Synthesis and Cheminformatics-Directed Antibacterial Evaluation of Echinosulfonic Acid-Inspired Bis-Indole Alkaloids
Abstract
1. Introduction
2. Results
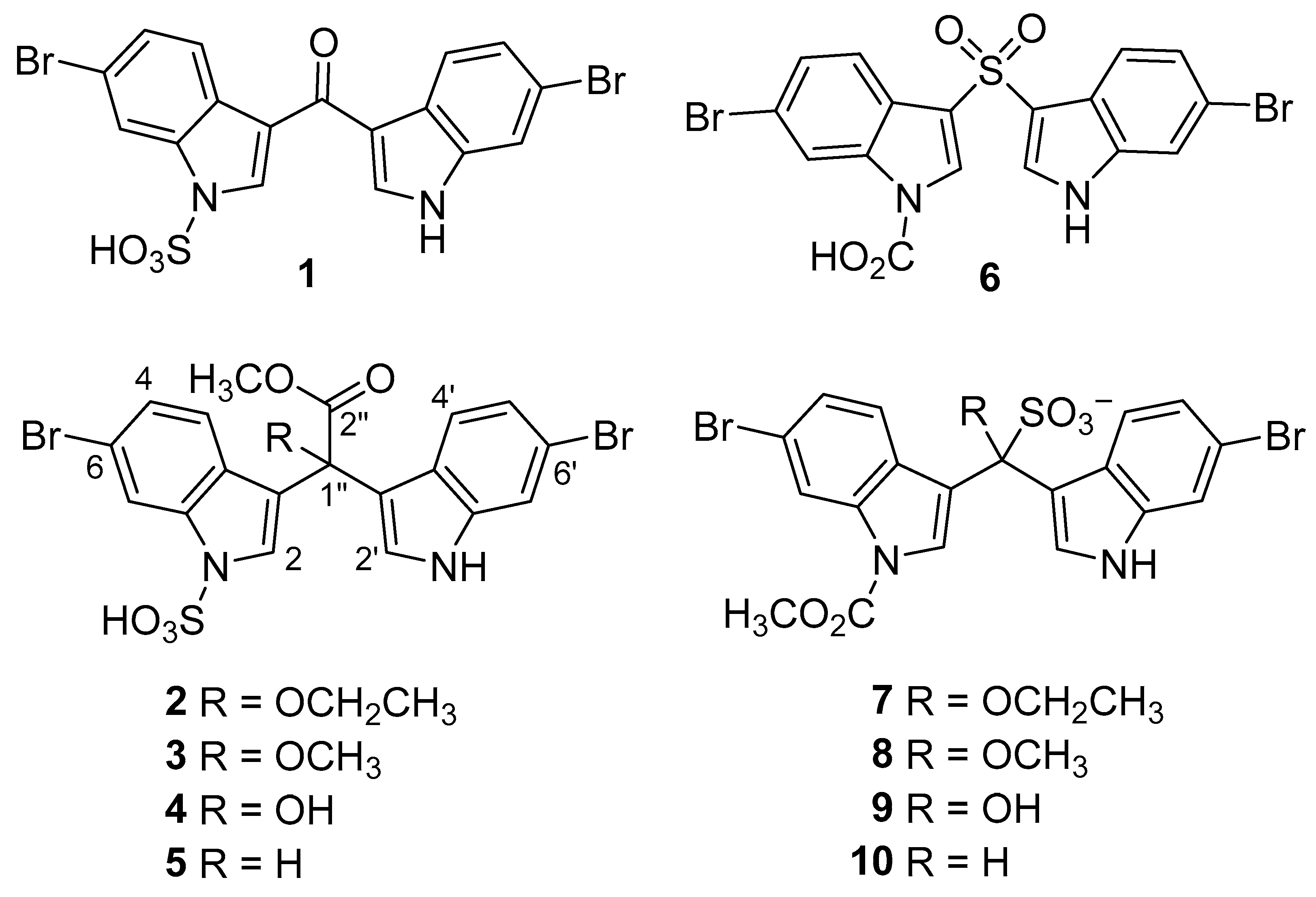
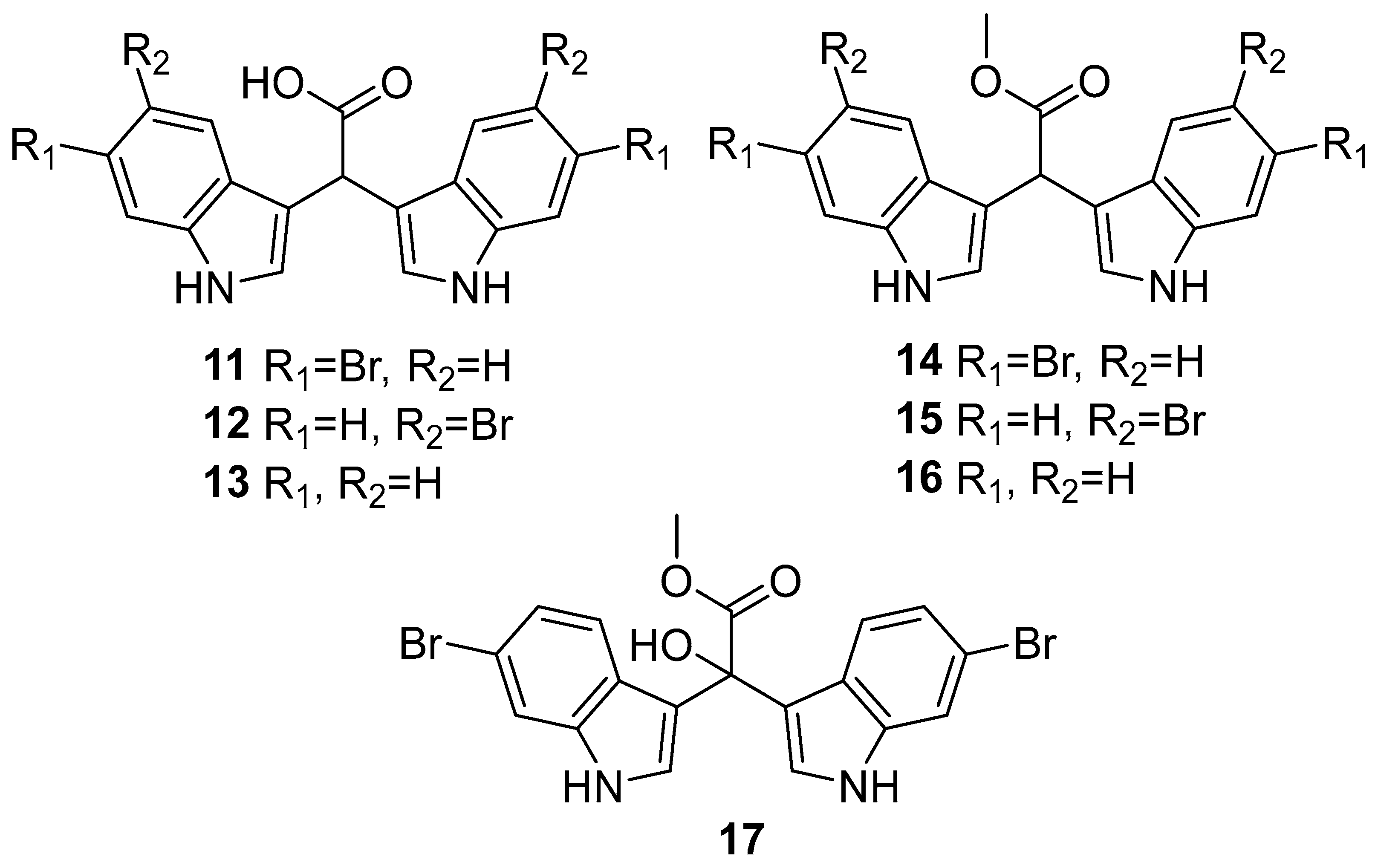
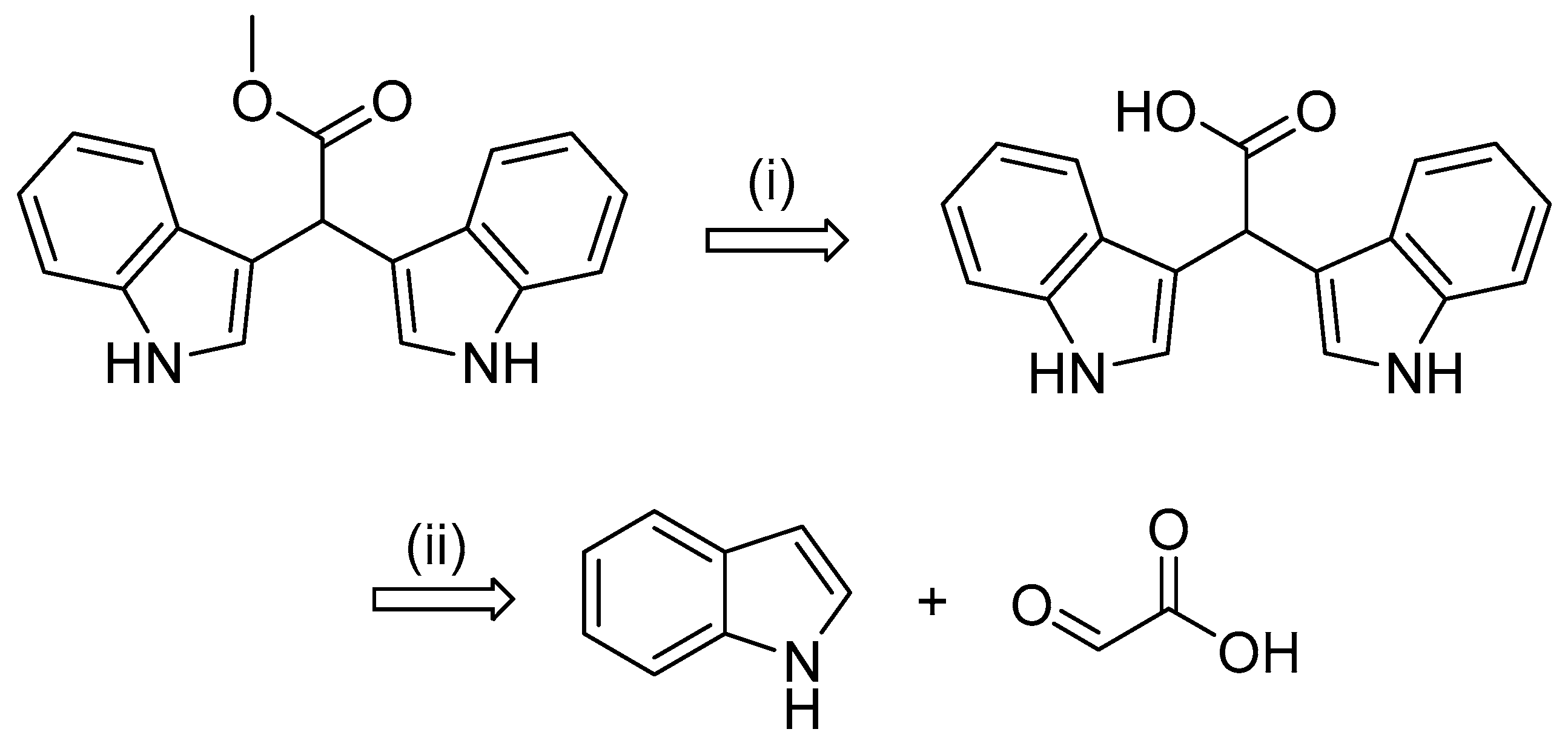
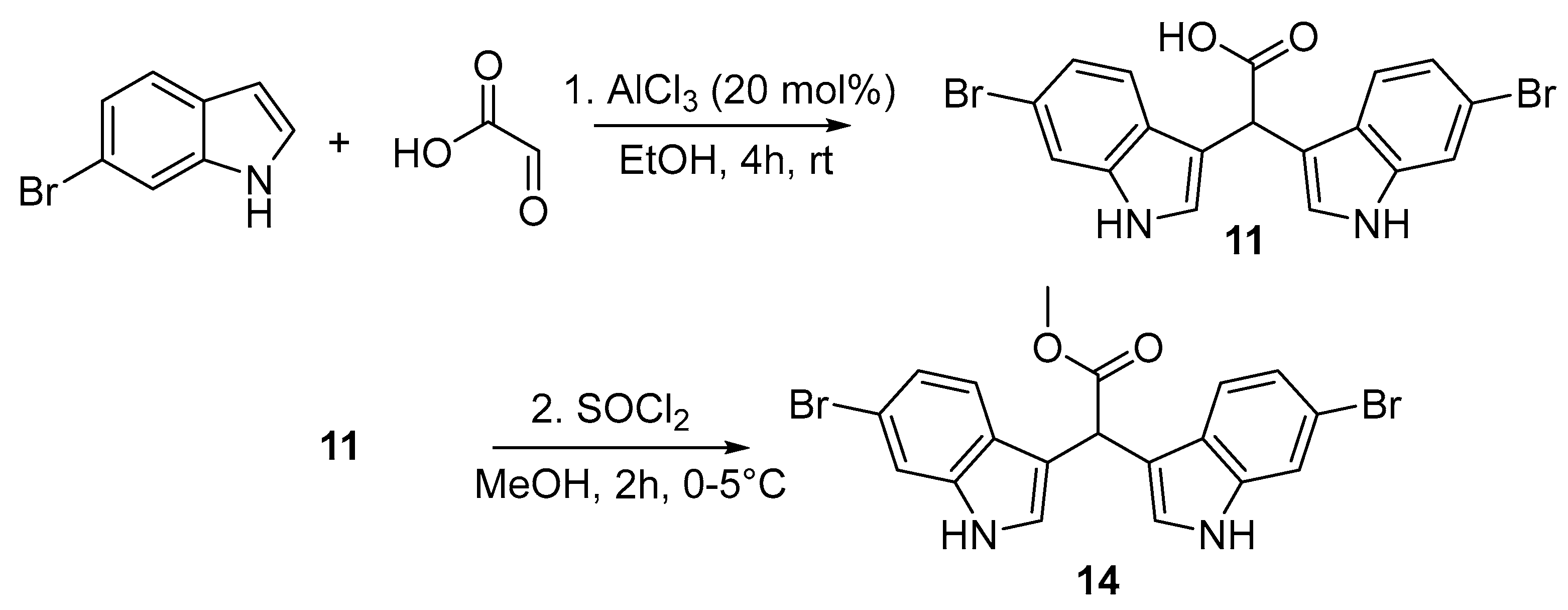
