Synthesis and Characterization of Sulfonamide-Containing Naphthalimides as Fluorescent Probes
Abstract
:1. Introduction
2. Results and Discussion
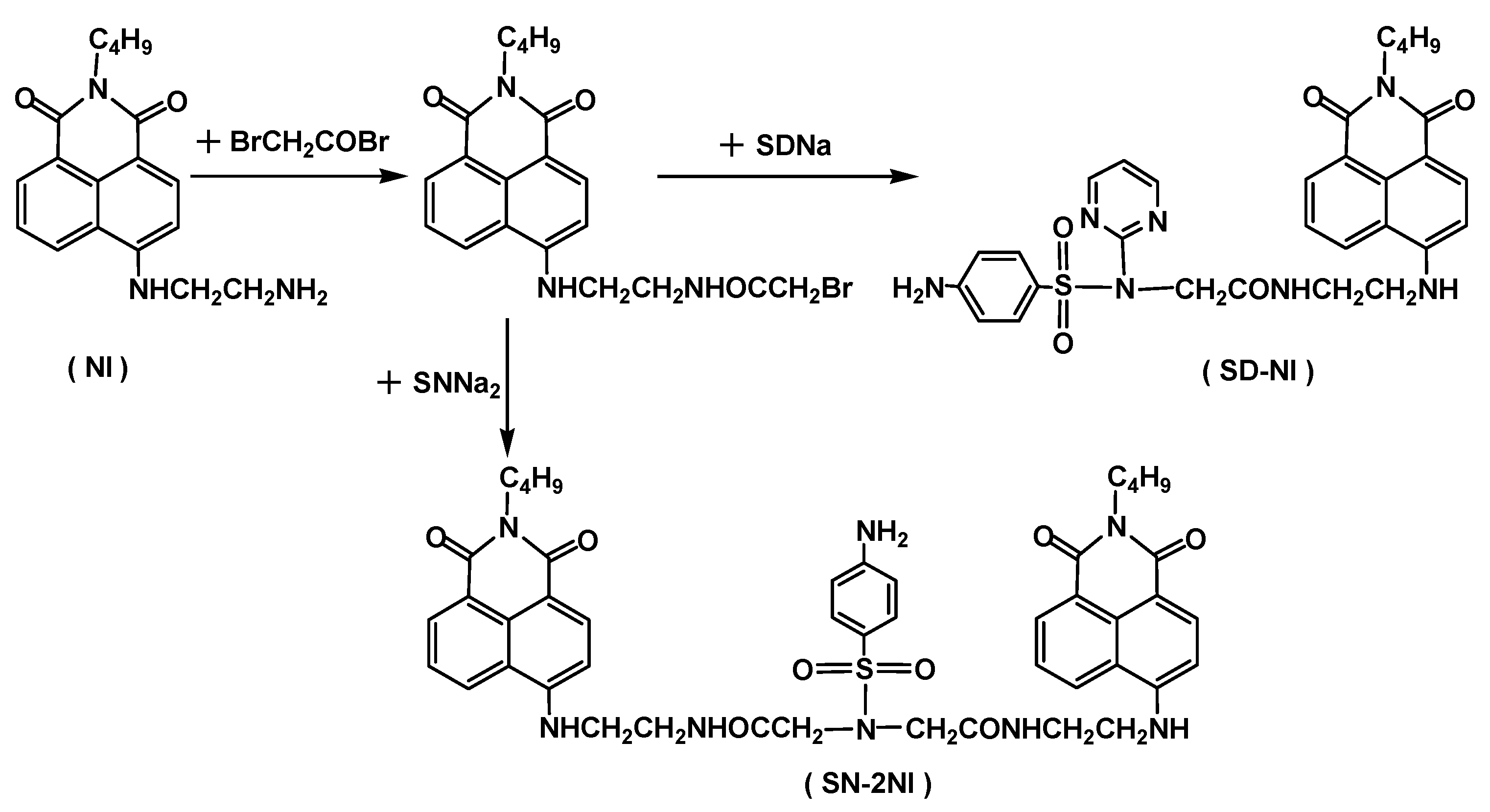
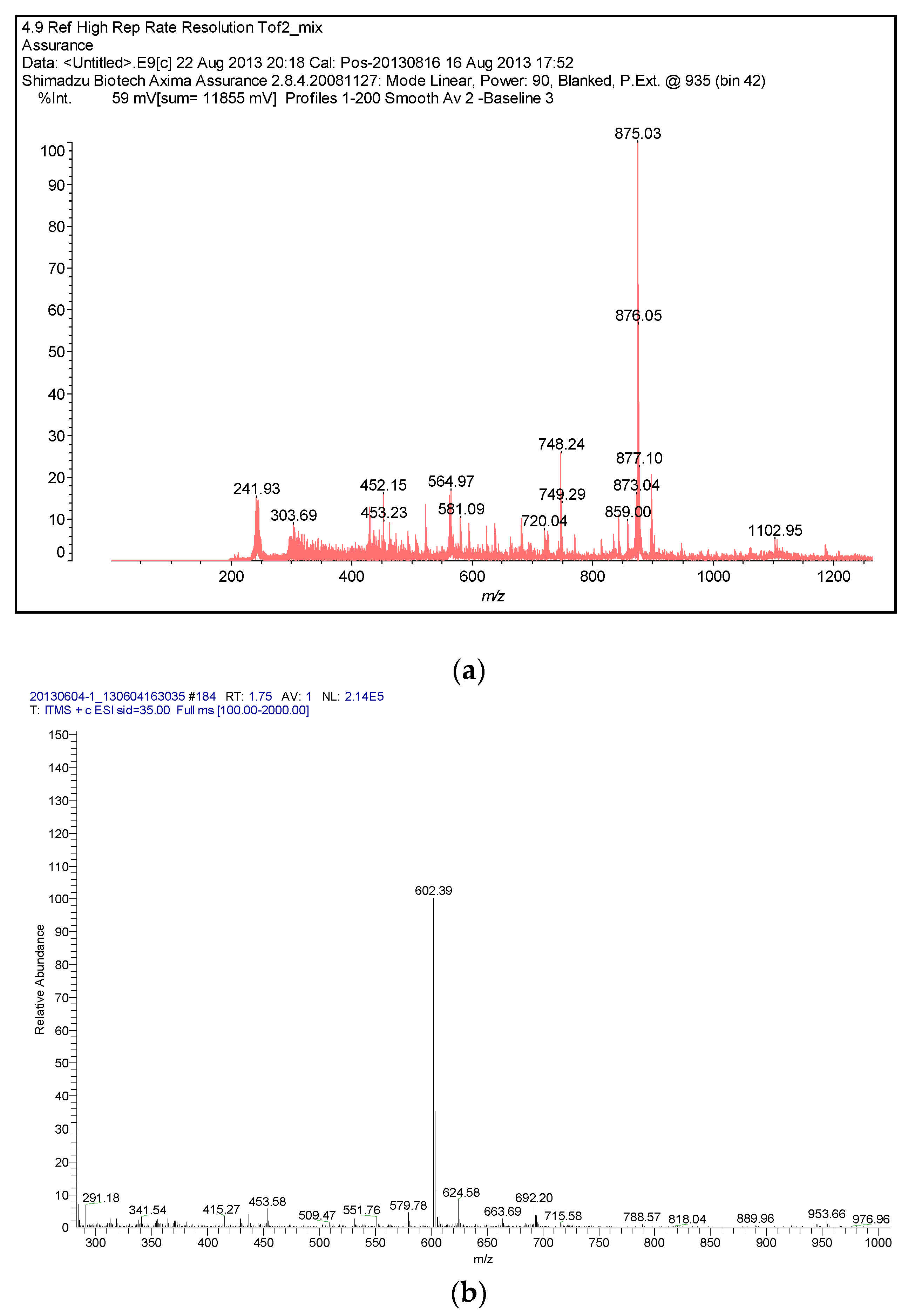
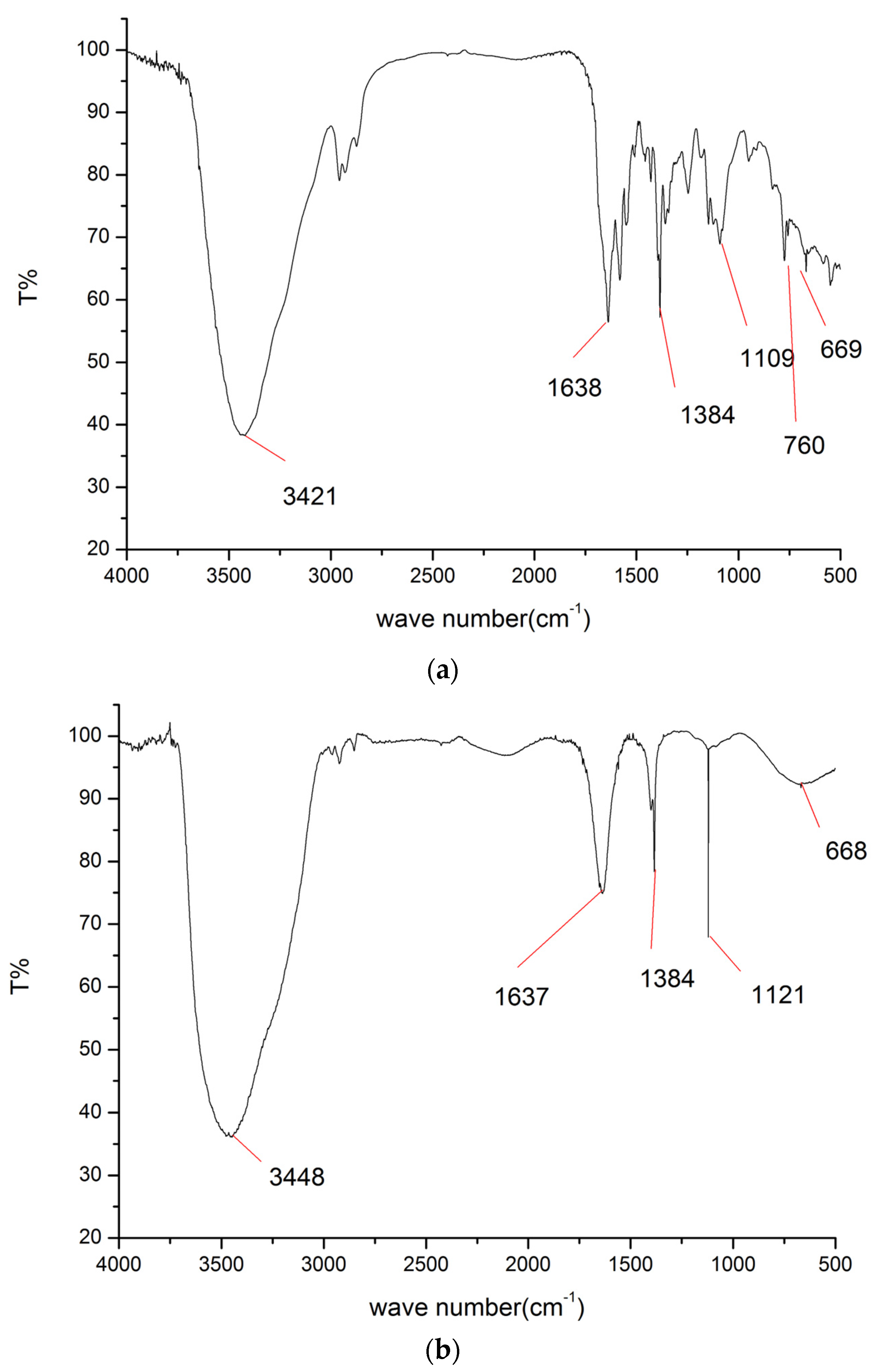
2.1. Synthesis
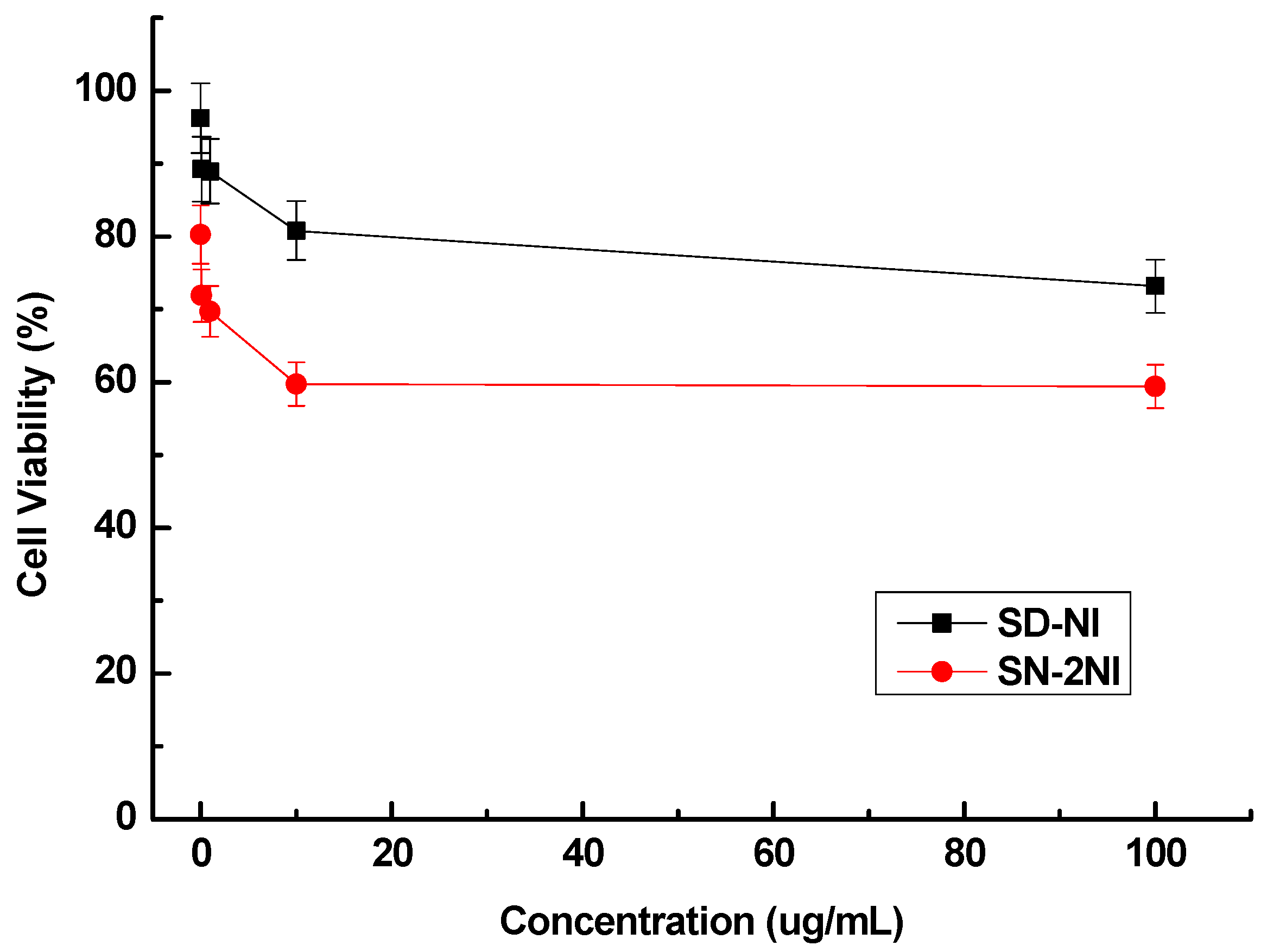
2.2. Cell Cytotoxicity
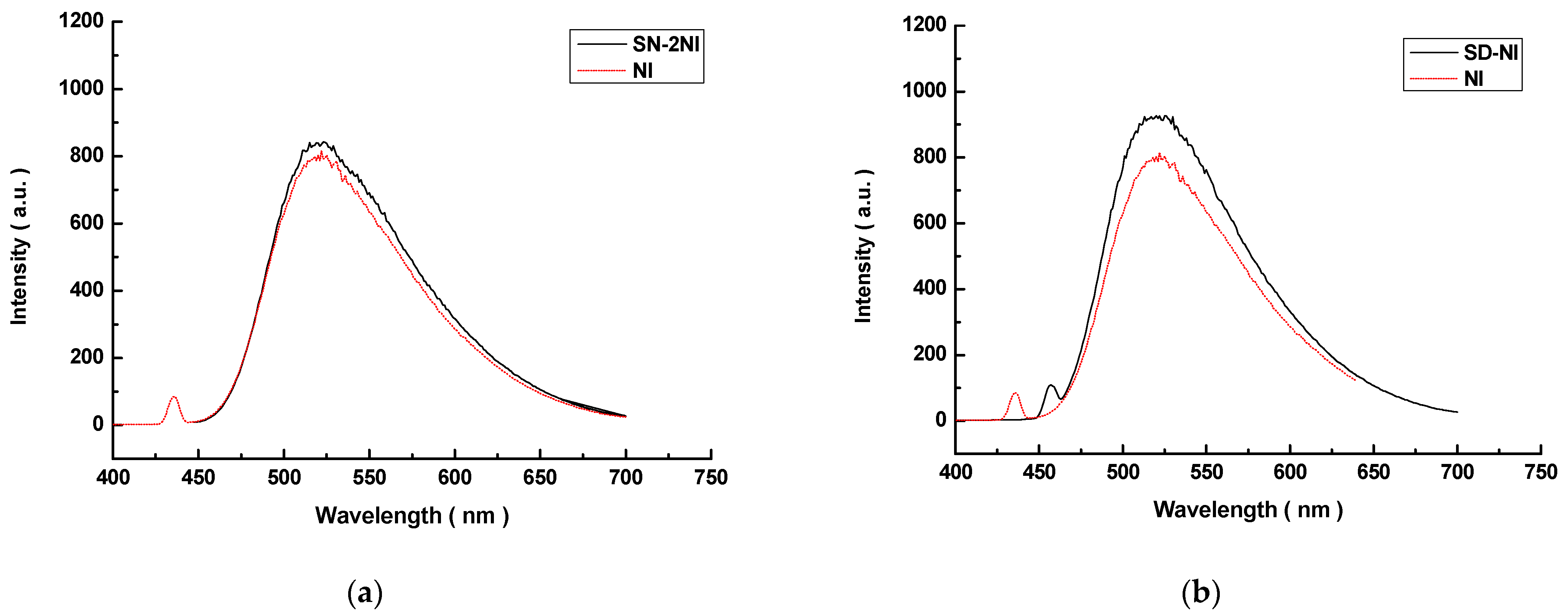
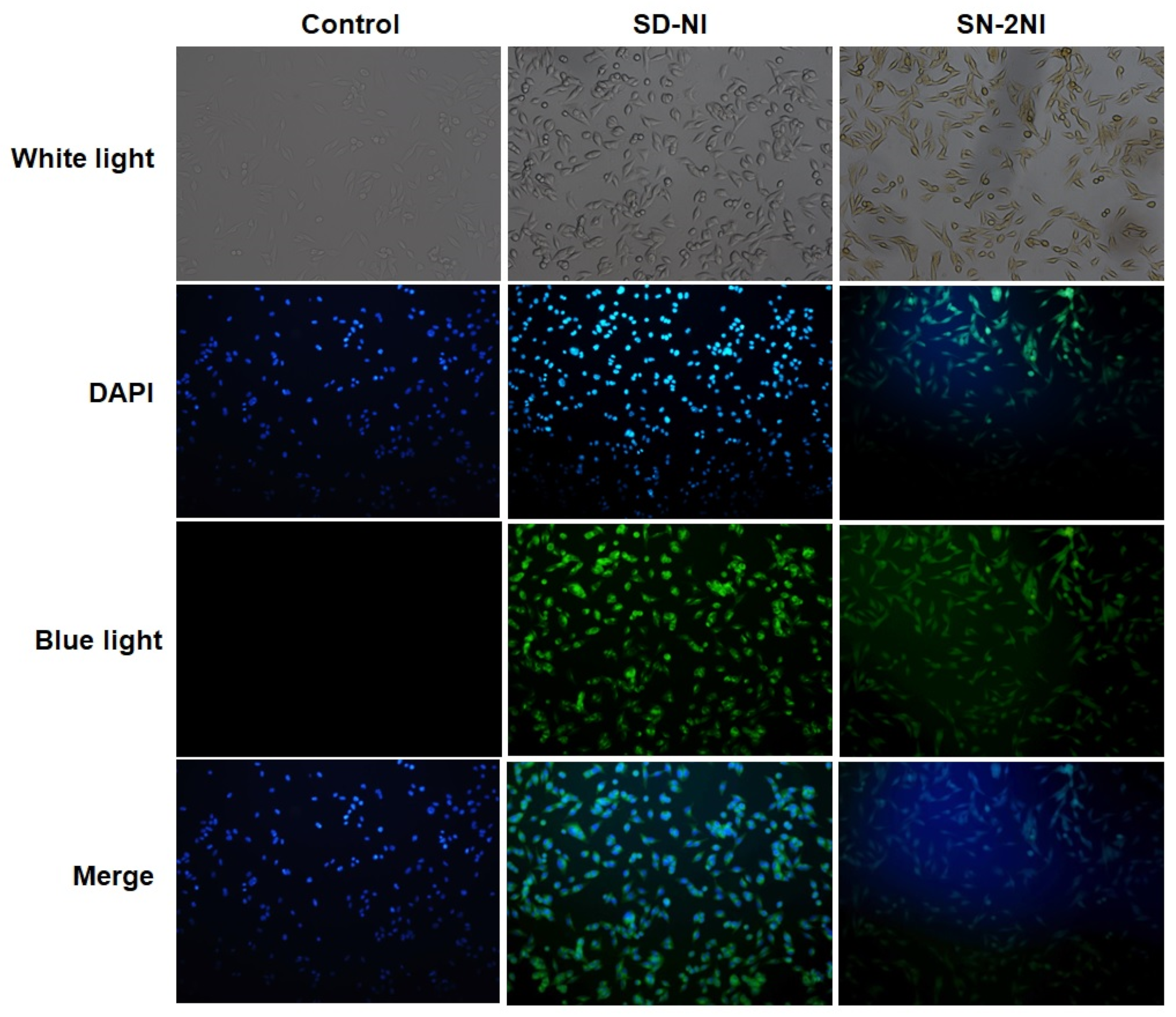
2.3. Fluorescent Imaging
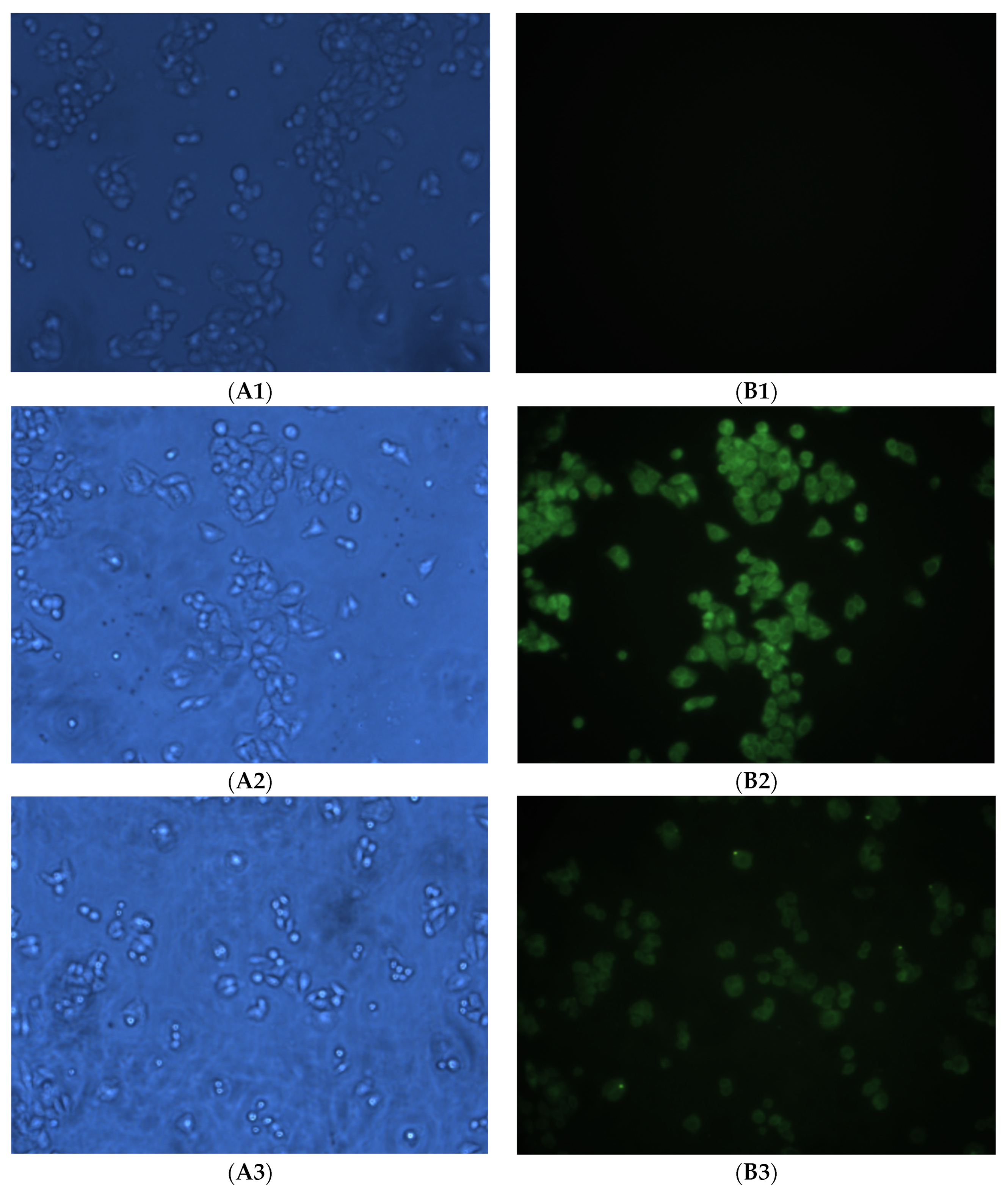
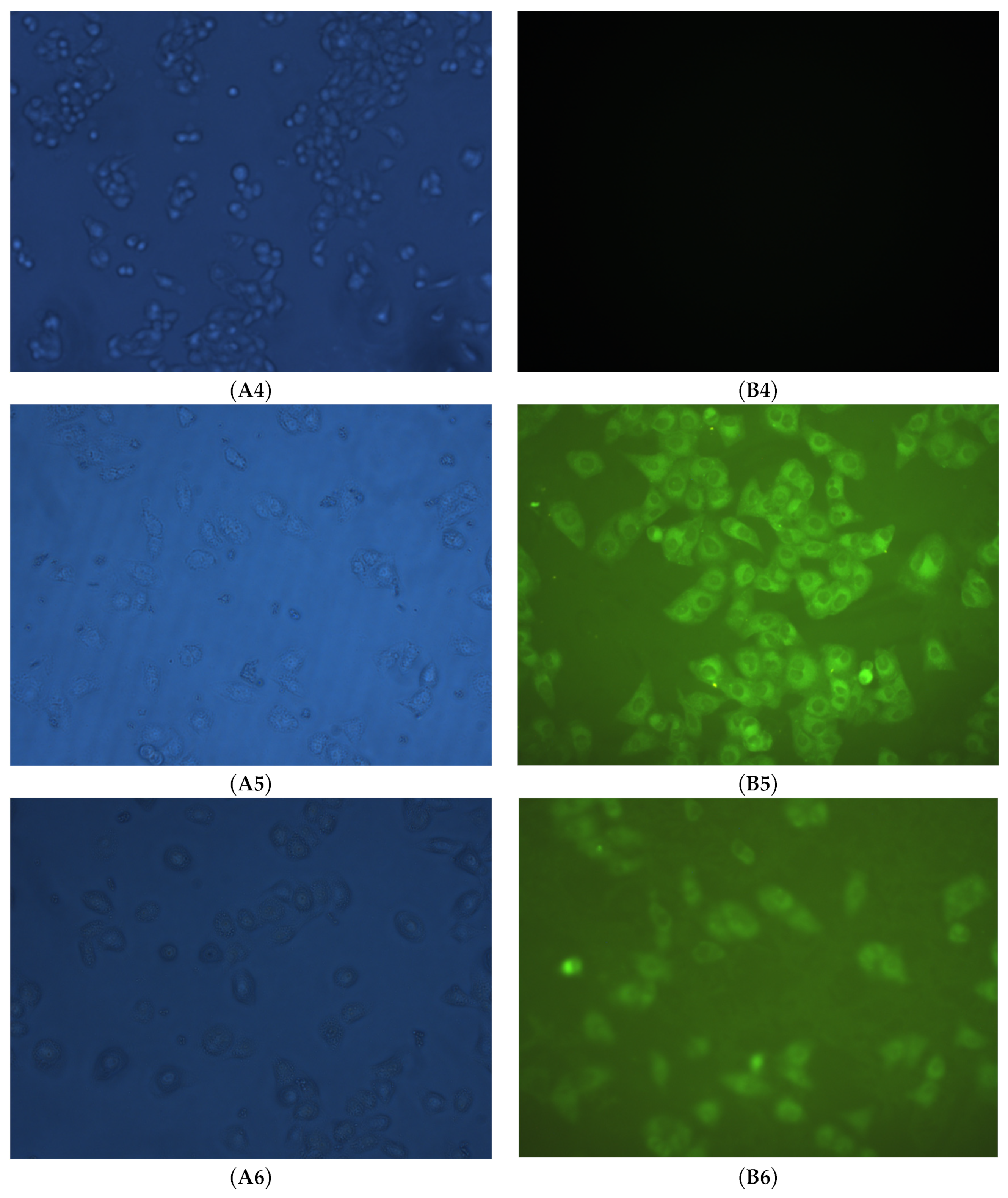
2.4. Cell Uptake and Fluorescent Imaging
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Sulfonamide-Containing Naphthalimide Derivatives (SN-2NI, SD-NI)
3.3. Fluorescent Spectroscopy
3.4. In Vitro Cell Cytotoxicity Assay
3.5. Fluorescent Imaging Assay
3.6. Cell Uptake Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.; Li, Y.; Zhou, A.; Zhang, G.; Jiang, C.S. A new endoplasmic reticulum (ER)-targeting fluorescent probe for the imaging of cysteine in living cells. J. Fluoresc. 