Composition Distribution of the Thermal Soluble Organics from Naomaohu Lignite and Structural Characteristics of the Corresponding Insoluble Portions
Abstract
:1. Introduction
2. Results and Discussion
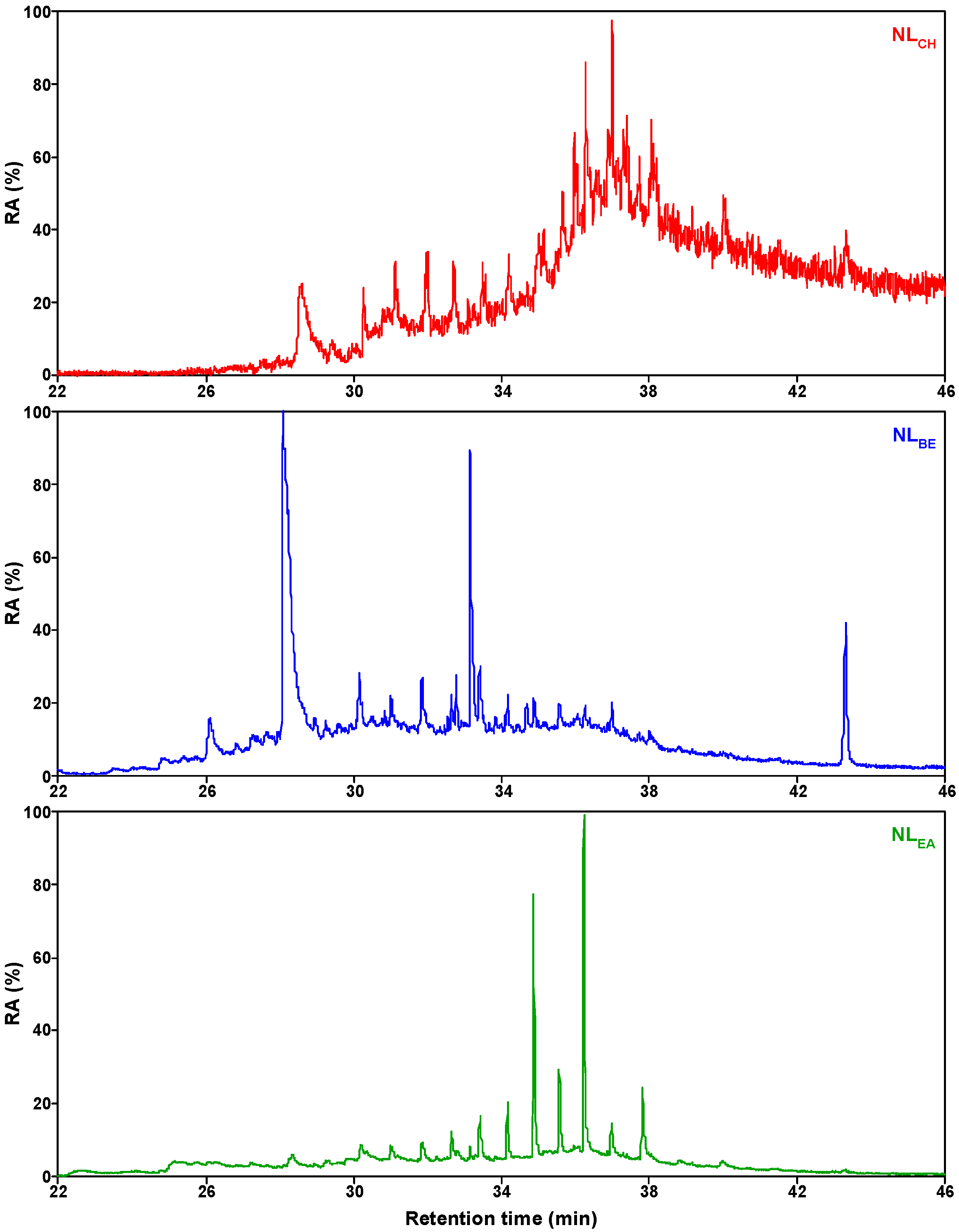
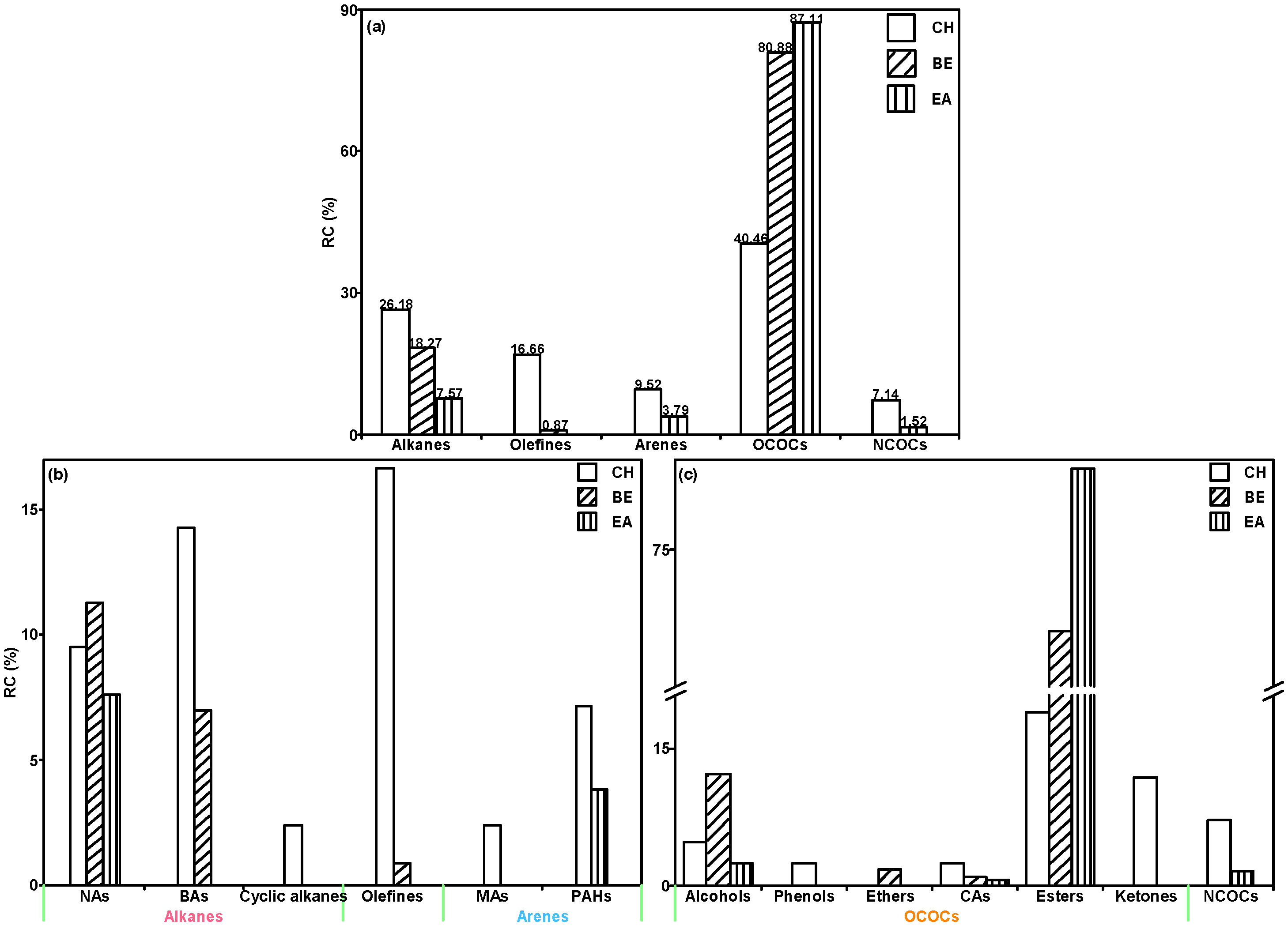
2.1. Composition Distribution of NL Thermal Soluble Organics
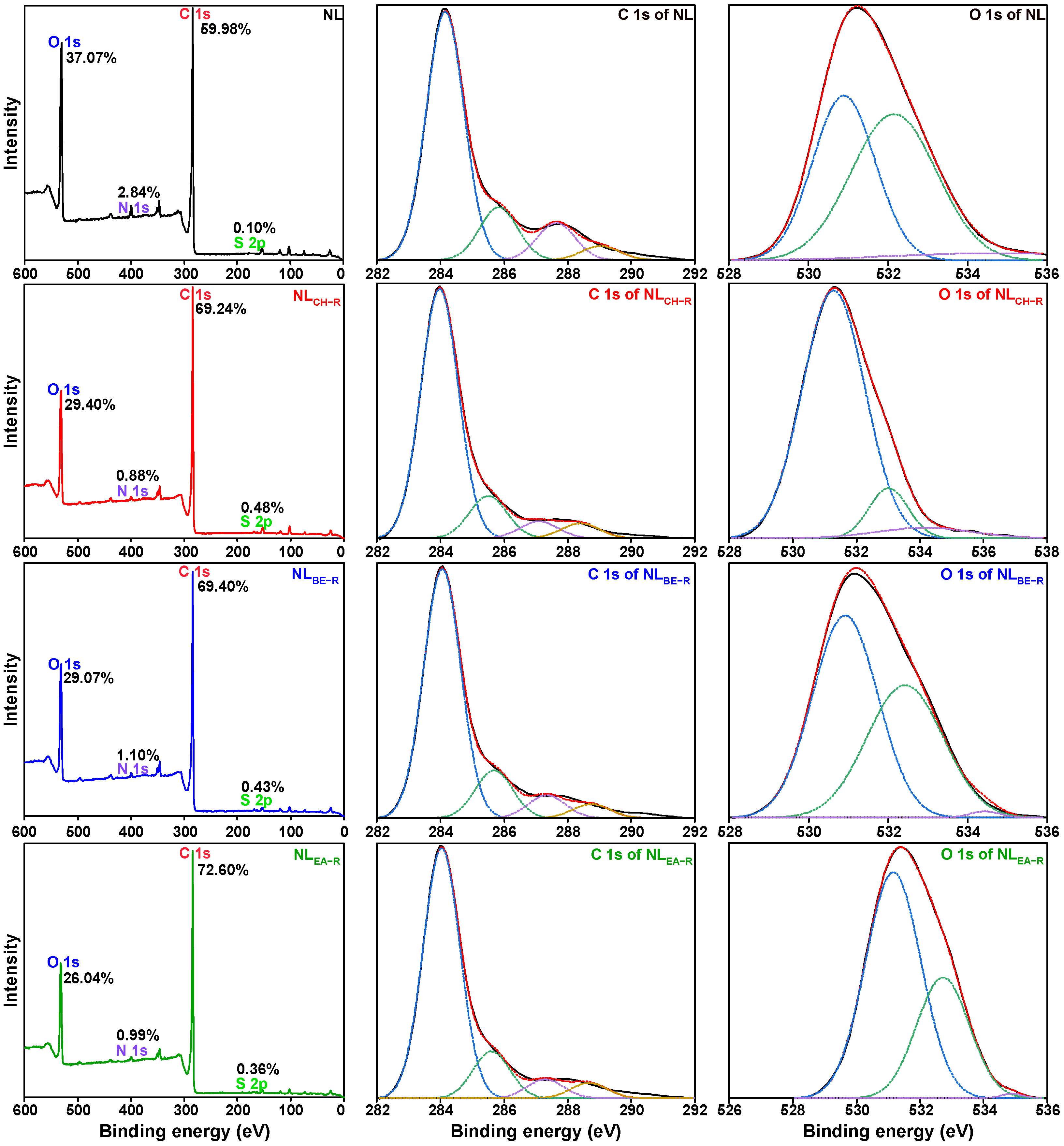
2.2. Impact of Thermal Dissolution on Element Distribution in NL
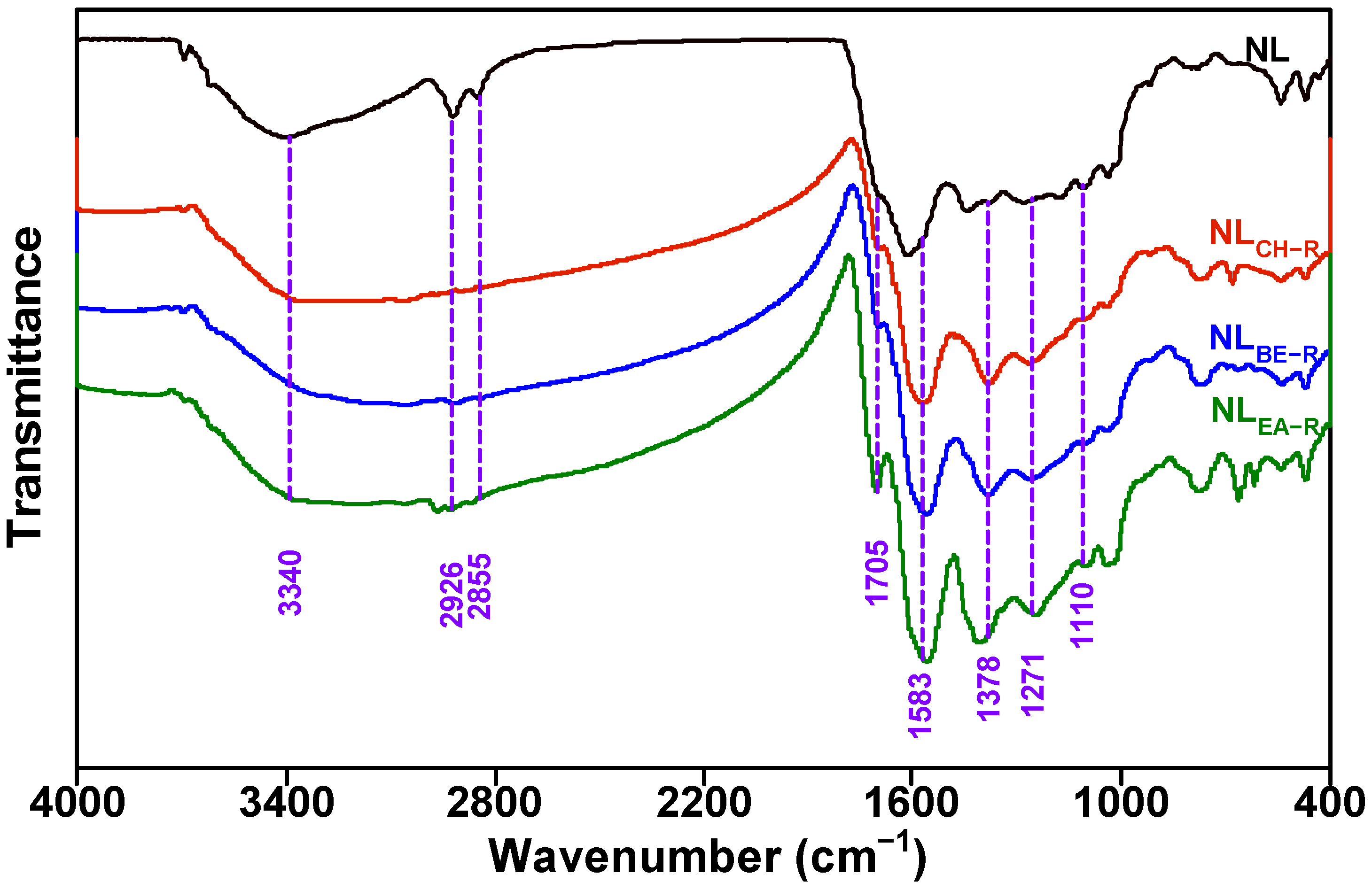
2.3. Changes in Functional Groups of Insoluble Portions
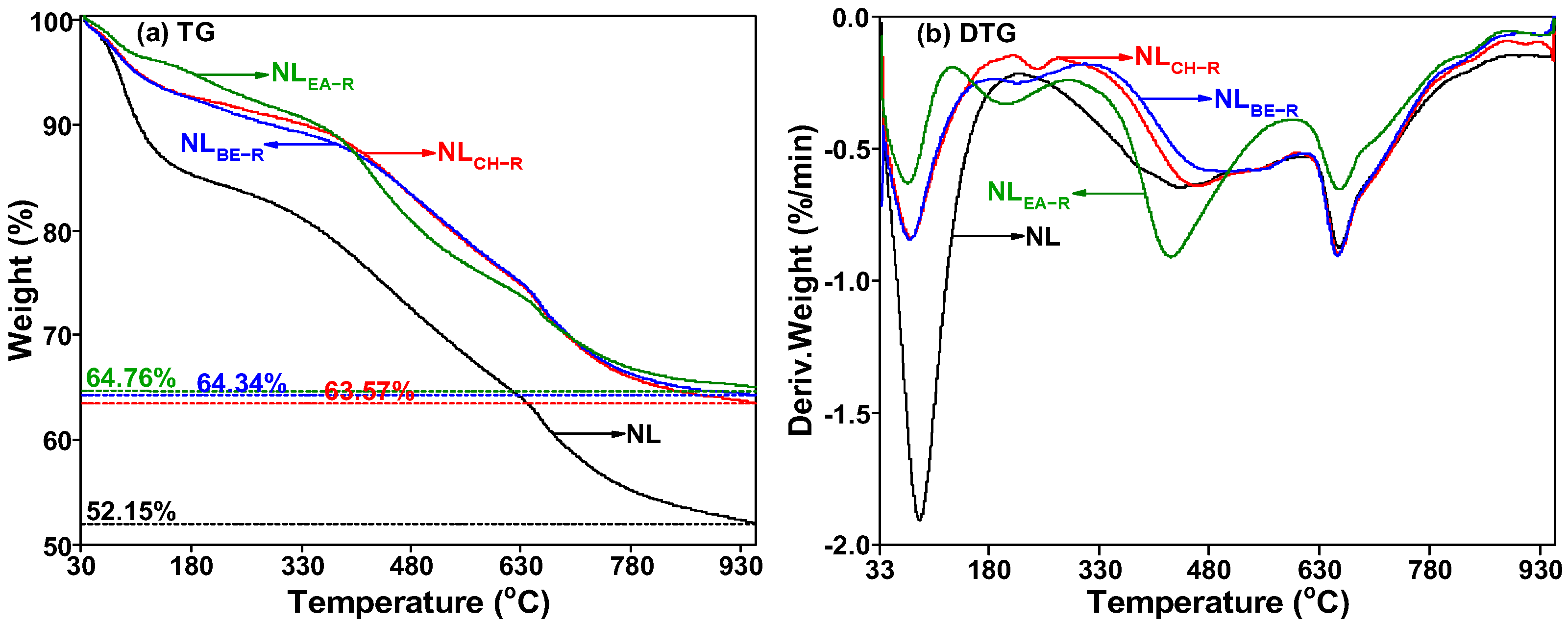
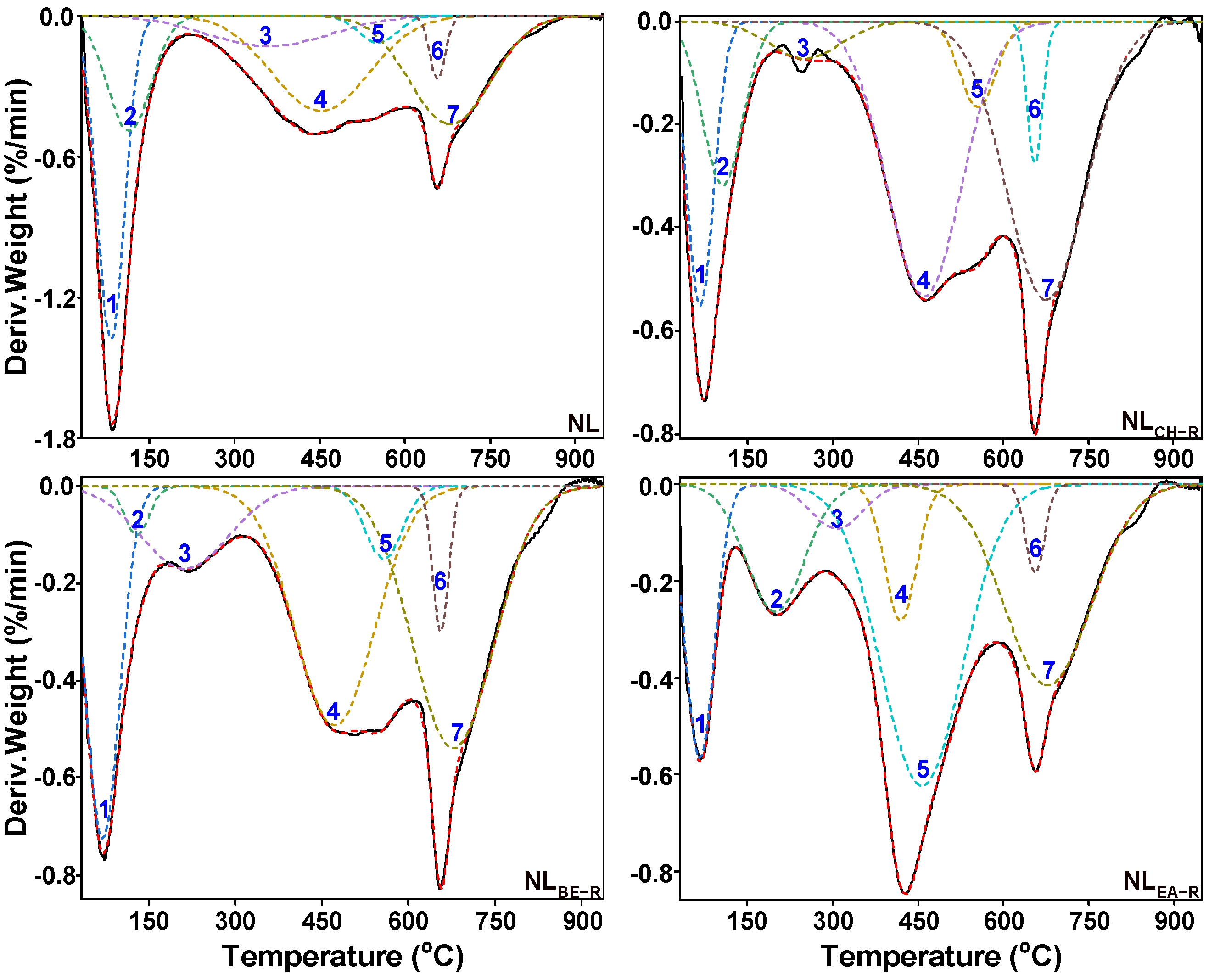
2.4. Thermal Decomposition Characteristics of Insoluble Portions
3. Experimental
3.1. Raw Materials
3.2. Thermal Dissolution
3.3. Analysis Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ju, Y.; Zhu, Y.; Zhou, H.W.; Ge, S.R.; Xie, H.P. Microwave pyrolysis and its applications to the insitu recovery and conversion of oil from tar-rich coal: An overview on fundamentals, methods, and challenges. Energy Rep. 2021, 7, 523–536. [Google Scholar] [CrossRef]
- Wang, S.M.; Shui, Q.M.; Wang, S.Q.; Shen, Y.J.; Sun, Q.; Cai, Y. Oil and gas resource attributes of oil-rich coal and green low-carbon development. J. China Coal Soc. 2021, 46, 1365–1377. [Google Scholar]
- Gao, Y.; Wei, X.Y.; Li, Y.J.; Bai, J.J.; Kang, Y.H.; Liu, G.H.; Ma, X.R.; Li, X.; Lu, C.Y.; Bai, H.C.; et al. Investigation on the composition of soluble portions from the extraction residue of Hanglaiwan subbituminous coal by thermal dissolution and alkanolyses. Fuel 2021, 306, 121747. [Google Scholar] [CrossRef]
