Synthetic Modifications of Andrographolide Targeting New Potential Anticancer Drug Candidates: A Comprehensive Overview
Abstract
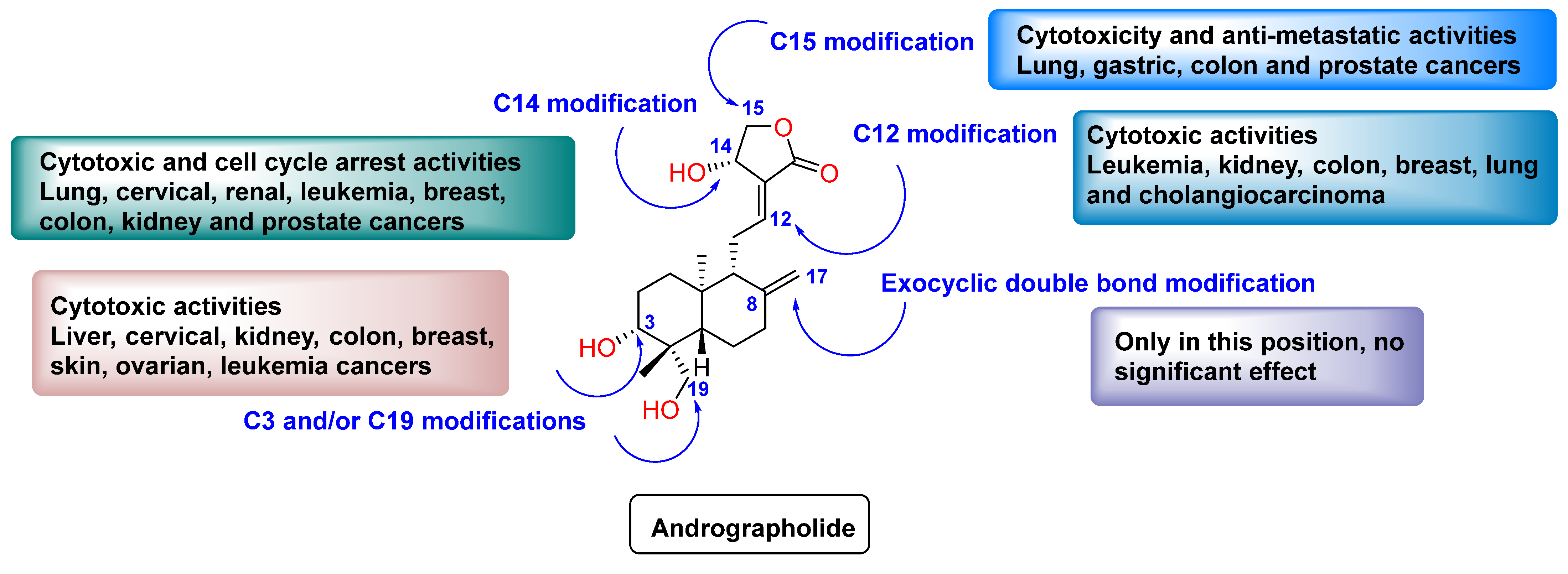
:1. Introduction
2. Results and Discussion
