Abstract
A series of phenyl β-carbonyl selenides with o-ester functionality substituted on the oxygen atom with chiral and achiral alkyl groups was synthesized. All compounds are the first examples of this type of organoselenium derivatives with an ester substituent in the ortho position. The obtained derivatives were tested as antioxidants and anticancer agents to see the influence of an ester functionality on the bioactivity of β-carbonyl selenides by replacing the o-amide group with an o-ester group. The best results as an antioxidant agent were observed for O-((1R,2S,5R)-(−)-2-isopropyl-5-methylcyclohexyl)-2-((2-oxopropyl)selanyl)benzoate. The most cytotoxic derivative against breast cancer MCF-7 cell lines was O-(methyl)-2-((2-oxopropyl)selanyl)benzoate and against human promyelocytic leukemia HL-60 was O-(2-pentyl)-2-((2-oxopropyl)selanyl)benzoate.
1. Introduction
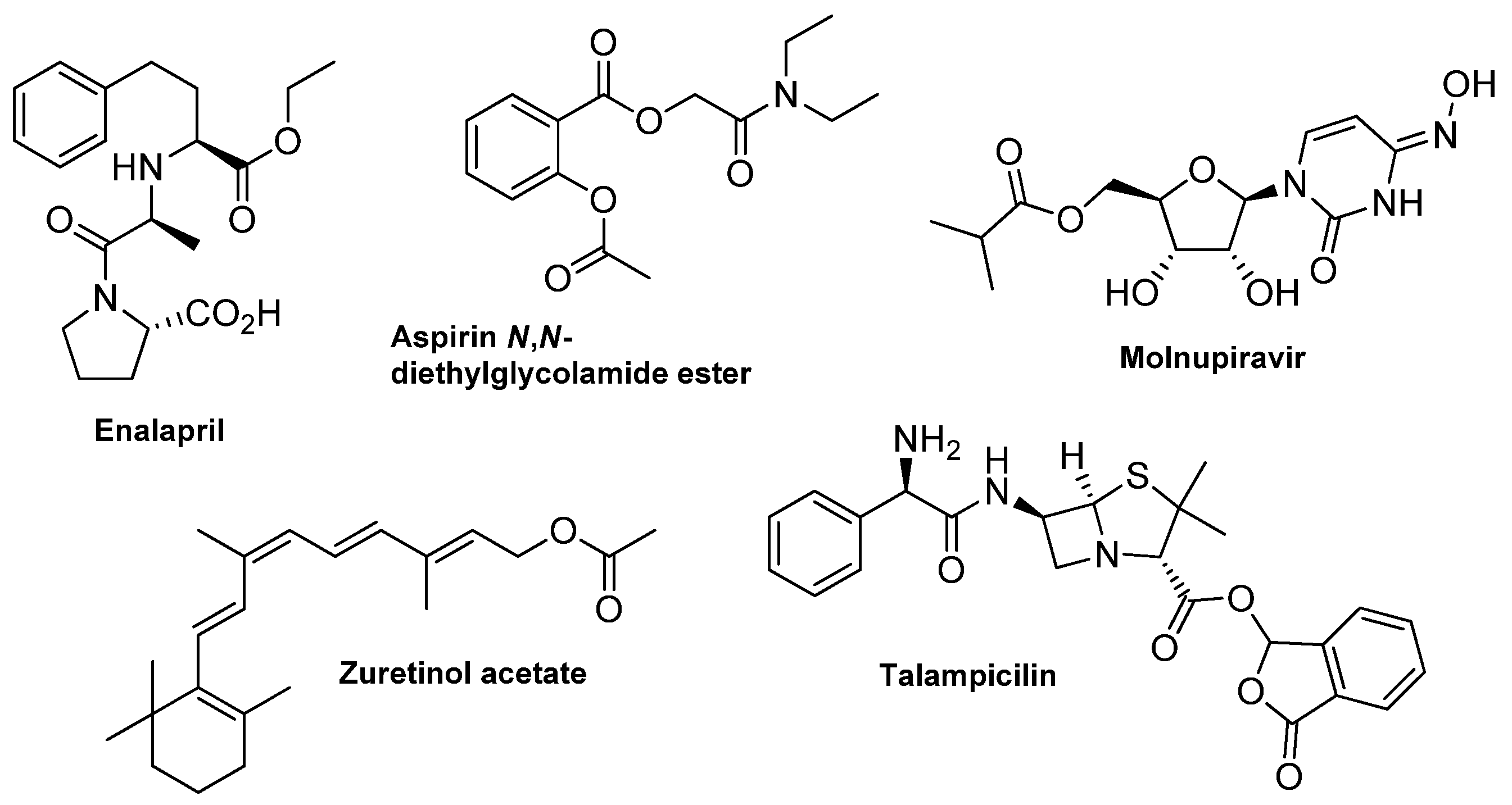
Organic molecules, both of a synthetic and natural origin, are utilized in almost every field of industry, depending on their applicability, determined mainly by the particular functional groups incorporated into their structure. Among those, esters are one of the most exploited compounds, commonly known for their characteristic fragrance properties and widely used in the perfume industry [1]. In addition to the natural presence in essential oils and pheromones, the ester bond is also a dominating functionality in many primary (e.g., lipids and carbohydrates [2]) and secondary (e.g., lactones, terpenoids and steroids [3]) metabolites [4]. The ester linkage is essential for several biochemical pathways due to its ability and facile cleavage by esterases, hydrolase-type enzymes that split the ester into a carboxylic acid and alcohol [5,6,7]. In the context of drug development, the introduction of an ester moiety bears several advantages. The transformation of bioactive compounds like polar carboxyl or hydroxyl groups into an ester prodrug improves its solubility and bioavailability [8]. Increased membrane permeability facilitates the delivery and installation inside the cells, where the molecule can be hydrolyzed to its water-soluble active form [9,10]. Using esterification in drug modification solves the bioavailability problem of many known bioactive agents. Examples of drugs applied in their ester form are presented in Figure 1 [11,12,13].
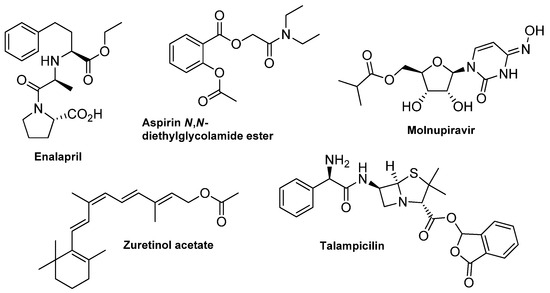
Figure 1.
Examples of ester-type drugs.
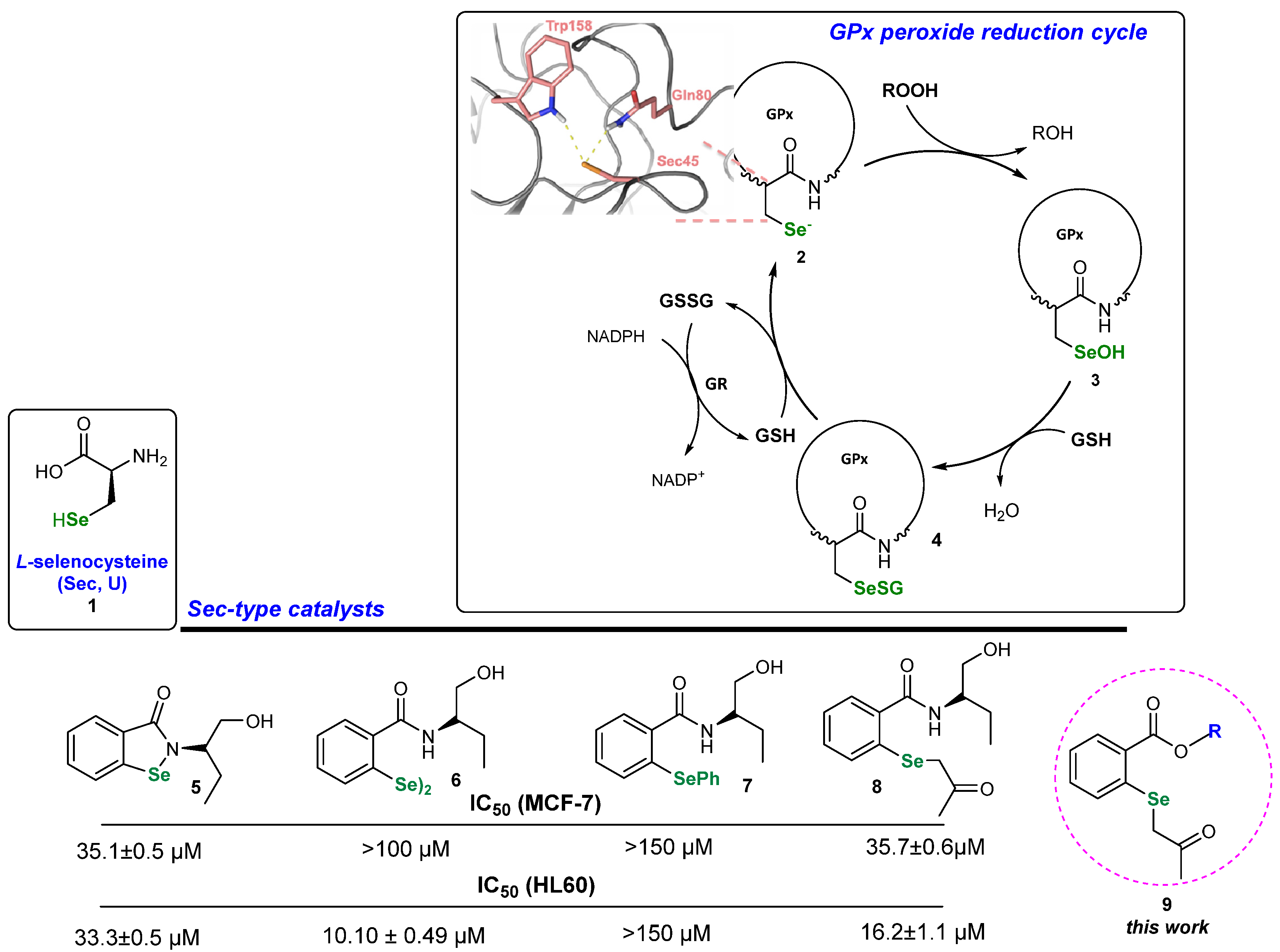
Organoselenium compounds like selenides, diselenides and selenols are well known to possess a multitude of possible biological activities due to the ability to mimic the activity of several selenoenzymes, including the antioxidant enzyme glutathione peroxidase (GPx), and they are involved in thyroid physiology, thioredoxin reductase (TRx) and iodothyronine deiodinase (ID) [14,15,16]. A catalytic reduction in peroxides by GPx is presented in Scheme 1 [17]. L-selenocysteine 1 builds up the active side of the enzyme and catalyzes the ROOH reduction to ROH [18]. The Se atom of 2 is maintained in its active ionized form due to the specific amino acid environment—glutamine and tryptophan—creating the catalytic triad with L-Sec [19]. The formed seleninic acid 3 is reduced to the starting RSe− in a two-step process by two glutathione (GHS) molecules. Se-derivatives acting as artificial L-Sec can exhibit various bioactivities [20]. Until now, we have synthesized a diversified series of GPx mimetics, including benzisoselenazolones 5, corresponding diselenides 6 [21] and phenyl selenides 7 [22]. An evaluation of their antiproliferative activity revealed that installing a -SePh functionality significantly decreases the reactivity. To address this issue, we created a new type of selenide-type catalyst possessing a 2-(2-oxopropyl)selanyl group 8 and showed that this modification significantly enhanced the reactivity of the tested compounds [23].