- Yin, J.N.; Lin, X.C.; Wang, C.H.; Dai, J.Z.; Wang, Y.G.; Xu, Z.G. Identification of the transformation features of heteroatomic compounds in a low rank coal by combining thermal extraction and various analytical approaches. Fuel 2020, 270, 117480. [Google Scholar] [CrossRef]
- Liu, G.H.; Zong, Z.M.; Ma, Z.H.; Liu, F.J.; Wei, X.Y.; Kang, Y.H.; Fan, X.; Ma, F.Y.; Liu, J.M.; Mo, W.L. Observing the structural variation of Dahuangshan lignite and four derived residues by non-destructive techniques and flash pyrolysis. Fuel 2020, 269, 117335. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Tian, Y.J.; Ding, M.; Dou, Y.Q.; Wei, X.Y.; Fan, X.; He, X.F.; Zong, Z.M. Difference in molecular composition of soluble organic species from two Chinese lignites with different geologic ages. Fuel 2015, 148, 120–126. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Wang, P.; Wu, G.G.; Meng, X.L.; Chu, R.Z.; Wan, Y.Z.; Bai, Z.Q.; Li, W. Structural changes and sodium species redistribution of a typical sodium-rich coal during thermal dissolution with aromatic solvents. Fuel 2021, 286, 119410. [Google Scholar] [CrossRef]
- Rahman, M.; Pudasainee, D.; Gupta, R. Review on chemical upgrading of coal: Production processes, potential applications and recent developments. Fuel Process. Technol. 2017, 158, 35–56. [Google Scholar] [CrossRef]
