Measurement of Zinc Ions in Seawater Samples Using a Microfluidic System Based on the GR/CeO2/Nafion Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Instruments and Reagents
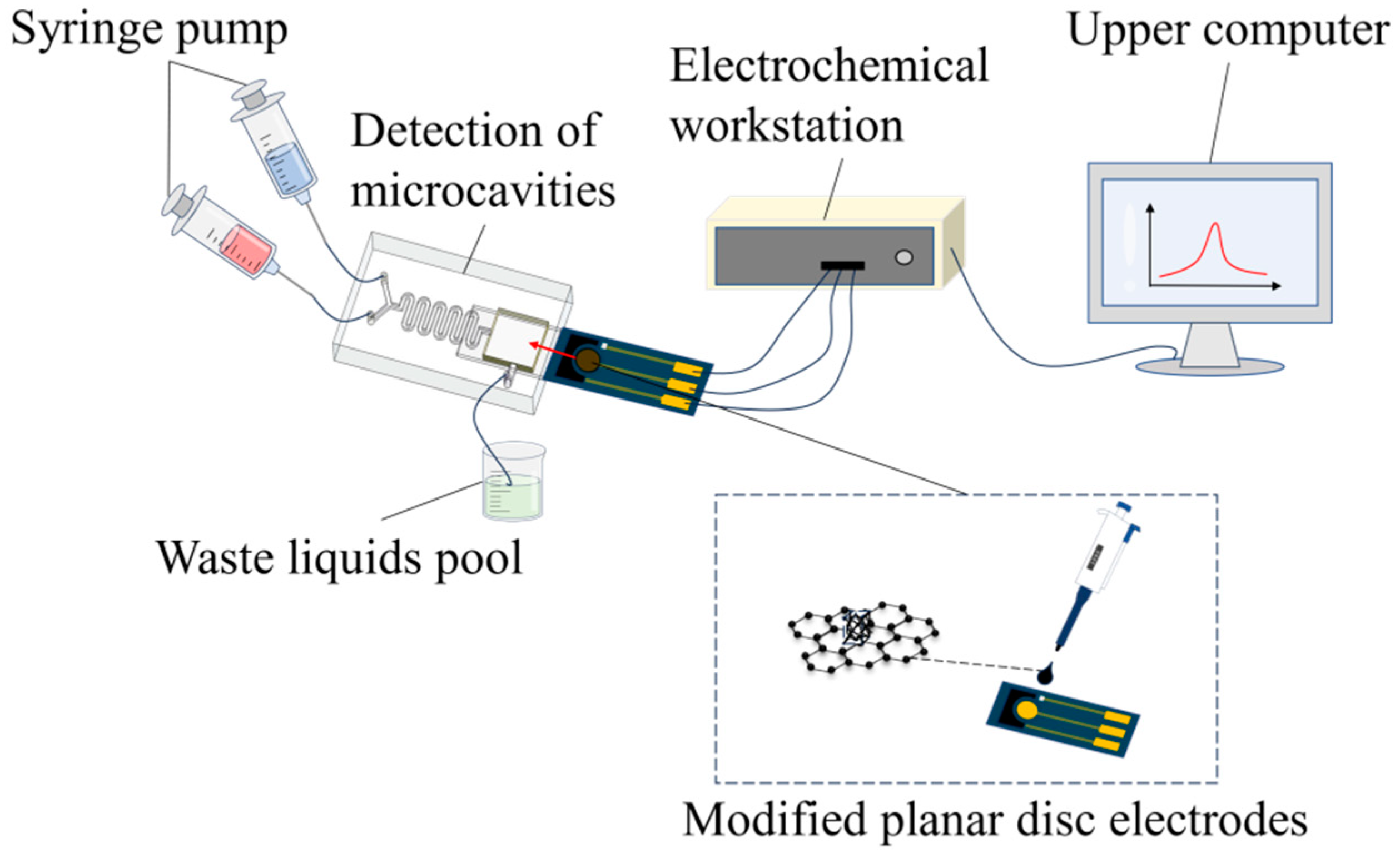
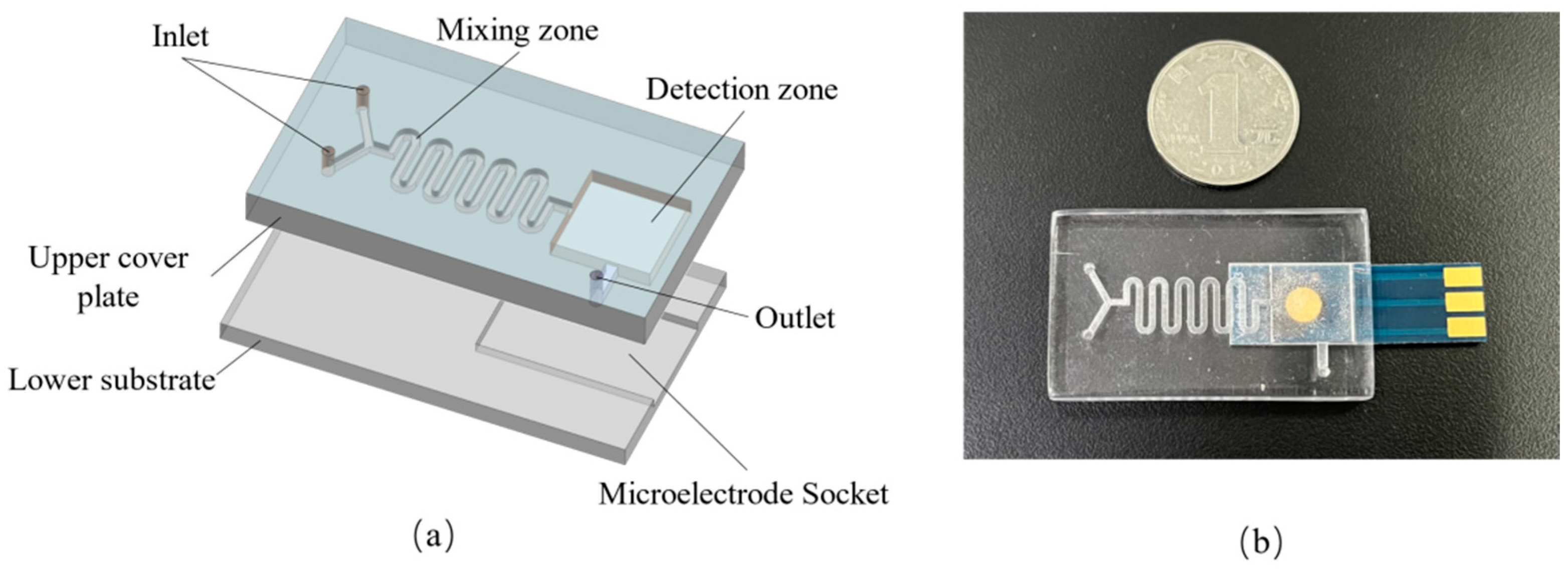
2.2. Design and Construction of Microfluidic System: General Design of the System
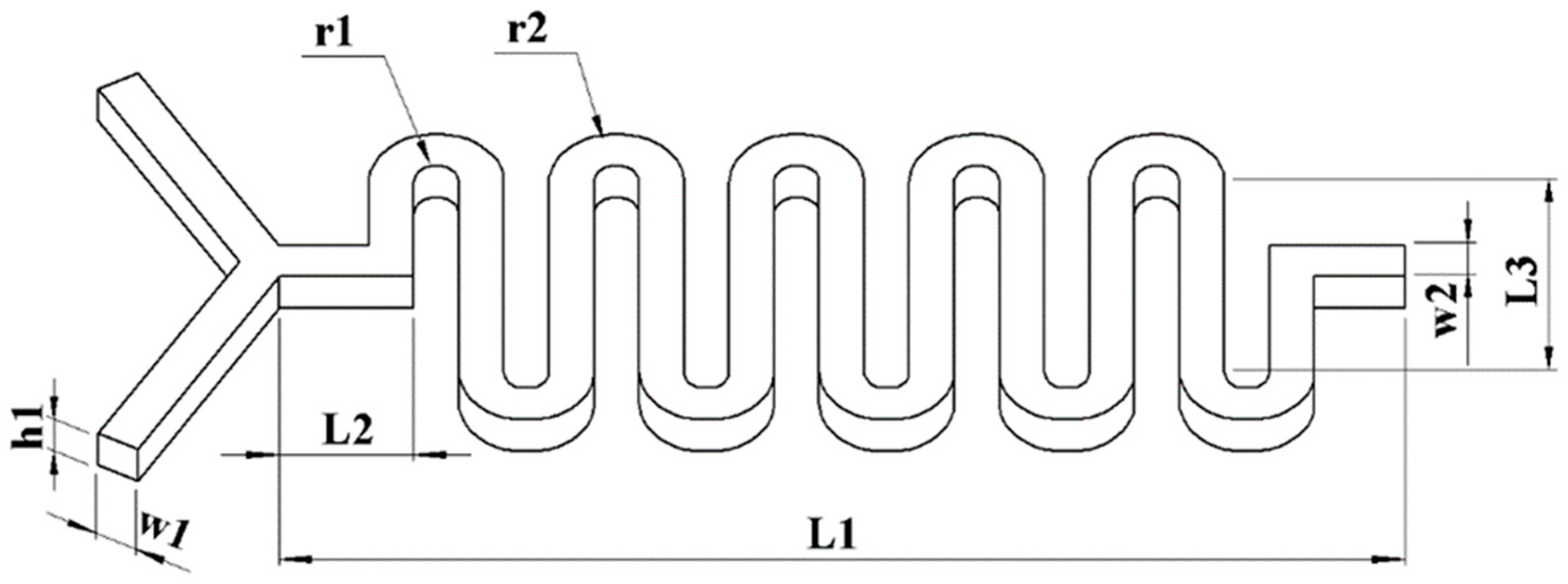
2.3. Planar Disc Electrode Design
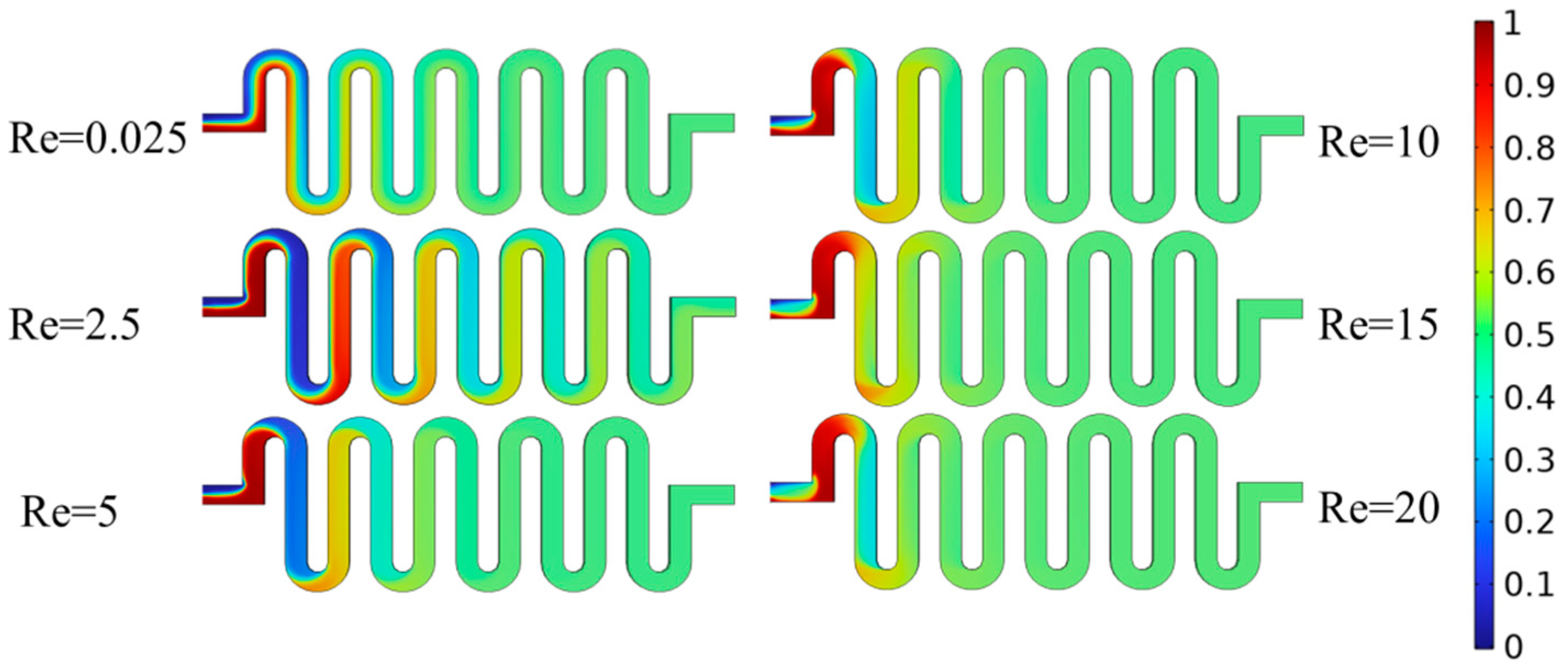
2.4. Detection of Micro-Cavity Mixing Zone Design
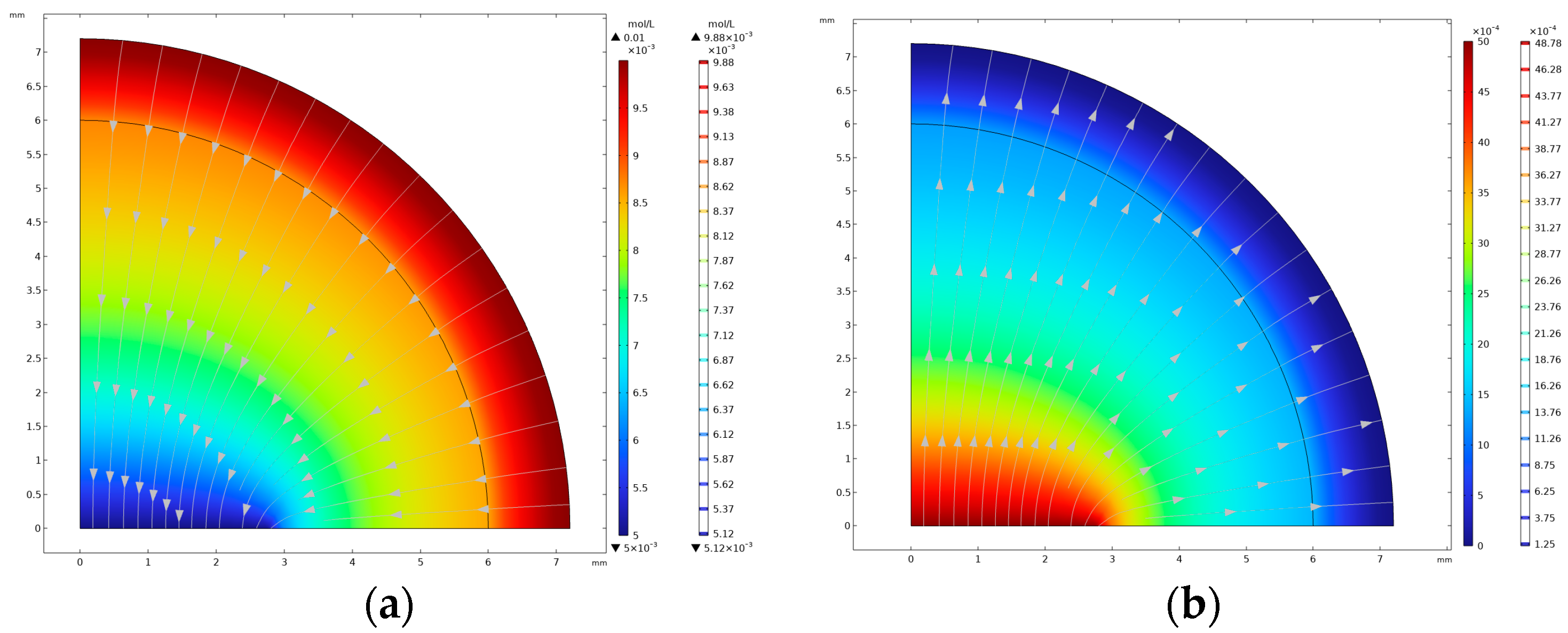
2.5. Physical Field Simulation Analysis
2.6. Construction of the Microfluidic System
2.7. Preparation of GR Solution
2.8. Preparation of the GR/CeO2/Nafion-Modified Electrode
2.9. Electrochemical Detection
3. Results and Discussion
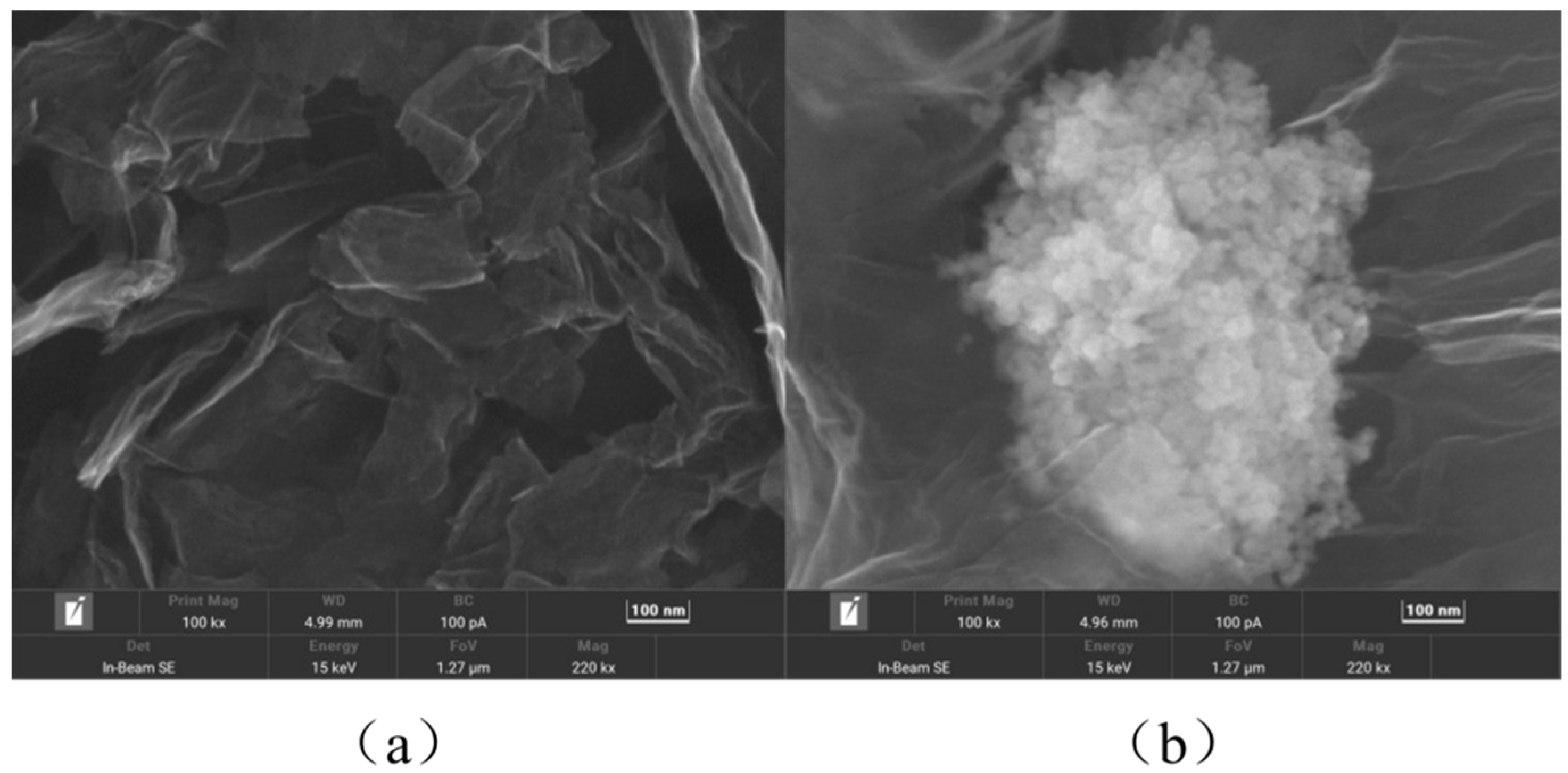
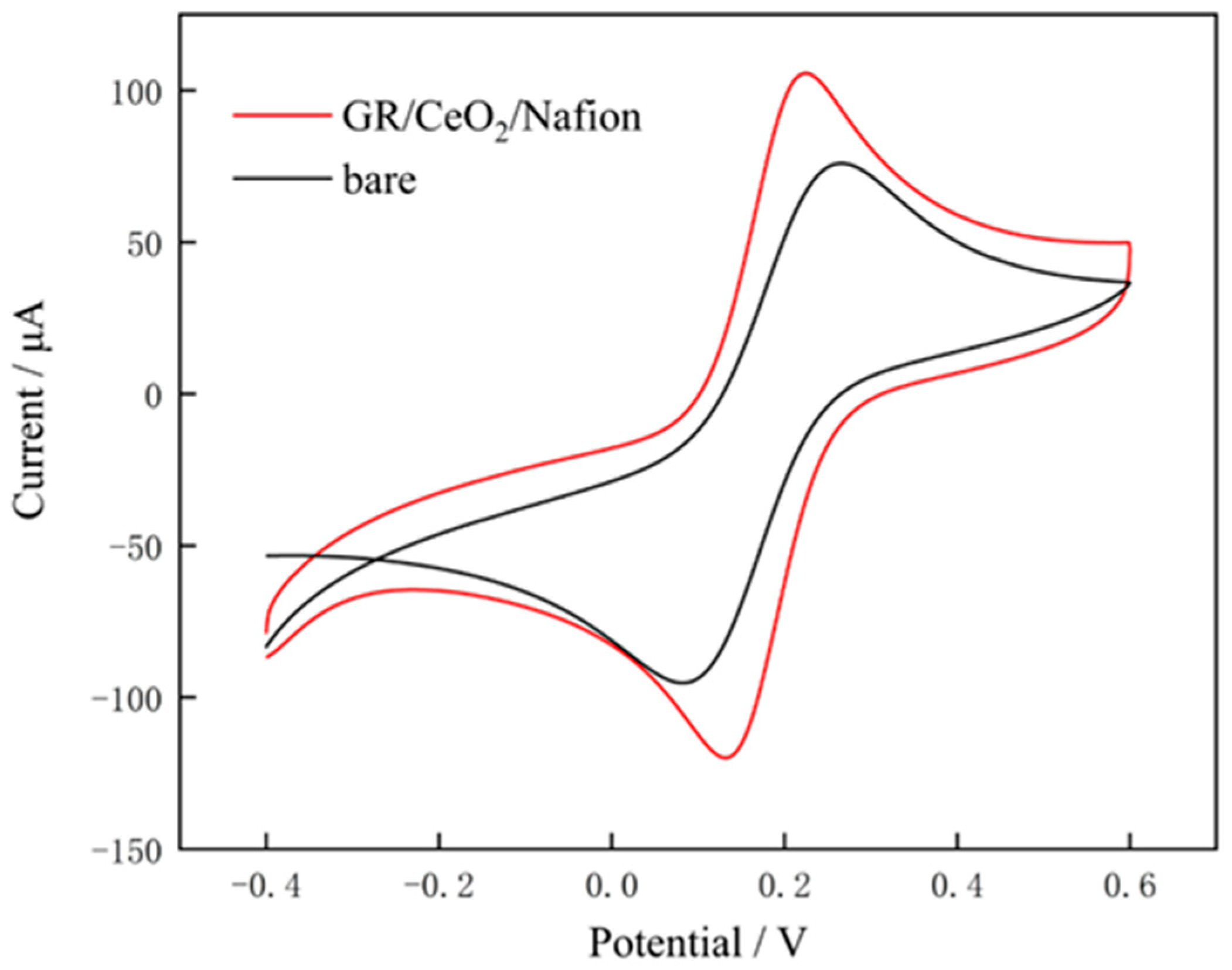
3.1. Morphology and Electrochemical Characterisation of Modified Materials
3.2. Detection Performance of Modified Electrodes
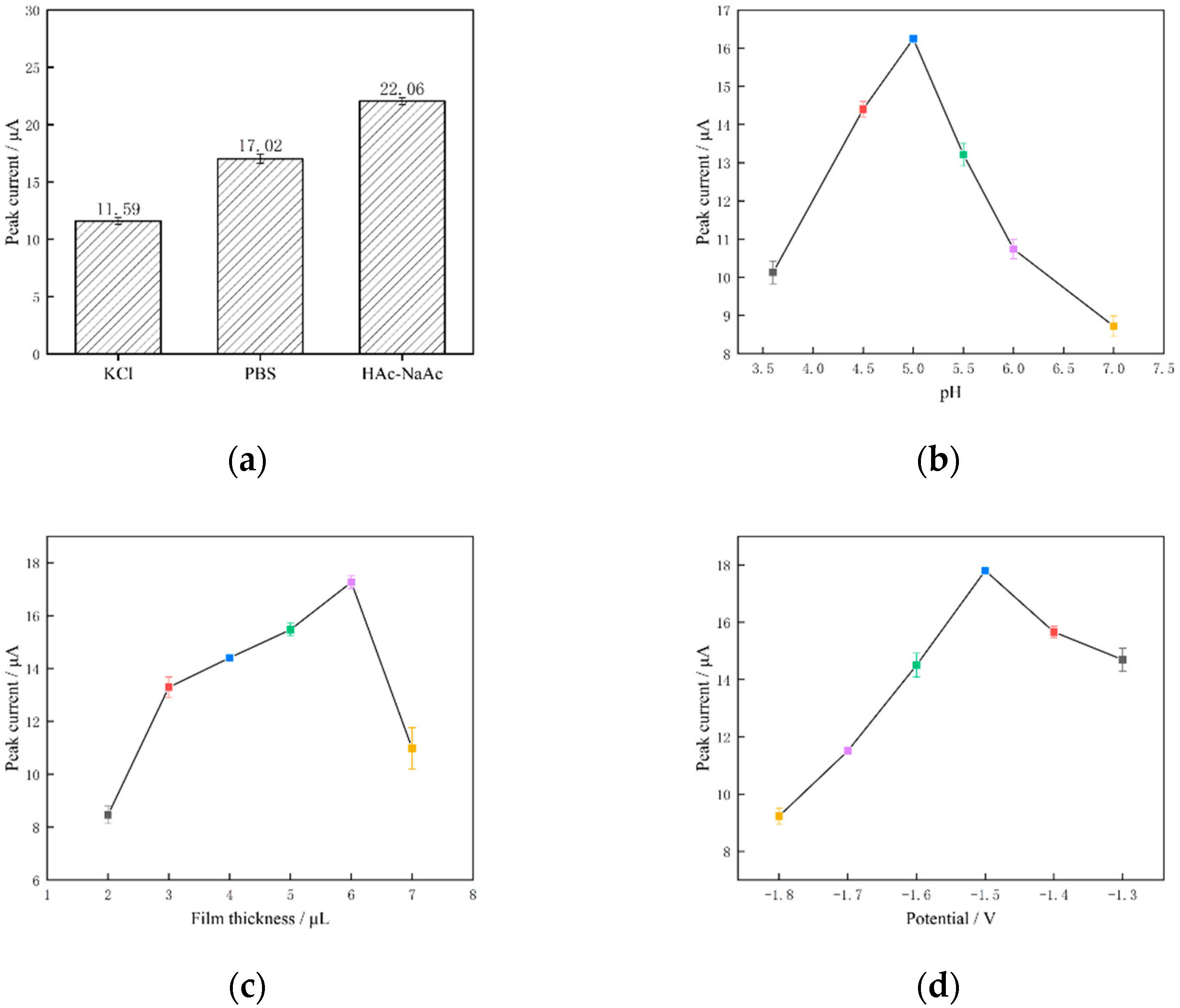
3.3. Optimisation of Electrochemical Detection Conditions
3.3.1. Optimisation of Buffer Solution pH
3.3.2. Optimisation of Film Thickness
3.3.3. Optimisation of Enrichment Potentials
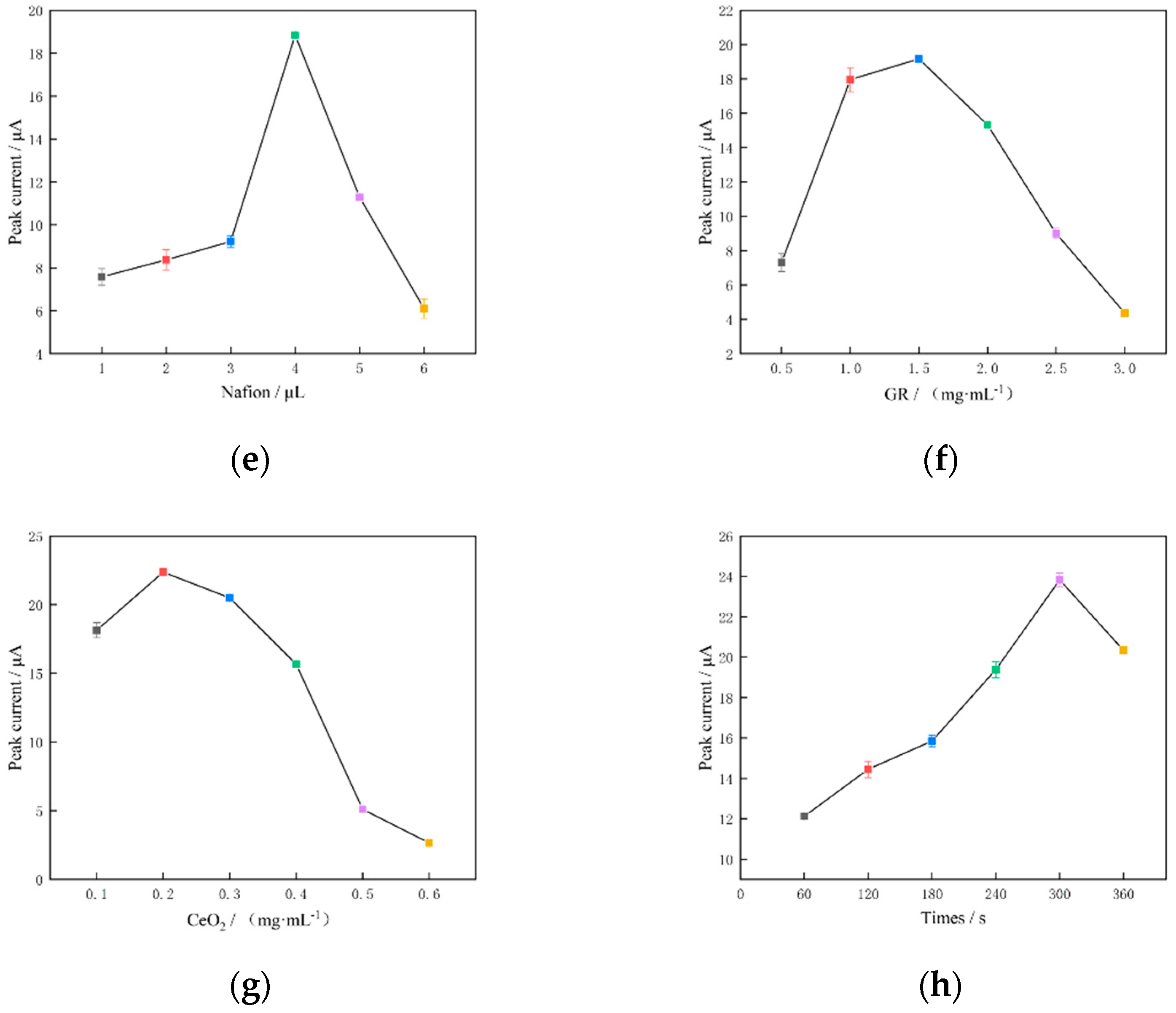
3.3.4. Optimisation of the Nafion Modification Amount