2020, 30, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Song, J.B.; Yung, B.C.; Huang, X.H.; Xiong, Y.H.; Chen, X.Y. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Liu, S.; Xia, X.; Chai, Y.; Cai, S.; Liu, H.; Lu, C.; Ma, C.; Nie, J.; Zeng, F.; et al. Near-infrared fluorescent read-out probe for ultra-sensitive imaging of leucine aminopeptidase in vitro and in vivo. Tetrahedron 2021, 99, 132449. [Google Scholar] [CrossRef]
- He, S.; Song, J.; Qu, J.; Cheng, Z. Crucial breakthrough of second near-infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018, 47, 4258–4278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Sun, L.; Yan, Q.; Qiu, X.Y.; Cheng, Y.T.; Wang, B.L.; Tan, X.P.; Fang, M.X.; Luck, R.L.; Liu, H.Y. Near-infrared fluorescent probe based on cyanine scaffold for sensitive detection of uranyl ions in living cells and water samples. Microchem. J. 2022, 180, 107619. [Google Scholar] [CrossRef]
- Su, D.D.; Teoh, C.L.; Wang, L.; Liu, X.G.; Chang, Y.T. Motion-induced change in emission (MICE) for developing fluorescent probes. Chem. Soc. Rev. 2017, 46, 4833–4844. [Google Scholar] [CrossRef] [PubMed]
- Wysockia, L.M.; Lavis, L.D. Advances in the chemistry of small molecule fluorescent probes. Curr. Opin. Chem. Biol. 2011, 15, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.T.; Liu, C.; Jiao, X.J.; Zhao, L.C.; Zeng, X.H. A rational design of fluorescent probes for specific detection and imaging of endogenous formaldehyde in living cells. Tetrahedron 2020, 76, 131617. [Google Scholar]
- Erten-Ela, S.; Ozcelik, S.; Eren, E. Synthesis and photophysical characterizations of thermal-stable naphthalene benzimidazoles. J. Fluoresc. 2011, 21, 1565–1573. [Google Scholar] [CrossRef]
- Paudel, S.; Nandhikonda, P.; Heagy, M.D. A comparative study into two dual fluorescent mechanisms via positional isomers of N-hydroxyarene-1,8-naphthalimides. J. Fluoresc. 2009, 19, 681–691. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.C.; Chen, S.; Yan, G.P.; Zhang, Q.; Guo, Q.Z.; Gu, Y.T. Tumor-targeting fluorescent probe based on 1,8-naphthalimide and porphyrin groups. Chemistryselect 2020, 5, 7680–7684. [Google Scholar] [CrossRef]
- Mahounga, D.M.; Shan, L.L.; Jie, C.; Du, C.L.; Wan, S.N.; Gu, Y.Q. Synthesis of a novel L-methyl-methionine–ICGDer-02 fluorescent probe for in vivo near infrared imaging of tumors. Mol. Imaging Biol. 2012, 14, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.J.; Chong, L.; Petryayeva, E.; Algar, W.R.; Krull, U.J. Quantum dots as contrast agents for in vivo tumor imaging: Progress and issues. Anal. Bioanal. Chem. 2011, 399, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cai, W.B.; Chen, X.Y. Near-Infrared fluorescence imaging of tumor integrin avβ3 expression with Cy7-labeled RGD multimers. Mol. Imaging Biol. 2006, 8, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.B.; Wu, Z.H.; Chen, K.; Ryu, E.K.; Chen, X.Y. 18F-Labeled BBN-RGD heterodimer for prostate cancer imaging. J. Nucl. Med. 2008, 49, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.T.; Hall, D.J.; Liss, M.A.; Hoh, C.K.; Kane, C.J.; Wallace, A.M.; Verab, D.R. Optimization via specific fluorescence brightness of a receptor-targeted probe for optical imaging and positron emission tomography of sentinel lymph nodes. J. Biomed. Opt. 2013, 18, 101315. [Google Scholar] [CrossRef] [PubMed]
- Waschkau, B.; Faust, A.; Schäfers, M.; Bremer, C. Performance of a new fluorescence-labeled MMP inhibitor to image tumor MMP activity in vivo in comparison to an MMP-activatable probe. Contrast Media Mol. Imaging 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Xu, J.X.; Tang, J.B.; Sui, M.H.; Shen, Y.Q. Folate-targeted optical and magnetic resonance dual modality PCL-b-PEG micelles for tumor imaging. Chin. J. Polym. Sci. 2011, 29, 427–430. [Google Scholar] [CrossRef]
