Tunability of Photovoltaic Functions via Halogen Substitution [(Ade)2 CdX4](X = Cl, Br): A Class of Three-Dimensional Organic–Inorganic Hybrid Materials
Abstract
:1. Introduction
2. Results and Discussion
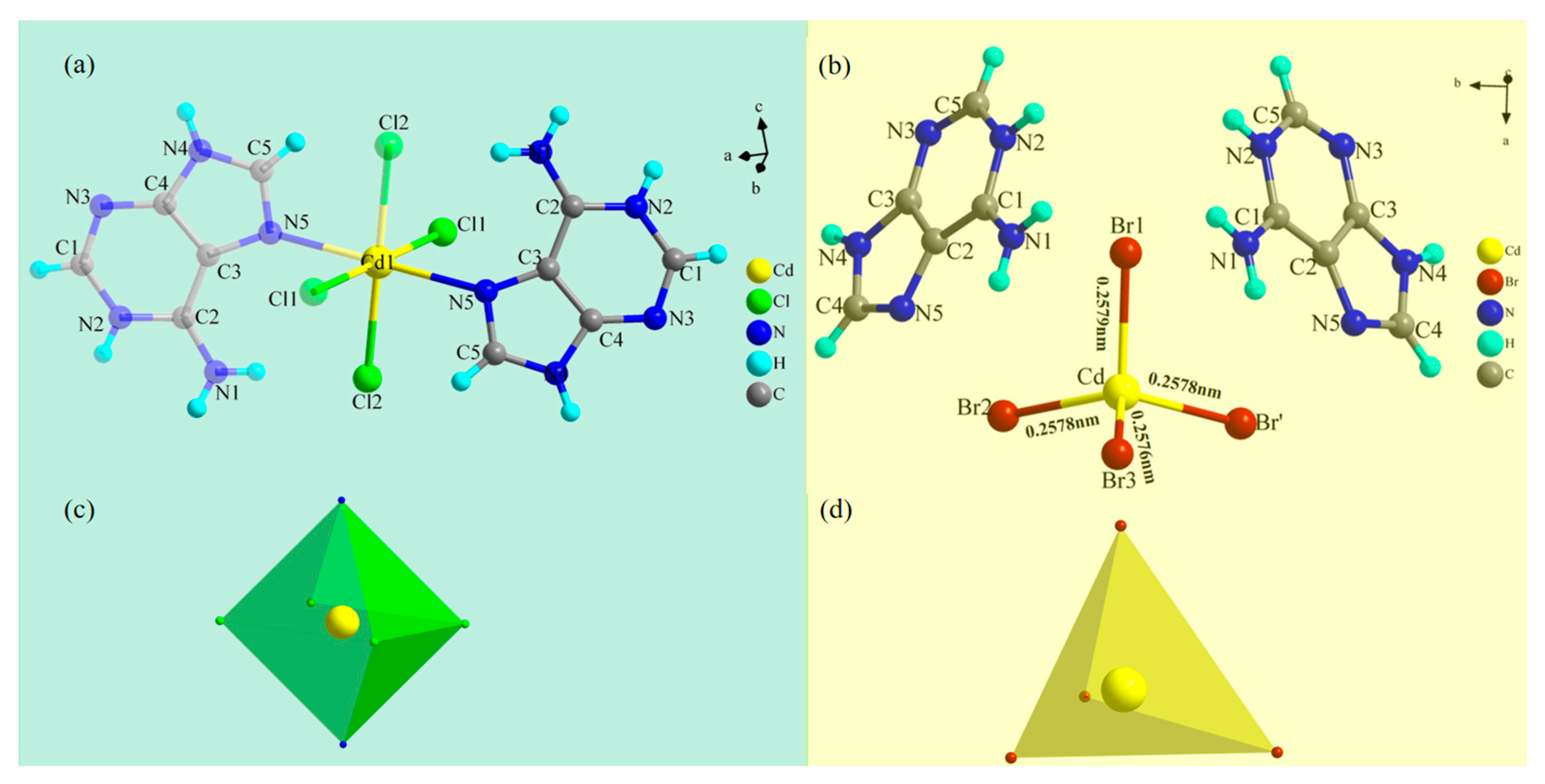
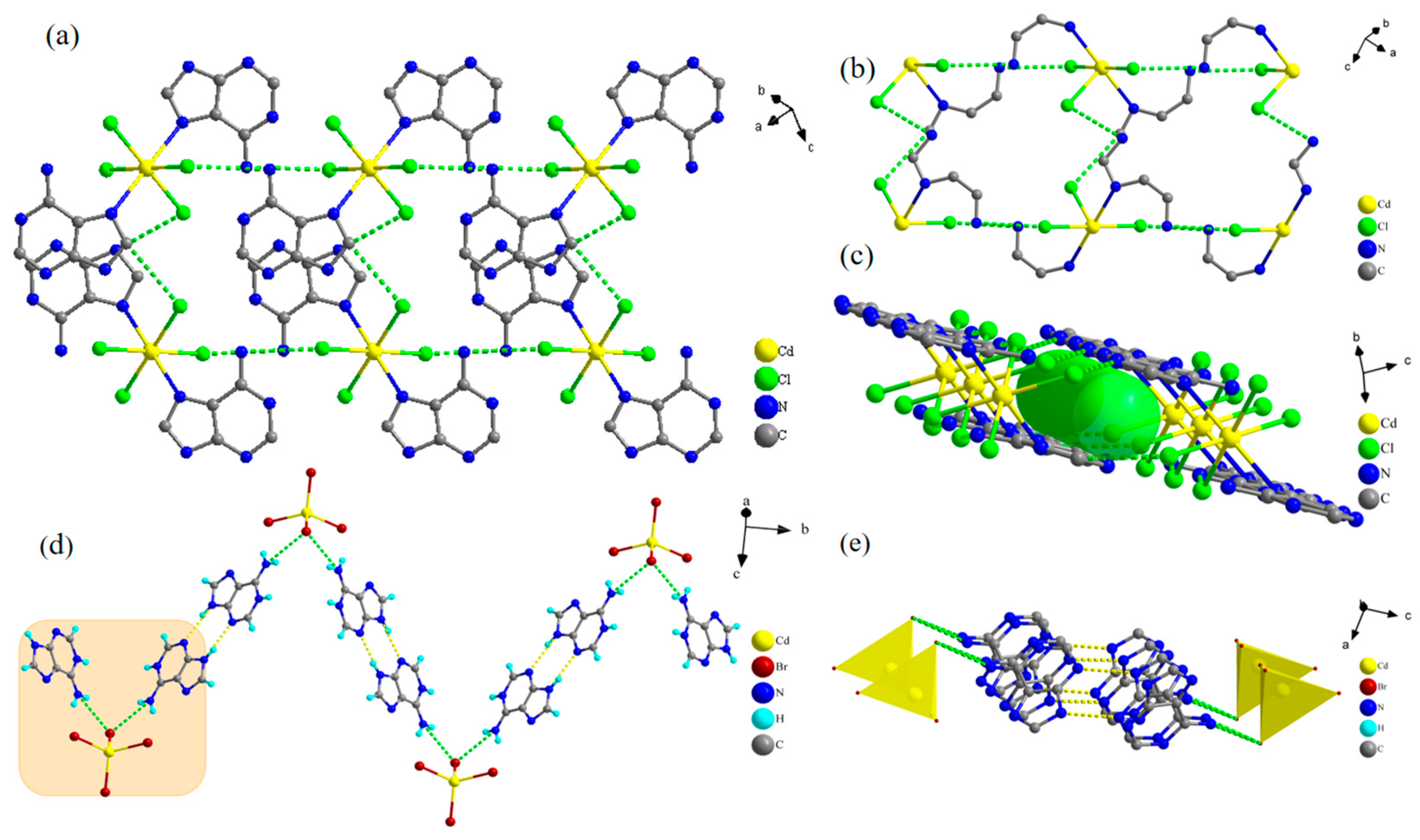
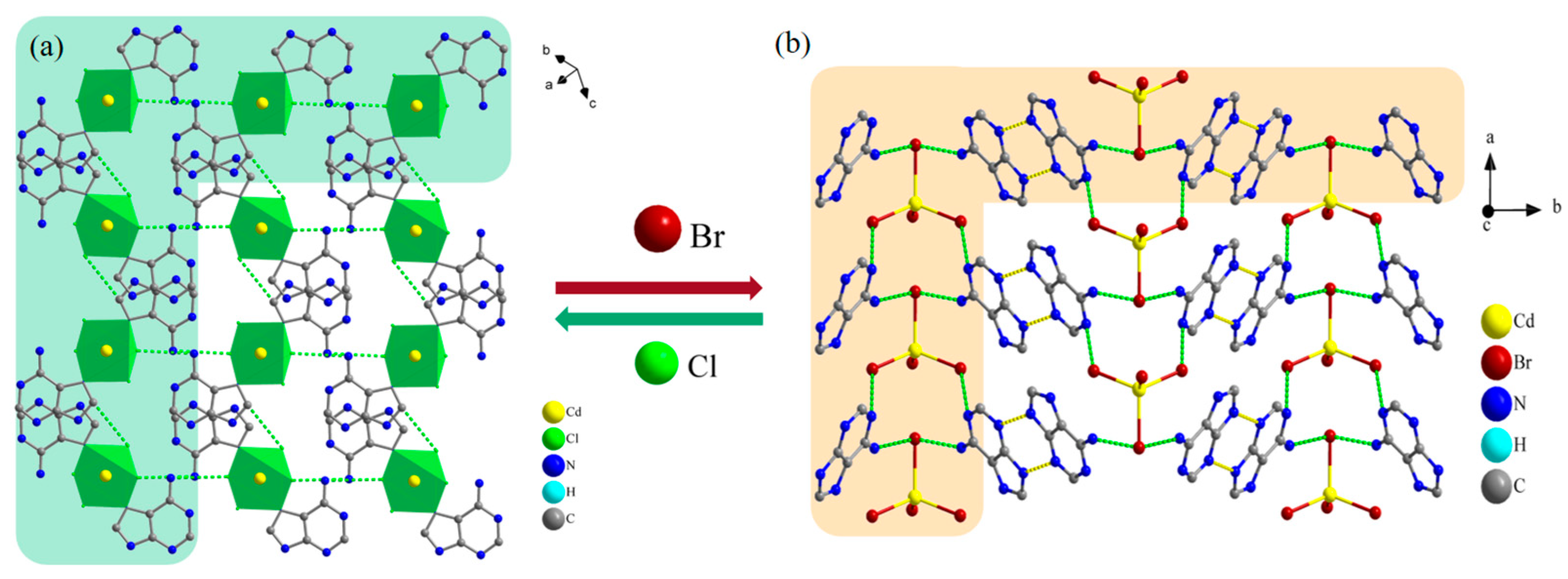
2.1. Single-Crystal Structures of Compounds 1 and 2
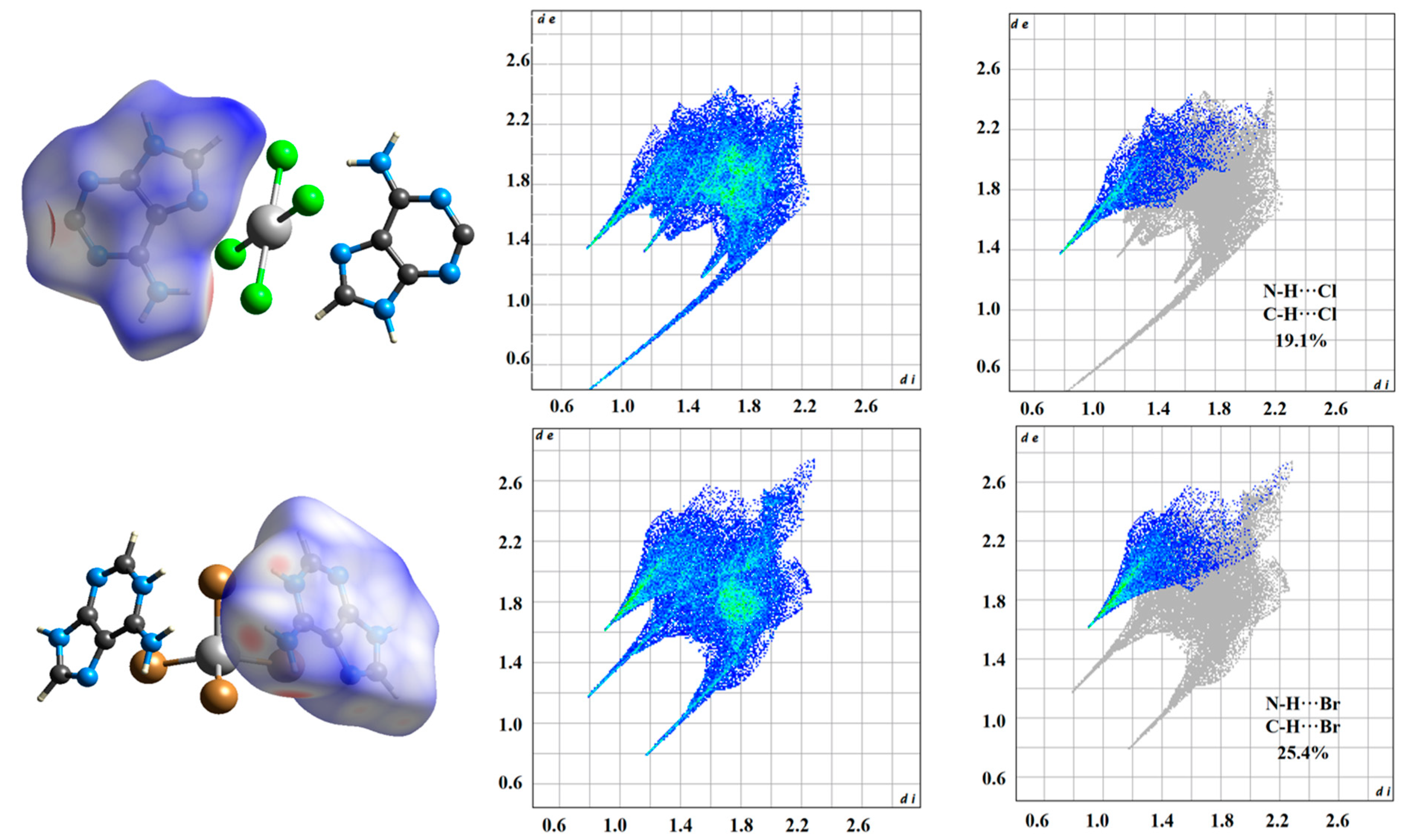
2.2. Hirshfeld Surface Analyses of Compounds 1 and 2
2.3. Infrared Spectra of Compounds 1 and 2
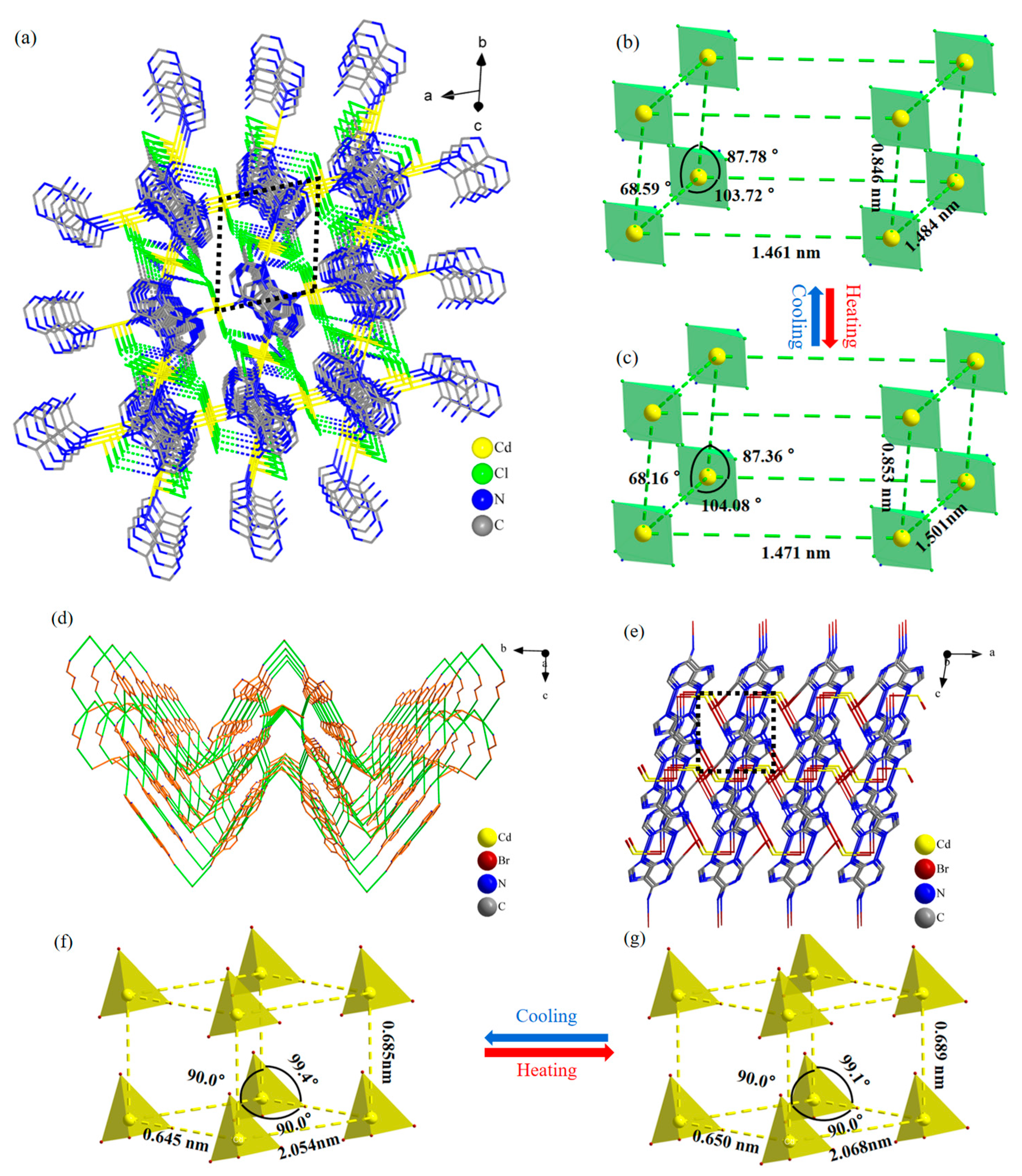
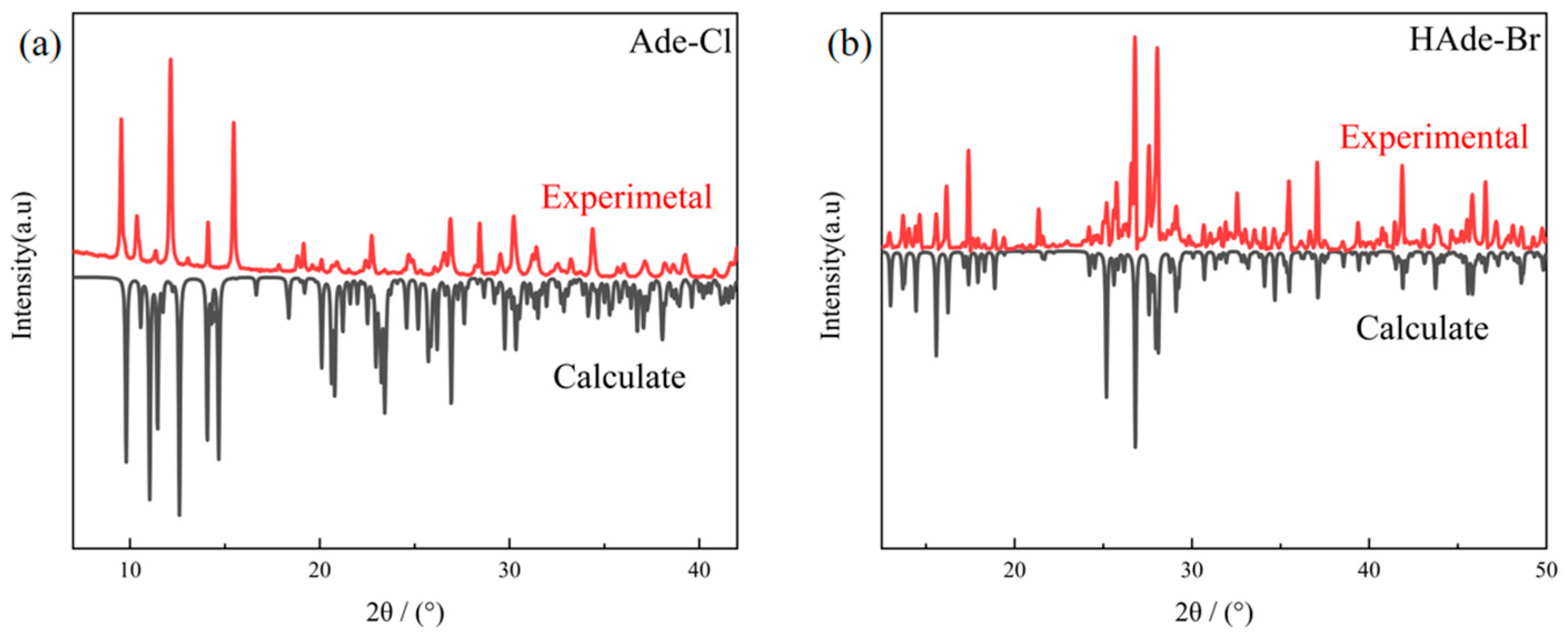
2.4. XRD Analysis of Compounds 1 and 2
2.5. Photoluminescence Properties of Compounds 1 and 2
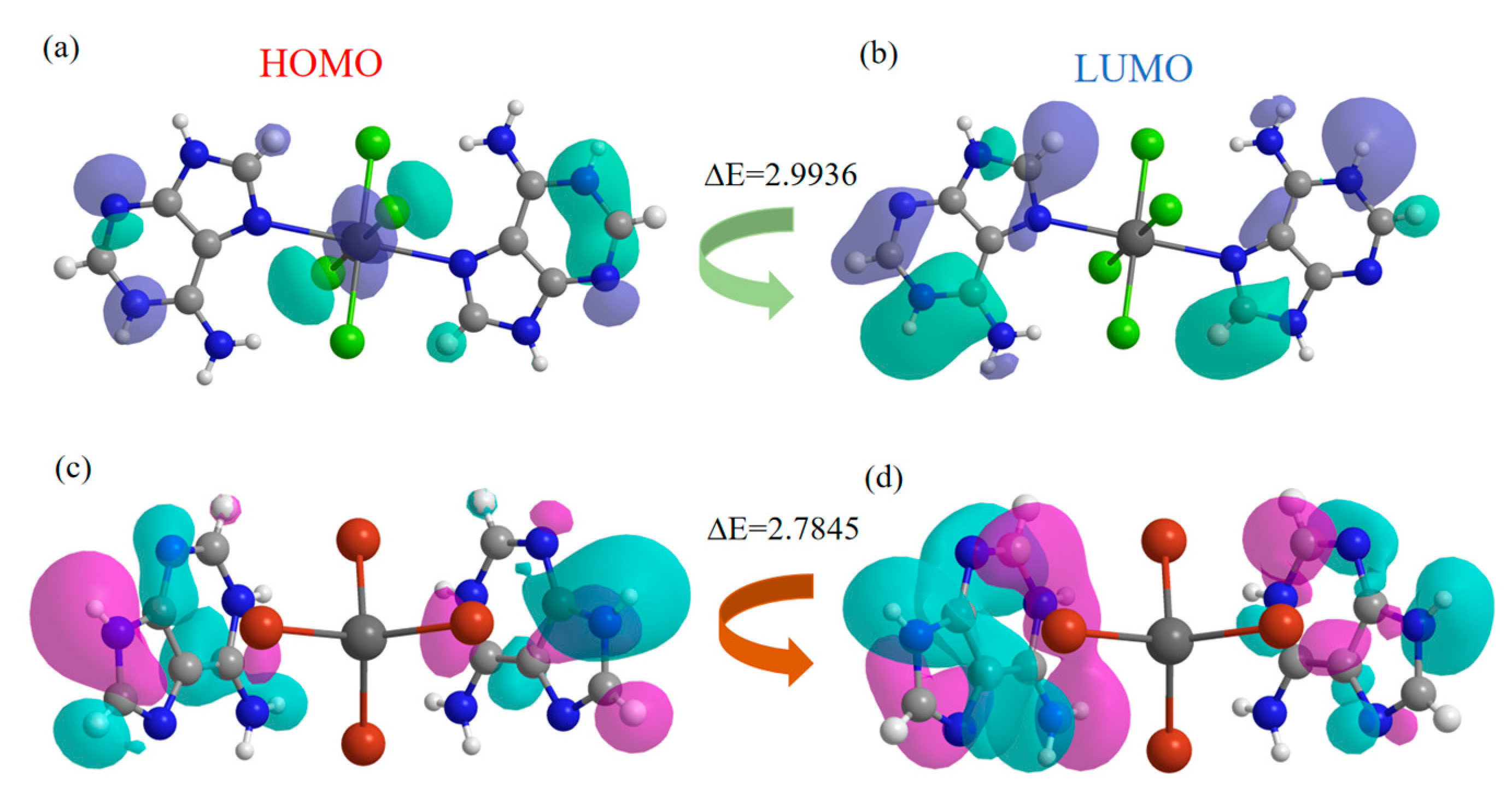
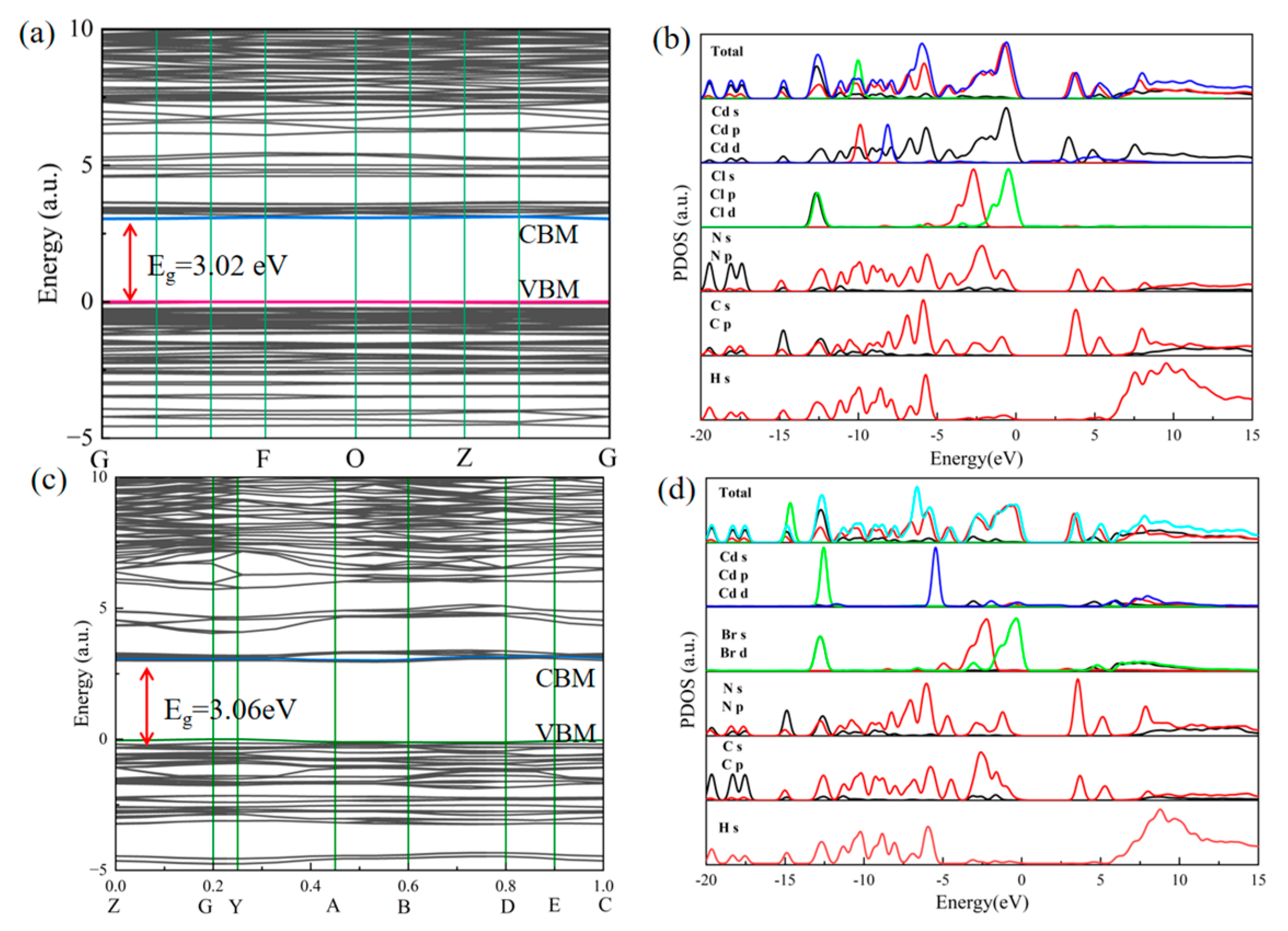
2.6. Electronic Structure Calculations of Compounds 1 and 2
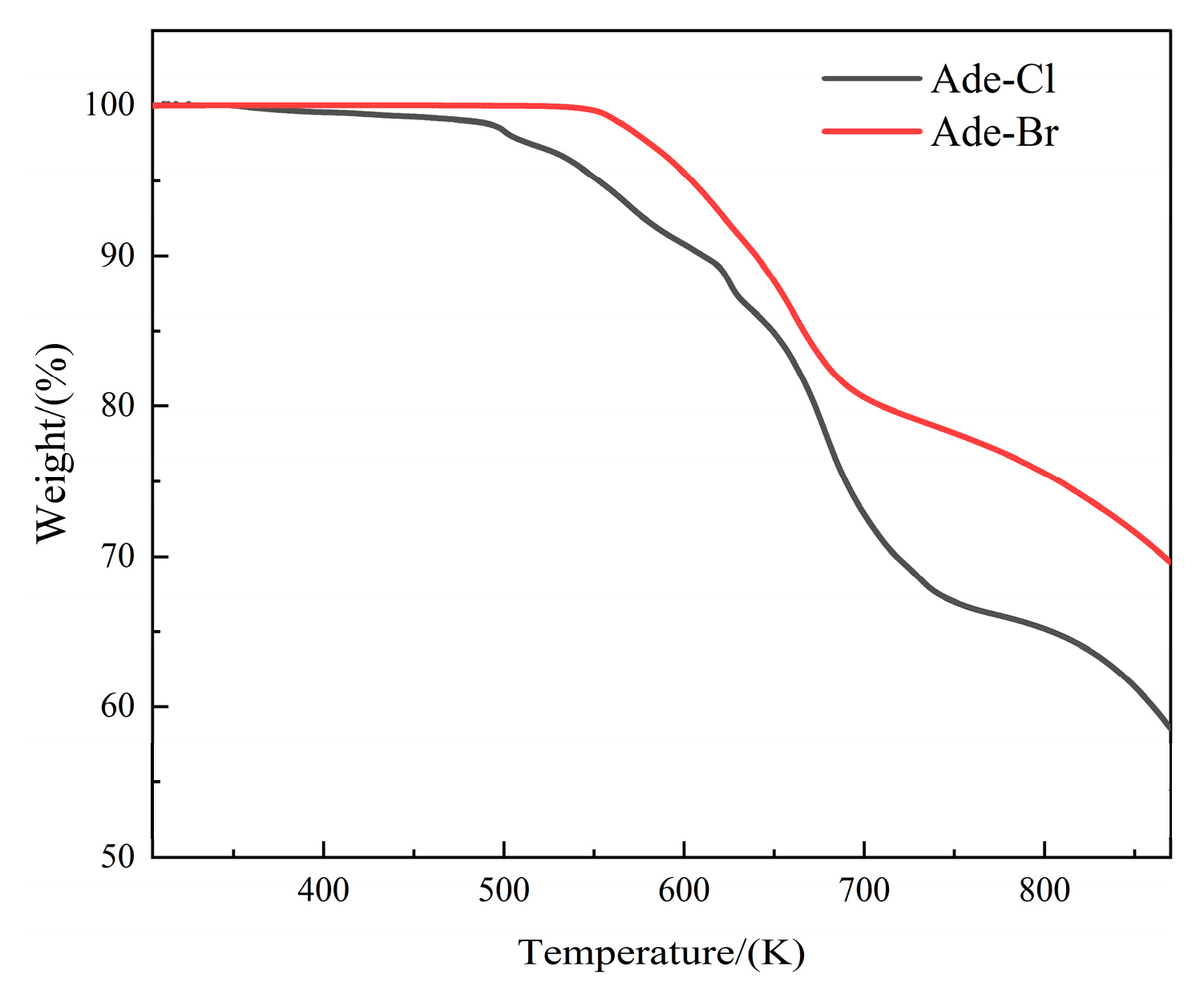
2.7. Thermal Analysis of Compounds 1 and 2
2.8. Dielectric Properties of Compounds 1 and 2
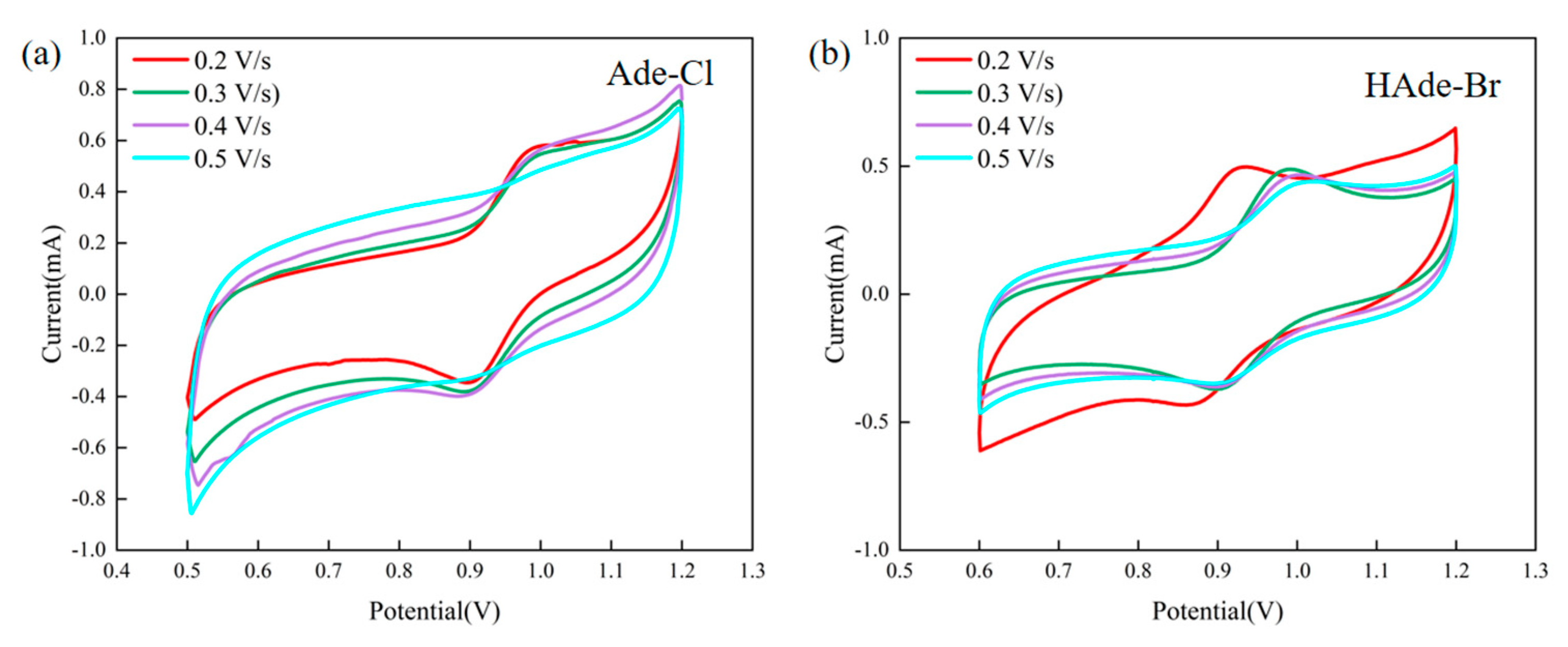
2.9. Electrochemical Properties of Compounds 1 and 2
3. Materials and Methods
3.1. Reagents and Instruments
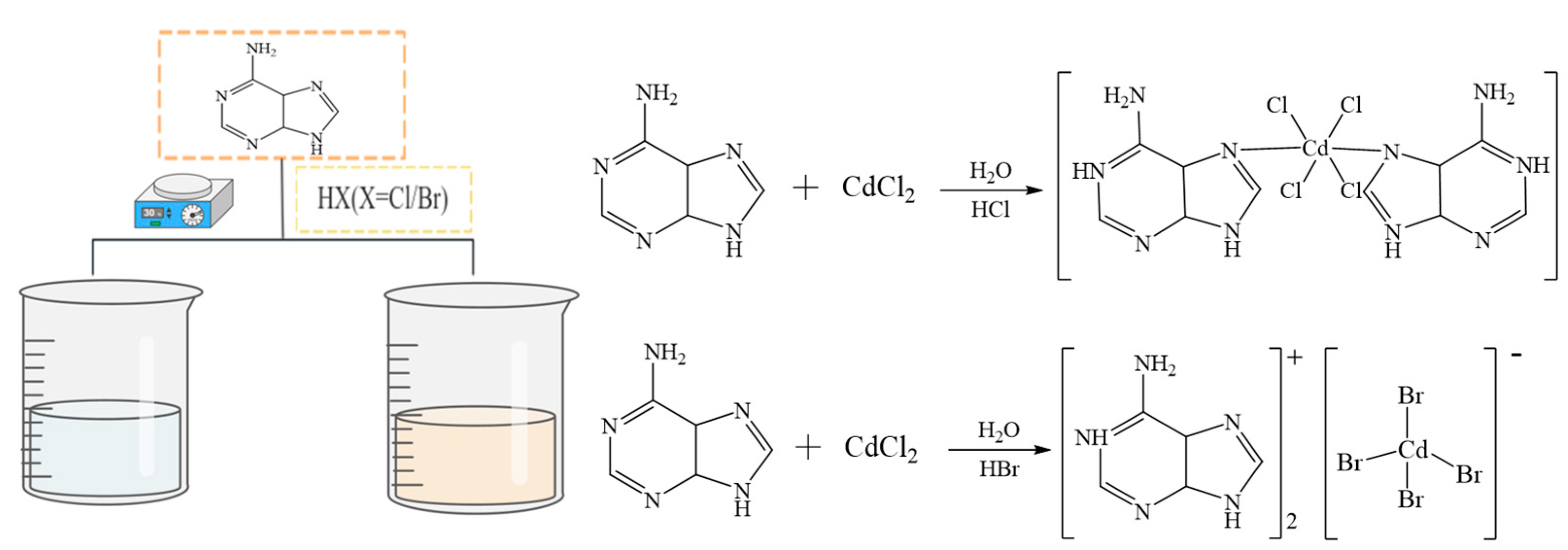
3.2. Synthesis of Compounds
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Ji, C.; Liu, X.; Han, S.; Zhang, J.; Sun, Z.; Khan, A.; Luo, J. (1,4-Butyldiammonium)CdBr4: A layered organic–inorganic hybrid perovskite with a visible-blind ultraviolet photoelectric response. Inorg. Chem. Front. 2018, 5, 2450–2455. [Google Scholar] [CrossRef]
- Shao, T.; Gong, J.M.; Liu, J.; Han, L.J.; Chen, M.; Jia, Q.; Fu, D.W.; Lu, H.-F. 2D lead-free organic–inorganic hybrid exhibiting dielectric and structural phase transition at higher temperatures. CrystEngComm 2022, 24, 4346–4350. [Google Scholar] [CrossRef]
- Gao, J.-X.; Hua, X.-N.; Li, P.-F.; Chen, X.-G.; Liao, W.-Q. High-Temperature Ferroelastic Phase Transition in an Organic–Inorganic Hybrid: [(CH3)3NCH2Br]2–ZnBr4. J. Phys. Chem. C 2018, 122, 23111–23116. [Google Scholar] [CrossRef]
- Chen, N.-N.; Ni, J.-N.; Wang, J. A new two-dimensional CdII coordination polymer based on 1,3-bis(2-methyl-1 H -imidazol-1-yl)benzene and 1,3-phenylenediacetic acid: Synthesis, crystal structure and physical properties. Acta Crystallogr. C Struct. Chem. 2018, 74, 1576–1580. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Ye, S.-Y.; Wan, M.; Wang, Y.-N.; Tong, L.; Chen, L.-Z. A novel organic–inorganic hybrid phase transition compound based on 4-ethylmorpholine with switchable dielectric and luminescent properties. New J. Chem. 2022, 46, 1054–1059. [Google Scholar] [CrossRef]