3.3.5. Optimisation of the GR Concentration
3.3.6. Optimisation of the CeO2 Concentration
3.3.7. Optimisation of Enrichment Time
3.3.8. Orthogonal Experiments
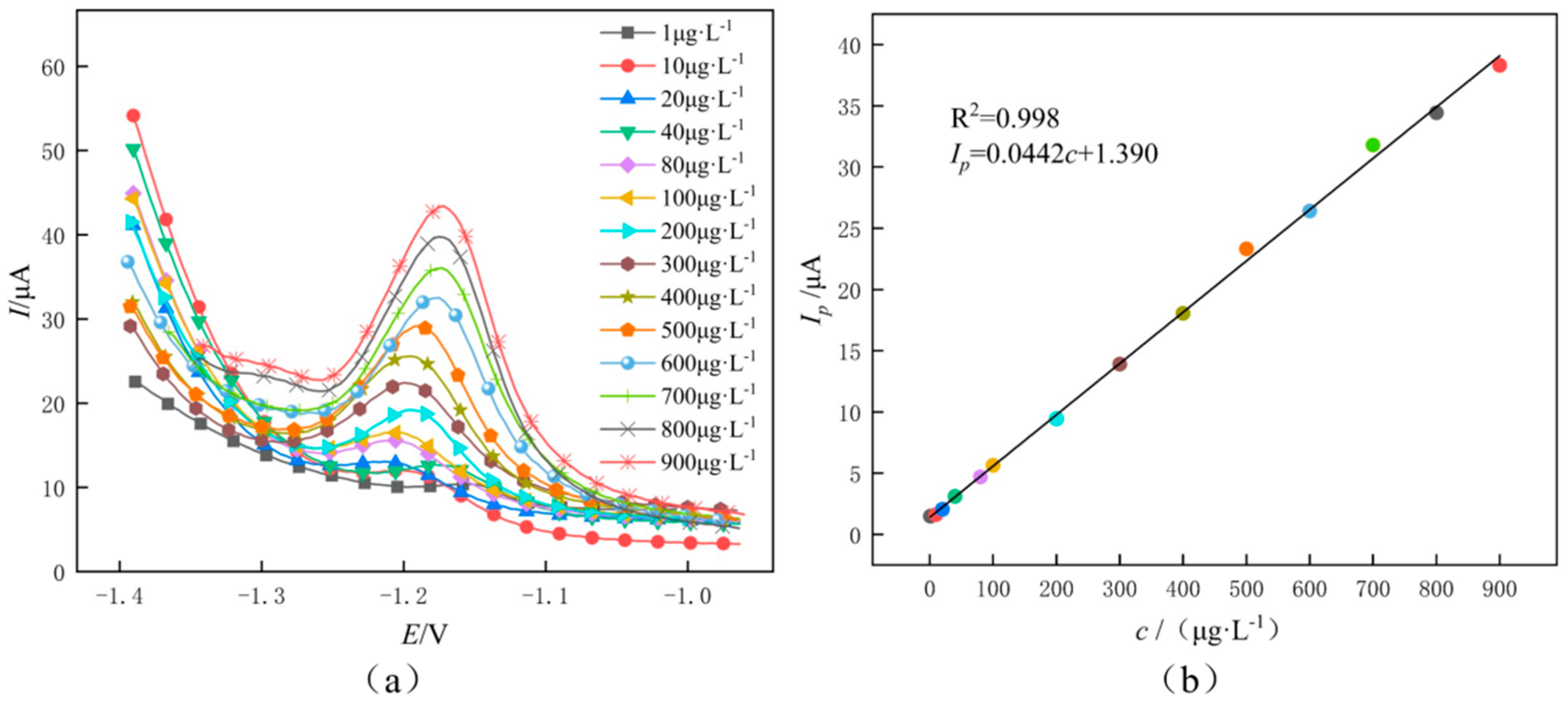
3.4. Detection of Zinc Ions
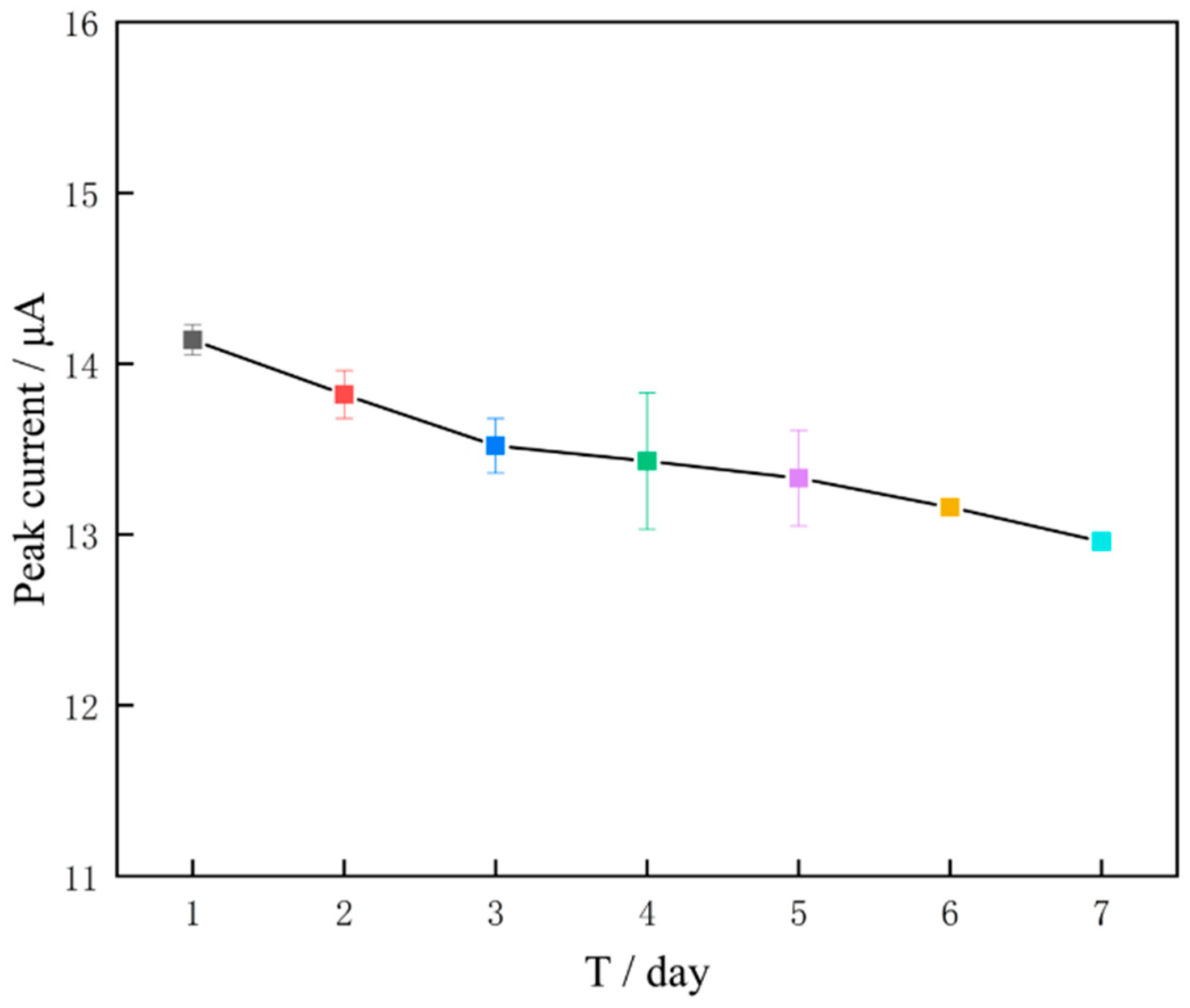
3.5. Stability, Reproducibility and Anti-Interference Experiments of Modified Electrodes
3.6. Sensitivity Analysis
3.7. Real Sample Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.H.; Xie, X.Y.; Pan, S.; Ke, Z.Z. Differential pulsed voltammetry determination of zinc and cadmium in tap water by polyL-valine modified electrode. Chin. J. Health Lab. Lab. 2019, 29, 2198. [Google Scholar]
- Zeng, W.; Zhang, D.Q.; Liu, B.; Yang, Y.P.; Wu, D.Y.; Yang, X.L. Distribution characteristics, main control factors and pollution evaluation of heavy metals in surface seawater of the northern bay of Hainan Island. J. Trop. Oceanogr. 2023, 42, 156–167. [Google Scholar]
- Luo, M.H.; Xiao, K.; Li, H.L.; Lin, D.B. Evaluation of heavy metal contamination in seawater, sediment and organisms in Hailing Island sea area, Guangdong. Mar. Lakes Marshes Bull. 2023, 45, 82–90. [Google Scholar]
- Kong, Q.Y.; Xia, C.B. Determination of trace zinc in municipal sludge by Rhodamine B spectrophotometry. Metall. Anal. 2012, 32, 57. [Google Scholar]
