Asymmetrical Diketopyrrolopyrrole Derivatives with Improved Solubility and Balanced Charge Transport Properties
Abstract
1. Introduction
2. Results and Discussion
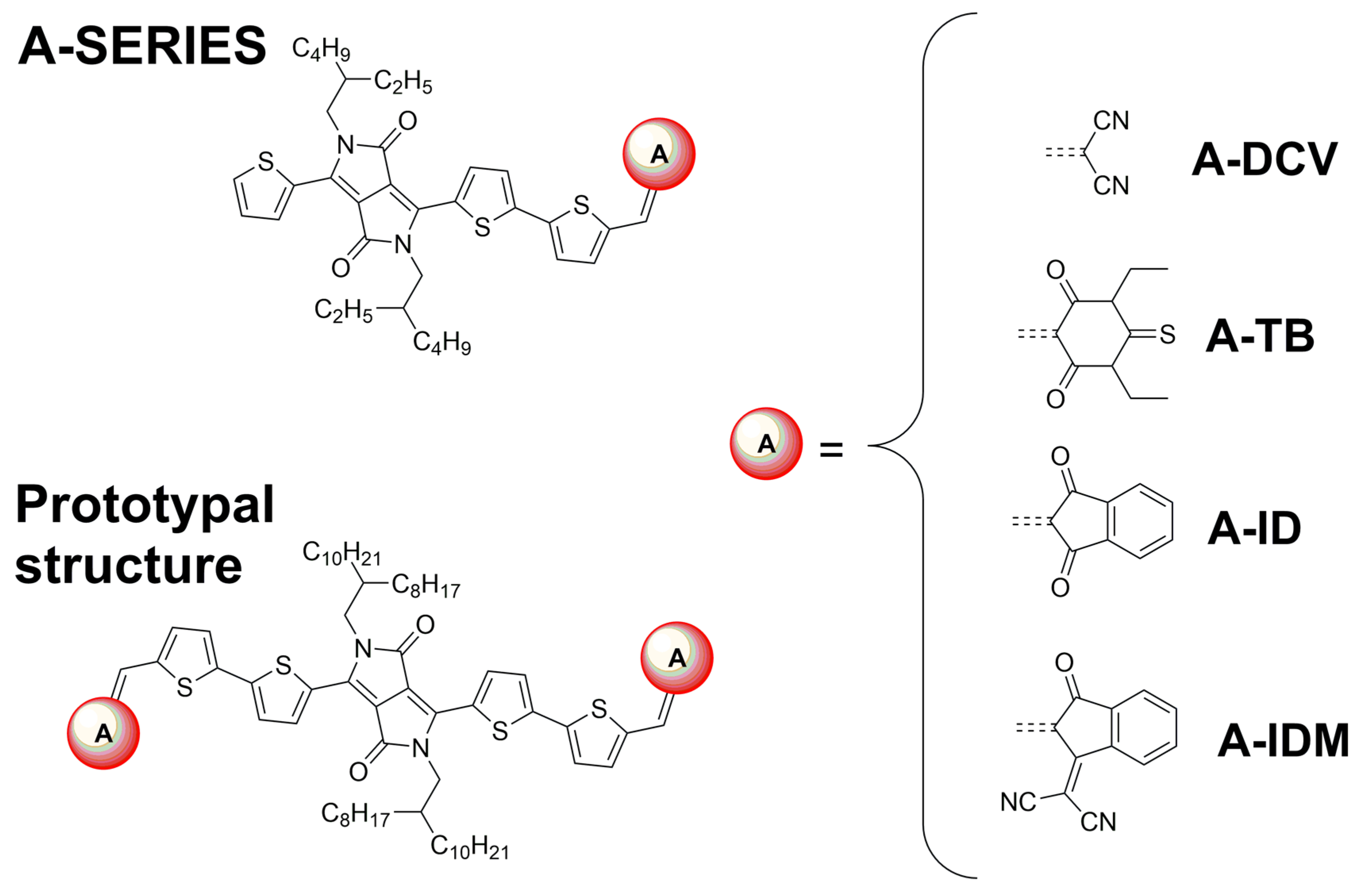
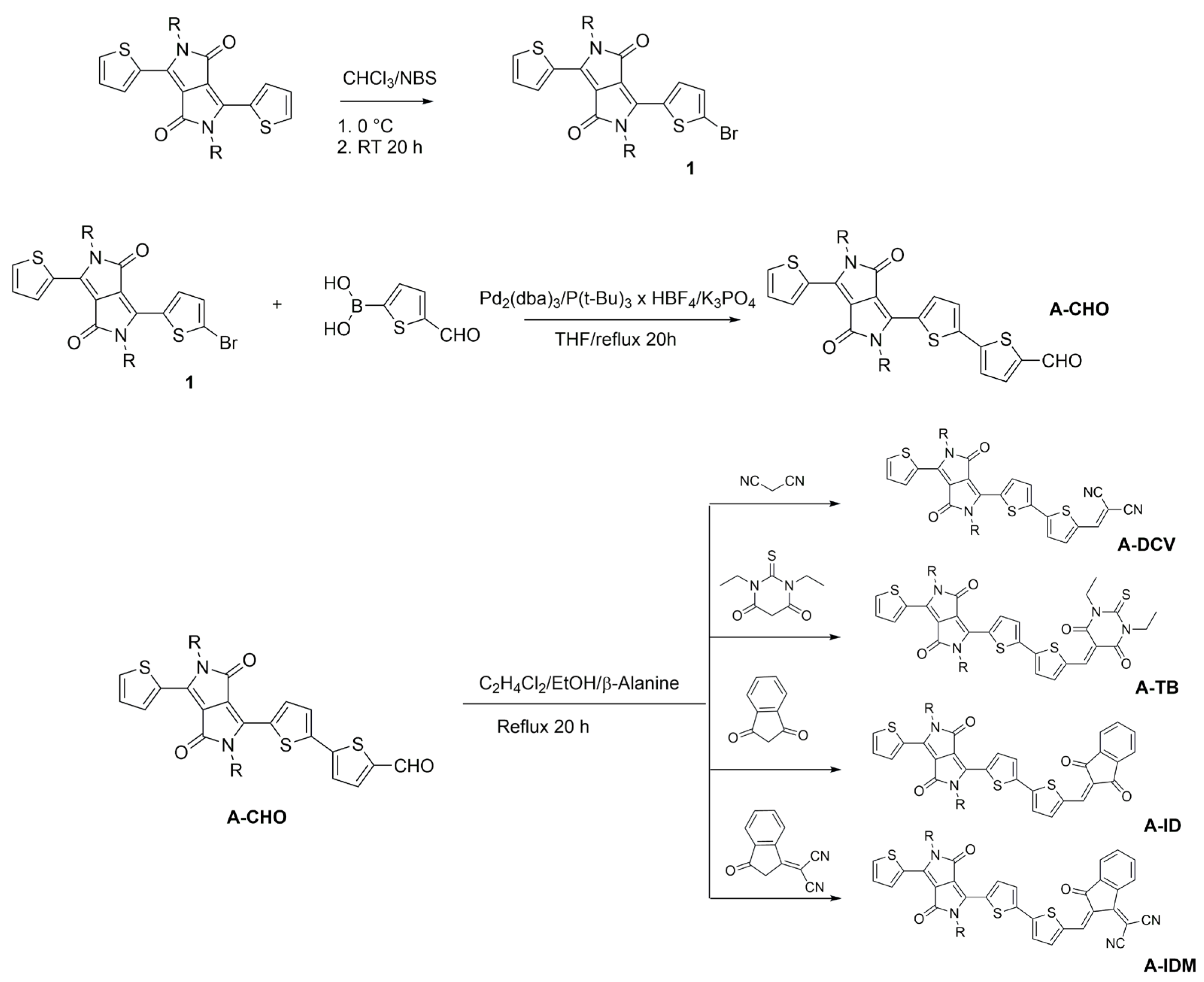
2.1. Synthesis, Structural Properties, and Solubility
2.2. Optical Characterization
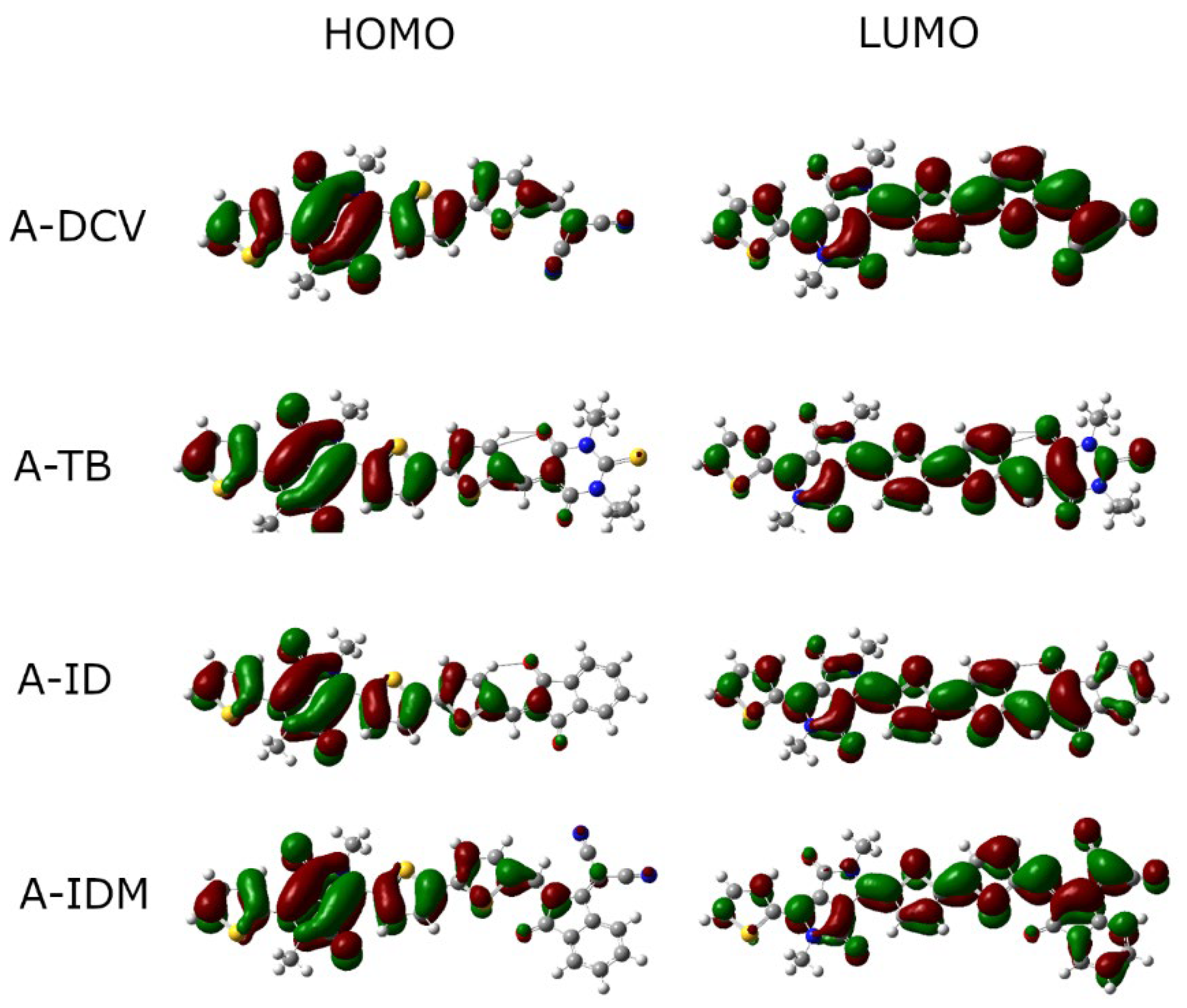
2.3. DFT Analysis
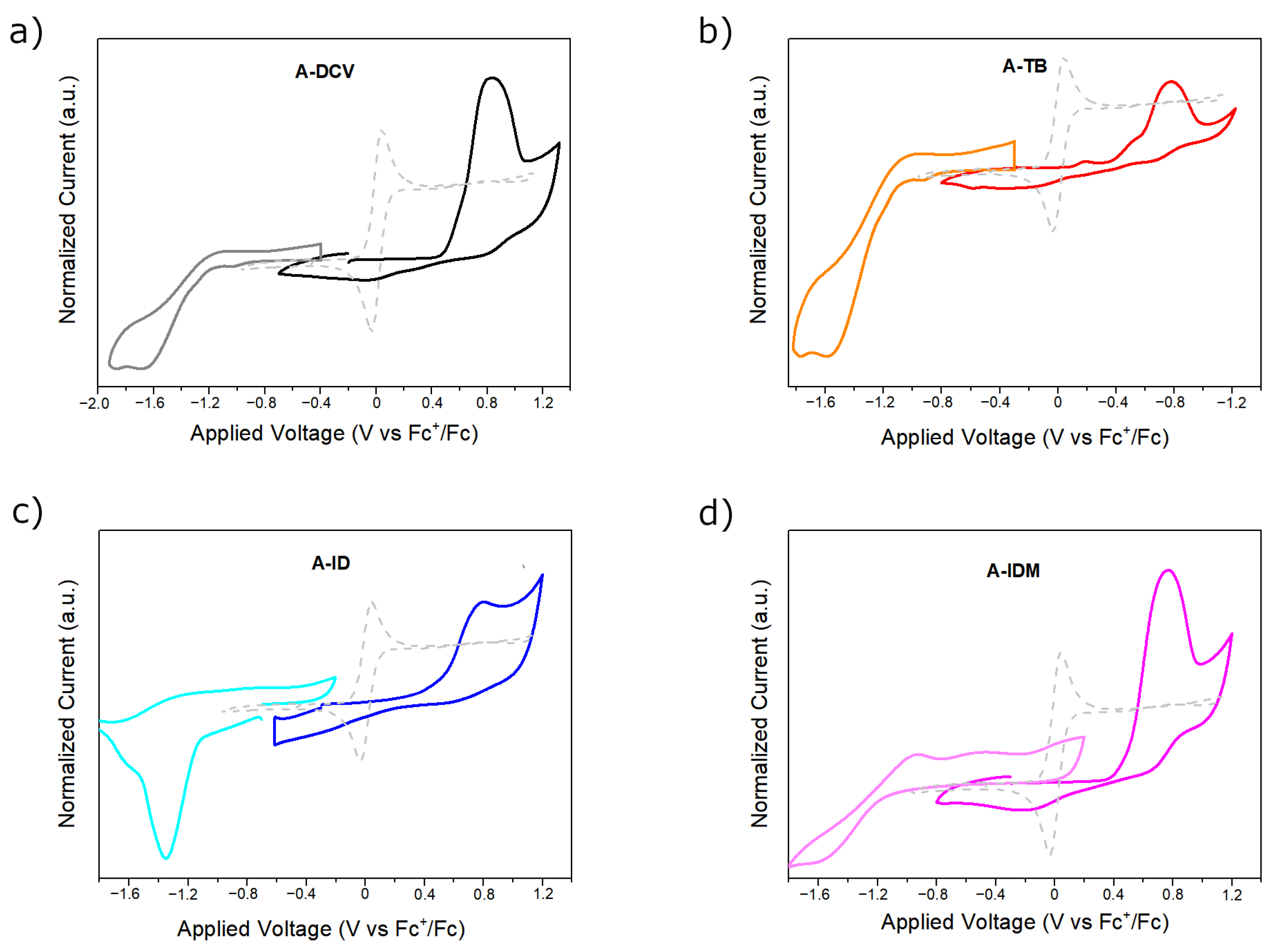
2.4. Electrochemical Characterization
2.5. Electrical Characterization
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of 3-(5-Bromothiophen-2-yl)-2,5-bis(2-ethylhexyl)-6-(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (1)
3.2.2. Synthesis of 5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophene-2-carbaldehyde (A-CHO)
3.2.3. Synthesis of 2-((5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)methylene)malononitrile (A-DCV)
3.2.4. Synthesis of 3-(5-((1,3-Diethyl-4,6-dioxo-5-thioxotetrahydropyrimidin-2(1H)-ylidene)methyl)thiophen-2-yl)-2,5-bis(2-ethylhexyl)-6-(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (A-TB)
3.2.5. Synthesis of 3-(5-((1,3-Dioxo-1,3-dihydro-2H-inden-2-ylidene)methyl)thiophen-2-yl)-2,5-bis(2-ethylhexyl)-6-(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (A-ID)
3.2.6. Synthesis of (E)-2-(2-((5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophen-2-yl)methylene)-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (A-IDM)
3.3. Solubility Measurements
3.4. DFT Studies
3.5. Electrical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Facchetti, A. The Journey of Conducting Polymers from Discovery to Application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef] [PubMed]
- 35 Years of Organic Transistors. Nat. Electron. 2021, 4, 541. [CrossRef]
- Basiricò, L.; Mattana, G.; Mas-Torrent, M. Editorial: Organic Electronics: Future Trends in Materials, Fabrication Techniques and Applications. Front. Phys. 2022, 10, 888155. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, W.; Sirringhaus, H.; Müllen, K. Recent Progress in Emerging Organic Semiconductors. Adv. Mater. 2022, 34, 2108701. [Google Scholar] [CrossRef]