- Lv, H.-P.; Xu, Z.-K.; Yu, H.; Huang, C.-R.; Wang, Z.-X. A Photochromic Organic–Inorganic Hybrid Schiff Base Metal Halide Ferroelectric. Chem. Mater. 2022, 34, 1737–1745. [Google Scholar] [CrossRef]
- Li, C.; Li, L.-S.; Wei, W.-J.; Tan, Y.-H. A Temperatural Semi-Memorized Phase Transition in a 1D Organic–Inorganic Hybrid Material of Sb|||-Based [(CH2)3NH2S]2 SbCl5. Inorg. Chem. 2019, 58, 9733–9737. [Google Scholar] [CrossRef] [PubMed]
- Rachuri, Y.; Kurisingal, J.F.; Chitumalla, R.K.; Vuppala, S.; Gu, Y.; Jang, J.; Choe, Y.; Suresh, E.; Park, D.-W. Adenine-Based Zn(II)/Cd(II) Metal–Organic Frameworks as Efficient Heterogeneous Catalysts for Facile CO2 Fixation into Cyclic Carbonates: A DFT-Supported Study of the Reaction Mechanism. Inorg. Chem. 2019, 58, 11389–11403. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Riaz, M.; Ashafaq, M.; Gao, Z.-Y.; Varma, R.S.; Li, D.-C.; Cui, P.; Tung, C.-H.; Sun, D. Adenine-incorporated metal–organic frameworks. Coord. Chem. Rev. 2022, 464, 214558. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Z.; Jiao, P.; Wu, Y.; Tang, Z.; Li, D.; Gao, Z.; Sun, X.; Cai, H.-L.; Wu, X.S. An Organic-Inorganic Hybrid Pyrrolidinium Ferroelectric Based on Solvent Selective Effect. Inorg. Chem. 2021, 60, 17212–17218. [Google Scholar] [CrossRef]
- Qi, Z.; Gao, H.; Zhu, X.; Lu, Z.; Zhang, X.-M. Blue Light-Excitable Broadband Yellow Emission in a Zero-Dimensional Hybrid Bismuth Halide with Type-II Band Alignment. Inorg. Chem. 2022, 61, 19483–19491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, L.; Ma, S.; Hu, T. Cd (II) coordination polymer as a strip based fluorescence sensor for sensing Fe3+ ions in aqueous system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120525. [Google Scholar] [CrossRef] [PubMed]
- Kumar Das, D.; Bakthavatsalam, R.; Anilkumar, V.; Mali, B.P.; Ahmed, M.S.; Raavi, S.S.K.; Pallepogu, R.; Kundu, J. Controlled Modulation of the Structure and Luminescence Properties of Zero-Dimensional Manganese Halide Hybrids through Structure-Directing Metal-Ion (Cd2+ and Zn2+) Centers. Inorg. Chem. 2022, 61, 5363–5372. [Google Scholar] [CrossRef] [PubMed]