- He, F.D.; Li, H.C.; Feng, X.J. Determination of Cd, Co, Cu, Mg, Mn, Ni, Pb, Zn, Ca in laterite nickel ore by inductively coupled plasma atomic emission spectrometry (ICP-AES). Chin. J. Inorg. Anal. Chem. 2011, 1, 39. [Google Scholar]
- Wang, Z.H.; Wang, X.N. Determination of cadmium, copper, lead, zinc, chromium-nickel in seawater by extractive separation-atomic absorption spectrometry. Metall. Anal. 2014, 34, 44. [Google Scholar]
- Liu, Z.-M.; Yang, Y.; Du, Y.; Pang, Y. Advances in droplet-based microfluidic technology and its applications. Chin. J. Anal. Chem. 2017, 45, 282–296. [Google Scholar] [CrossRef]
- Yew, M.; Ren, Y.; Koh, K.S.; Sun, C.; Snape, C. A Review of State-of-the-Art Microfluidic Technologies for Environmental Applications: Detection and Remediation. Glob. Chall. 2019, 3, 1800060. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Islam, M.; Gupta, A. Microfluidic Devices for the Detection of Contamination in Water Samples: A Review. Sens. Actuators A Phys. 2022, 347, 113926. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Wang, S.; Liu, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. [Google Scholar] [CrossRef]
- Tang, F.J.; Zhang, F.; Jin, Q.H.; Zhao, J.L. Determination of trace heavy metals cadmium and lead in water by graphene-modified platinum electrode sensor. Chin. J. Anal. Chem. 2013, 41, 278. [Google Scholar]