- Lv, F.; Li, Y.Z.; Cao, B.; Liu, T.J. Galactose substituted zinc phthalocyanines as near infrared fluorescence probes for liver cancer imaging. J. Mater. Sci. Mater. Med. 2013, 24, 811–819. [Google Scholar] [CrossRef]
- Lolak, N.; Akocak, S.; Bua, S.; Sanku, R.; Supuran, C. Discovery of new ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as carbonic anhydrase I, II, IX and XII inhibitors. Bioorg. Med. Chem. 2019, 27, 1588–1594. [Google Scholar] [CrossRef]
- Meleddu, R.; Distinto, S.; Cottiglia, F.; Angius, R.; Caboni, P.; Angeli, A.; Melis, C.; Deplano, S.; Alcaro, S.; Ortuso, F.; et al. New dihydrothiazole benzensulfonamides: Looking for selectivity toward carbonic anhydrase isoforms I, II, IX, and XII. ACS Med. Chem. Lett. 2020, 11, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Wang, H.Y.; Zhou, C.L. Ring-opening polymerization of ethylene oxide by anion initiation using sulfadiazine as parent compound. J. Appl. Polym. Sci. 1995, 58, 8–11. [Google Scholar] [CrossRef]
- Pal, R.R.; Kim, M.S.; Lee, D.S. Synthesis and pH-dependent micellization of sulfonamide-modified diblock copolymer. Macrom. Res. 2005, 13, 467–476. [Google Scholar] [CrossRef]
