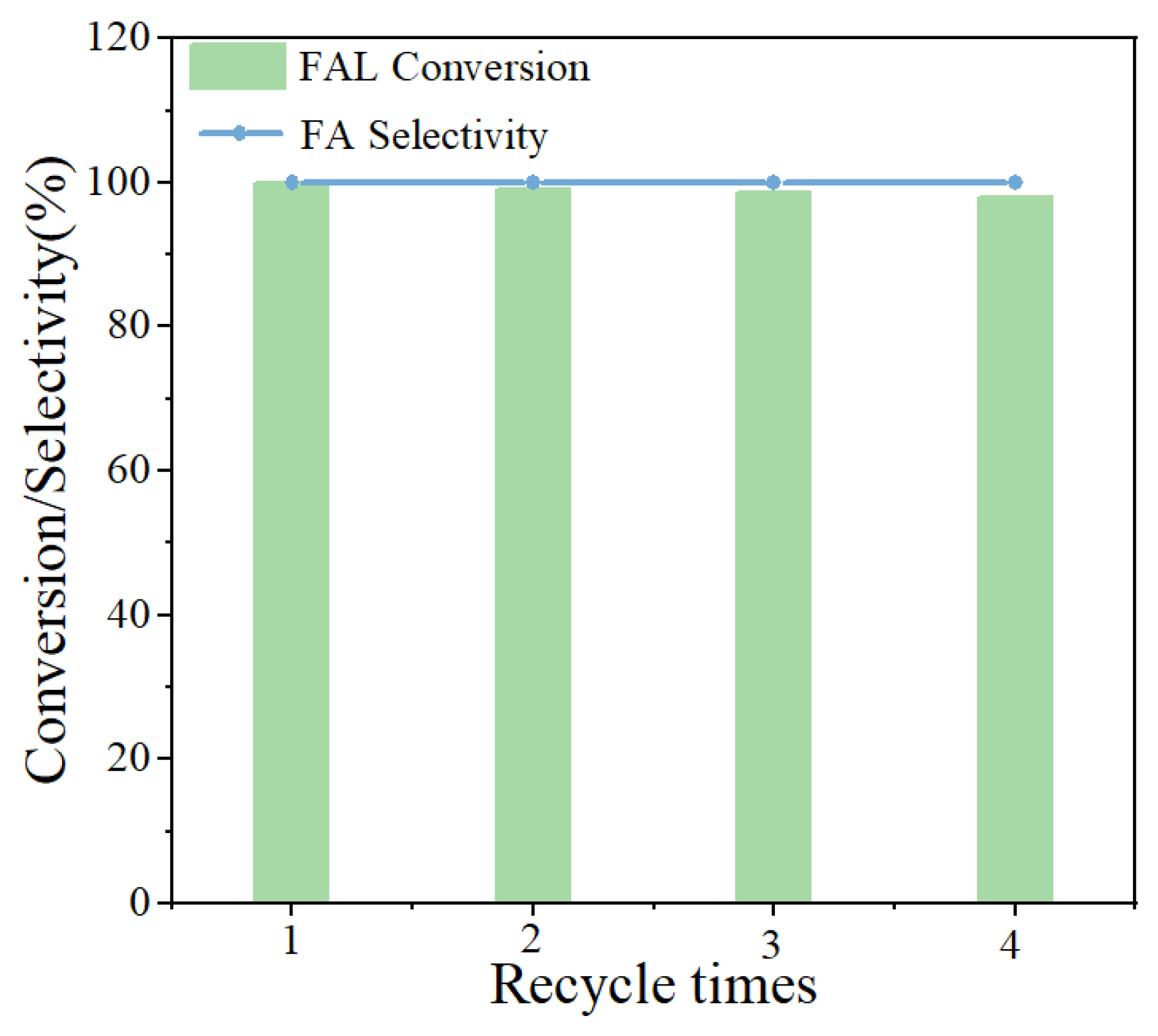
3.3. Catalytic Performance
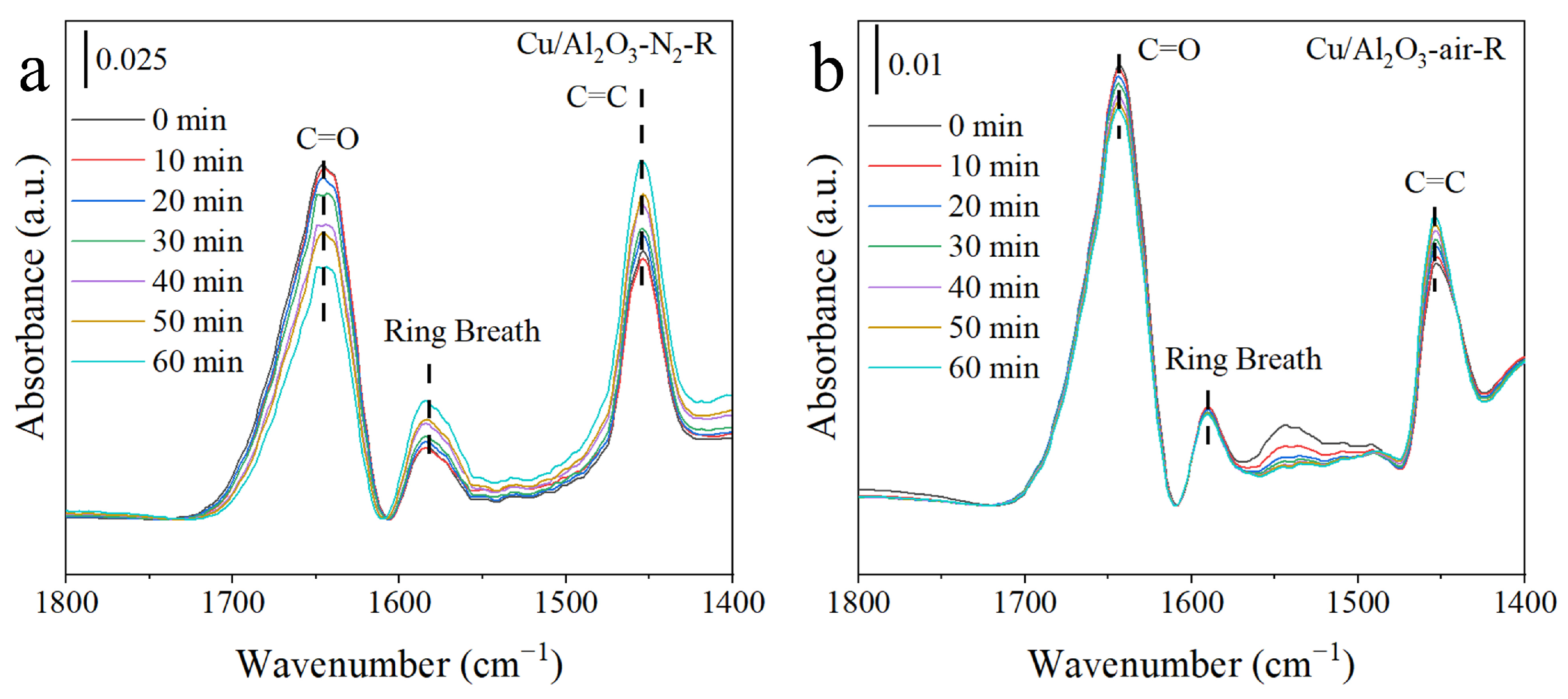
3.4. Reaction Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, Z.; Li, J. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts. Green Chem. 2022, 24, 1780–1808. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- He, D.; Li, T.; Dai, X.; Liu, S.; Cui, X.; Shi, F. Construction of highly active and selective molecular imprinting catalyst for hydrogenation. J. Am. Chem. Soc. 2023, 145, 20813–20824. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural-a versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent advances in the conversion of furfural into bio-chemicals through chemo- and bio-catalysis. RSC Adv. 2021, 11, 27042–27058. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Liu, Q.; Zhang, Q.; Wang, T.; Ma, L. High yield production of 5-hydroxymethylfurfural from cellulose by high concentration of sulfates in biphasic system. Green Chem. 2013, 15, 1967–1974. [Google Scholar] [CrossRef]
- Chen, H.; Ruan, H.; Lu, X.; Fu, J.; Langrish, T.; Lu, X. Efficient catalytic transfer hydrogenation of furfural to furfuryl alcohol in near-critical isopropanol over Cu/MgO-Al2O3 catalyst. Mol. Catal. 2018, 445, 94–101. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Ranaware, V.; Kurniawan, R.G.; Verma, D.; Kwak, S.K.; Ryu, B.C.; Kang, J.W.; Kim, J. Solvent-mediated selectivity control of furfural hydrogenation over a N-doped carbon-nanotube-supported Co/CoOx catalyst. Appl. Catal., B 2022, 318, 121838. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Chen, H.; Ding, J.; Liang, H.; Yu, H. Synthesis and application of sustainable furfuryl alcohol-based plasticizer. ChemistrySelect 2020, 5, 4085–4090. [Google Scholar] [CrossRef]
- Merat, N.; Godawa, C.; Gaset, A. High selective production of tetrahydrofurfuryl alcohol: Catalytic hydrogenation of furfural and furfuryl alcohol. J. Chem. Technol. Biotechnol. 1990, 48, 145–159. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Durán-Martín, D.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Mariscal, R.; Maireles-Torres, P. Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu/ZnO catalysts. J. Catal. 2016, 336, 107–115. [Google Scholar] [CrossRef]