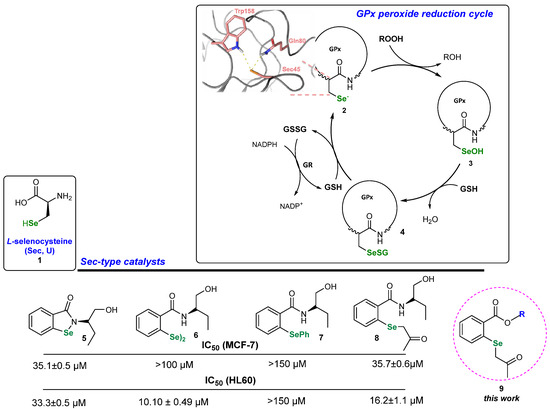
Scheme 1.
GPx activity cycle and examples of Sec-type catalysts 5–9.
Herein, we wanted to examine the influence of an ester functionality on the bioactivity of β-carbonyl selenides by replacing the o-amide group with an o-ester substituent. For this purpose, we synthesized the first β-carbonyl phenyl selenides possessing an o-ester group and evaluated if this modification changes their antioxidant and anticancer properties.
2. Results and Discussion
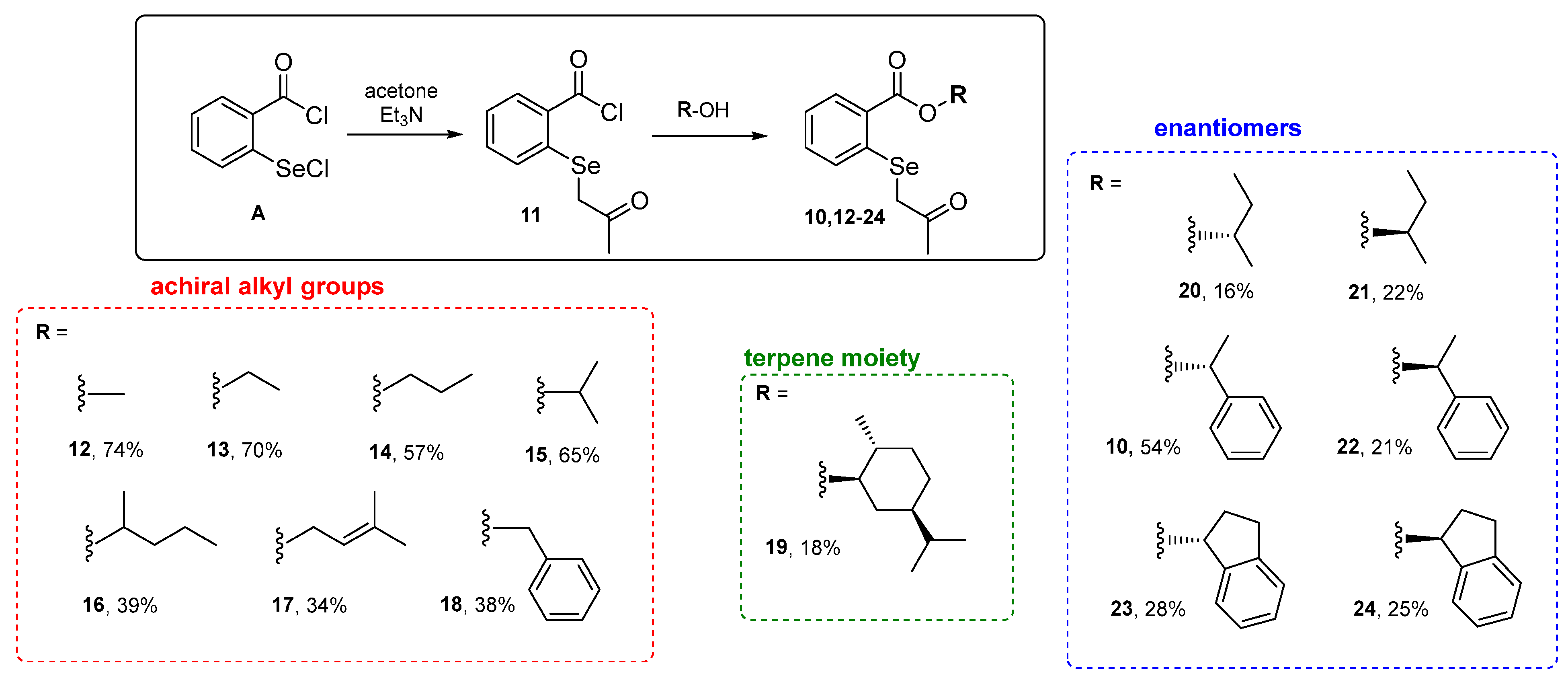
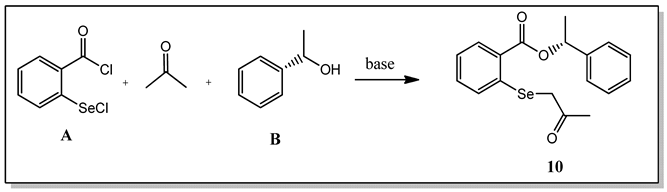
The first step in the research involved synthesizing a new class of compounds—O-substituted 2-((2-oxopropyl)selanyl)-benzoate derivatives. The procedure was based on the reaction of acetylated selenide 11, formed in situ from 2-(chloroseleno)-benzoyl chloride A and acetone, with commercially available alcohols. Initially, the reaction of acetone with sodium bicarbonate yielded a carbanion which was used to acylate the selenium atom. A subsequent reaction with alcohol B furnished the final product 10 with only a 16% yield (entry 1, Table 1). In the case of a reaction with several other alcohols, the product was not formed. In the next attempt, NaHCO3 was replaced with triethylamine. In this way, the reaction efficiency increased to 54% (entry 2, Table 1). The reaction’s key step was mixing the ketone with triethylamine to form the carbanion and remove the hydrogen chloride produced in the reaction.

Table 1.
Optimization of reaction conditions.
The selected conditions (entry 2, Table 1) were applied in further reactions with commercially available alkyl achiral and chiral alcohols, including monocyclic terpene alcohol. A series of β-carbonyl selenides, seven alkyl derivatives 12–18 and seven optically active compounds, including O-terpenyl selenide 19 and three pairs of enantiomers 10 and 20–24, were obtained with good yields (Scheme 2).
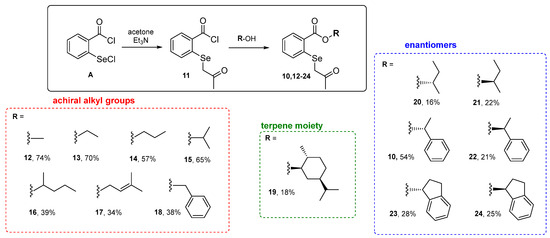
Scheme 2.
Synthetized β-carbonyl selenides with o-ester groups 10 and 12–24.