- Majumder, I.; Chakraborty, P.; Dasgupta, S.; Massera, C.; Escudero, D.; Das, D. A Deep Insight into the Photoluminescence Properties of Schiff Base CdII and ZnII Complexes. Inorg. Chem. 2017, 56, 12893–12901. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.; Su, C.; Li, J.; Jia, Q.; Lu, H.; Fu, D.; Zhang, Y.; Zhang, Z. Introducing Ferroelasticity into 1D Hybrid Lead Halide Semiconductor by Halogen Substitution Strategy. Small 2023, 19, 2303127. [Google Scholar] [CrossRef] [PubMed]
- Rok, M.; Zarychta, B.; Janicki, R.; Witwicki, M.; Bieńko, A.; Bator, G. Dielectric-Optical Switches: Photoluminescent, EPR, and Magnetic Studies on Organic–Inorganic Hybrid (azetidinium)2 MnBr4. Inorg. Chem. 2022, 61, 5626–5636. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Vázquez, L.D.; Dorazco-González, A.; Sánchez-Mendieta, V. Efficient chemosensors for toxic pollutants based on photoluminescent Zn(ii) and Cd(ii) metal–organic networks. Dalton Trans. 2021, 50, 4470–4485. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-F.; Ni, H.-F.; Huang, P.-Z.; Zhu, M.; Wang, C.-F.; Zhuo, Q.-H.; Fu, D.-W.; Zhang, Y.; Zhang, Z.-X. Multifunctional dielectric/optical response with broadband white light emission in a hybrid stannic halide crystal. Mater. Chem. Front. 2023, 7, 6247–6253. [Google Scholar] [CrossRef]
- Berezin, A.S.; Vinogradova, K.A.; Nadolinny, V.A.; Sukhikh, T.S.; Krivopalov, P.; Nikolaenkova, E.B.; Bushuev, M.B. Temperature- and excitation wavelength-dependent emission in a manganese(II) complex. Dalton Trans. 2018, 47, 1657–1665. [Google Scholar] [CrossRef]
- Cheng, J.; Deng, Y.; Dong, X.; Li, J.; Huang, L.; Zeng, H.; Zou, G.; Lin, Z. Homochiral Hybrid Organic–Inorganic Cadmium Chlorides Directed by Enantiopure Amino Acids. Inorg. Chem. 2022, 61, 11032–11035. [Google Scholar] [CrossRef]
- Gómez, P.; Georgakopoulos, S.; Más-Montoya, M.; Cerdá, J.; Pérez, J.; Ortí, E.; Aragó, J.; Curiel, D. Improving the Robustness of Organic Semiconductors through Hydrogen Bonding. ACS Appl. Mater. Interfaces 2021, 13, 8620–8630. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Zhang, S.; Khan, T.; Sun, Z.; Zeb, A.; Ji, C.; Li, L.; Zhao, S.; Luo, J. Reversible phase transition driven by orderâdisorder transformations of metal-halide moieties in [(C6H14)NH2]2·CuBr4. J. Mater. Chem. C 2016, 4, 7537–7540. [Google Scholar] [CrossRef]