- Arundhathi, R.; Reddy, P.L.; Samanta, C.; Newalkar, B.L. Chromium-free Cu@Mg/γ-Al2O3-an active catalyst for selective hydrogenation of furfural to furfuryl alcohol. RSC Adv. 2020, 10, 41120–41126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Zhang, C.; Arai, M.; Zhou, L.; Zhao, F. Selective hydrogenation of furfural to furfuryl alcohol over Pd/TiH2 catalyst. Mol. Catal. 2021, 508, 111599. [Google Scholar] [CrossRef]
- Ruan, L.; Zhu, L.; Zhang, X.; Guo, G.; Shang, C.; Hui Chen, B.; Guo, Z. Porous SiO2 nanosphere-supported PtCuCo trimetallic nanoparticles for highly efficient and selective furfural hydrogenation. Fuel 2023, 335, 126935. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.; Yuan, Q.; Zhang, P.; Guan, Y. Selective hydrogenation of furfural on Ru/Al-MIL-53: A comparative study on the effect of aromatic and aliphatic organic linkers. RSC Adv. 2016, 6, 92299–92304. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.-L.; Zhou, H.-j.; Fu, Y. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over nitrogen-doped carbon-supported iron catalysts. ChemSusChem 2016, 9, 1339–1347. [Google Scholar] [CrossRef]
- Ruan, L.; Pei, A.; Liao, J.; Zeng, L.; Guo, G.; Yang, K.; Zhou, Q.; Zhao, N.; Zhu, L.; Chen, B.H. Nitrogen-doped carbon nanotubes-supported PdNiCo nanoparticles as a highly efficient catalyst for selective hydrogenation of furfural. Fuel 2021, 284, 119015. [Google Scholar] [CrossRef]
- Sheng, Y.; Tian, F.; Wang, X.; Jiang, N.; Zhang, X.; Chen, X.; Liang, C.; Wang, A. Carbon-encapsulated Ni catalysts derived from citrate complexes for highly efficient hydrogenation of furfural to tetrahydrofurfuryl alcohol. Energy 2024, 292, 130360. [Google Scholar] [CrossRef]
- Islam, M.J.; Granollers Mesa, M.; Osatiashtiani, A.; Taylor, M.J.; Manayil, J.C.; Parlett, C.M.A.; Isaacs, M.A.; Kyriakou, G. The effect of metal precursor on copper phase dispersion and nanoparticle formation for the catalytic transformations of furfural. Appl. Catal. B 2020, 273, 119062. [Google Scholar] [CrossRef]