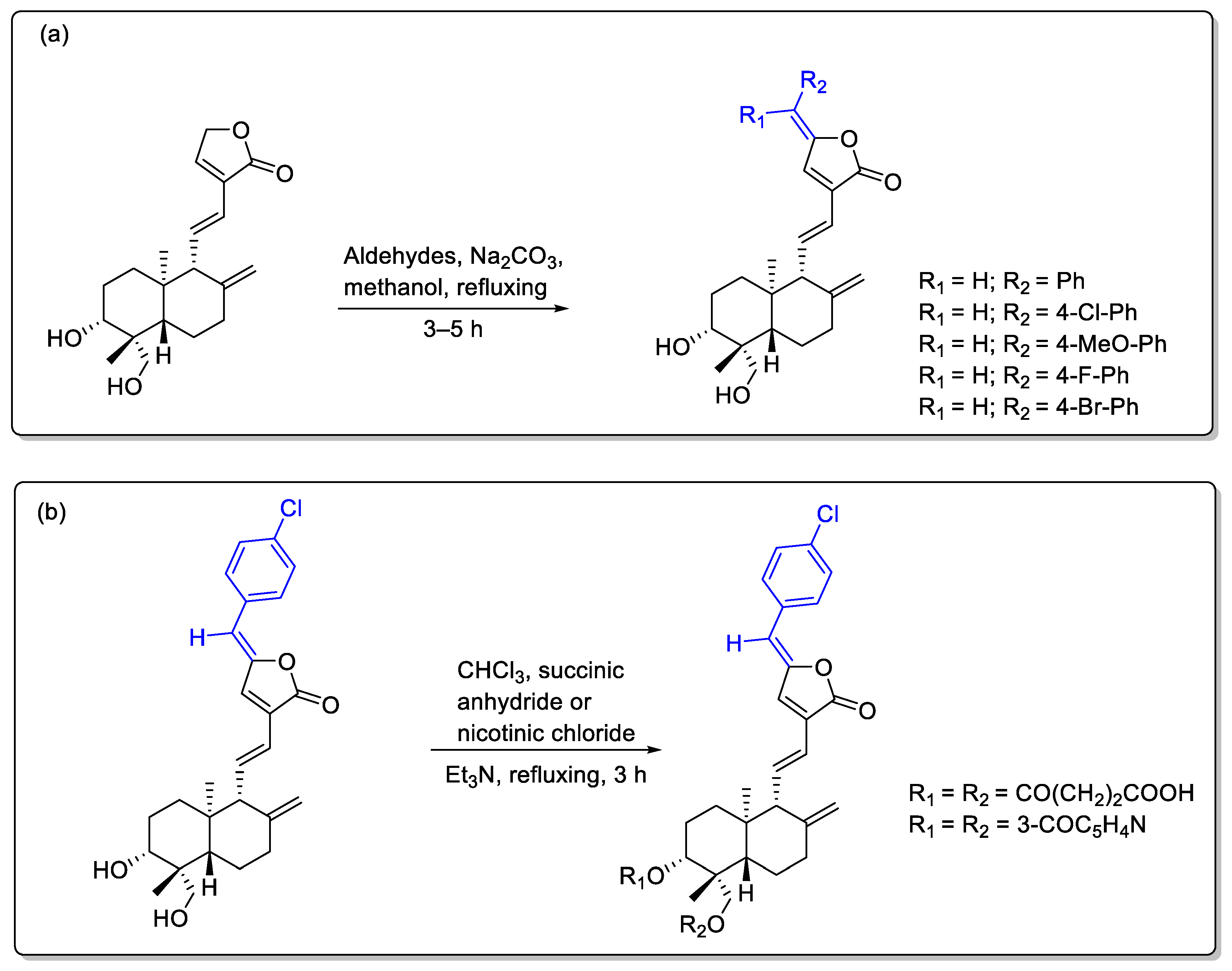
2.1. Lactone Modification at Position C15