- Fan, X.; Yu, G.; Wang, M.; Zhao, Y.P.; Wei, X.Y.; Ma, F.Y.; Zhong, M. Insight into the molecular distribution of soluble components from Dayan lignite through mass spectrometers with four ionization methods. Fuel 2018, 227, 177–182. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, X.Y.; Yan, H.L.; Yu, X.Y.; Zong, Z.M. Characterization of soluble portions from thermal dissolution of Zhaotong lignite in cyclohexane and methanol. Fuel Process. Technol. 2016, 151, 131–138. [Google Scholar] [CrossRef]
- Masaki, K.; Yoshida, T.; Li, C.Q.; Takanohashi, T.; Saito, I. The Effects of Pretreatment and the Addition of Polar Compounds on the Production of “HyperCoal” from Subbituminous Coals. Energy Fuels 2004, 18, 995–1000. [Google Scholar] [CrossRef]
- Wang, Z.C.; Li, L.; Shui, H.F.; Lei, Z.P.; Ren, S.B.; Kang, S.G.; Pan, C.X. Thermal dissolution of Xianfeng lignite and infrared spectrum characterization of thermal dissolution. J. Fuel Chem. Technol. 2011, 39, 401–406. [Google Scholar] [CrossRef]
- Wang, T.M.; Zong, Z.M.; Liu, F.J.; Liu, C.; Lv, J.H.; Liu, J.; Zhang, D.D.; Qu, M.; Gui, J.; Liu, X.X.; et al. Investigation on compositional and structural features of Xianfeng lignite through sequential thermal dissolution. Fuel Process. Technol. 2015, 138, 125–132. [Google Scholar] [CrossRef]
- Yang, Z.S.; Zong, Z.M.; Chen, B.; Liu, C.; Zhao, Y.P.; Fan, X.; Wei, X.Y.; Hayashi, J.I. Thermal dissolution of Shengli lignite in ethyl acetate. Chin. Sci. Bull. 2014, 7, 308–321. [Google Scholar] [CrossRef]
- Hao, M.L.; Liang, P.; Zhang, W.R.; Li, S.Z.; Gao, Y.; Jiao, T.T.; Zhang, Y.Q. Investigation on selective separation of oxygen-containing compounds in lignite and sub-bitumite. J. Anal. Appl. Pyrolysis 2023, 171, 105953. [Google Scholar] [CrossRef]