- Li, J.; Niu, X.; Zhu, Y. Synergistic effect of surface Cu0 and Cu+ species on improved selective hydrogenation of furfural to furfuryl alcohol over hydrotalcite-derived CuxMg3Al oxides. Appl. Surf. Sci. 2023, 635, 157774. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Yu, S.; Liu, S.; Li, L.; Xie, C.; Wu, Q.; Zhang, Y.; Yu, H.; Liu, Y.; et al. Regulating the Cu0-Cu+ ratio to enhance metal-support interaction for selective hydrogenation of furfural under mild conditions. Chem. Eng. J. 2023, 468, 143955. [Google Scholar] [CrossRef]
- Yi, W.-J.; Gao, Y.; Yang, J.; Zhou, X.; Liu, Z.; Zhang, M. Synergistic effect of surface Cu0 and Cu+ species over hydrotalcite-derived CuxCo3-xAlOy mixed-metal oxides toward efficient hydrogenation of furfural to furfuryl alcohol. Appl. Surf. Sci. 2023, 641, 158559. [Google Scholar] [CrossRef]
- Tan, J.; He, J.; Gao, K.; Zhu, S.; Cui, J.; Huang, L.; Zhu, Y.; Zhao, Y. Catalytic hydrogenation of furfural over Cu/CeO2 catalyst: The effect of support morphology and exposed facet. Appl. Surf. Sci. 2022, 604, 154472. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Alba-Rubio, A.C.; Cassidy, A.; Moreno-Tost, R.; García-Sancho, C.; Maireles-Torres, P. Tailoring the selectivity of Cu-based catalysts in the furfural hydrogenation reaction: Influence of the morphology of the silica support. Fuel 2022, 319, 123827. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Gu, B.; Tang, Q.; Cao, Q.-E.; Fang, W. Regulating the interaction within Pd-Cu dual metal sites for selective hydrogenation of furfural using ambient H2 pressure. ACS Sustain Chem. Eng. 2023, 11, 12798–12808. [Google Scholar] [CrossRef]
- Kumar, A.; Bal, R.; Srivastava, R. Modulation of Ru and Cu nanoparticle contents over CuAlPO-5 for synergistic enhancement in the selective reduction and oxidation of biomass-derived furan based alcohols and carbonyls. Catal. Sci. Technol. 2021, 11, 4133–4148. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]
- Wang, S.; Lv, Y.; Ren, J.; Xu, Z.; Yang, Q.; Zhao, H.; Gao, D.; Chen, G. Ultrahigh selective hydrogenation of furfural enabled by modularizing hydrogen dissociation and substrate activation. ACS Catal. 2023, 13, 8720–8730. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, R.; Yang, X.; Fang, Y.-X. Potassium carbonate (K2CO3)-assisted copper-catalyzed liquid-phase hydrogenation of furfural: Striking promotion synergy enables a superior high furfuryl alcohol yield at mild reaction conditions. Ind. Eng. Chem. Res. 2022, 61, 16643–16652. [Google Scholar] [CrossRef]