- Ma, S. Construction of Graphene-Modified Electrode and Its Application in Metal Detection in Coastal Water. Master’s Thesis, Shandong Normal University, Jinan, China, 2020. [Google Scholar]
- Guo, Z.Y.; Yue, Z.J.; Pu, C. Detection of zinc content in chrysanthemum and almond by graphene-based electrochemical sensor. J. Shanxi Datong Univ. (Nat. Sci. Ed.) 2022, 38, 5. [Google Scholar]
- Tan, F.N.; Li, L. Determination of trace zinc in milk powder by graphene/nanotitanium dioxide/lauroyl-carboxymethyl chitosan. Food Res. Dev. 2018, 39, 113. [Google Scholar]
- Sobahi, N.; Imran, M.; Khan, M.E.; Mohammad, A.; Alam, M.M.; Yoon, T.; Mehedi, I.M.; Hussain, M.A.; Abdulaal, M.J.; Jiman, A.A. Electrochemical Sensing of H2O2 by Employing a Flexible Fe3O4/Graphene/Carbon Cloth as Working Electrode. Materials 2023, 16, 2770. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.K.; Alamier, W.M.; Hasan, N.; Ahmed, S.; Ansari, A.; Imran, M. Synergistic nanomaterials: Zinc sulfide-polyaniline for ciprofloxacin electrochemical sensing. Appl. Phys. A 2023, 129, 859. [Google Scholar] [CrossRef]
- Jiang, J.X.; Duan, X.J. Preparation of nano-cerium oxide and its application in water treatment. Rare Earth Inf. 2021, 9, 18–21. [Google Scholar]