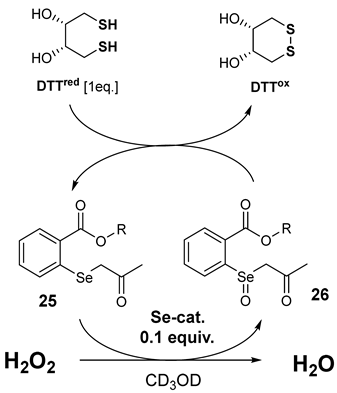
All derivatives were evaluated as antioxidants by two assays. Firstly, the NMR-based method was used, as presented by Iwaoka and co-workers [24]. The ability to reduce hydrogen peroxide was measured by the rate of dithiothreitol oxidation to disulphide (DTTred to DTTox). The active Se-catalyst is formed through the oxidation of selenide 25 to selenoxide 26 by hydrogen peroxide. Dithiothreitol (DTTred) reduces compound 26 to the starting selenide 25. The substrate (DTTred) disappearance rate was estimated by recording changes in the 1H NMR spectrum in specific time intervals. The reaction equation and antioxidant test results (mean value ± standard deviation) are shown below (Table 2), and all obtained results are presented in the Supplementary Materials (Tables S1 and S2).

Table 2.
Results of the antioxidant activity measurement.
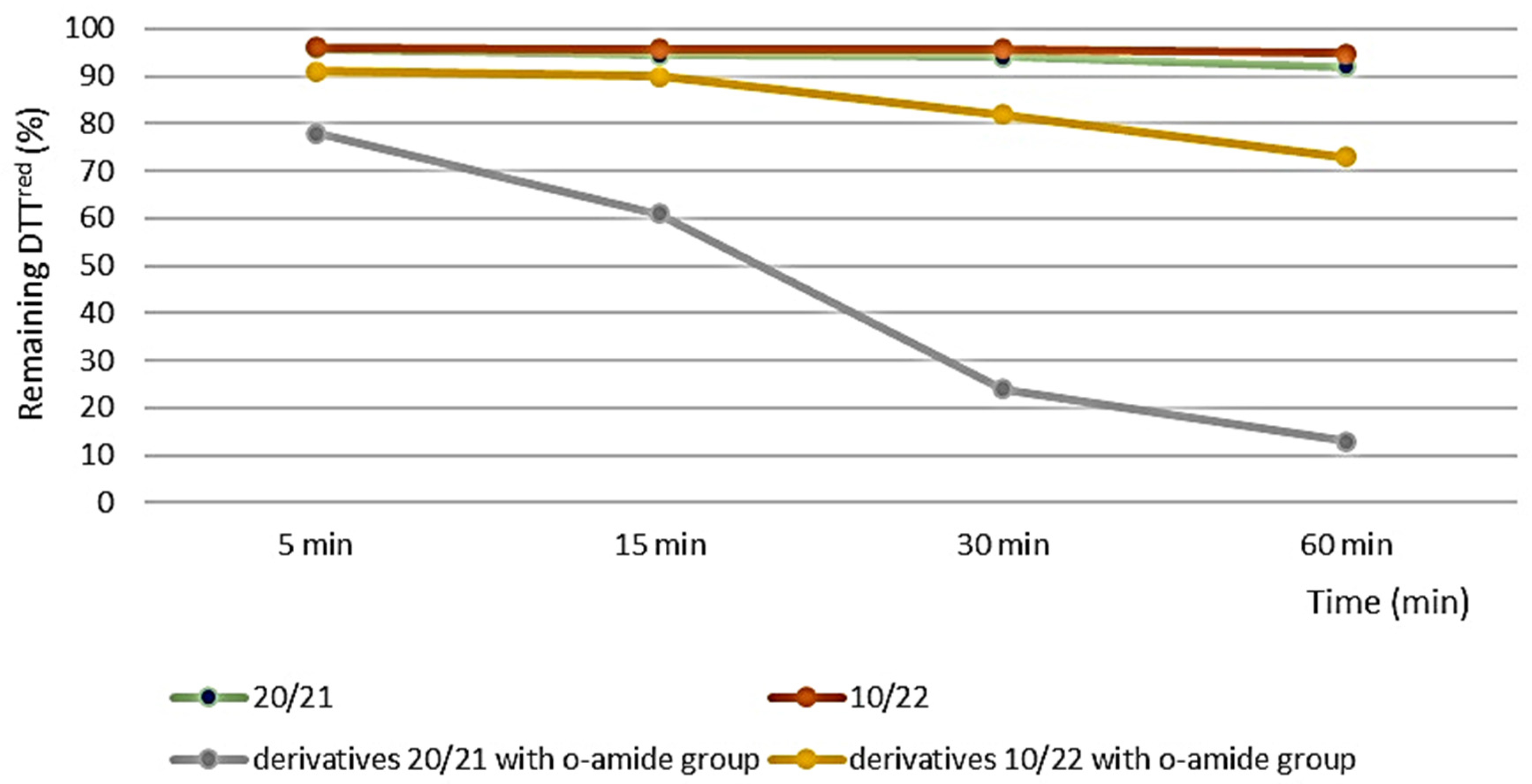
The best result was observed for O-(propyl)-2-((2-oxopropyl)selanyl)benzoate 14. However, the conversion obtained for all derivatives was less efficient than for ebselen and the previously obtained derivatives with the o-amide group. As presented in Figure 2, it can be observed that the presence of an o-ester substituent instead of an o-amide group in the structure of β-carbonyl selenides significantly lowers the ability to reduce H2O2. In the case of enantiomers 20/21 and their counterparts with the o-amide group, these properties are reduced by as much as 9 times.
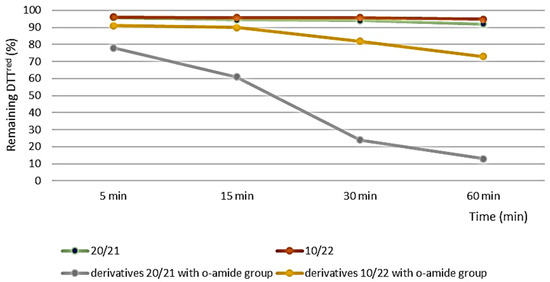
Figure 2.
Results of the antioxidant activity measurements of 20/21, 10/22 and corresponding Se-derivatives with o-amide group.
During our previous research, we also obtained a series of benzisoselenazolones and diselenides N-functionalized with long carbon chains terminated with ester groups. The obtained results indicate high reactivity of compounds possessing a Se–N bond along with the ester functionality. This suggests that the isoselenazolone core is essential for the elevated antioxidant potential of the ester-type Se-derivatives [25].
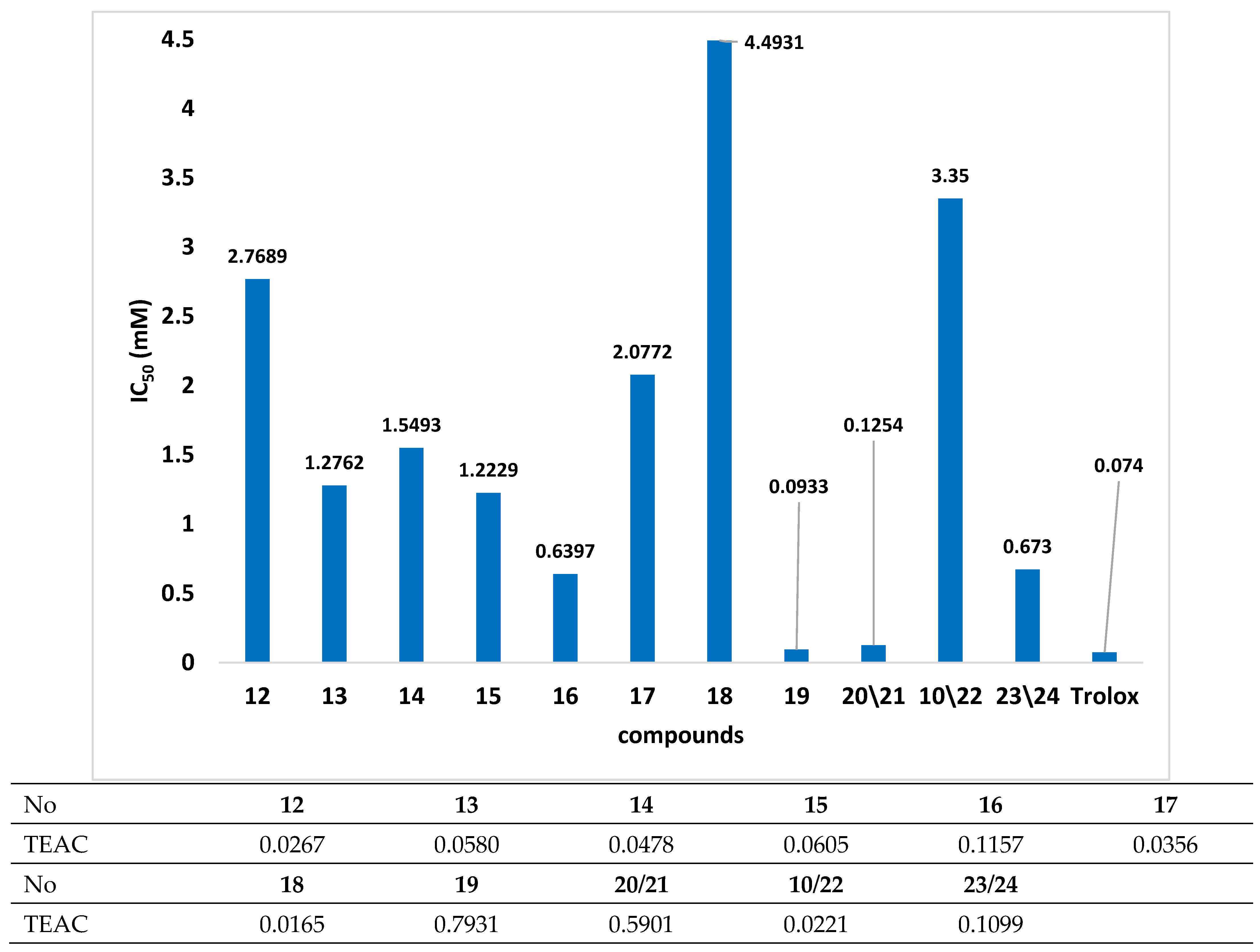
Because organoselenium compounds are well known for their ability to act as antioxidants and/or pro-oxidants, the compounds obtained were tested for their capacities to scavenge free radicals. The results obtained for the DPPH assay are presented in Figure 3 and listed in Table S1 (Supplementary Materials).