2.1. Synthesis of α-Methine Bis(3′-indolyl) Acetic Acids and Methyl Acetates (11–16)
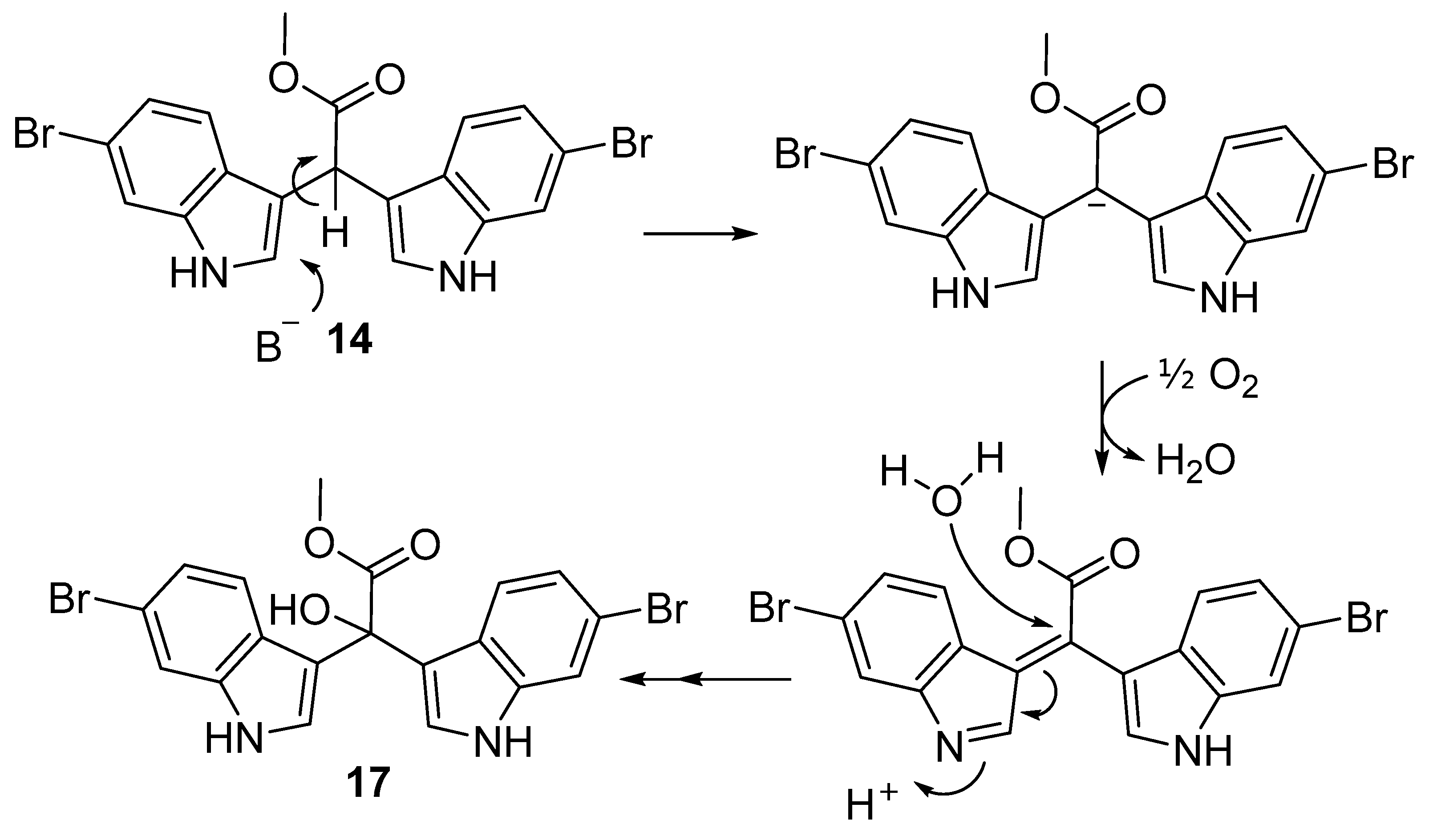
2.2. Synthesis of α-Hydroxy Bis(3′-indolyl) Methylacetates (17−19)
2.3. Structure Revision of α-Hydroxy Bis(3′-indolyl) Compounds 3o′, 5a–5n, and 5ab′
2.4. Cheminformatics-Directed Prediction of Synthetic Bis-Indole Bioactivities
2.5. Antibacterial Testing of Synthetic Bis-Indoles 1, 11–17, and 19–22
3. Materials and Methods
3.1. Chemistry
3.2. Synthetic Procedures
3.2.1. Synthesis of Bis(1H-indol-3-yl)acetic Acids (11–13)
3.2.2. Synthesis of Bis(1H-indol-3-yl)acetates (14–16)
3.2.3. Synthesis of Methyl 2,2-Bis(6-bromo-1H-indol-3-yl)-2-hydroxyacetate (17 and 18)
3.2.4. Synthesis of Bis(1H-indol-3-yl)methanones (19–22)
3.3. Cheminformatics Analyses of Marine and Synthetic Indole Chemical Diversity
3.4. Antibacterial Testing of Synthetic Bis-Indoles 1, 11–17, and 19–22
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holland, D.C.; Carroll, A.R. Marine Indole Alkaloid Diversity and Bioactivity. What Do We Know and What Are We Missing? Nat. Prod. Rep. 2023, 40, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- MarinLit. Available online: http://pubs.rsc.org/marinlit/ (accessed on 11 May 2024).