- Duan, S.; He, J.Y.; Zhan, K.; Gong, Z.Y.; Chen, J.W. Cerium nano dioxide for electrochemical detection of hypoxanthine, a freshness characteristic of Procrayfish cruzi. J. Food Saf. Qual. Detect. 2022, 13, 2426. [Google Scholar]
- Zarinkamar, M.; Farahmandjou, M.; Firoozabadi, T.P. One-step synthesis of ceria (CeO2) nano-spheres by a simple wet chemical method. J. Ceram. Process Res. 2016, 17, 166. [Google Scholar]
- NS, A.K.; Adarakatti, P.S.; Ashoka, S.; Malingappa, P. CeO2 nanoparticle-modified electrode as a novel electrochemical interface in the quantification of Zn(II) ions at trace level: Application to real sample analysis. J. Solid State Electrochem. 2018, 22, 1711. [Google Scholar]
- Zhao, X.; Wang, K.; Li, B.; Li, C.; Lin, Y. Preparation, Surface Modification and in vivo/Single Cell Electroanalytical Application of Microelectrode. Prog. Chem. 2017, 29, 1173–1183. [Google Scholar]
- Tian, Q.K.; Zheng, H.P.; Zhang, S.B.; Zhang, J.; Yu, Z.Y. Advances in hybrid-enhanced microfluidic channels. Adv. Chem. Eng. 2023, 42, 1677–1687. [Google Scholar]
- Seanghirun, W.; Samoson, K.; Cotchim, S.; Kongkaew, S.; Limbut, W. Green electrochemical sensor for Zn (II) ions detection in human seminal fluid. Microchem. J. 2020, 157, 104958. [Google Scholar] [CrossRef]