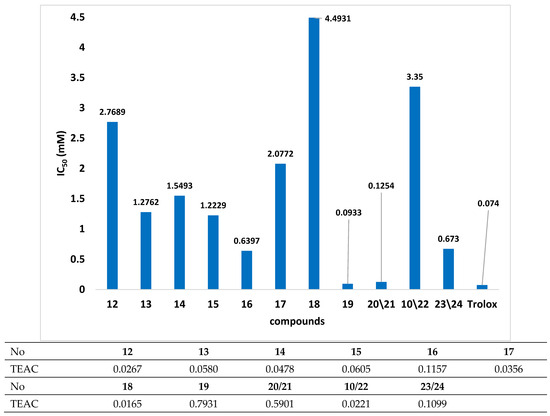
Figure 3.
The results of DPPH test for tested compounds where TEAC–Trolox equivalent antioxidant capacity (IC50Trolox/IC50 compounds) was calculated.
In this study, the selected β-carbonyl selenides with the o-ester group were tested, and the loss of DPPH radical absorbance was detected in the presence of all. As observed, compound 19 exhibited the best free-radical scavenging capabilities. The calculated IC50 value was 0.0933 mM at 15 min post-reaction initiation. Given the IC50 value obtained for Trolox (0.0744 mM) and the remaining tested compounds, the free-radical scavenging capacity of compound 19 can be considered noticeable.
All tested organoselenium derivatives contained the same 2-((2-oxopropyl)selenyl)benzoate core but differed in the attached esterification functional substituents. O-((1R,2S,5R)-(−)-2-isopropyl-5-methylcyclohexyl)-2-((2-oxopropyl)selanyl)benzoate 19 differed from the other studied derivatives by the presence of a terpene moiety in its structure. It surprised us because monoterpenes without conjugated π bonds, such as menthol, did not have satisfactory free-radical scavenging properties in the DPPH assay [26] and antioxidant activity in the ABTS test [27]. In our previous research, we obtained a new group of chiral benzisoselenazol-3(2H)-ones substituted on the nitrogen atom with monoterpene moieties, among others, p-menthane [28]. The antioxidant activities of synthesized benzisoselenazolones (such as N-menthyl-1,2-benzisoselenazol-3(2H)-one) were evaluated based on the Iwaoka test [24]. The pinene derivatives were the most effective, whereas the substrate conversion observed for the menthane derivative after 60 min was only 67%. However, this compound was characterized by the best anticancer activity against MCF-7 cells [28]. The latter, the literature data and the values of IC50 obtained in this study suggest that the presence of terpene moiety [26,27] in the structure may allow compounds with interesting antioxidant and anticancer properties to be obtained.
In the case of the remaining tested compounds, the organoselenium derivatives of chiral alcohols (20/21, 10/22 and 23/24) exhibited better free-radical scavenging properties compared to those derived from aliphatic alcohols (12–17) or benzyl alcohol (18). Moreover, the capacity for free-radical inhibition among this group of compounds was the highest for butyl esters and the lowest for products incorporating methylbenzyl in their structure.
The inferior properties of inhibiting free radicals for organoselenium derivatives of aliphatic alcohols may stem from the absence of π bonds in the functional moiety. However, compound 17, containing a double bond, was not characterized by better free-radical quenching properties. The lowest TEAC value (0.0166) was determined for compound 18, which may be due to the appearance of an aromatic ring in the structure. A similar situation was observed by Wojtunik et al. [26] for p-cymene. It indicates that further research on these compounds should concentrate on elucidating their mechanisms of action as free-radical inhibitors.
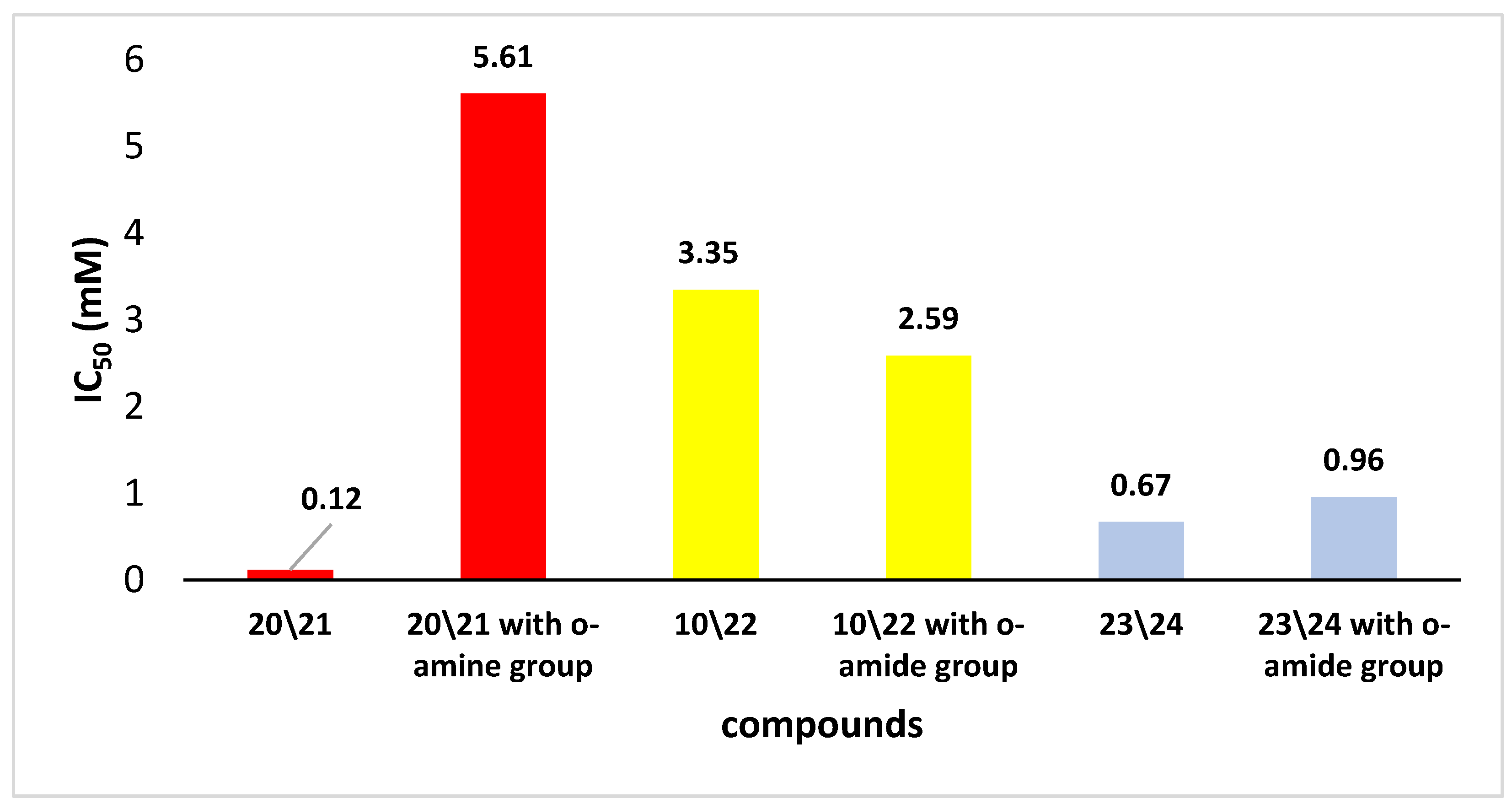
The literature extensively discusses the relationship between the structures of well-known antioxidants (such as polyphenols) and their antioxidant activity. However, for the discussed compounds, the literature data are very scarce. For this reason, the IC50 values for the selected and obtained derivatives were compared with the results for analogous organoselenium compounds described previously [23] (Figure 4).