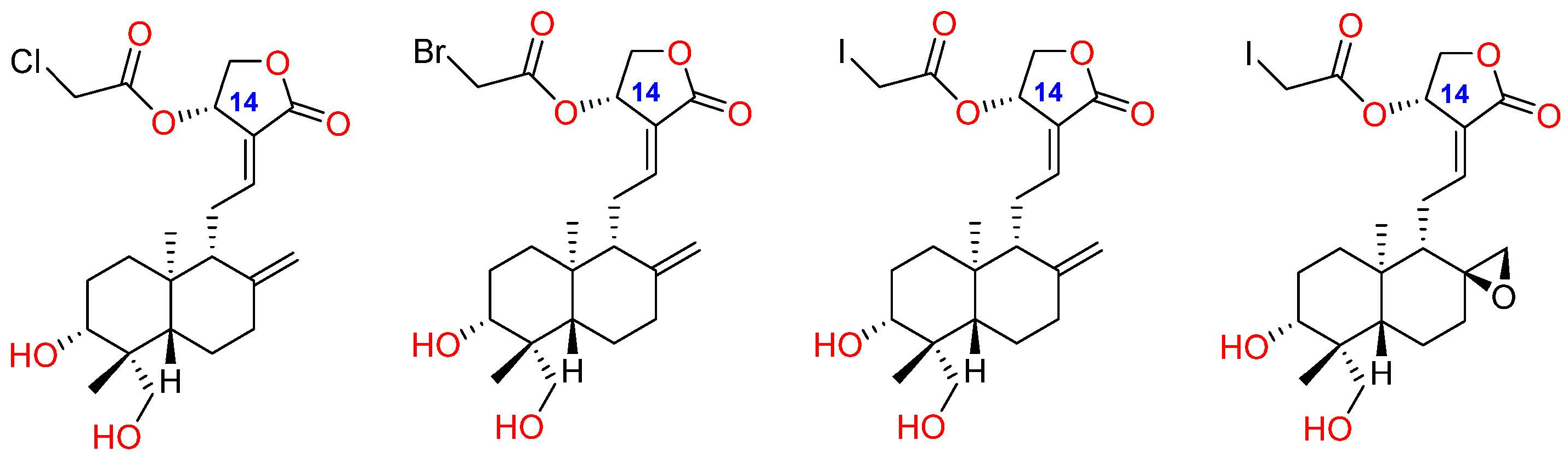
2.2. Lactone Modification at Position C14
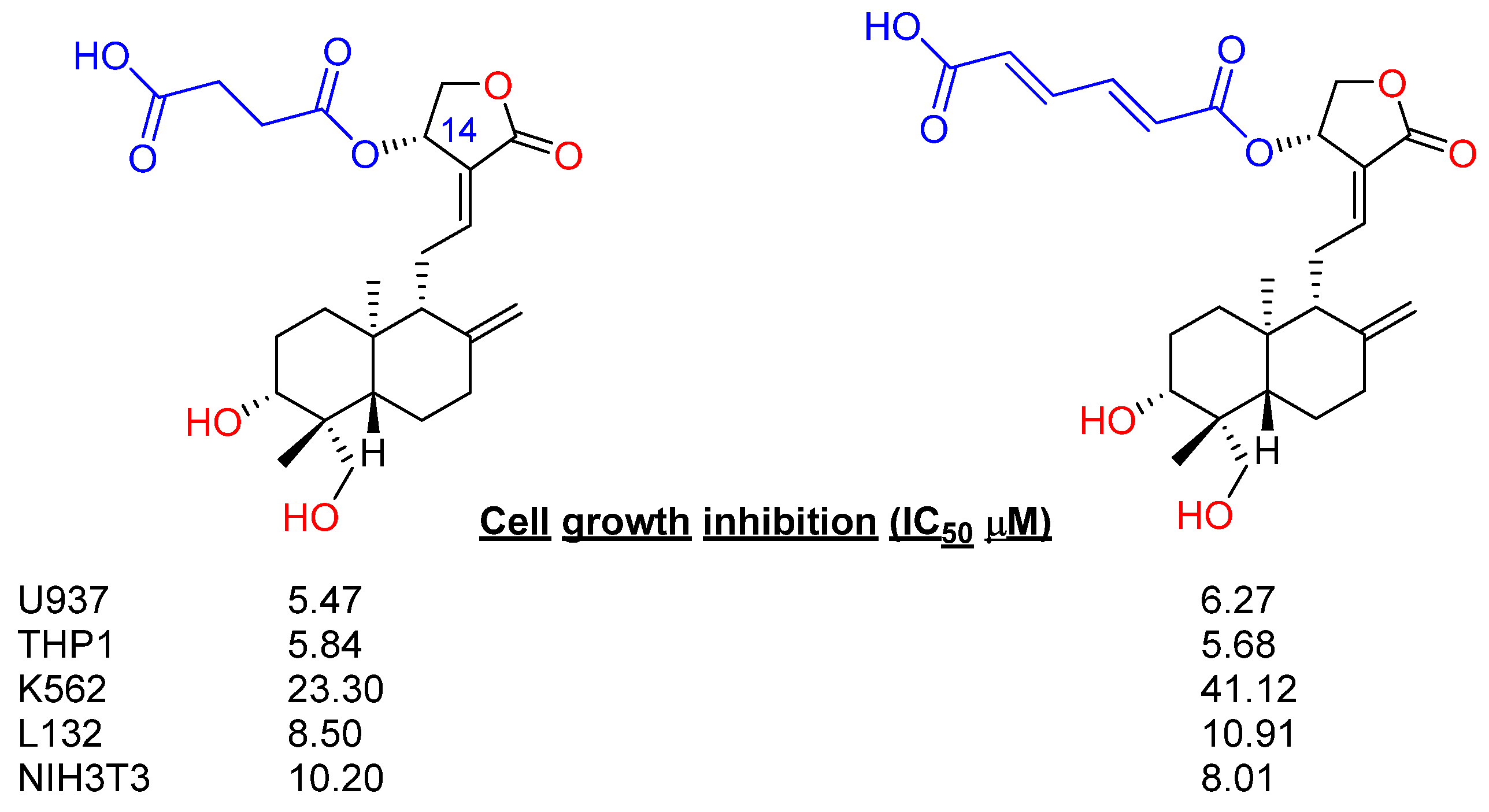