- Qin, J.; Li, W.; Cai, K.; Wang, D.; Peng, C.; Luo, L.; Song, S.; Mei, Y.; Wang, Y. Simultaneous electrochemical detection of zinc and copper in fruit juice using Hg/CMWCNTs@ ZIF-8 modified glassy carbon electrode. Microporous Mesoporous Mater. 2023, 360, 112721. [Google Scholar] [CrossRef]
- Aziz, S.F.N.A.; Ainliah Alang Ahmad, S. Electrochemical detection of nickel (II) and zinc (II) ions by a dicarboxyl-Calix [4] arene-based sensor (Calix/MPA/Au) through differential pulse voltammetry analysis. J. Water Supply Res. Technol.-Aqua 2023, 72, 160–172. [Google Scholar] [CrossRef]
- Akhtar, M.; Sohail, M.; Warsi, M.F.; Ibrahim, M.M.; Amin, M.A.; Shahid, M. Fe3O4 nanochips loaded MXenes/g-C3N4 nanocomposite for ultrasensitive electrochemical detection of zinc (II), cadmium (II), lead (II), copper (II) and mercury (II) metal ions. FlatChem 2023, 41, 100537. [Google Scholar] [CrossRef]
- Zeng, Z.X.; Qu, W.; Qiu, C.J.; Zhuang, Y.; Wang, P.; Wu, Y.X.; Gao, J.Q. Determination of seawater-based zinc ions based on graphene-cerium oxide nano-modified electrode. Anal. Lab. 2023, 1–8. Available online: http://kns.cnki.net/kcms/detail/11.2017.TF.20230920.1912.024.html (accessed on 4 June 2024).




| Experiment | GR (mg·mL−1) | CeO2 (mg·mL−1) | Nafion (μL) | Film Thickness (μL) | Peak Current (μA) |
|---|---|---|---|---|---|