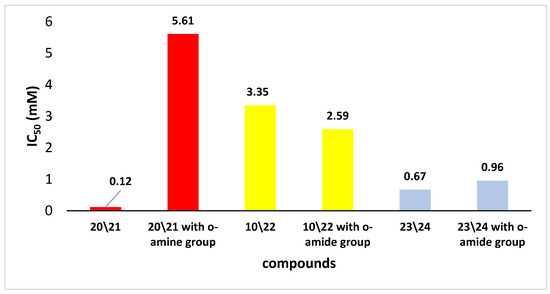
Figure 4.
The IC50 for the DPPH test of 20/21, 10/22 and 23/24 and corresponding Se-derivatives with the o-amide group.
Given the obtained results, it is difficult to estimate how the replacement of functional groups allowed for organoselenium derivatives with better properties to inhibit the action of free radicals. For example, in the case of compounds 23/24, these properties increased modestly, while for compounds with a sec-butyl group (20/21), they increased significantly. On the other hand, we did not notice a decrease in the IC50 value for derivatives with an α-methyl benzyl group 10/22. Generally, one of the compounds with the highest reactivity for derivatives from the o-amide group was observed for N-(trans)-indanyl selenide, for which the IC50 value was 0.96 mM. Similar compounds synthesized in this work with an indanyl moiety 23/24 but without a hydroxyl group showed an even higher ability to scavenge free radicals.
Finally, the cytotoxic activity of the obtained compounds 10 and 12–24 against breast cancer MCF-7 and human promyelocytic leukemia HL-60 cell lines were assessed using the cell viability assay MTT [29]. The highest cytotoxic potential was observed for the methyl β-carbonyl selenoesters 12 for the MCF-7 cell line and sec-amyl β-carbonyl selenoesters 16 for the HL-60 cell line. All results are shown in Table 3.

Table 3.
The antiproliferative activity of compounds 10 and 12–24.
Previously, we determined the IC50 values for selenium derivatives like benzisoselenazolones, diselenides, phenyl selenides and β-carbonyl selenides with the o-amide group substituted with different achiral and chiral moieties, also in the form of enantiomeric and diastereomeric pairs. Through the analysis of these results, we can see the differentiation of values between individual groups of compounds and between enantiomers and diastereoisomers. As a representative example, a comparison of the results obtained for enantiomers 10/22 and corresponding Se-derivatives is presented (Table 4).

Table 4.
The antiproliferative activity of α-methylbenzyl Se-derivatives.
Comparing the IC50 values obtained for the same α-methylbenzyl derivatives, we can notice that for both molecules with an o-ester and an o-amide group, the best activity was achieved for the S configuration for the HL-60 cell line. Additionally, a greater sensitivity of the HL-60 cell line compared to the MCF-7 cell line to the obtained derivatives with an α-methylbenzyl group can be noticed. We can also see an increase in the anticancer properties of o-ester selenides compared to phenyl selenides. In general, we can observe that the exchange of the o-amide to o-ester moiety enhances the cytotoxic potential of the obtained β-carbonyl selenides.
An important scaffold in our research, which is particularly interesting due to the chirality of compounds and the change in biological activity for individual enantiomers and diastereoisomers, was the hydroxyindanyl moiety. In this work, for β-carbonyl selenide with the o-ester group, we obtained derivatives with an indanyl substituent without an additional hydroxyl group; therefore, the values can not be compared in a direct way. Looking at the results for the other groups of compounds, we can notice lower sensitivity of cancer cells to compounds 23 and 24, but it is difficult to determine whether this is the effect of replacing the amide group with an ester group in the ortho position or the lack of an additional hydroxyl group.
Looking ahead, our primary goals will involve advancing our understanding of apoptosis and DNA damage pathways, which are fundamental to the cytotoxicity mechanisms. By unravelling the molecular mechanisms governing these pathways, we will aim to elucidate the underlying basis for the anticancer effects of our compounds. Understanding apoptosis and DNA damage pathways is crucial because they regulate cell death and survival, especially in cancer.
3. Materials and Methods
3.1. General
The 1H, 13C NMR and 77Se NMR spectra were recorded on Bruker Avance III/400 or Bruker Avance III/700 (Karlsruhe, Germany) and 176.1 MHz or 100.6 MHz (Supplementary Materials). The spectra were made in d6-DMSO solvent (CD3OD δ3.31), and chemical shifts were recorded relative to SiMe4 or diphenyl diselenide as an external standard. NMR spectra were edited using ACD/NMR Processor Academic Edition. Multiplicities were given as s (singlet), d (doublet), dd (double doublet), t (triplet), dt (double triplet), q (quartet), sextet, septet and m (multiplet). Melting points were measured on the Büchi Tottoli SPM-20 heating unit (Büchi Labortechnik AG, Flawil, Switzerland) and were uncorrected. Vario MACRO CHN analyzer was used for elemental analyses. Optical rotations were measured with a polAAr 3000 polarimeter (Bury Road Industrial Estate, Ramsey, UK).
For column chromatography, Merck 40-63D 60Å silica gel (Merck, Darmstadt, Germany) was used. Alcohols were commercially available from Merck (Merck, Darmstadt, Germany). Their chemical purities were above 98%, and optical purities were above 97%. The MCF-7 (human breast adenocarcinoma) cell line was obtained from the European Collection of Cell Cultures (ECACC, Nice, France), and the HL-60 cell line (human leukemia) was obtained from the European Collection of Authenticated Cell Cultures (ECACC Nice, France).
3.2. General Procedure and Analysis Data
To acetone (2 mL) was added NaHCO3 (0.063 g, 0.75 mmol, 1 eq.) (12–15) or Et3N (0.095 g, 0.75 mmol, 1 eg) (10, 16–24) with stirring for 30 min; then, the solution of 2-(chloroseleno)-benzoyl chloride (0.190 g, 0.75 mmol, 1 eq.) in acetone (1 mL) was added. After 1 h, alcohol (1 mmol, 1.3 eq.) was added, and the reaction was continued for 15 h at room temperature. The reaction was completed with water (10 mL) and was extracted with DCM. The combined organic layers were dried under magnesium sulfate, and the solvent was evaporated. The product was purified by column chromatography (silica gel, DCM).
O-(methyl)-2-((2-oxopropyl)selanyl)benzoate 12
Yield: 74%; mp 74–75 °C;
1H NMR (400 MHz, DMSO) δ = 2.27 (s, 3H), 3.87 (s, 2H), 3.90 (s, 1H), 7.32 (t, J = 7.7 Hz, 1Har), 7.52–7.59 (m, 2Har), 7.98 (d, J = 7.7 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 28.92 (CH3), 35.44 (CH2), 52.88 (CH2), 125.75 (CHar), 127.98 (Car), 128.98 (CHar), 131.69 (CHar), 133.65 (CHar), 136.83 (Car), 167.04 (C=O), 204.90 (C=O) 77Se NMR (400 MHz, DMSO) δ = 339.57 ppm; IR: 2951, 2913, 1693, 1583, 1460, 1433, 1397, 1357, 1288, 1274, 1252, 1193, 1145, 1101, 1056, 1031, 958, 747, 688 cm−1. Elemental Anal. Calcd for C11H12O3Se (271.17): C, 48.72; H, 4.46; Found C, 48.63; H, 4.57.
O-(ethyl)-2-((2-oxopropyl)selanyl)benzoate 13
Yield: 70%; mp 60–61 °C;
1H NMR (400 MHz, DMSO) δ = 1.32 (t, J = 7.2 Hz, 3H), 2.25 (s, 3H), 3.87 (s, 2H), 4.99 (q, J = 7.2 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1Har), 7.47–7.56 (m, 2Har), 7.96 (d, J = 7.2 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 14.59 (CH3), 28.93 (CH3), 35.46 (CH2), 61.69 (CH2), 125.74 (CHar), 128.31 (Car), 128.97 (CHar), 131.64 (CHar), 133.57 (CHar), 136.70 (Car), 166.59 (C=O), 204.92 (C=O) 77Se NMR (400 MHz, DMSO) δ = 338.99 ppm; IR: 2990, 2976, 2910, 2852, 1688, 1582, 1561, 1457, 1398, 1358, 1304, 1286, 1265, 1248, 1145, 1097, 1053, 1031, 1020, 961, 745, 687 cm−1. Elemental Anal. Calcd for C12H14O3Se (285.20): C, 53.98; H, 4.65; Found C, 53.81; H, 4.71.
O-(propyl)-2-((2-oxopropyl)selanyl)benzoate 14
Yield: 57%;
1H NMR (700 MHz, DMSO) δ = 0.98 (t, J = 7.0 Hz, 3H), 1.74 (sextet, J = 6.3 Hz, 2H), 2.27 (s, 3H), 3.90 (s, 2H), 4.25 (t, J = 7.0 Hz, 2H), 7.33 (t, J = 7.0 Hz, 1Har), 7.50–7.58 (m, 2Har), 7.99 (d, J = 7.7 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 10.86 (CH3), 22.04 (CH2), 28.93 (CH3), 35.45 (CH2), 67.01 (CH2), 125.77 (CHar), 128.26 (Car), 128.98 (CHar), 131.62 (CHar), 133.60 (CHar), 136.74 (Car), 166.64 (C=O), 204.93 (C=O) 77Se NMR (700 MHz, DMSO) δ = 338.76 ppm; IR: 2969, 2878, 1696, 1584, 1535, 1459, 1354, 1305, 1272, 1227, 1143, 1100, 1056, 1032, 739 cm−1. Elemental Anal. Calcd for C13H16O3Se (299.22): C, 52.18; H, 5.39; Found C, 52.31; H, 5.55.