- Xiao, R.H. Coal Tar Chemical Engineering; Metallurgical Industry Press: Beijing, China, 2022. [Google Scholar]
- Hu, X.B.; Xu, H.; Mo, W.L.; Fan, X.; Guo, W.C.; Guo, J.; Niu, J.M.; Mi, H.Y.; Ma, Y.Y.; Wei, X.Y. Effect of sequential thermal dissolution on the structure and pyrolysis characteristics of Naomaohu lignite. Fuel 2023, 331, 125930. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.B.; Yang, H.Y.; Liu, Q. Chemical Composition and Structural Characteristics of Oil Shales and Their Kerogens Using Fourier Transform Infrared (FTIR) Spectroscopy and Solid-State 13C Nuclear Magnetic Resonance (NMR). Energy Fuels 2016, 30, 6271–6280. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Fernández-González, M.C.; Cuerda-Correa, E.M.; Macías-García, A.; Alexandre-Franco, M.F.; Rojas-Cervantes, M.L. Physico-chemical properties of low-rank coals: Thermal and demineralisation effects. Powder Technol. 2004, 148, 38–42. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, P.H.; Chen, G.; Pei, J.T.; Sun, S.Z.; Liu, L.; Liu, H.P. Selective enrichment of chemical structure during first grinding of Zhundong coal and its effect on pyrolysis reactivity. Fuel 2017, 189, 46–56. [Google Scholar] [CrossRef]
- Xiong, G.; Li, Y.S.; Jin, L.J.; Hu, H.Q. In situ FT-IR spectroscopic studies on thermal decomposition of the weak covalent bonds of brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 262–267. [Google Scholar] [CrossRef]