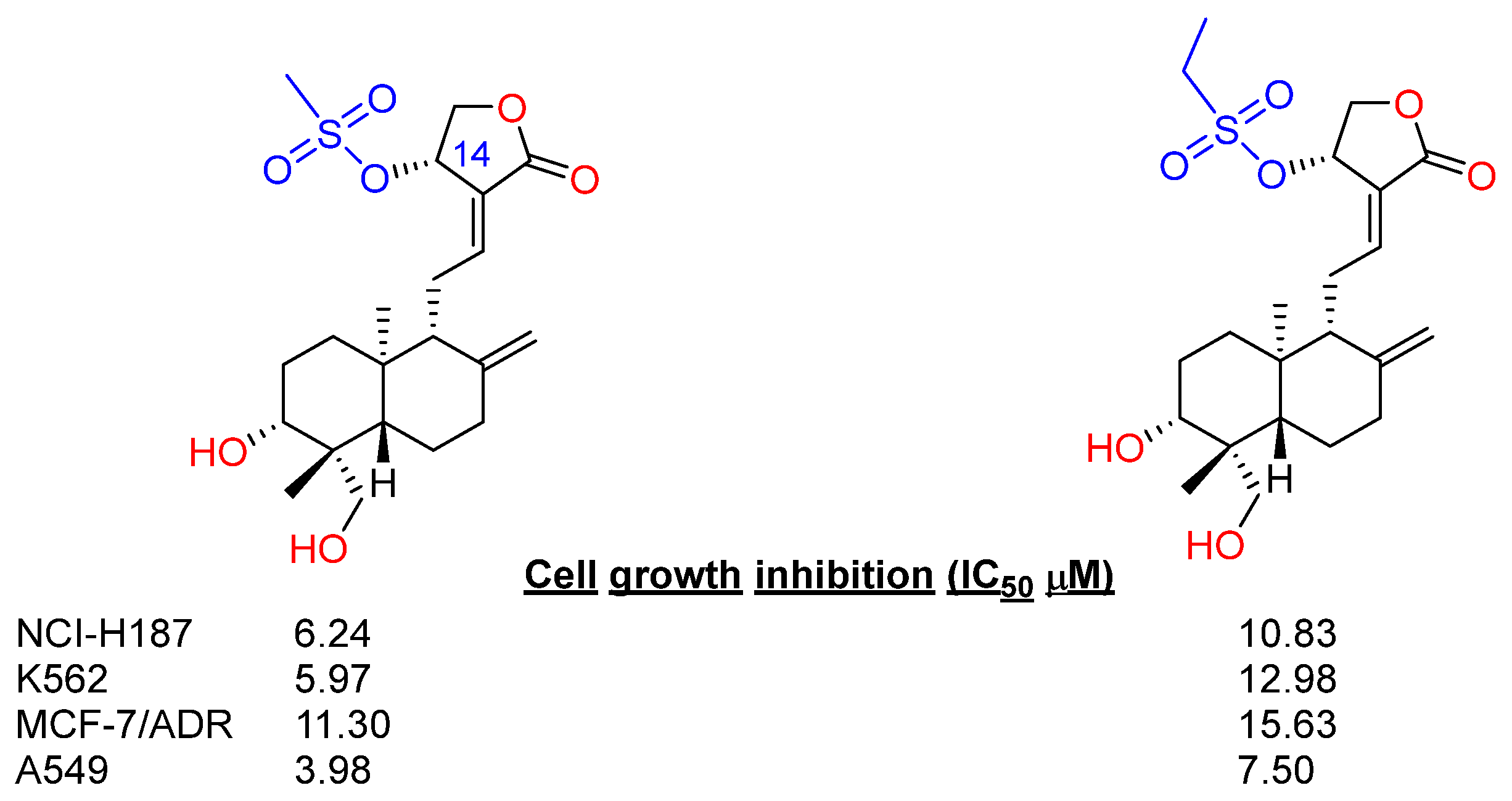
2.2.1. C14, C3 and C19 Modifications