- Landi, A.; Peluso, A.; Troisi, A. Quantitative Prediction of the Electro-Mechanical Response in Organic Crystals. Adv. Mater. 2021, 33, 2008049. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A Large-Area, Flexible Pressure Sensor Matrix with Organic Field-Effect Transistors for Artificial Skin Applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef]
- Li, Y.; Wan, J.; Smilgies, D.M.; Miller, R.; Headrick, R.L. Enhancement of Charge Transfer in Thermally-Expanded and Strain-Stabilized TIPS-Pentacene Thin Films. Phys. Rev. Res. 2020, 2, 033294. [Google Scholar] [CrossRef]
- Riede, M.; Spoltore, D.; Leo, K. Organic Solar Cells—The Path to Commercial Success. Adv. Energy Mater. 2021, 11, 2002653. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible Polymer Transistors with High Pressure Sensitivity for Application in Electronic Skin and Health Monitoring. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Buga, C.S.; Viana, J.C. A Review on Materials and Technologies for Organic Large-Area Electronics. Adv. Mater. Technol. 2021, 6, 2001016. [Google Scholar] [CrossRef]
- Park, S.; Takakuwa, M.; Fukuda, K.; Lee, S.; Yokota, T.; Someya, T. Toward Ultraflexible Organic Electronic Devices. MRS Bull. 2023, 48, 999–1012. [Google Scholar] [CrossRef]
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; García-Frutos, E.M.; Hennrich, G.; Gómez-Lor, B. Organic Semiconductors toward Electronic Devices: High Mobility and Easy Processability. J. Phys. Chem. Lett. 2012, 3, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C.; Sariciftci, N.S.; Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857. [Google Scholar] [CrossRef]
- Schweda, B.; Reinfelds, M.; Hofstadler, P.; Trimmel, G.; Rath, T. Recent Progress in the Design of Fused-Ring Non-Fullerene Acceptors-Relations between Molecular Structure and Optical, Electronic, and Photovoltaic Properties. ACS Appl. Energy Mater. 2021, 4, 11899–11981. [Google Scholar] [CrossRef]