- Kan, H.; Wang, Y.; Mo, W.L.; Wei, X.Y.; Mi, H.Y.; Ma, K.J.; Zhu, M.X.; Guo, W.C.; Guo, J.; Niu, J.M.; et al. Effect of solvent swelling with different enhancement methods on the microstructure and pyrolysis performance of Hefeng subbituminous coal. Fuel 2023, 332, 126066. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.Y.; Mo, W.L.; Gong, W.T.; Ma, F.Y.; Wei, X.Y.; Fan, X.; Zhang, S.P. FT-IR analysis of functional groups of step-by-step extract and raffinate of Hefeng bituminous coal. J. Fuel Chem. Technol. 2021, 49, 890–901. [Google Scholar] [CrossRef]
- Hu, X.B.; Yang, X.Q.; Mo, W.L.; Zhang, S.P.; Gao, J.; Wei, X.Y.; Fan, X. Structural characteristics and thermal conversion performance of ash and slag in circulating fluidized bed coal gasifier. J. Fuel Chem. Technol. 2022, 50, 1361–1370. [Google Scholar] [CrossRef]
- Li, H.; Liang, S.S.; Hou, Y.C.; Wang, Y.P.; Ren, S.H.; Wu, W.Z. A study on the structure of Naomaohu coal and its suitability for direct coal liquefaction. Fuel Process. Technol. 2022, 227, 107135. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wei, X.Y.; Lv, J.H.; Zong, Z.M. Study on the oxygen forms in soluble portions from thermal dissolution and alkanolyses of the extraction residue from Baiyinhua lignite. Fuel 2020, 260, 116301. [Google Scholar] [CrossRef]
- Liu, C.M.; Zong, Z.M.; Jia, J.X.; Liu, G.F.; Wei, X.Y. An evidence for the strong association of N-methyl-2-pyrrolidinone with some organic species in three Chinese bituminous coals. Chin. Sci. Bull. 2008, 53, 1157–1164. [Google Scholar] [CrossRef]