| 1 | 1 | 0.1 | 3 | 5 | 15.75 |
| 2 | 1 | 0.2 | 4 | 6 | 17.02 |
| 3 | 1 | 0.3 | 5 | 7 | 9.32 |
| 4 | 1.5 | 0.1 | 4 | 7 | 18.57 |
| 5 | 1.5 | 0.2 | 5 | 5 | 22.06 |
| 6 | 1.5 | 0.3 | 3 | 6 | 21.93 |
| 7 | 2 | 0.1 | 5 | 6 | 17.83 |
| 8 | 2 | 0.2 | 3 | 7 | 14.03 |
| 9 | 2 | 0.3 | 4 | 5 | 16.26 |
| Average | GR (mg·mL−1) | Peak Current (μA) | CeO2 (mg·mL−1) | Peak Current (μA) | Nafion (μL) | Peak Current (μA) | Film Thickness (μL) | Peak Current (μA) |
|---|---|---|---|---|---|---|---|---|
| I | 1 | 14.030 | 0.1 | 17.383 | 3 | 17.237 | 5 | 18.023 |
| II | 1.5 | 20.853 | 0.2 | 17.703 | 4 | 17.283 | 6 | 18.927 |
| III | 2 | 16.040 | 0.3 | 15.837 | 5 | 16.403 | 7 | 13.973 |
| Range R | – | 6.823 | – | 1.866 | – | 0.880 | – | 4.954 |
| Electrodes | Linear Range (μg·L−1) | LOD (μg·L−1) | Reference |
|---|---|---|---|
| Bi/GO/GCE | 20–8000 | 6 | [23] |
| Hg/CMWCNTs@ZIF-8/GCE | 50–1000 | 5.23 | [24] |
| Calix/MPA/Au | 2850–6650 | 1500 | [25] |
| Ti3C2(HF)/Fe3O4/gC3N4 | 0.325–32.5 | 0.0169 | [26] |
| GR/CeO2/Nafion/GCE | 5–800 | 1.20 | [27] |
| GR/CeO2/Nafion/Au | 1–900 | 0.87 | This work |
| Seawater Samples | Found (μg·L−1) | ICP-MS | Added (μg·L−1) | Found (μg·L−1) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|---|---|
| 1 | 16.04 ± 0.39 | 16.0 | 0 | — | — | — |