- Tang, C.G.; Hou, K.; Leong, W.L. The Quest for Air Stability in Organic Semiconductors. Chem. Mater. 2024, 36, 28–53. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for Organic Transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Li, M.; Zheng, J.; Wang, X.; Yu, R.; Wang, Y.; Qiu, Y.; Cheng, X.; Wang, G.; Chen, G.; Xie, K.; et al. Light-Responsive Self-Strained Organic Semiconductor for Large Flexible OFET Sensing Array. Nat. Commun. 2022, 13, 4912. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Rogatch, M.; Chen, H.; Guo, X.; Tang, J. Supramolecular Design and Assembly Engineering toward High-Performance Organic Field-Effect Transistors. Acc. Mater. Res. 2024, 5, 505–517. [Google Scholar] [CrossRef]
- Liu, K.; Ouyang, B.; Guo, X.; Guo, Y.; Liu, Y. Advances in Flexible Organic Field-Effect Transistors and Their Applications for Flexible Electronics. npj Flex. Electron. 2022, 6, 1. [Google Scholar] [CrossRef]
- Gómez-Bombarelli, R.; Aguilera-Iparraguirre, J.; Hirzel, T.D.; Duvenaud, D.; Maclaurin, D.; Blood-Forsythe, M.A.; Chae, H.S.; Einzinger, M.; Ha, D.G.; Wu, T.; et al. Design of Efficient Molecular Organic Light-Emitting Diodes by a High-Throughput Virtual Screening and Experimental Approach. Nat. Mater. 2016, 15, 1120–1127. [Google Scholar] [CrossRef]
- Landi, A.; Borrelli, R.; Capobianco, A.; Velardo, A.; Peluso, A. Second-Order Cumulant Approach for the Evaluation of Anisotropic Hole Mobility in Organic Semiconductors. J. Phys. Chem. C 2018, 122, 25849–25857. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Atahan-Evrenk, S.; Mondal, R.; Akkerman, H.B.; Sánchez-Carrera, R.S.; Granados-Focil, S.; Schrier, J.; Mannsfeld, S.C.B.; Zoombelt, A.P.; Bao, Z.; et al. From Computational Discovery to Experimental Characterization of a High Hole Mobility Organic Crystal. Nat. Commun. 2011, 2, 437. [Google Scholar] [CrossRef]
- Robb, M.J.; Ku, S.Y.; Brunetti, F.G.; Hawker, C.J. A Renaissance of Color: New Structures and Building Blocks for Organic Electronics. J. Polym. Sci. A Polym. Chem. 2013, 51, 1263–1271. [Google Scholar] [CrossRef]
- Gsänger, M.; Bialas, D.; Huang, L.; Stolte, M.; Würthner, F. Organic Semiconductors Based on Dyes and Color Pigments. Adv. Mater. 2016, 28, 3615–3645. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882. [Google Scholar] [CrossRef]