- Liang, S.S.; Hou, Y.C.; Wu, W.Z.; Li, L.; Ren, S.H. New Insights into the Primary Reaction Products of Naomaohu Coal via Breaking Weak Bonds with Supercritical Ethanolysis. Energy Fuels 2019, 33, 6294–6301. [Google Scholar] [CrossRef]
- Kang, Y.H.; Ma, Z.Y.; Zhang, X.Q.; Wei, X.Y.; Li, Y.J.; Liu, G.H.; Wang, A.M.; Ma, X.R.; Yan, L.; Zong, Z.M.; et al. Investigation on the structural features of Hecaogou subbituminous coal and its residues by multiple technical strategies. Fuel 2022, 309, 122111. [Google Scholar] [CrossRef]
- Kang, Y.H.; Chen, T.; Gao, J.; Li, F.; Hu, L.; Liu, G.H.; Lu, C.Y.; Li, Y.J.; Wei, X.Y.; Ma, Y.J.; et al. Comprehensive investigation of the mechanisms for pyrolyzing macromolecular networks in Hecaogou subbituminous coal by comparing the ethanolysis and flash pyrolysis. Fuel 2022, 324, 124619. [Google Scholar] [CrossRef]
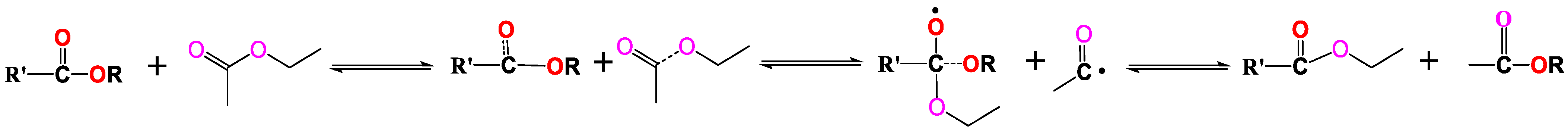

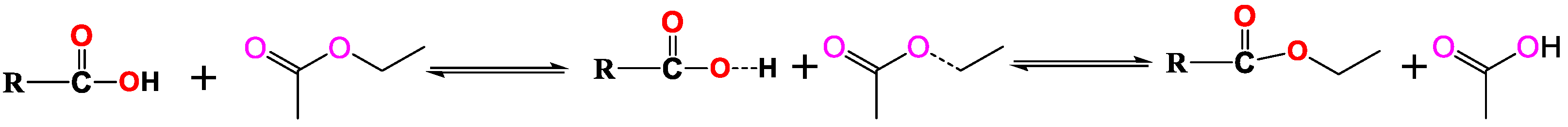

| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (daf, wt.%) | H/C | O/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | FCdaf | C | H | N | S | Odiff | |||
| NL | 10.77 | 11.48 | 53.41 | 46.59 | 67.43 | 3.92 | 0.94 | 0.26 | 27.45 | 0.70 | 0.31 |
| NLCH-R | 5.83 | 12.75 | 57.86 | 42.14 | 74.91 | 3.21 | 1.03 | 0.26 | 20.59 | 0.51 | 0.21 |
| NLBE-R | 5.94 | 11.88 | 56.63 | 43.37 | 74.54 | 3.25 | 1.02 | 0.24 | 20.95 | 0.52 | 0.21 |
| NLEA-R | 5.00 | 10.78 | 67.39 | 32.61 | 75.55 | 3.87 | 0.93 | 0.19 | 19.46 | 0.61 | 0.19 |
| Wavenumber/cm−1 | Functional Group | Content (Area, %) | |||
|---|---|---|---|---|---|
| NL | NLCH-R | NLBE-R | NLEA-R | ||
| 3600–3500 | OH-π | 5.15 | 3.39 | 12.50 | 11.12 |