- Popy, D.A.; Evans, B.N.; Jiang, J.; Creason, T.D.; Banerjee, D.; Loftus, L.M.; Pachter, R.; Glatzhofer, D.T.; Saparov, B. Intermolecular arrangement facilitated broadband blue emission in group-12 metal (Zn, Cd) hybrid halides and their applications. Mater. Today Chem. 2023, 30, 101502. [Google Scholar] [CrossRef]
- Elgahami, H.; Ajili, M.; Roisnel, T.; Oueslati, A. Investigation of structural and ionic conductivity in the new organic-inorganic bromide:[(C3H7)4P]2Cd2Br6. J. Solid State Chem. 2022, 311, 123108. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zhang, Z.-X.; Chen, X.-G.; Song, X.-J.; Zhang, Y.; Xiong, R.-G. Large Electrostrictive Coefficient in a Two-Dimensional Hybrid Perovskite Ferroelectric. J. Am. Chem. Soc. 2021, 143, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yin, X.; Tu, B.; Pang, Q.; Li, H.; Ren, X.; Wang, B.; Li, Q. Metal–organic frameworks constructed from mixed infinite inorganic units and adenine. CrystEngComm 2014, 16, 3082. [Google Scholar] [CrossRef]
- Portela, S.; Fernández, I. Nature of the Hydrogen Bond Enhanced Halogen Bond. Molecules 2021, 26, 1885. [Google Scholar] [CrossRef]
- Peng, H.; Huang, T.; Zou, B.; Tian, Y.; Wang, X.; Guo, Y.; Dong, T.; Yu, Z.; Ding, C.; Yang, F.; et al. Organic-inorganic hybrid manganese bromine single crystal with dual-band photoluminescence from polaronic and bipolaronic excitons. Nano Energy 2021, 87, 106166. [Google Scholar] [CrossRef]
- Kassou, S.; Kaiba, A.; Guionneau, P.; Belaaraj, A. Organic-inorganic hybrid perovskite (C6H5(CH2)2NH3)2CdCl4: Synthesis, structural and thermal properties. J. Struct. Chem. 2016, 57, 737–743. [Google Scholar] [CrossRef]
- Msalmi, R.; Elleuch, S.; Hamdi, B.; Abd El-Fattah, W.; Ben Hamadi, N.; Naïli, H. Organically tuned white-light emission from two zero-dimensional Cd-based hybrids. RSC Adv. 2022, 12, 10431–10442. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Li, M.; Xu, G.C. Phase Transition and Dielectric Response Originating from Disorder-Order Transition in the In-Based Organic-Inorganic Hybrid Material [NH3(CH2)5NH3][InCl5(H2O)] H2O. Eur. J. Inorg. Chem. 2021, 2021, 1251–1255. [Google Scholar] [CrossRef]