- Grzybowski, M.; Gryko, D.T. Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties. Adv. Opt. Mater. 2015, 3, 280–320. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent Advances in the Development of Semiconducting DPP-Containing Polymers for Transistor Applications. Adv. Mater. 2013, 25, 1859–1880. [Google Scholar] [CrossRef]
- Bao, W.W.; Li, R.; Dai, Z.C.; Tang, J.; Shi, X.; Geng, J.T.; Deng, Z.F.; Hua, J. Diketopyrrolopyrrole (DPP)-Based Materials and Its Applications: A Review. Front. Chem. 2020, 8, 567625. [Google Scholar] [CrossRef]
- Naik, M.A.; Patil, S. Diketopyrrolopyrrole-Based Conjugated Polymers and Small Molecules for Organic Ambipolar Transistors and Solar Cells. J. Polym. Sci. A Polym. Chem. 2013, 51, 4241–4260. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, N.; Ni, W.; Kan, B.; Li, M.; Zhang, Q.; Wan, X.; Chen, Y. Diketopyrrolopyrrole Based Small Molecules with near Infrared Absorption for Solution Processed Organic Solar Cells. Dye. Pigment. 2016, 126, 173–178. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Zhang, Y.; Yan, N.; You, S.; Li, W. Diketopyrrolopyrrole-Based Conjugated Materials for Non-Fullerene Organic Solar Cells. J. Mater. Chem. A Mater. 2019, 7, 10174–10199. [Google Scholar] [CrossRef]
- Hendriks, K.H.; Heintges, G.H.L.; Gevaerts, V.S.; Wienk, M.M.; Janssen, R.A.J. High-Molecular-Weight Regular Alternating Diketopyrrolopyrrole-Based Terpolymers for Efficient Organic Solar Cells. Angew. Chem.-Int. Ed. 2013, 52, 8341–8344. [Google Scholar] [CrossRef]
- Ko, E.Y.; Choi, S.; Park, G.E.; Lee, D.H.; Cho, M.J.; Choi, D.H. Diketopyrrolopyrrole-Based Conjugated Small Molecules Bearing Two Different Acceptor Moieties for Organic Solar Cells. Synth. Met. 2016, 221, 39–47. [Google Scholar] [CrossRef]
- Li, W.; Hendriks, K.H.; Wienk, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar] [CrossRef]
- Suraru, S.-L.; Zschieschang, U.; Klauk, H.; Würthner, F. Diketopyrrolopyrrole as a P-Channel Organic Semiconductor for High Performance OTFTs. Chem. Commun. 2011, 47, 1767–1769. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Zhang, K.; Zhang, Z.; Sun, Q.; Yang, W. Thionating Iso-Diketopyrrolopyrrole-Based Polymers: From p-Type to Ambipolar Field Effect Transistors with Enhanced Charge Mobility. Polym. Chem. 2018, 9, 1807–1814. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Y.; Du, T.; Wang, Z.; Liang, Z.; Han, Y.; Deng, Y.; Tian, H.; Geng, Y. Diketopyrrolopyrrole-Based Small Molecules for Solution-Processed n-Channel Organic Thin Film Transistors. J. Mater. Chem. C Mater. 2019, 7, 13939–13946. [Google Scholar] [CrossRef]