| 3500–3350 | Self-associated OH | 39.75 | 29.29 | 34.79 | 35.18 |
| 3350–3260 | OH-ether O | 25.94 | 35.05 | 17.69 | 27.90 |
| 3260–3000 | Cyclic OH | 29.16 | 32.27 | 35.01 | 25.80 |
| 3000–2930 | Asymmetric aliphatic −CH3 | 11.90 | 25.27 | 32.78 | 30.79 |
| 2930–2900 | Asymmetric aliphatic −CH2 | 44.10 | 38.70 | 17.43 | 21.35 |
| 2900–2870 | Aliphatic −CH | 15.97 | 7.89 | 15.42 | 21.38 |
| 2870–2810 | Symmetric aliphatic −CH2 | 28.03 | 28.14 | 34.37 | 26.49 |
| 1700 | Carboxylic acids C=O | 8.00 | 3.68 | 5.34 | 8.63 |
| 1650 | Conjugated C=O | 14.37 | 10.61 | 10.78 | 17.50 |
| 1600–1480 | C=C in ARs | 29.34 | 27.32 | 22.63 | 18.74 |
| 1480–1400 | Asymmetric −CH3, −CH2 | 13.03 | 15.41 | 14.65 | 13.39 |
| 1400–1240 | Symmetric −CH3 | 4.76 | 24.22 | 33.48 | 22.85 |
| 1240–1160 | Phenols C−OH | 19.50 | 9.47 | 9.83 | 10.43 |
| 1160–1090 | Grease C−O | 7.96 | 5.91 | 2.48 | 5.60 |
| 1090–1030 | Alkyl ethers | 3.05 | 3.38 | 0.80 | 2.86 |
| 860–810 | Four adjacent H deformation | 24.31 | 9.56 | 11.87 | 9.06 |
| 810–750 | Three adjacent H deformation | 40.12 | 62.89 | 72.10 | 84.39 |
| 750–720 | Two adjacent H deformation | 35.57 | 27.55 | 16.03 | 6.55 |
| Elemental Peak | Functionality | Binding Energy (eV) | Molar Content (%) | |||
|---|---|---|---|---|---|---|
| NL | NLCH-R | NLBE-R | NLEA-R | |||
| C 1s | C−C | 284.0 | 70.45 | 77.19 | 74.68 | 75.55 |
| C−H | 285.6 | 14.99 | 13.04 | 14.30 | 14.17 | |
| C=O | 287.3 | 10.54 | 5.18 | 6.82 | 5.67 | |
| COO | 288.7 | 4.01 | 4.59 | 4.20 | 4.61 | |
| O 1s | C=O | 531.1 | 42.41 | 84.06 | 56.16 | 65.94 |