- Li, M.; Xu, G.-C.; Zhang, Y.-Q.; Xin, W.-B. Phase transition, dielectric switching property of an In (III)-based organic-inorganic hybrid compound: (C5H16N2)InBr5. J. Solid State Chem. 2020, 287, 121329. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Dong, X.; Huang, L.; Zeng, H.; Lin, Z.; Zou, G. Corrugated 1D Hybrid Metal Halide [C6H7ClN]CdCl3 Exhibiting Broadband White-Light Emission. Inorg. Chem. 2022, 61, 4752–4759. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Luo, D.; Liu, T.; Zheng, W.; Chen, T.; Li, D. Self-Assembly of Copper Polypyridyl Supramolecular Metallopolymers to Achieve Enhanced Anticancer Efficacy. ChemistryOpen 2019, 8, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pan, J.; Chen, X.; Chen, H.; Pan, S. Solution growth and optical properties of lead-free highly efficient green-emitting [Emim]4[Cu4I8] single crystals with 0D structure. J. Lumin. 2023, 253, 119467. [Google Scholar] [CrossRef]
- Han, S.; Zhang, J.; Teng, B.; Ji, C.; Zhang, W.; Sun, Z.; Luo, J. Inorganic–organic hybrid switchable dielectric materials with the coexistence of magnetic anomalies induced by reversible high-temperature phase transition. J. Mater. Chem. C 2017, 5, 8509–8515. [Google Scholar] [CrossRef]
- Gong, Y.-Y.; Zhang, T.; Li, J.; Fu, D.-W.; Zhang, Y.; Lu, H.-F. Structural Optimization and Property Tunability by Halogen Regulation in Zero-Dimensional Zinc Halide Organic–Inorganic Hybrid Materials. Cryst. Growth Des. 2022, 22, 6801–6808. [Google Scholar] [CrossRef]
- Lim, A.R.; Joo, Y.L. Study on structural geometry and dynamic property of [NH3(CH2)5NH3]CdCl4 crystal at phases I, II, and III. Sci. Rep. 2022, 12, 4251. [Google Scholar] [CrossRef] [PubMed]
- Roccanova, R.; Ming, W.; Whiteside, V.R.; McGuire, M.A.; Sellers, I.R.; Du, M.-H.; Saparov, B. Synthesis, Crystal and Electronic Structures, and Optical Properties of (CH3NH3)2 CdX4 (X = Cl, Br, I). Inorg. Chem. 2017, 56, 13878–13888. [Google Scholar] [CrossRef]
- Dadi, A.; Mazzeo, P.P.; Bacchi, A.; Loukil, M. Synthesis, crystal structure, structural phase transition and dielectric properties of new organic-inorganic hybrid compound: (C6H5CH2N(C2H5)3)CdCl3. J. Mol. Struct. 2022, 1258, 132617. [Google Scholar] [CrossRef]
- Wang, D.-L.; Sun, D.-F.; Xu, L.-Y.; Liu, J.; Wang, J.-Y.; Shen, C.-Y. The synthesis, structure and photoluminescence of new (C8H18N)2CdCl4 crystals. J. Mol. Struct. 2023, 1282, 135222. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Guo, L.; Wang, B.; Li, F.; Cao, J.; Li, W.; Song, Y. Three new Cd(II)/Zn(II) coordination polymers assembled via dual-ligand strategy: Crystal structures and luminescent properties. Z. Anorg. Allg. Chem. 2021, 647, 770–776. [Google Scholar] [CrossRef]