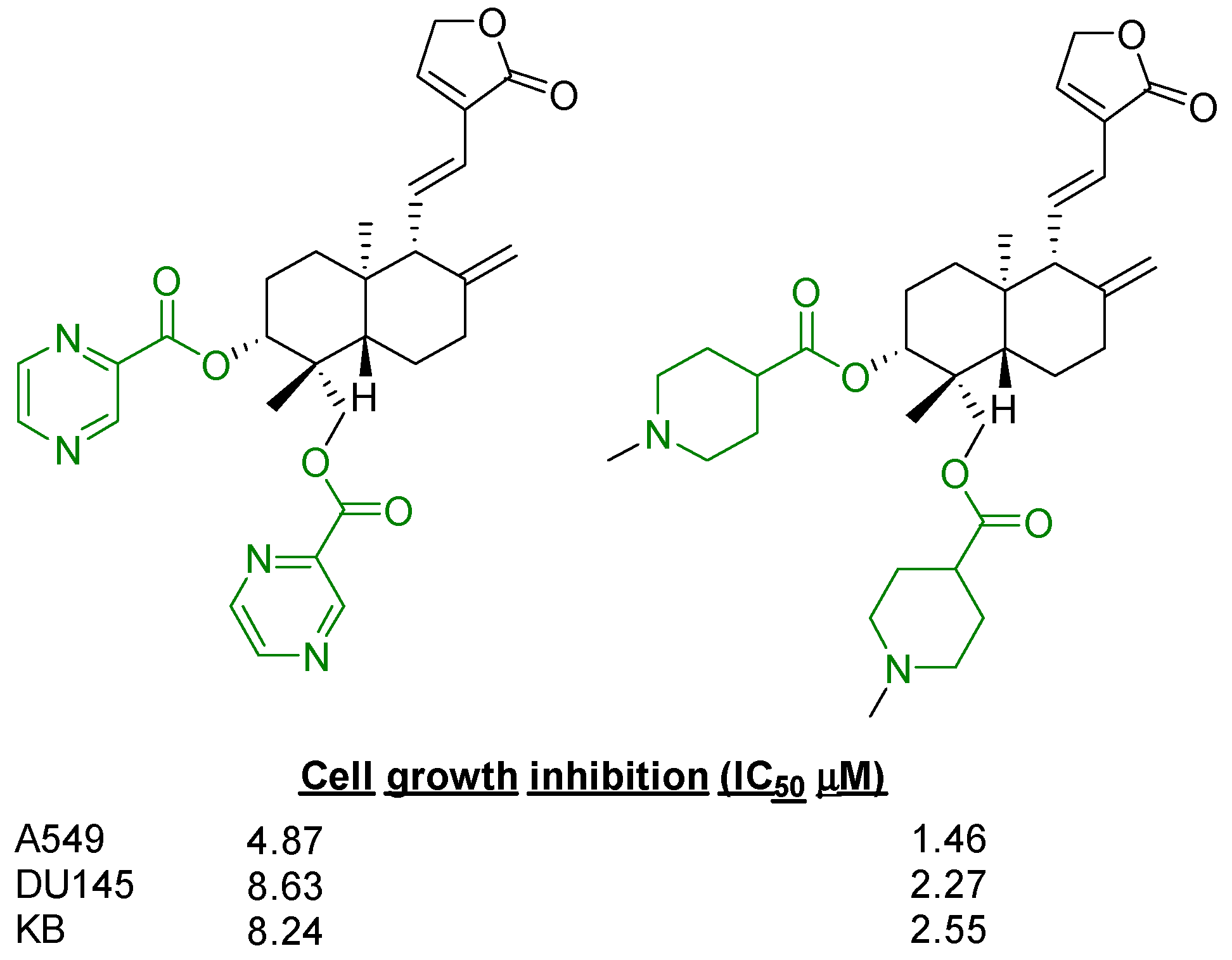
2.2.2. C14, C12, C3 and C19 Modifications
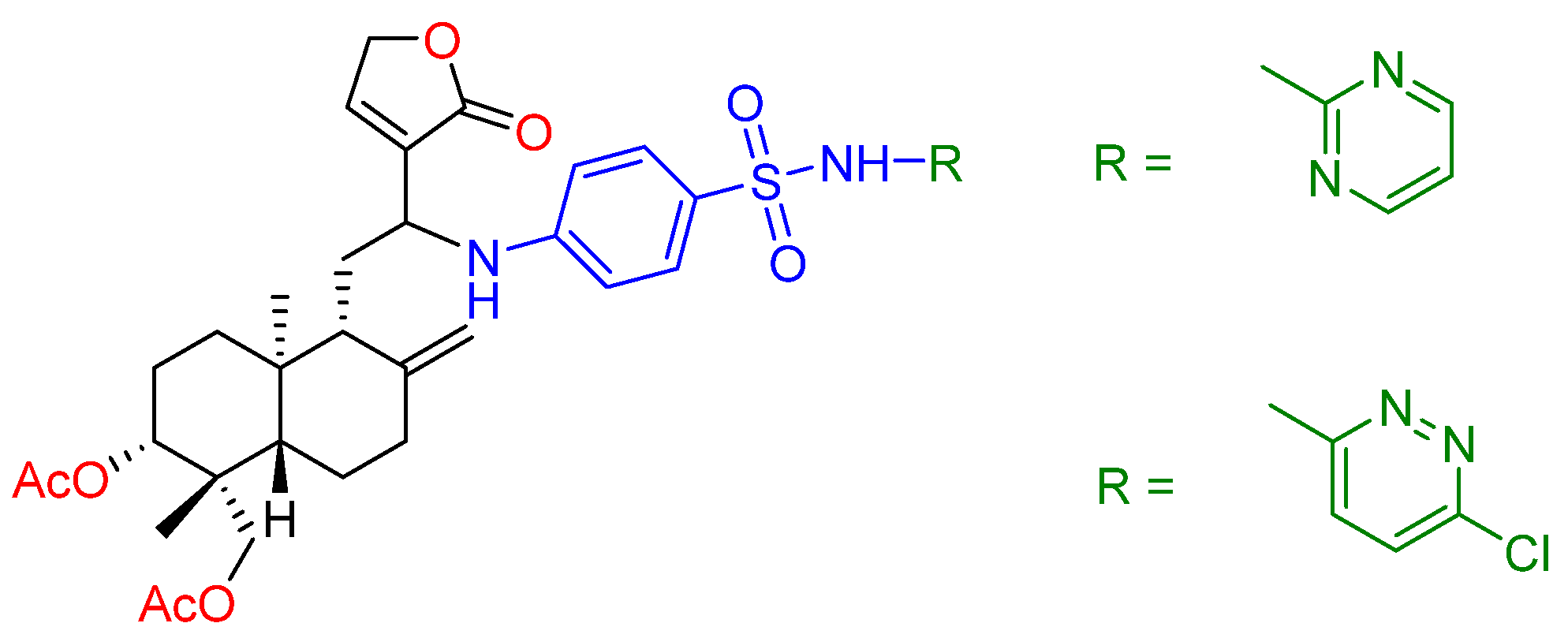