O-(2-propyl)-2-((2-oxopropyl)selanyl)benzoate 15
Yield: 65%;
1H NMR (400 MHz, DMSO) δ = 1.31 (d, J = 6.4 Hz, 6H), 2.25 (s, 3H), 3.86 (s, 2H), 5.13 (septet, J = 6.0 Hz, 1H), 7.30 (t, J = 6.4 Hz, 1Har), 7.46–7.55 (m, 2Har), 7.93 (d, J = 6.8 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 22.11 (2×CH3), 28.94 (CH3), 35.49 (CH2), 69.36 (CH), 125.72 (CHar), 128.73 (Car), 128.97 (CHar), 131.57 (CHar), 133.49 (CHar), 136.54 (Car), 166.13 (C=O), 204.92 (C=O) 77Se NMR (400 MHz, DMSO) δ = 337.89 ppm; IR: 2980, 2937, 1693, 1584, 1455, 1354, 1273, 1254, 1226, 1146, 1098, 1054, 1032, 738 cm−1. Elemental Anal. Calcd for C13H16O3Se (299.22): C, 52.18; H, 5.39; Found C, 52.42; H, 5.48.
O-(2-pentyl)-2-((2-oxopropyl)selanyl)benzoate 16
Yield: 39%;
1H NMR (700 MHz, DMSO) δ = 0.91 (t, J = 7.0 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H), 1.33–1.45 (m, 2H), 1.56–1.64 (m, 1H), 1.65–1.73 (m, 1H), 2.27 (s, 3H), 3.89 (s, 2H), 5.08 (sextet, J = 7.0 Hz, 1H), 7.32 (t, J = 7.0 Hz, 1Har), 7.50–7.58 (m, 2Har), 7.96 (d, J = 7.7 Hz, 1Har) 13C NMR (700 MHz, DMSO) δ = 14.22 (CH3), 18.62 (CH2), 20.28 (CH3), 28.94 (CH3), 35.50 (CH2), 37.92 (CH2), 72.26 (CH), 125.76 (CHar), 128.66 (Car), 128.99 (CHar), 131.51 (CHar), 133.51 (CHar), 136.61 (Car), 166.23 (C=O), 204.94 (C=O) 77Se NMR (700 MHz, DMSO) δ = 339.07 ppm; IR: 2958, 2931, 2872, 1693, 1583, 1458, 1356, 1303, 1275, 1254, 1226, 1183, 1145, 1099, 1054, 1031, 738, 688 cm−1. Elemental Anal. Calcd for C15H20O3Se (327.28): C, 55.05; H, 6.16; Found C, 55.23; H, 6.24.
O-(3-methylbut-2-en-1-yl)-2-((2-oxopropyl)selanyl)benzoate 17
Yield: 34%;
1H NMR (700 MHz, DMSO) δ = 1.75 (d, J = 9.1 Hz, 6H), 2.27 (s, 3H), 3.89 (s, 2H), 4.80 (d, J = 7.0 Hz, 2H), 5.44 (t, J = 7.0 Hz, 1H), 7.32 (t, J = 7.0 Hz, 1Har), 7.49–7.58 (m, 2Har), 7.96 (d, J = 7.7 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 18.42 (CH3), 25.91 (CH3), 28.93 (CH3), 35.46 (CH2), 62.27 (CH2), 118.95 (CH), 125.74 (CHar), 128.23 (Car), 128.95 (CHar), 131.67 (CHar), 133.59 (CHar), 136.77 (Car), 139.59 (Car), 166.52 (C=O), 204.92 (C=O) 77Se NMR (400 MHz, DMSO) δ = 339.21 ppm; IR: 2971, 2938, 1690, 1585, 1439, 1358, 1270, 1228, 1146, 1095, 1053, 1031, 942, 738, 687 cm−1. Elemental Anal. Calcd for C15H18O3Se (325.26): C, 55.39; H, 5.56; Found C, 55.61; H, 5.72.
O-(benzyl)-2-((2-oxopropyl)selanyl)benzoate 18
Yield: 38%;
1H NMR (700 MHz, DMSO) δ = 2.27 (s, 3H), 3.91 (s, 2H), 5.37 (s, 2H), 7.30–7.35 (m, 2Har), 7.35–7.39 (m, 1Har) 7.42 (t, J = 7.7 Hz, 2Har), 7.47–7.50 (m, 2Har), 7.52–7.58 (m, 2Har), 8.02 (d, J = 7.7 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 28.94 (CH3), 35.46 (CH2), 67.07 (CH), 125.83 (CHar), 127.88 (Car), 128.58 (2×CHar), 128.71 (CHar), 129.03 (2×CHar), 129.45 (CHar), 131.79 (CHar), 133.79 (CHar), 136.32 (Car), 137.01 (Car), 166.38 (C=O), 204.96 (C=O) 77Se NMR (400 MHz, DMSO) δ = 339.53 ppm; IR: 3030, 2924, 2853, 1696, 1584, 1631, 1455, 1355, 1303, 1270, 1251, 1227, 1141, 1096, 1053, 1031, 967, 738, 696 cm−1. Elemental Anal. Calcd for C17H16O3Se (347.27): C, 58.80; H, 4.64; Found C, 59.04; H, 4.71.
O-((1R,2S,5R)-(−)-2-isopropyl-5-methylcyclohexyl)-2-((2-oxopropyl)selanyl)benzoate 19
Yield: 18%; = −29 (c = 0.20, CHCl3);
1H NMR (700 MHz, DMSO) δ = 0.76 (d, J = 7 Hz, 3H), 0.89 (d, J = 7 Hz, 3H), 0.91 (d, J = 7 Hz, 3H), 1.08–1.18 (m, 2H), 1.22–1.29 (m, 1H), 1.48–1.60 (m, 2H), 1.84–1.94 (m, 2H), 1.97–2.05 (m, 1H), 2.22–2.28 (m, 1H), 2.27 (s, 3H), 3.89 (s, 2H), 4.86 (td, J1 = 4.9 Hz, J2 = 11.2 Hz, 1H), 7.33 (t, J = 7.0 Hz, 1Har), 7.47–7.56 (m, 2Har), 7.95 (d, J = 7.7 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 16.86 (CH3), 20.94 (CH3), 22.33 (CH3), 23.62 (CH2), 26.64 (CH), 28.95 (CH3), 31.37 (CH), 34.15 (CH2), 35.54 (CH2), 40.98 (CH2), 47.05 (CH), 75.30 (CH), 125.81 (CHar), 128.49 (Car), 129.04 (CHar), 131.46 (CHar), 133.56 (CHar), 136.76 (Car), 166.08 (C=O), 204.93 (C=O) 77Se NMR (400 MHz, DMSO) δ = 338.30 ppm; IR: 2953, 2923, 2868, 1692, 1585, 1460, 1358, 1272, 1254, 1228, 1142, 1098, 1052, 1031, 958, 738 cm−1. Elemental Anal. Calcd for C20H28O3Se (395.39): C, 60.75; H, 7.14; Found C, 60.89; H, 7.22.
O-((S)-(+)-sec-butyl)-2-((2-oxopropyl)selanyl)benzoate 20
Yield: 16%; = 50 (c = 0.40, CHCl3);
1H NMR (700 MHz, DMSO) δ = 0.90 (t, J = 7.0 Hz, 3H), 1.28 (d, J = 11.2 Hz, 3H), 1.58–1.72 (m, 2H), 2.25 (s, 3H), 3.85 (s, 2H), 4.99 (sextet, J = 7.0 Hz 1H), 7.26–7.32 (m, 1Har), 7.44–7.57 (m, 2Har), 7.91–7.99 (dd, J1 = 2.1 Hz, J2 = 9.8 Hz, 1Har) 13C NMR (700 MHz, DMSO) δ = 10.00 (CH3), 19.75 (CH3), 28.73 (CH2), 28.95 (CH3), 35.48 (CH2), 73.71 (CH), 125.77 (CHar), 128.66 (Car), 128.98 (CHar), 131.52 (CHar), 133.52 (CHar), 136.59 (Car), 167.27 (C=O), 204.99 (C=O) 77Se NMR (700 MHz, DMSO) δ = 338.89 ppm; IR: 2969, 2924, 1693, 1584, 1457, 1434, 1355, 1304, 1273, 1254, 1226, 1144, 1127, 1099, 1053, 1029, 965, 739, 688 cm−1. Elemental Anal. Calcd for C14H18O3Se (313.25): C, 53.68; H, 5.79; Found C, 53.97; H, 5.86.