- Cheng, S.; Lei, Q.; Deng, C.; Liang, L.; Chen, Y.; Meng, H.; Lei, W.; Chen, H. Mild and selective transfer hydrogenation of biomass-derived furfural to furfuryl alcohol over Cu/ZnO/Al2O3 with methanediol as the hydrogen donor. Green Chem. 2024. Advance Article. [Google Scholar] [CrossRef]
- Lee, J.; Seo, J.H.; Nguyen-Huy, C.; Yang, E.; Lee, J.G.; Lee, H.; Jang, E.J.; Kwak, J.H.; Lee, J.H.; Lee, H.; et al. Cu2O(100) surface as an active site for catalytic furfural hydrogenation. Appl. Catal. B 2021, 282, 119576. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Y.; Yang, M.; Wu, Y. Effects of pretreatment atmospheres on the catalytic performance of Pd/γ-Al2O3 catalyst in benzene degradation. Catal. Commun. 2014, 46, 11–16. [Google Scholar] [CrossRef]
- Gao, X.; Ashok, J.; Kawi, S. A review on roles of pretreatment atmospheres for the preparation of efficient Ni-based catalysts. Catal. Today 2022, 397–399, 581–591. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Shi, D.; Jia, X.; Wang, X.; Borgna, A.; Lau, R.; Yang, Y. Methane reforming with carbon dioxide over a Ni/ZiO2-SiO2 catalyst: Influence of pretreatment gas atmospheres. Int. J. Hydrogen Energy 2012, 37, 10135–10144. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, L.; Ye, R.-P.; Sun, M.-L.; Yang, J.-X.; Li, F.; Yao, Y.-G. Mannitol as a novel dopant for Cu/SiO2: A low-cost, environmental and highly stable catalyst for dimethyl oxalate hydrogenation without hydrogen prereduction. J. Catal. 2020, 389, 421–431. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, X.; Wang, H.; Li, Z.; Wang, W.; Yu, Q.; Zheng, L.; Wang, Y.; Zhu, J.; Wei, M. ZrO2−x modified Cu nanocatalysts with synergistic catalysis towards carbon-oxygen bond hydrogenation. Appl. Catal. B 2021, 280, 119406. [Google Scholar] [CrossRef]
- Cui, G.; Meng, X.; Zhang, X.; Wang, W.; Xu, S.; Ye, Y.; Tang, K.; Wang, W.; Zhu, J.; Wei, M.; et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid-base sites. Appl. Catal. B 2019, 248, 394–404. [Google Scholar] [CrossRef]
- Kurniawan, R.G.; Karanwal, N.; Park, J.; Verma, D.; Kwak, S.K.; Kim, S.K.; Kim, J. Direct conversion of furfural to 1,5-pentanediol over a nickel-cobalt oxide-alumina trimetallic catalyst. Appl. Catal., B 2023, 320, 121971. [Google Scholar] [CrossRef]