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2022, 39, 1111–1368. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Steinbeck, C. Review on Natural Products Databases: Where to Find Data in 2020. J. Cheminform. 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT online: Collection of Open Natural Products database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products. Available online: https://dnp.chemnetbase.com/chemical/ChemicalSearch.xhtml?dswid=5053 (accessed on 22 March 2024).
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How Different Are Marine Microbial Natural Products Compared to Their Terrestrial Counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Idelbayev, Y.; Roberts, N.; Tao, Y.; Nannapaneni, Y.; Duggan, B.M.; Min, J.; Lin, E.C.; Gerwick, E.C.; Cottrell, G.W.; et al. Small Molecule Accurate Recognition Technology (SMART) to Enhance Natural Products Research. Sci. Rep. 2017, 7, 14243. [Google Scholar] [CrossRef] [PubMed]
- Robien, W. Computer-Assisted Peer Reviewing of Spectral Data: The CSEARCH Protocol. Monatsh. Chem. 2019, 150, 927–932. [Google Scholar] [CrossRef]
- Guan, Y.; Shree Sowndarya, S.V.; Gallegos, L.C.; St. John, P.C.; Paton, R.S. Real-time prediction of 1H and 13C chemical shifts with DFT accuracy using a 3D graph neural network. Chem. Sci. 2021, 12, 12012–12026. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.C.; Kiefel, M.J.; Carroll, A.R. Structure Revisions of the Sponge-Derived Dibrominated Bis-Indole Alkaloids, Echinosulfone A and the Echinosulfonic Acids A to D. J. Org. Chem. 2020, 85, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.B.; Capon, R.J. Echinosulfonic Acids A-C and Echinosulfone A: Novel Bromoindole Sulfonic Acids and a Sulfone from a Southern Australian Marine Sponge, Echinodictyum. J. Nat. Prod. 1999, 62, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Rubnov, S.; Chevallier, C.; Thoison, O.; Debitus, C.; Laprevote, O.; Guénard, D.; Sévenet, T. Echinosulfonic Acid D: An ESI MSn Evaluation of a New Cytotoxic Alkaloid from the New-Caledonian Sponge Psammoclemma sp. Nat. Prod. Res. 2005, 19, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sala, S.; Nealon, G.L.; Sobolev, A.N.; Fromont, J.; Gomez, O.; Flematti, G.R. Structure Reassignment of Echinosulfone A and the Echinosulfonic Acids A − D Supported by Single-Crystal