- Sun, X.-T.; Zhang, Y.-Y.; Han, Y.; Wang, X.-P.; Li, J.; Li, J.-Y.; Ni, H.-F.; Fu, D.-W.; Zhang, Z.-X. The halogen substitution strategy of inorganic skeletons triggers dielectric and band gap regulation of hybrid perovskites. Dalton Trans. 2023, 52, 16406–16412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, Y.-H.; Tang, Y.-Z.; Fan, X.-W.; Peng, X.-L.; Han, R.-R.; Li, Y.-K.; Wang, F.-X. Two Manganese(II)-Based Hybrid Multifunctional Phase Transition Materials with Strong Photoluminescence, High Quantum Yield, and Switchable Dielectric Properties: (C6NH16)2 MnBr4 and (C7NH18)2 MnBr4. Inorg. Chem. 2022, 61, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- Akrout, F.; Hajlaoui, F.; Karoui, K.; Audebrand, N.; Roisnel, T.; Zouari, N. Two-dimensional copper (II) halide-based hybrid perovskite templated by 2-chloroethylammonium: Crystal structures, phase transitions, optical and electrical properties. J. Solid State Chem. 2020, 287, 121338. [Google Scholar] [CrossRef]
- Ma, H.-F.; Ding, L.-W.; Chen, L.; Wang, Y.-L.; Liu, Q.-Y. Two cadmium compounds with adenine and carboxylate ligands: Syntheses, structures and photoluminescence. J. Coord. Chem. 2017, 70, 145–155. [Google Scholar] [CrossRef]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal–Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Cizman, A.; Kowalska, D.; Trzebiatowska, M.; Medycki, W.; Krupiński, M.; Staniorowski, P.; Poprawski, R. The structure and switchable dielectric properties of dabco complex with chromium chloride. Dalton Trans. 2020, 49, 10394–10401. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.F.; Elkhiyami, S.S.; Alal, S.A. Discontinuous transition from insulator to semiconductor induced by phase change of the new organic- inorganic hybrid [(CH2)7(NH3)2]CoBr4. Mater. Chem. Phys. 2017, 199, 454–463. [Google Scholar] [CrossRef]
- Van Den Berg, J.-A.; Seddon, K.R. Critical Evaluation of C-H···X Hydrogen Bonding in the Crystalline State. Cryst. Growth Des. 2003, 3, 643–661. [Google Scholar] [CrossRef]
- Pramanik, S.; Pathak, S.; Frontera, A.; Mukhopadhyay, S. Syntheses, crystal structures and supramolecular assemblies of two Cu(II) complexes based on a new heterocyclic ligand: Insights into C-H···Cl and π···π interactions. CrystEngComm 2022, 24, 1598–1611. [Google Scholar] [CrossRef]