- Gao, G.; Remón, J.; Jiang, Z.; Yao, L.; Hu, C. Selective hydrogenation of furfural to furfuryl alcohol in water under mild conditions over a hydrotalcite-derived Pt-based catalyst. Appl. Catal. B 2022, 309, 121260. [Google Scholar] [CrossRef]
- Thongratkaew, S.; Luadthong, C.; Kiatphuengporn, S.; Khemthong, P.; Hirunsit, P.; Faungnawakij, K. Cu-Al spinel-oxide catalysts for selective hydrogenation of furfural to furfuryl alcohol. Catal. Today 2021, 367, 177–188. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, Y.; Chen, L.; Wang, L.; Shi, Y.; Yin, P.; Wang, W.; Shao, M.; Zhang, X.; Wei, M. Synergetic effect of Cu0-Cu+ derived from layered double hydroxides toward catalytic transfer hydrogenation reaction. Appl. Catal. B 2022, 314, 121515. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, S.; Chen, Y.; Wei, Y.; Zhang, M.; Li, C.; Sun, Y.; Zhang, S.; Jiang, Y. Epitaxial growth of Cu2−xSe on Cu (220) crystal plane as high property anode for sodium storage. Chin. Chem. Lett. 2023, 35, 108922. [Google Scholar] [CrossRef]
- Islam, M.J.; Granollers Mesa, M.; Osatiashtiani, A.; Manayil, J.C.; Isaacs, M.A.; Taylor, M.J.; Tsatsos, S.; Kyriakou, G. PdCu single atom alloys supported on alumina for the selective hydrogenation of furfural. Appl. Catal. B 2021, 299, 120652. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M. A control over hydrogenation selectivity of furfural via tuning exposed facet of Ni catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial structure-determined reaction pathway and selectivity for 5-(hydroxymethyl)furfural hydrogenation over Cu-based catalysts. ACS Catal. 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Q.; Dong, X.; Wang, J.; Sun, K.; Zhang, L.; Zhang, S.; Xu, L.; Yuan, X.; Hu, X. Cooperation between hydrogenation and acidic sites in Cu-based catalyst for selective conversion of furfural to γ-valerolactone. Fuel 2021, 293, 120457. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, X.; Cui, H.; Zhu, M.; Hao, F.; Xiong, W.; Luo, H.; Lv, Y.; Liu, P. Efficient Cu-Co bimetallic catalysts for the selective hydrogenation of furfural to furfuryl alcohol. Fuel 2023, 351, 128887. [Google Scholar] [CrossRef]