| C−O | 532.7 | 51.51 | 10.60 | 43.03 | 33.52 | |
| COO | 534.5 | 6.08 | 5.35 | 0.81 | 0.54 | |
| Peak | Possible Origin | PTR (°C) |
|---|---|---|
| 1–3 | Cleavage of weak bonds (e.g., >Cal−O−, >Cal−N<, and >Cal−S−), release of combined water and decarboxylation | <400 |
| 4 | Breakage of relatively strong bonds (e.g., >Cal−Cal<, >Cal−H, >Cal−O and >Car−N) | 380–500 |
| 5 | Dissociation of strong bonds (e.g., >Car−Cal<, > Car−O− and >Car−S−) | 440–610 |
| 6–7 | CO2 release from carbonate decomposition and condensation of aromatic rings to release H2 | >610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Ma, Y.; Mo, W.; Hao, S.; Wei, X.; Fan, X.; Ren, T.; Ma, K.; Guo, J. Composition Distribution of the Thermal Soluble Organics from Naomaohu Lignite and Structural Characteristics of the Corresponding Insoluble Portions. Molecules 2024, 29, 2776. https://doi.org/10.3390/molecules29122776
Zhu M, Ma Y, Mo W, Hao S, Wei X, Fan X, Ren T, Ma K, Guo J. Composition Distribution of the Thermal Soluble Organics from Naomaohu Lignite and Structural Characteristics of the Corresponding Insoluble Portions. Molecules. 2024; 29(12):2776. https://doi.org/10.3390/molecules29122776
Chicago/Turabian StyleZhu, Meixia, Yaya Ma, Wenlong Mo, Shihao Hao, Xianyong Wei, Xing Fan, Tiezhen Ren, Kongjun Ma, and Jia Guo. 2024. "Composition Distribution of the Thermal Soluble Organics from Naomaohu Lignite and Structural Characteristics of the Corresponding Insoluble Portions" Molecules 29, no. 12: 2776. https://doi.org/10.3390/molecules29122776