- Qiao, Y.; Guo, Y.; Yu, C.; Zhang, F.; Xu, W.; Liu, Y.; Zhu, D. Diketopyrrolopyrrole-Containing Quinoidal Small Molecules for High-Performance, Air-Stable, and Solution-Processable n-Channel Organic Field-Effect Transistors. J. Am. Chem. Soc. 2012, 134, 4084–4087. [Google Scholar] [CrossRef]
- Chen, H.Y.; Nikolka, M.; Wadsworth, A.; Yue, W.; Onwubiko, A.; Xiao, M.; White, A.J.P.; Baran, D.; Sirringhaus, H.; McCulloch, I. A Thieno[2,3-b]Pyridine-Flanked Diketopyrrolopyrrole Polymer as an n-Type Polymer Semiconductor for All-Polymer Solar Cells and Organic Field-Effect Transistors. Macromolecules 2018, 51, 71–79. [Google Scholar] [CrossRef]
- Lin, G.; Qin, Y.; Zhang, J.; Guan, Y.S.; Xu, H.; Xu, W.; Zhu, D. Ambipolar Organic Field-Effect Transistors Based on Diketopyrrolopyrrole Derivatives Containing Different π-Conjugating Spacers. J. Mater. Chem. C Mater. 2016, 4, 4470–4477. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, D.; Huang, J.; Mao, Z.; Zhang, W.; Yu, G. Thiazole-Flanked Diketopyrrolopyrrole Polymeric Semiconductors for Ambipolar Field-Effect Transistors with Balanced Carrier Mobilities. ACS Appl. Mater. Interfaces 2016, 8, 34725–34734. [Google Scholar] [CrossRef]
- Riaño, A.; Mayorga Burrezo, P.; Mancheño, M.J.; Timalsina, A.; Smith, J.; Facchetti, A.; Marks, T.J.; López Navarrete, J.T.; Segura, J.L.; Casado, J.; et al. The Unusual Electronic Structure of Ambipolar Dicyanovinyl-Substituted Diketopyrrolopyrrole Derivatives. J. Mater. Chem. C Mater. 2014, 2, 6376–6386. [Google Scholar] [CrossRef]
- Fusco, S.; Barra, M.; Bonomo, M.; Cassinese, A.; Centore, R.; Chiarella, F.; Senneca, F.; Carella, A. Novel DPP Derivatives Functionalized with Auxiliary Electron-Acceptor Groups and Characterized by Narrow Bandgap and Ambipolar Charge Transport Properties. Dye. Pigment. 2021, 186, 109026. [Google Scholar] [CrossRef]