- Park, S.; Kannapu, H.P.R.; Jeong, C.; Kim, J.; Suh, Y.-W. Highly active mesoporous Cu-Al2O3 catalyst for the hydrodeoxygenation of furfural to 2-methylfuran. ChemCatChem 2020, 12, 105–111. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.; Qu, Z. Cu/ZnAl2O4 catalysts prepared by ammonia evaporation method: Improving methanol selectivity in CO2 hydrogenation via regulation of metal-support interaction. Chem. Eng. J. 2023, 469, 144008. [Google Scholar] [CrossRef]
- Fang, W.; Liu, S.; Steffensen, A.K.; Schill, L.; Kastlunger, G.; Riisager, A. On the role of Cu+ and CuNi alloy phases in mesoporous CuNi catalyst for furfural hydrogenation. ACS Catal. 2023, 13, 8437–8444. [Google Scholar] [CrossRef]
- Luo, L.; Yuan, F.; Zaera, F.; Zhu, Y. Catalytic hydrogenation of furfural to furfuryl alcohol on hydrotalcite-derived CuxNi3−xAlOy mixed-metal oxides. J. Catal. 2021, 404, 420–429. [Google Scholar] [CrossRef]
- Chen, S.; de Souza, P.M.; Ciotonea, C.; Marinova, M.; Dumeignil, F.; Royer, S.; Wojcieszak, R. Micro-/mesopores confined ultrasmall Cu nanoparticles in SBA-15 as a highly efficient and robust catalyst for furfural hydrogenation to furfuryl alcohol. Appl. Catal. A 2022, 633, 118527. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Cui, Z.; Yu, X.; Zhang, X.; Li, Y.; Zhang, Q.; Chen, L.; Ma, L. In situ synthesis of Cu nanoparticles on carbon for highly selective hydrogenation of furfural to furfuryl alcohol by using pomelo peel as the carbon source. ACS Sustain. Chem. Eng. 2020, 8, 12944–12955. [Google Scholar] [CrossRef]
- Ghashghaee, M.; Shirvani, S.; Ghambarian, M. Kinetic models for hydroconversion of furfural over the ecofriendly Cu-MgO catalyst: An experimental and theoretical study. Appl. Catal. A 2017, 545, 134–147. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D. Aqueous phase catalytic hydrogenation of furfural to furfuryl alcohol over in-situ synthesized Cu-Zn/SiO2 catalysts. Mater. Chem. Phys. 2021, 260, 124152. [Google Scholar] [CrossRef]
- Cao, P.; Lin, L.; Qi, H.; Chen, R.; Wu, Z.; Li, N.; Zhang, T.; Luo, W. Zeolite-encapsulated Cu nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol. ACS Catal. 2021, 11, 10246–10256. [Google Scholar] [CrossRef]
- Li, X.-L.; Deng, J.; Shi, J.; Pan, T.; Yu, C.-G.; Xu, H.-J.; Fu, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu-Co catalysts. Green Chem. 2015, 17, 1038–1046. [Google Scholar] [CrossRef]