| 10 | 26.02 ± 0.34 | 99.9 | 1.37 | |||
| 20 | 35.76 ± 0.37 | 99.2 | 1.05 | |||
| 2 | 546.23 ± 0.72 | 546.0 | 0 | — | — | — |
| 10 | 554.15 ± 0.78 | 99.6 | 0.14 | |||
| 20 | 566.21 ± 0.91 | 100.0 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Zeng, Z.; Qiu, C.; Qu, W.; Zhuang, Y.; Gu, Y.; Hao, H.; Zhao, Z. Measurement of Zinc Ions in Seawater Samples Using a Microfluidic System Based on the GR/CeO2/Nafion Material. Molecules 2024, 29, 2867. https://doi.org/10.3390/molecules29122867
Tao W, Zeng Z, Qiu C, Qu W, Zhuang Y, Gu Y, Hao H, Zhao Z. Measurement of Zinc Ions in Seawater Samples Using a Microfluidic System Based on the GR/CeO2/Nafion Material. Molecules. 2024; 29(12):2867. https://doi.org/10.3390/molecules29122867
Chicago/Turabian StyleTao, Wei, Zexi Zeng, Chengjun Qiu, Wei Qu, Yuan Zhuang, Yang Gu, Huili Hao, and Zizi Zhao. 2024. "Measurement of Zinc Ions in Seawater Samples Using a Microfluidic System Based on the GR/CeO2/Nafion Material" Molecules 29, no. 12: 2867. https://doi.org/10.3390/molecules29122867
APA StyleTao, W., Zeng, Z., Qiu, C., Qu, W., Zhuang, Y., Gu, Y., Hao, H., & Zhao, Z. (2024). Measurement of Zinc Ions in Seawater Samples Using a Microfluidic System Based on the GR/CeO2/Nafion Material. Molecules, 29(12), 2867. https://doi.org/10.3390/molecules29122867