- Fusco, S.; Barra, M.; Gontrani, L.; Bonomo, M.; Chianese, F.; Galliano, S.; Centore, R.; Cassinese, A.; Carbone, M.; Carella, A. Novel Thienyl DPP Derivatives Functionalized with Terminal Electron-Acceptor Groups: Synthesis, Optical Properties and OFET Performance. Chem.—A Eur. J. 2022, 28, e202104552. [Google Scholar] [CrossRef]
- MacHui, F.; Abbott, S.; Waller, D.; Koppe, M.; Brabec, C.J. Determination of Solubility Parameters for Organic Semiconductor Formulations. Macromol. Chem. Phys. 2011, 212, 2159–2165. [Google Scholar] [CrossRef]
- Yao, X.; Shao, W.; Xiang, X.; Xiao, W.J.; Liang, L.; Zhao, F.G.; Ling, J.; Lu, Z.; Li, J.; Li, W.S. Side Chain Engineering on a Small Molecular Semiconductor: Balance between Solubility and Performance by Choosing Proper Positions for Alkyl Side Chains. Org. Electron. 2018, 61, 56–64. [Google Scholar] [CrossRef]
- He, Y.; Kukhta, N.A.; Marks, A.; Luscombe, C.K. The Effect of Side Chain Engineering on Conjugated Polymers in Organic Electrochemical Transistors for Bioelectronic Applications. J. Mater. Chem. C Mater. 2022, 10, 2314–2332. [Google Scholar] [CrossRef]
- Pinal, R. Effect of Molecular Symmetry on Melting Temperature and Solubility. Org. Biomol. Chem. 2004, 2, 2692–2699. [Google Scholar] [CrossRef]
- Hu, H.; Ge, J.; Chen, Z.; Song, W.; Xie, L.; Ge, Z. The Asymmetric Strategy of Small-Molecule Materials for Organic Solar Cells. Adv. Energy Mater. 2024, 14, 2304242. [Google Scholar] [CrossRef]
- Carella, A.; Franzini, M.; Fusco, S.; Centore, R.; Barra, M.; Chiarella, F.; Cassinese, A.; Bonomo, M.; Nejrotti, S.; Carbone, M.; et al. Isoindigo Dyes Functionalized with Terminal Electron-Withdrawing Groups: Computational, Optical and Electrical Characterization. Dye. Pigment. 2023, 208, 110866. [Google Scholar] [CrossRef]
- Coluccini, C.; Manfredi, N.; Salamone, M.M.; Ruffo, R.; Lobello, M.G.; De Angelis, F.; Abbotto, A. Quaterpyridine Ligands for Panchromatic Ru(II) Dye Sensitizers. J. Org. Chem. 2012, 77, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; Padula, D. Multiple Charge Separation Pathways in New-Generation Non-Fullerene Acceptors: A Computational Study. J. Mater. Chem. A Mater. 2021, 9, 24849–24856. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Zaumseil, J.; Sirringhaus, H. Electron and Ambipolar Transport in Organic Field-Effect Transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.D. Charge Carrier Traps in Organic Semiconductors: A Review on the Underlying Physics and Impact on Electronic Devices. J. Mater. Chem. C Mater. 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilizaion of AB Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Landi, A.; Landi, A.; Velardo, A.; Peluso, A. Efficient Charge Dissociation of Triplet Excitons in Bulk Heterojunction Solar Cells. ACS Appl. Energy Mater. 2022, 5, 10815–10824. [Google Scholar] [CrossRef]
- Paternò, G.M.; Barbero, N.; Galliano, S.; Barolo, C.; Lanzani, G.; Scotognella, F.; Borrelli, R. Excited State Photophysics of Squaraine Dyes for Photovoltaic Applications: An Alternative Deactivation Scenario. J. Mater. Chem. C Mater. 2018, 6, 2778–2785. [Google Scholar] [CrossRef]
- Riccio, M.; Irace, A.; Breglio, G.; Rossi, L.; Barra, M.; Di Girolamo, F.V.; Cassinese, A. Current Distribution Effects in Organic Sexithiophene Field Effect Transistors Investigated by Lock-in Thermography: Mobility Evaluation Issues. Appl. Phys. Lett. 2008, 93, 243504. [Google Scholar] [CrossRef]
- Li, S.; Li, C.Z.; Shi, M.; Chen, H. New Phase for Organic Solar Cell Research: Emergence of Y-Series Electron Acceptors and Their Perspectives. ACS Energy Lett. 2020, 5, 1554–1567. [Google Scholar] [CrossRef]
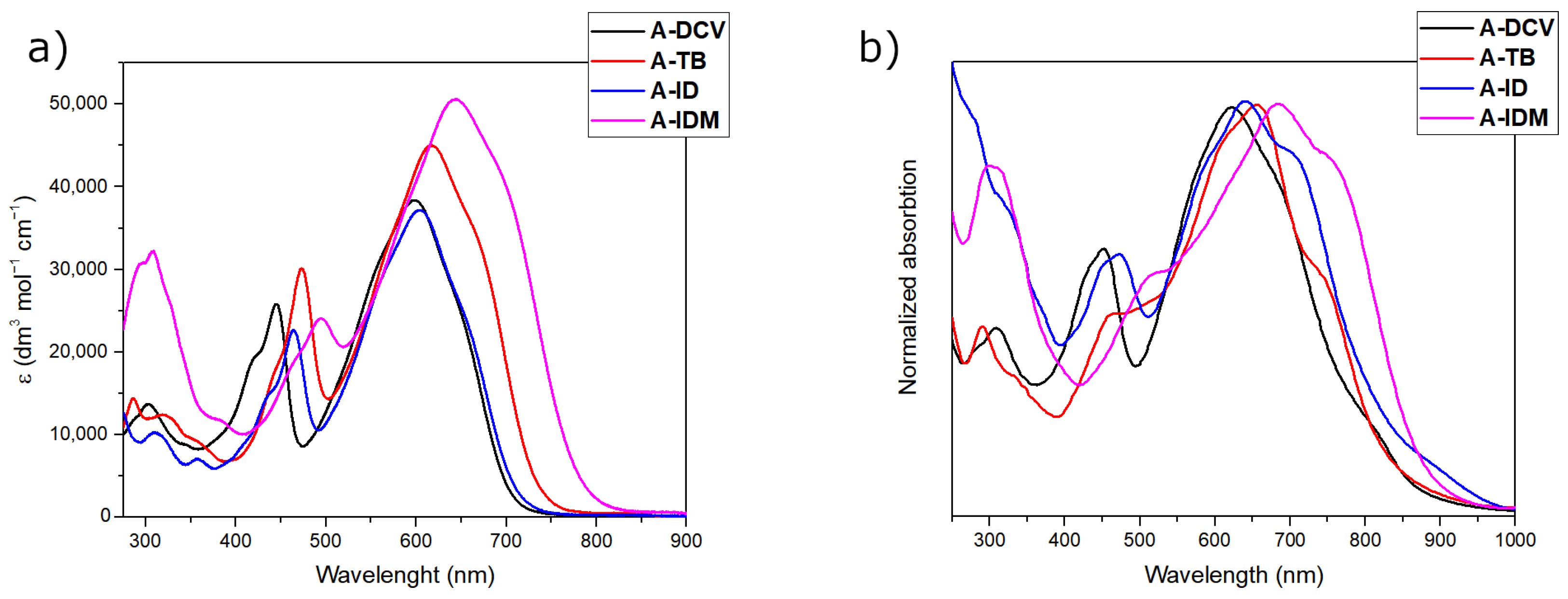

| Dyes | λmax (nm) in CHCl3 | ε (dm3·mol−1·cm−1) in CHCl3 | λmax (nm) in THF | ε (dm3·mol−1·cm−1) in THF | λmax (nm) as Thin Film 1 | Eg(eV) 2 |
|---|---|---|---|---|---|---|
| A-DCV | 598/445 | 3.35 × 104/2.23 × 104 | 592/439 | 4.51 × 104/2.87 × 104 | 667/620/451 | 1.63 |
| A-TB | 616/473 | 4.01 × 104/2.72 × 104 | 610/468 | 4.88 × 104/3.12 × 104 | 732/655/515 | 1.53 |
| A-ID | 605/464 | 4.36 × 104/2.75 × 104 | 599/457 | 4.46 × 104/2.66 × 104 | 702/640/473 | 1.51 |
| A-IDM | 644/495 | 4.63 × 104/2.18 × 104 | 631/489 | 4.85 × 104/2.19 × 104 | 746/685/520 | 1.48 |
| Dyes | λabs (nm) | F | µGS (D) | µES (D) | HOMO (eV) | LUMO (eV) | Eg (eV) | EgEXP(eV) 1 |
|---|---|---|---|---|---|---|---|---|
| A-DCV | 682 | 1.27 | 11.90 | 13.58 | −5.64 | −3.45 | 1.82 | 1.76 |