O-((R)-(−)-sec-butyl)-2-((2-oxopropyl)selanyl)benzoate 21
Yield: 22%; = −46 (c = 0.35, CHCl3);
1H NMR (700 MHz, DMSO) δ = 0.92 (t, J = 7.0 Hz, 3H), 1.30 (d, J = 7.0 Hz, 3H), 1.59–1.72 (m, 2H), 2.27 (s, 3H), 3.88 (s, 2H), 5.01 (sextet, J = 7.0 Hz, 1H), 7.31–7.36 (m, 1Har), 7.47–7.56 (m, 2Har), 7.91–7.99 (dd, J1 = 1.4 Hz, J2 = 7 Hz, 1Har) 13C NMR (700 MHz, DMSO) δ = 10.00 (CH3), 19.75 (CH3), 28.74 (CH2), 28.95 (CH3), 35.49 (CH2), 73.71 (CH), 125.77 (CHar), 128.67 (Car), 128.99 (CHar), 131.52 (CHar), 133.52 (CHar), 136.59 (Car), 167.27 (C=O), 204.98 (C=O) 77Se NMR (700 MHz, DMSO) δ = 338.91 ppm; IR: 2969, 2924, 1693, 1584, 1457, 1355, 1304, 1273, 1254, 1226, 1144, 1127, 1099, 1052, 1028, 966, 739, 688 cm−1. Elemental Anal. Calcd for C14H18O3Se (313.25): C, 53.68; H, 5.79; Found C, 53.54; H, 5.71.
O-((R)-(+)-α-methylbenzyl)-2-((2-oxopropyl)selanyl)benzoate 10
Yield: 54%; = 58 (c = 0.24, CHCl3);
1H NMR (400 MHz, DMSO) δ = 1.60 (d, J = 6.4 Hz, 3H), 2.23 (s, 3H), 3.86 (s, 2H), 6.04 (q, J = 6.8 Hz, 1H), 7.27–7.35 (m, 2Har), 7.38 (t, J = 6.8 Hz, 2Har), 7.42–7.49 (m, 2Har), 7.51–7.56 (m, 2Har), 8.07 (d, J = 7.2 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 22.78 (CH3), 28.94 (CH3), 35.47 (CH2), 73.71 (CH), 125.82 (CHar), 126.39 (2×CHar), 128.11 (Car), 128.38 (CHar), 128.98 (CHar), 129.02 (2×CHar), 131.80 (CHar), 133.75 (CHar), 136.99 (Car), 141.99 (Car), 165.77 (C=O), 204.91 (C=O) 77Se NMR (400 MHz, DMSO) δ = 339.53 ppm; IR: 2980, 2929, 1695, 1584, 1456, 1355, 1302, 1270, 1252, 1144, 1099, 1053, 1029, 993, 760, 698 cm−1. Elemental Anal. Calcd for C14H18O3Se (361.29): C, 59.84; H, 5.02; Found C, 59.98; H, 4.95.
O-((S)-(−)-α-methylbenzyl)-2-((2-oxopropyl)selanyl)benzoate 22
Yield: 21%; = −59 (c = 0.32, CHCl3);
1H NMR (700 MHz, DMSO) δ = 1.62 (d, J = 5.6 Hz, 3H), 2.25 (s, 3H), 3.88 (s, 2H), 6.05 (q, J = 6.3 Hz, 1H), 7.29–7.36 (m, 2Har), 7.40 (t, J = 6.3 Hz, 2Har), 7.46–7.52 (m, 2Har), 7.53–7.58 (m, 2Har), 8.09 (d, J = 6.3 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 22.77 (CH3), 28.94 (CH3), 35.47 (CH2), 73.71 (CH), 125.82 (CHar), 126.38 (2×CHar), 128.13 (Car), 128.32 (CHar), 129.00 (CHar), 129.01 (2×CHar), 131.79 (CHar), 133.74 (CHar), 136.97 (Car), 141.98 (Car), 165.77 (C=O), 204.89 (C=O) 77Se NMR (400 MHz, DMSO) δ = 339.57 ppm; IR: 2979, 2929, 1694, 1583, 1456, 1355, 1301, 1270, 1252, 1143, 1099, 1052, 1028, 993, 760, 698 cm−1. Elemental Anal. Calcd for C14H18O3Se (361.29): C, 59.84; H, 5.02; Found C, 60.02; H, 5.12.
O-((R)-(−)-2,3-dihydro-(1H)-inden-1-yl)-2-((2-oxopropyl)selanyl)benzoate 23
Yield: 28%; = −27 (c = 0.31, CHCl3);
1H NMR (700 MHz, DMSO) δ = 2.14–2.23 (m, 1H), 2.27 (s, 3H), 2.55–2.61 (m, 1H), 2.88–2.97 (m, 1H), 3.09–3.17 (m, 1H), 3.91 (s, 2H), 6.36 (q, J = 2.1 Hz, 1H), 7.22–7.30 (m, 2Har), 7.33–7.40 (m, 2Har), 7.48 (d, J = 1.4 Hz, 1Har), 7.51–7.57 (m, 2Har), 7.91 (d, J = 7.7 Hz, 1Har) 13C NMR (700 MHz, DMSO) δ = 28.97 (CH3), 30.24 (CH2), 32.37 (CH2), 35.55 (CH2), 79.71 (CH), 125.37 (CHar), 125.77 (CHar), 125.92 (CHar), 127.17 (CHar), 128.37 (Car), 129.03 (CHar), 129.58 (CHar), 131.76 (CHar), 133.64 (CHar), 136.73 (Car), 141.04 (Car), 144.86 (Car), 166.56 (C=O), 204.91 (C=O) 77Se NMR (400 MHz, DMSO) δ = 337.85 ppm; IR: 2970, 2927, 2852, 1692, 1584, 1460, 1355, 1271, 1251, 1227, 1141, 1099, 1053, 1031, 738 cm−1. Elemental Anal. Calcd for C19H18O3Se (373.30): C, 61.13; H, 4.86; Found C, 61.01; H, 4.97.
O-((S)-(+)-2,3-dihydro-(1H)-inden-1-yl)-2-((2-oxopropyl)selanyl)benzoate 24
Yield: 25%; = 24 (c = 0.37, CHCl3);
1H NMR (700 MHz, DMSO) δ = 2.14–2.22 (m, 1H), 2.27 (s, 3H), 2.53–2.60 (m, 1H), 2.90–2.97 (m, 1H), 3.08–3.18 (m, 1H), 3.91 (s, 2H), 6.38 (q, J = 3.5 Hz, 1H), 7.21–7.30 (m, 2Har), 7.30–7.40 (m, 2Har), 7.48 (d, J = 7.7 Hz, 1Har), 7.52–7.58 (m, 2Har), 7.91 (d, J = 7.7 Hz, 1Har) 13C NMR (400 MHz, DMSO) δ = 28.97 (CH3), 30.24 (CH2), 32.36 (CH2), 35.54 (CH2), 79.70 (CH), 125.36 (CHar), 125.77 (CHar), 125.92 (CHar), 127.17 (CHar), 128.36 (Car), 129.02 (CHar), 129.58 (CHar), 131.76 (CHar), 133.64 (CHar), 136.74 (Car), 141.03 (Car), 144.86 (Car), 166.55 (C=O), 204.90 (C=O) 77Se NMR (400 MHz, DMSO) δ = 337.85 ppm; IR: 2970, 2925, 2851, 1694, 1584, 1460, 1354, 1271, 1251, 1227, 1142, 1099, 1053, 1032, 740 cm−1. Elemental Anal. Calcd for C19H18O3Se (373.30): C, 61.13; H, 4.86; Found C, 61.26; H, 4.93.
3.3. Antioxidant Activity Evaluation
3.3.1. DTT Activity Assay
DDT activity assay for compounds 10 and 11–24 was performed according to the Iwaoka procedure [24].
3.3.2. The 2,2-di(4-tert-Octyl phenyl)-1-picrylhydrazyl) (DPPH) Test
The assay for the neutralization of radicals was executed following the methodology delineated in our previous work [23]. The 50% decline in absorbance of the DPPH solution was derived from the curve with the tested compound concentration (mM) plotted against the absorbance. The obtained results are presented as the inhibitory concentration (IC50) of the tested compounds. The absorbance value was measured after 15 min post-initiation. In addition to the compounds that were obtained, the antioxidant activity of Trolox was assessed for comparison purposes. The efficacy of the tested compounds in scavenging DPPH radicals was quantified and expressed as Trolox equivalent antioxidant capacity (TEAC). All details regarding the procedure are included in the Supplementary Materials.
3.4. MTT Viability Assay
The MTT (3-(4,5-didiazol-2-yl)-2,5 diphenyl tetrazolium bromide) assay, which measures the activity of methylcellular dehydrogenases, was based on the method of Mosmann [29]. The MTT assay was executed following the methodology delineated in our previous work [23].