- Sun, D.; Wang, D.; Dang, Y.; Zhang, S.; Chen, H.; Hou, R.; Wu, K.; Shen, C. Organic-Inorganic Hybrid Noncentrosymmetric (Morpholinium)2Cd2Cl6 Single Crystals: Synthesis, Nonlinear Optical Properties, and Stability. Inorg. Chem. 2022, 61, 8076–8082. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Guerfel, T. Crystal structure, vibrational studies, optical properties and TG-DTA investigations of a new chlorocadmate templated by 1-methylimidazolium. Chem. Res. Chin. Univ. 2016, 32, 253–260. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Yoon, D.K.; Kim, Y.G.; Ko, Y.G. Fabrication of organic-inorganic hybrid materials on metal surface for optimizing electrochemical performance. J. Colloid Interface Sci. 2020, 573, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Kouraichi, C.; Guerfel, T. Modelling, structural, thermal, optical and vibrational studies of a new organic–inorganic hybrid material (C5H16N2)Cd1.5Cl5. Bull. Mater. Sci. 2017, 40, 55–66. [Google Scholar] [CrossRef]
- Alzahrani, S.; Morad, M.; Bayazeed, A.; Aljohani, M.M.; Alkhatib, F.; Shah, R.; Katouah, H.; Abumelha, H.M.; Althagafi, I.; Zaky, R.; et al. Ball milling approach to prepare new Cd(II) and Zn(II) complexes; characterization, crystal packing, cyclic voltammetry and MOE-docking agrees with biological assay. J. Mol. Struct. 2020, 1218, 128473–128484. [Google Scholar] [CrossRef]
- Fahim, A.M.; Magar, H.S.; Mahmoud, N.H. Synthesis, antimicrobial, antitumor activity, docking simulation, theoretical studies, and electrochemical analysis of novel Cd(II), Co(II), Cu(II), and Fe(III) complexes containing barbituric moiety. Appl. Organomet. Chem. 2023, 37, 7023–7045. [Google Scholar] [CrossRef]
- Yadav, J.K.; Mishra, A.; Mishra, G.K.; Pal, S.K.; Narvekar, K.U.; Rahaman, A.; Singh, N.; Lama, P.; Kumar, K. Isonicotinate-Zn(II)/Cd(II) bridged dicobaloximes: Synthesis, characterization and electrocatalytic proton reduction studies. New J. Chem. 2023, 47, 20583–20593. [Google Scholar] [CrossRef]
- Jaiswal, S.; Pandey, S.K.; Prajapati, J.; Chandra, S.; Gond, M.K.; Bharty, M.K.; Tiwari, I.; Butcher, R.J. Cd(II) complexes derived from thiazoline, hydrazide and carbodithioate ligands: Synthesis, crystal structures and electrochemical sensing of uric acid. Appl. Organomet. Chem. 2023, 37, 7085–7101. [Google Scholar] [CrossRef]