| A-TB | 697 | 1.57 | 9.19 | 11.82 | −5.62 | −3.46 | 1.78 | 1.70 |
| A-ID | 674 | 1.60 | 3.27 | 5.15 | −5.56 | −3.34 | 1.84 | 1.75 |
| A-IDM | 744 | 1.46 | 9.58 | 11.67 | −5.60 | −3.57 | 1.66 | 1.60 |
| Dyes 1 | Eox (V) 2 | Ered(V) 3 | HOMO (eV) 4 | LUMO (eV) 5 | Egec (eV) 6 |
|---|---|---|---|---|---|
| A-DCV | 0.58 | −1.25 | −5.68 | −3.85 | 1.83 |
| A-TB | 0.52 | −1.14 | −5.62 | −3.96 | 1.66 |
| A-ID | 0.50 | −1.16 | −5.60 | −3.94 | 1.66 |
| A-IDM | 0.48 | −1.15 | −5.58 | −3.95 | 1.63 |
| Dyes | µeaver (cm2/V·s) | µemax (cm2/V·s) | Vth,eaver (V) | µhaver (cm2/V·s) | µhmax (cm2/V·s) | Vth,haver (V) |
|---|---|---|---|---|---|---|
| A-DCV | (6.5 ± 3.7)·10−5 | 1.1·10−4 | 21.3 ± 1.5 | (1.8 ± 0.9)·10−5 | 2.8·10−5 | −22.9 ± 0.3 |
| A-TB | (1.7 ± 0.7)·10−4 | 2.6·10−4 | 18.4 ± 1.7 | (3.7 ± 1.4)·10−5 | 5.2·10−5 | −16.5 ± 2.7 |
| A-IDM | (1.8 ± 0.5)·10−4 | 2.4·10−4 | 15.1 ± 4.8 | (6.6 ± 0.4)·10−5 | 7.1·10−5 | −21.0 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carella, A.; Landi, A.; Bonomo, M.; Chiarella, F.; Centore, R.; Peluso, A.; Nejrotti, S.; Barra, M. Asymmetrical Diketopyrrolopyrrole Derivatives with Improved Solubility and Balanced Charge Transport Properties. Molecules 2024, 29, 2805. https://doi.org/10.3390/molecules29122805
Carella A, Landi A, Bonomo M, Chiarella F, Centore R, Peluso A, Nejrotti S, Barra M. Asymmetrical Diketopyrrolopyrrole Derivatives with Improved Solubility and Balanced Charge Transport Properties. Molecules. 2024; 29(12):2805. https://doi.org/10.3390/molecules29122805
Chicago/Turabian StyleCarella, Antonio, Alessandro Landi, Matteo Bonomo, Fabio Chiarella, Roberto Centore, Andrea Peluso, Stefano Nejrotti, and Mario Barra. 2024. "Asymmetrical Diketopyrrolopyrrole Derivatives with Improved Solubility and Balanced Charge Transport Properties" Molecules 29, no. 12: 2805. https://doi.org/10.3390/molecules29122805
APA StyleCarella, A., Landi, A., Bonomo, M., Chiarella, F., Centore, R., Peluso, A., Nejrotti, S., & Barra, M. (2024). Asymmetrical Diketopyrrolopyrrole Derivatives with Improved Solubility and Balanced Charge Transport Properties. Molecules, 29(12), 2805. https://doi.org/10.3390/molecules29122805