X-Ray Diffraction and Density Functional Theory Analysis. J. Nat. Prod. 2020, 83, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Neupane, P.; Salim, A.A.; Capon, R.J. Structure Revision of the Rare Sponge Metabolite Echinosulfone A, and Biosynthetically Related Echinosulfonic Acids A–D. Tetrahedron Lett. 2020, 61, 151651. [Google Scholar] [CrossRef]
- Abe, T.; Nakajima, R.; Yamashiro, T.; Sawada, D. First Total Synthesis of Reassigned Echinosulfonic Acid D. J. Nat. Prod. 2022, 85, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, X.X.; Zhang, W.; Han, M.Y. Friedel–Crafts Reaction of Acylsilanes: Highly Chemoselective Synthesis of 1-Hydroxy-Bis(Indolyl)Methanes and 1-Silyl-Bis(Indolyl)Methanes Derivatives. Molecules 2023, 28, 5685. [Google Scholar] [CrossRef] [PubMed]
- Sathieshkumar, P.P.; Latha, P.; Nagarajan, R. Total Synthesis of Pseudellone C. Eur. J. Org. Chem. 2017, 2017, 3161–3164. [Google Scholar] [CrossRef]
- Elayaraja, R.; Joel Karunakaran, R. Cesium Carbonate Mediated Synthesis of 3-(α-Hydroxyaryl)Indoles. Tetrahedron Lett. 2012, 53, 6901–6904. [Google Scholar] [CrossRef]
- Deb, M.L.; Bhuyan, P.J. An Efficient and Clean Synthesis of Bis(Indolyl)Methanes in a Protic Solvent at Room Temperature. Tetrahedron Lett. 2006, 47, 1441–1443. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Hitora, Y.; Takada, K.; Ise, Y.; Okada, S.; Matsunaga, S. Dragmacidins G and H, Bisindole Alkaloids Tethered by a Guanidino Ethylthiopyrazine Moiety, from a Lipastrotethya sp. Marine Sponge. J. Nat. Prod. 2016, 79, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- McConnell, O.J.; Koehn, F.; Rinehart, K.L.; Kohmoto, S.; Wright, A.; Kashman, Y. Dragmacidin, a New Cytotoxic Bis(Indole) Alkaloid from a Deep Water Marine Sponge, Dragmacidon sp. J. Org. Chem. 2005, 53, 3116–3118. [Google Scholar] [CrossRef]
- Wright, A.E.; Killday, K.B.; Chakrabarti, D.; Guzmán, E.A.; Harmody, D.; McCarthy, P.J.; Pitts, T.; Pomponi, S.A.; Reed, J.K.; Roberts, B.F.; et al. Dragmacidin G, a Bioactive Bis-Indole Alkaloid from a Deep-Water Sponge of the Genus Spongosorites. Mar. Drugs 2017, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Lauro, G.; O’Connor, R.D.; Lohith, K.; Kelly, M.; Colin, P.; Bifulco, G.; Bewley, C.A. Tulongicin, an Antibacterial Tri-Indole Alkaloid from a Deep-Water Topsentia sp. Sponge. J. Nat. Prod. 2017, 80, 2556–2560. [Google Scholar] [CrossRef] [PubMed]