4. Conclusions
We developed an efficient method for synthesizing a new group of organoselenium compounds, β-carbonyl phenyl selenides, possessing an ester group in the ortho position. The key step in the synthesis was using triethylamine during the acylation of selenide. The first derivatives with alkyl achiral and chiral scaffolds were obtained. The obtained derivatives were tested for antioxidant and cytotoxic activity. The antioxidant activity was tested in two ways: the reduction in peroxide to water (Iwaoka test) and the quenching of free radicals (DPPH test). As a result of these studies, we observed that the exchange of the amide group to an ester group significantly lowers the H2O2 reduction properties. However, it was observed that the obtained ester derivatives are better free-radical scavengers. The best results were obtained for the compound O-((1R,2S,5R)-(−)-2-isopropyl-5-methylcyclohexyl)-2-((2-oxopropyl)selanyl)benzoate, for which the IC50 value was close to the value for Trolox. Very good results of the DPPH test were also obtained for the sec-butyl and indanyl derivatives. In the case of cytotoxic activity, which was tested on two cell lines, MCF-7 (breast cancer) and HL-60 (promyelocytic leukemia), we did not notice a relevant improvement in these properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29122866/s1, Figure S1: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(methyl)- 2-((2-oxopropyl)selanyl)benzoate 12; Figure S2: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(ethyl)- 2-((2-oxopropyl)selanyl)benzoate 13; Figure S3: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(propyl)- 2-((2-oxopropyl)selanyl)benzoate 14; Figure S4: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(2-propyl)- 2-((2-oxopropyl)selanyl)benzoate 15; Figure S5: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(2-pentyl)- 2-((2-oxopropyl)selanyl)benzoate 16; Figure S6: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(3-methylbut-2-en-1-yl)- 2-((2-oxopropyl)selanyl)benzoate 17; Figure S7: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-(benzyl)- 2-((2-oxopropyl)selanyl)benzoate 18; Figure S8; (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((1R,2S,5R)-(-)-2-isopropyl-5-methylcyclohexyl)-2-((2-oxopropyl)selanyl)benzoate 19; Figure S9: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((S)-(+)-sec-butyl)- 2-((2-oxopropyl)selanyl)benzoate 20; Figure S10: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((R)-(-)-sec-butyl)- 2-((2-oxopropyl)selanyl)benzoate 21; Figure S11: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((R)-(+)-α-methylbenzyl)- 2-((2-oxopropyl)selanyl)benzoate 10; Figure S12: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((S)-(-)-α-methylbenzyl)- 2-((2-oxopropyl)selanyl)benzoate 22; Figure S13: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((R)-(-)-2,3-dihydro-(1H)-inden-1-yl)-2-((2-oxopropyl)selanyl)benzoate 23; Figure S14: (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of O-((S)-(+)-2,3-dihydro-(1H)-inden-1-yl)-2-((2-oxopropyl)selanyl)benzoate 24; Table S1: Results of antioxidant activity measurement of integration from 1H NMR spectra after reaction time 5 min and 15 min for all compounds; Table S2: Results of antioxidant activity measurement of integration from 1H NMR spectra after reaction time 30 min and 60 min for all compounds; Table S3: The results of DPPH Radical Scavenging Assay.
Author Contributions
Conceptualization, J.Ś.; Methodology, A.J.P.-M., K.G.-J. and J.Ś.; Formal analysis, A.L., M.O.-F., A.J. and A.D.-P.; Investigation, A.L., A.J.P.-M., A.J. and A.D.-P.; Data curation, A.L., M.O.-F. and A.D.-P.; Writing—original draft, A.L., A.J.P.-M. and A.J.; Writing—review & editing, A.D.-P., K.G.-J. and J.Ś.; Supervision, K.G.-J. and J.Ś.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Lodz grant number 503/1-156-02/503-11-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.-M.; Shin, W.H.; Oh, J.-M.; Park, C. Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse. Polymers 2020, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined Bead Milling and Enzymatic Hydrolysis for Efficient Fractionation of Lipids, Proteins, and Carbohydrates of Chlorella Vulgaris Microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Secondary Metabolites. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2023; pp. 765–808. [Google Scholar]
- Tsakos, M.; Schaffert, E.S.; Clement, L.L.; Villadsen, N.L.; Poulsen, T.B. Ester Coupling Reactions—An Enduring Challenge in the Chemical Synthesis of Bioactive Natural Products. Nat. Prod. Rep. 2015, 32, 605–632. [Google Scholar] [CrossRef]
- Takahashi, M.; Hirota, I.; Nakano, T.; Kotani, T.; Takani, D.; Shiratori, K.; Choi, Y.; Haba, M.; Hosokawa, M. Effects of Steric Hindrance and Electron Density of Ester Prodrugs on Controlling the Metabolic Activation by Human Carboxylesterase. Drug Metab. Pharmacokinet. 2021, 38, 100391. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Yokoi, T. The Emerging Role of Human Esterases. Drug Metab. Pharmacokinet. 2012, 27, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Casey Laizure, S.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The Role of Human Carboxylesterases in Drug Metabolism: Have We Overlooked Their Importance? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Ohura, K. Evaluation of the Oral Absorption of Ester-Type Prodrugs. Yakugaku Zasshi 2020, 140, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs—From Serendipity to Rational Design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Nunoya, K.; Nakamura, Y.; Bi, J.; Mukunoki, A.; Takeo, T.; Nakagata, N.; Hitoshi, M.; Yamaura, Y.; Imawaka, H.; et al. Species Difference in Hydrolysis of an Ester-Type Prodrug of Levodopa in Human and Animal Plasma: Different Contributions of Alpha-1 Acid Glycoprotein. Mol. Pharm. 2021, 18, 1985–1991. [Google Scholar] [CrossRef]
- Lavis, L.D. Ester Bonds in Prodrugs. ACS Chem. Biol. 2008, 3, 203–206. [Google Scholar] [CrossRef]
- Noronha, G.; Paul, P.; Katz, B.; Teuscher, N. PK Model with Concentration-Dependent Clearance for Zuretinol Acetate, an Oral Agent in Development for Treatment of Inherited Retinal Dystrophy Caused by LRAT or RPE65 Mutations. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4942. [Google Scholar]
- dos Santos Fernandes, G.F.; Prokopczyk, I.M.; Chin, C.M.; dos Santos, J.L. The Progress of Prodrugs in Drug Solubility. Recent Adv. Prodrugs 2020, 165, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [CrossRef] [PubMed]
- Başeğmez, M. An Overview of the Antioxidant and Anti-Inflammatory Activity of Selenium. In Selenium and Human Health; IntechOpen: London, UK, 2023. [Google Scholar]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, D.; Mugesh, G. Introduction of a Catalytic Triad Increases the Glutathione Peroxidase-like Activity of Diaryl Diselenides. Org. Biomol. Chem. 2015, 13, 9072–9082. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, M.A.; Nelson, S.J.; O’Leary, C.; Self, W.T. Exploring the Selenium-over-Sulfur Substrate Specificity and Kinetics of a Bacterial Selenocysteine Lyase. Biochimie 2021, 182, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Chotana, G.A.; Faisal, A.; Zaib Saleem, R.S. Chemical Synthesis of Selenium-Containing Peptides. Mini-Rev. Med. Chem. 2023, 23, 1090–1117. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in Health and Diseases. In Glutathione System and Oxidative Stress in Health and Disease; IntechOpen: London, UK, 2020. [Google Scholar]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Długosz-Pokorska, A.; Janecka, A.; Wojtczak, A.; Ścianowski, J. Attachment of Chiral Functional Groups to Modify the Activity of New GPx Mimetics. Materials 2022, 15, 2068. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Gach-Janczak, K.; Janecka, A.; Ścianowski, J. Facile Synthesis of Chiral Phenylselenides as Novel Antioxidants and Cytotoxic Agents. RSC Adv. 2023, 13, 14698–14702. [Google Scholar] [CrossRef]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. Synthesis of New Chiral β-Carbonyl Selenides with Antioxidant and Anticancer Activity Evaluation—Part I. Materials 2024, 17, 899. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Jastrzębska, A.; Długosz-Pokorska, A.; Gach-Janczak, K.; Ścianowski, J. The Influence of Long Carbon Chains on the Antioxidant and Anticancer Properties of N-Substituted Benzisoselenazolones and Corresponding Diselenides. Pharmaceuticals 2023, 16, 1560. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model Studies on the Antioxidant Activity of Common Terpenoid Constituents of Essential Oils by Means of the 2,2-Diphenyl-1-Picrylhydrazyl Method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Cieśla, Ł.M.; Waksmundzka-Hajnos, M. Approach to Determination a Structure—Antioxidant Activity Relationship of Selected Common Terpenoids Evaluated by ABTS •+ Radical Cation Assay. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Obieziurska, M.; Pacuła, A.J.; Długosz-Pokorska, A.; Krzemiński, M.; Janecka, A.; Ścianowski, J. Bioselectivity Induced by Chirality of New Terpenyl Organoselenium Compounds. Materials 2019, 12, 3579. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.-J.; Shao, X.-T.; Wang, S.; Lu, G.-H.; Xu, T.; Zhou, J.-Y. Sesquiterpene Lactone Parthenolide Markedly Enhances Sensitivity of Human A549 Cells to Low-Dose Oxaliplatin via Inhibition of NF-ΚB Activation and Induction of Apoptosis. Planta Med. 2010, 76, 258–264. [Google Scholar] [CrossRef]
- Marchetti, P.; Galla, D.A.P.; Russo, F.P.; Ricevuto, E.; Flati, V.; Porzio, G.; Ficorella, C.; Cifone, M.G. Apoptosis Induced by Oxaliplatin in Human Colon Cancer HCT15 Cell Line. Anticancer Res. 2004, 24, 219–226. [Google Scholar]
- Oliveira, M.d.S.; Barbosa, M.I.F.; de Souza, T.B.; Moreira, D.R.M.; Martins, F.T.; Villarreal, W.; Machado, R.P.; Doriguetto, A.C.; Soares, M.B.P.; Bezerra, D.P. A Novel Platinum Complex Containing a Piplartine Derivative Exhibits Enhanced Cytotoxicity, Causes Oxidative Stress and Triggers Apoptotic Cell Death by ERK/P38 Pathway in Human Acute Promyelocytic Leukemia HL-60 Cells. Redox Biol. 2019, 20, 182–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).