- Tang, F.; Wang, L.; Dessie Walle, M.; Mustapha, A.; Liu, Y.-N. An alloy chemistry strategy to tailoring the d-band center of Ni by Cu for efficient and selective catalytic hydrogenation of furfural. J. Catal. 2020, 383, 172–180. [Google Scholar] [CrossRef]
- Şebin, M.E.; Akmaz, S.; Koc, S.N. Hydrogenation of furfural to furfuryl alcohol over efficient sol-gel nickel-copper/zirconia catalyst. J. Chem. Sci. 2020, 132, 157. [Google Scholar] [CrossRef]
- Khromova, S.A.; Bykova, M.V.; Bulavchenko, O.A.; Ermakov, D.Y.; Saraev, A.A.; Kaichev, V.V.; Venderbosch, R.H.; Yakovlev, V.A. Furfural hydrogenation to furfuryl alcohol over bimetallic Ni-Cu sol-gel catalyst: A model reaction for conversion of oxygenates in pyrolysis liquids. Top. Catal. 2016, 59, 1413–1423. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Sener, C.; Jackson DH, K.; Kuech, T.F.; Dumesic, J.A. Control of thickness and chemical properties of atomic layer deposition overcoats for stabilizing Cu/γ-Al2O3 catalysts. ChemSusChem 2014, 7, 3247–3251. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Márquez-Rodríguez, I.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Maireles-Torres, P. Gas-phase hydrogenation of furfural over Cu/CeO2 catalysts. Catal. Today 2017, 279, 327–338. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, D.; Li, C.; Wang, S.; Hu, X.; Zheng, G.; Chen, G. Highly dispersed Pt on partial deligandation of Ce-MOFs for furfural selective hydrogenation. Appl. Catal. B 2023, 328, 122458. [Google Scholar] [CrossRef]
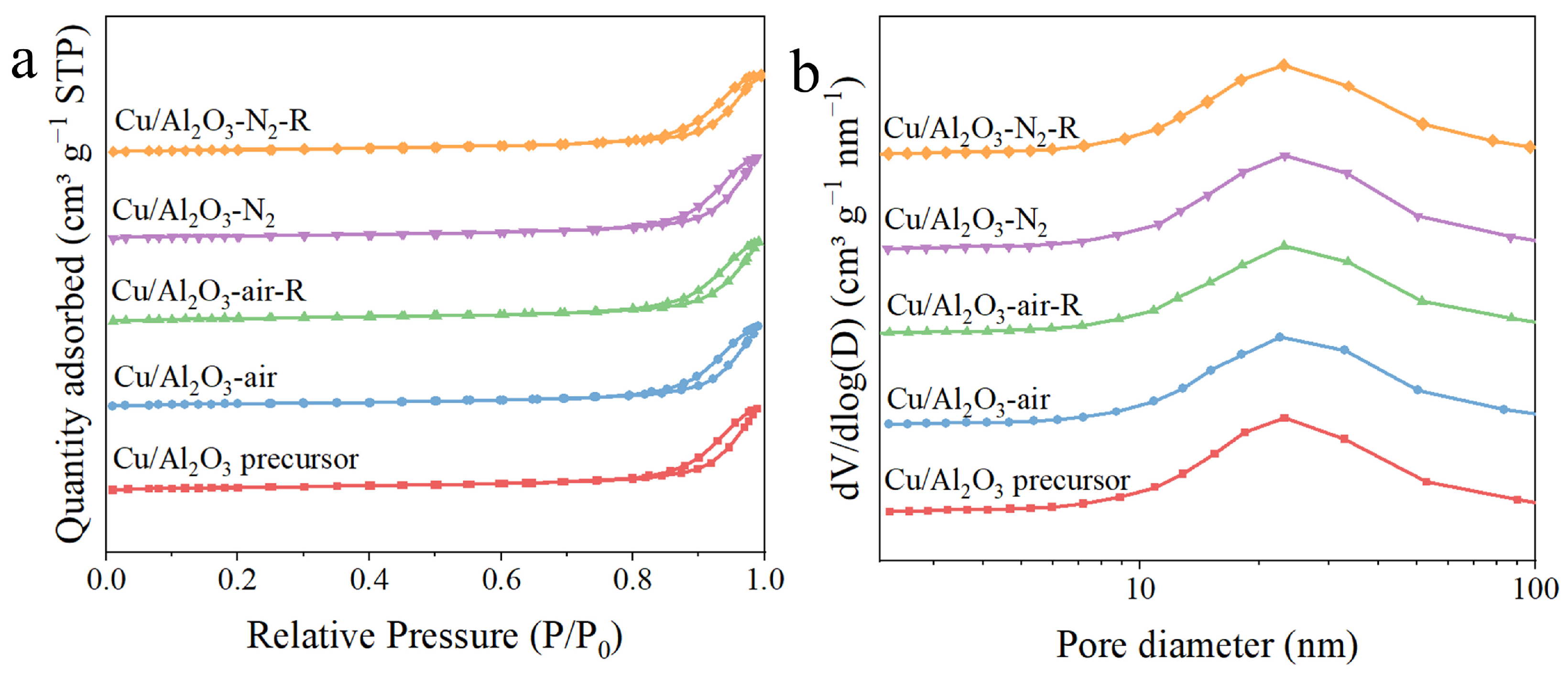

| Sample | SBET (m2/g) a | Vpore (cm3/g) a | Dpore (nm) a | Cu Loading (wt.%) b | DCu (%) c | SCu (m2Cu/gcat) c |
|---|---|---|---|---|---|---|
| Cu/Al2O3 precursor | 131.5 | 0.80 | 20.5 | - | - | - |
| Cu/Al2O3-N2 | 125.1 | 0.80 | 20.7 | 4.3 | - | - |
| Cu/Al2O3-N2-R | 128.5 | 0.76 | 19.9 | 4.3 | 48.3 | 14.1 |
| Cu/Al2O3-air | 125.0 | 0.78 | 21.1 | 4.3 | - | - |
| Cu/Al2O3-air-R | 127.2 | 0.77 | 20.8 | 4.3 | 21.5 | 6.3 |
| Catalyst | (Cu0 + Cu+)/Cu(%) a | Cu+/Cu(%) b | Cu0/Cu(%) b |
|---|---|---|---|
| Cu/Al2O3-N2 | 37.1 | 27.4 | 9.7 |
| Cu/Al2O3-N2-R | 60.8 | 45.3 | 15.5 |
| Cu/Al2O3-air | 21.8 | 13.8 | 8.0 |