- Veale, C.G.L.; Zoraghi, R.; Young, R.M.; Morrison, J.P.; Pretheeban, M.; Lobb, K.A.; Reiner, N.E.; Andersen, R.J.; Davies-Coleman, M.T. Synthetic Analogues of the Marine Bisindole Deoxytopsentin: Potent Selective Inhibitors of MRSA Pyruvate Kinase. J. Nat. Prod. 2015, 78, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
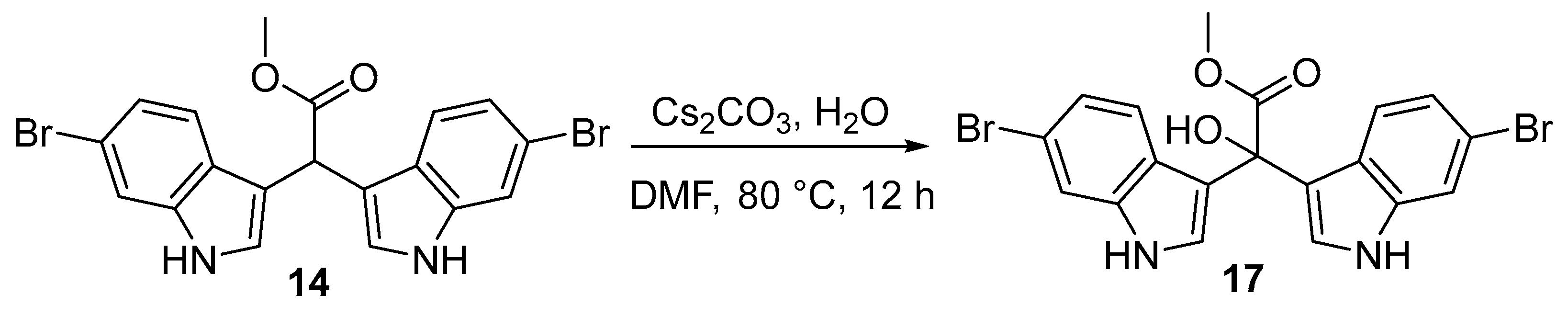




| Compound | MIC50 (µg/mL (µM)) | ||
|---|---|---|---|
| MSSA, ATCC 25923 | MRSA, ATCC 43300 | Pa, ATCC 27853 | |
| 1 | >64 (>128.5) | >64 (>128.5) | >32 (>64.2) |
| 11 | 16 (35.7) | 16 (35.7) | >32 (>71.4) |
| 12 | 16 (35.7) | 16 (35.7) | >32 (>71.4) |
| 13 | 64 (220.4) | >64 (>220.4) | >32 (>110.2) |
| 14 | 4 (8.7) | 8 (17.3) | >32 (>69.2) |
| 15 | 16 (34.6) | 16 (34.6) | >32 (>69.2) |
| 16 | >64 (>210.3) | >64 (>210.3) | >32 (>105.1) |
| 17 | >64 (>133.9) | >64 (>133.9) | >32 (>66.9) |
| 19 | >64 (>153.1) | >64 (>153.1) | >32 (>76.5) |
| 20 | 64 (188.7) | 64 (188.7) | >32 (>94.3) |
| 21 | >64 (>245.9) | >64 (>245.9) | >32(>122.9) |
| 22 | >64 (>153.1) | >64 (>153.1) | >32 (>75.5) |
| rifampicin | 0.25 (0.3) | <0.13 (<0.16) | >32 (>38.9) |
| ciprofloxacin | 0.5 (1.5) | 0.5 (1.5) | 0.5 (1.5) |
| tobramycin | - | - | 1 (2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holland, D.C.; Hayton, J.B.; Kiefel, M.J.; Carroll, A.R. Synthesis and Cheminformatics-Directed Antibacterial Evaluation of Echinosulfonic Acid-Inspired Bis-Indole Alkaloids. Molecules 2024, 29, 2806. https://doi.org/10.3390/molecules29122806
Holland DC, Hayton JB, Kiefel MJ, Carroll AR. Synthesis and Cheminformatics-Directed Antibacterial Evaluation of Echinosulfonic Acid-Inspired Bis-Indole Alkaloids. Molecules. 2024; 29(12):2806. https://doi.org/10.3390/molecules29122806
Chicago/Turabian StyleHolland, Darren C., Joshua B. Hayton, Milton J. Kiefel, and Anthony R. Carroll. 2024. "Synthesis and Cheminformatics-Directed Antibacterial Evaluation of Echinosulfonic Acid-Inspired Bis-Indole Alkaloids" Molecules 29, no. 12: 2806. https://doi.org/10.3390/molecules29122806
APA StyleHolland, D. C., Hayton, J. B., Kiefel, M. J., & Carroll, A. R. (2024). Synthesis and Cheminformatics-Directed Antibacterial Evaluation of Echinosulfonic Acid-Inspired Bis-Indole Alkaloids. Molecules, 29(12), 2806. https://doi.org/10.3390/molecules29122806