- Yan, G.P.; Zheng, C.Y.; Cao, W.; Li, W.; Li, L.Y.; Liu, M.L.; Zhang, Y.X.; Zhou, R.X. Synthesis and preliminary evaluation of gadolinium complexes containing sulfonamide groups as potential MRI contrast agents. Radiography 2003, 9, 35–41. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, F.; Ke, X.J.; Chen, S.; Yan, G.P.; Zhang, Q.; Liang, S.C.; Wang, Y.F.; Jiang, C. Polyaspartamide fluorescent probe containing rhodamine B and Sulfadiazine groups. Chinese J. Org. Chem. 2020, 40, 938–943. [Google Scholar] [CrossRef]
- Liang, S.C.; Yu, H.; Xiang, J.; Yang, W.; Chen, X.H.; Liu, Y.B.; Gao, C.; Yan, G.P. New naphthalimide modified polyethylenimine nanoparticles as fluorescent probe for DNA detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 359–365. [Google Scholar] [CrossRef]
- Rogers, J.E.; Weiss, S.J.; Kelly, L.A. Photoprocesses of naphthalene imide and diimide derivatives in aqueous solutions of DNA. J. Am. Chem. Soc. 2000, 122, 427–436. [Google Scholar] [CrossRef]
- Shi, F.Q. How to copy the disease model of animals. In Medical Animal Experiment Method; People’s Medical Publishing House: Beijing, China, 1990; Chapter 4; pp. 226–232. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-W.; Liu, F.; Shao, C.-T.; Yan, G.-P.; Wu, J.-Y. Synthesis and Characterization of Sulfonamide-Containing Naphthalimides as Fluorescent Probes. Molecules 2024, 29, 2774. https://doi.org/10.3390/molecules29122774
Liu Z-W, Liu F, Shao C-T, Yan G-P, Wu J-Y. Synthesis and Characterization of Sulfonamide-Containing Naphthalimides as Fluorescent Probes. Molecules. 2024; 29(12):2774. https://doi.org/10.3390/molecules29122774
Chicago/Turabian StyleLiu, Zhi-Wei, Fan Liu, Chun-Tao Shao, Guo-Ping Yan, and Jiang-Yu Wu. 2024. "Synthesis and Characterization of Sulfonamide-Containing Naphthalimides as Fluorescent Probes" Molecules 29, no. 12: 2774. https://doi.org/10.3390/molecules29122774