| Compounds | Ade-Cl | Ade-Br | ||
|---|---|---|---|---|
| Temperature | 100 K | 293 K | 100 K | 293 K |
| Chemical formula | C5H5Cd0.5Cl2N5 | C5H5Cd0.5Cl2N5 | C10H12N10CdBr4 | C10H12N10CdBr4 |
| Formula weight | 526.50 | 526.50 | 704.34 | 704.34 |
| Crystal size (mm)3 | 0.16 × 0.13 × 0.12 | 0.15 × 0.13 × 0.12 | 0.13 × 0.12 × 0.11 | 0.13 × 0.12 × 0.11 |
| Crystal system | triclinic | triclinic | monoclinic | monoclinic |
| Space group | P-1 | P-1 | P21/m | P21/m |
| a (Å) | 0.74465 (6) | 0.75040 (8) | 0.645740 (10) | 0.650350 (10) |
| b (Å) | 0.84468 (6) | 0.85271 (9) | 2.05480 (3) | 2.06785 (3) |
| c (Å) | 1.46159 (11) | 1.47168 (11) | 0.685480 (10) | 0.689280 (10) |
| α (°) | 93.221 (6) | 92.638 (7) | 90 | 90 |
| β (°) | 103.718 (7) | 104.088 (8) | 99.4100 (10) | 99.1640 (10) |
| γ (°) | 111.405 (6) | 111.840 (10) | 90 | 90 |
| V (Å)3 | 821.08 (11) | 838.00 (15) | 897.30 (2) | 915.13 (2) |
| Z | 4 | 4 | 2 | 2 |
| Dcalc (g-cm)−1 | 262.24 | 262.24 | 2.607 | 2.556 |
| F (000) | 512.0 | 512.0 | 660.0 | 660.0 |
| μ (mm)−1 | 2.000 | 1.959 | 20.358 | 19.961 |
| Measured 2 range (°) | 5.246–49.97 | 5.206–49.998 | 13.786–143.658 | 13.7–133.13 |
| Rint | 0.0289 | 0.0295 | 0.0209 | 0.0246 |
| R (I > 2 (I)) [a] | 0.0438 | 0.0408 | 0.0388 | 0.0381 |
| WR (all data) [b] | 0.1273 | 0.1031 | 0.1059 | 0.1074 |
| GOF | 1.117 | 1.102 | 1.058 | 1.086 |
| CCDC NO. | 2,326,393 | 2,326,394 | 2,326,404 | 2,326,405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Hu, H.; Adila, A.; Yan, Y.; Liu, Y.; Liu, Z. Tunability of Photovoltaic Functions via Halogen Substitution [(Ade)2 CdX4](X = Cl, Br): A Class of Three-Dimensional Organic–Inorganic Hybrid Materials. Molecules 2024, 29, 2773. https://doi.org/10.3390/molecules29122773
Lv M, Hu H, Adila A, Yan Y, Liu Y, Liu Z. Tunability of Photovoltaic Functions via Halogen Substitution [(Ade)2 CdX4](X = Cl, Br): A Class of Three-Dimensional Organic–Inorganic Hybrid Materials. Molecules. 2024; 29(12):2773. https://doi.org/10.3390/molecules29122773
Chicago/Turabian StyleLv, Meixia, Hongzhi Hu, Abuduheni Adila, Yibo Yan, Yang Liu, and Zunqi Liu. 2024. "Tunability of Photovoltaic Functions via Halogen Substitution [(Ade)2 CdX4](X = Cl, Br): A Class of Three-Dimensional Organic–Inorganic Hybrid Materials" Molecules 29, no. 12: 2773. https://doi.org/10.3390/molecules29122773