| Cu/Al2O3-air-R | 48.8 | 38.6 | 10.2 |
| Entry | Catalyst | FAL Conversion (%) | FA Selectivity (%) | FA Yield (%) |
|---|---|---|---|---|
| 1 | Cu/Al2O3-N2 | 42.7 | 99.9 | 42.7 |
| 2 | Cu/Al2O3-N2-R | 99.9 | 99.9 | 99.9 |
| 3 | Cu/Al2O3-air | 14.3 | 62.2 | 8.9 |
| 4 | Cu/Al2O3-air-R | 26.7 | 85.8 | 22.9 |
| Catalyst | Reaction Conditions | Con. (%) | Sel. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| Cu2Ni1AlOy | 120 °C, 1.6 MPa H2, 1.5 h | 98.0 | 99.0 | 97.0 | [58] |
| 10Cu-MCM-41-GLY | 150 °C, 2 MPa H2, 2 h | 98.1 | 80.0 | 78.0 | [59] |
| Cu/SiO2 | 110 °C, 1 MPa H2, 4 h | 66.0 | 100.0 | 66.0 | [60] |
| Cu/C-400 | 170 °C, 2 MPa H2, 3 h | 99.6 | 99.3 | 98.9 | [61] |
| Cu/MgO | 180 °C, 0.9 MPa | 95.0 | 94.0 | 89.3 | [62] |
| CuAlOx | 220 °C, 3.5 MPa H2, 5 h | 91.0 | 98.4 | 89.5 | [63] |
| Na-Cu@TS-1 | 110 °C, 1 MPa H2, 2 h | 93.0 | 98.1 | 91.2 | [64] |
| Cu/Co3O4 | 170 °C, 2 MPa H2, 1 h | 100.0 | 67.0 | 67.0 | [65] |
| NiCu0.33/C | 120 °C, 1.5 MPa H2, 12 h | 96.7 | 93.8 | 89.6 | [66] |
| 7NiCu/ZrO2 | 200 °C, 1.5 MPa H2, 4 h | 99.5 | 93.0 | 93.0 | [67] |
| Ni-Cu | 110 °C, 6 MPa H2, 2.3 h | 50.0 | 100.0 | 50.0 | [68] |
| Cu/γ-Al2O3 | 130 °C, 4 MPa H2, 4 h | 64.2 | 72.3 | 46.4 | [69] |
| Cu/CeO2 | 210 °C, 0.1 MPa H2 | 83 | 67 | 55.6 | [70] |
| Cu/Al2O3-N2-R | 120 °C, 1 MPa H2, 2 h | 99.9 | 99.9 | 99.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Yi, W.; Yang, J.; Jiang, K.; Yang, T.; Li, Z.; Zhang, M.; Liu, Z.; Wu, B. Effect of Calcination Atmosphere on the Performance of Cu/Al2O3 Catalyst for the Selective Hydrogenation of Furfural to Furfuryl Alcohol. Molecules 2024, 29, 2753. https://doi.org/10.3390/molecules29122753
Gao Y, Yi W, Yang J, Jiang K, Yang T, Li Z, Zhang M, Liu Z, Wu B. Effect of Calcination Atmosphere on the Performance of Cu/Al2O3 Catalyst for the Selective Hydrogenation of Furfural to Furfuryl Alcohol. Molecules. 2024; 29(12):2753. https://doi.org/10.3390/molecules29122753
Chicago/Turabian StyleGao, Yongzhen, Wenjing Yi, Jingyi Yang, Kai Jiang, Tao Yang, Zhihan Li, Meng Zhang, Zhongyi Liu, and Benlai Wu. 2024. "Effect of Calcination Atmosphere on the Performance of Cu/Al2O3 Catalyst for the Selective Hydrogenation of Furfural to Furfuryl Alcohol" Molecules 29, no. 12: 2753. https://doi.org/10.3390/molecules29122753