2.2.3. C14, C12, C8, C17, C3 and C19 Modifications
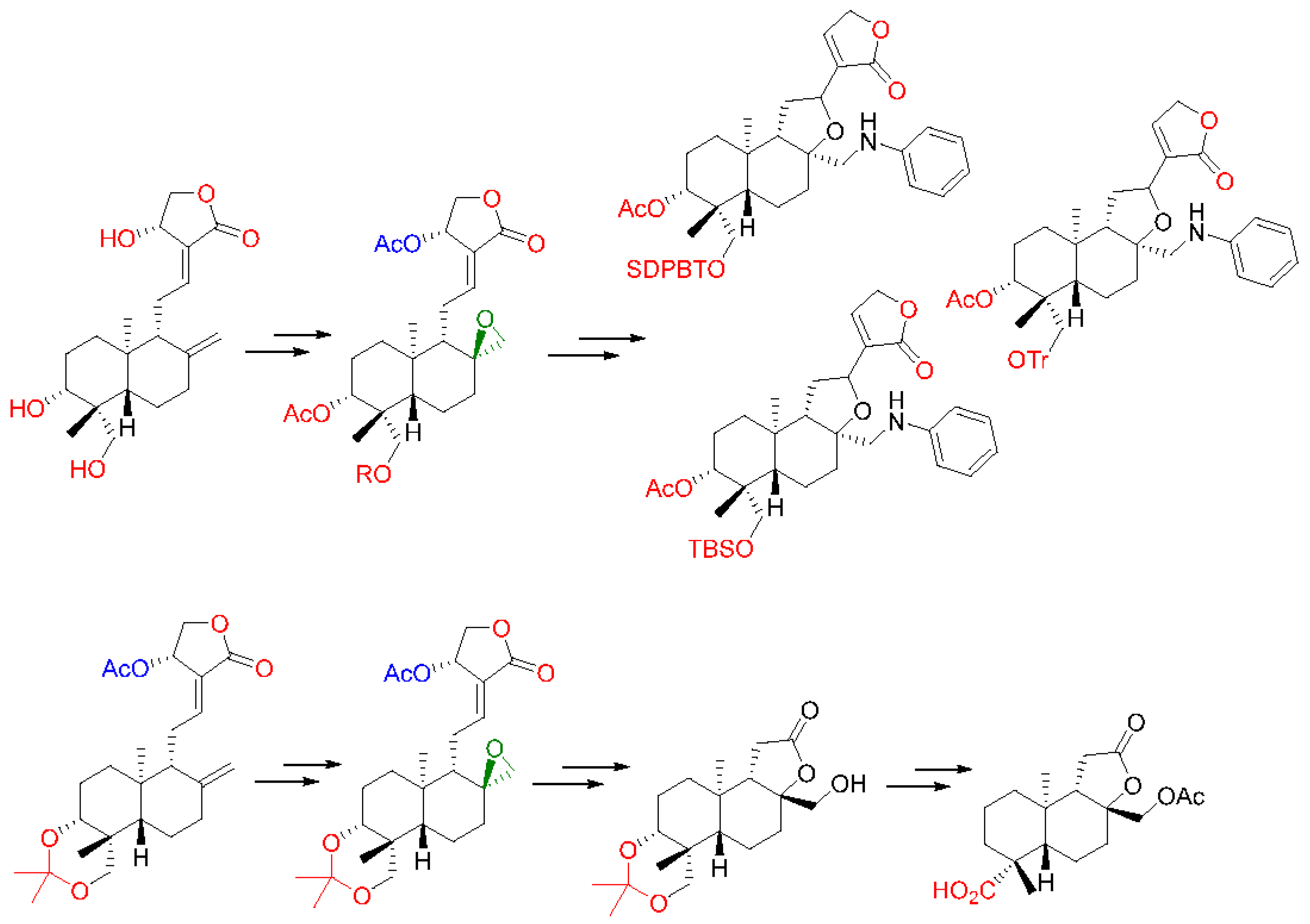
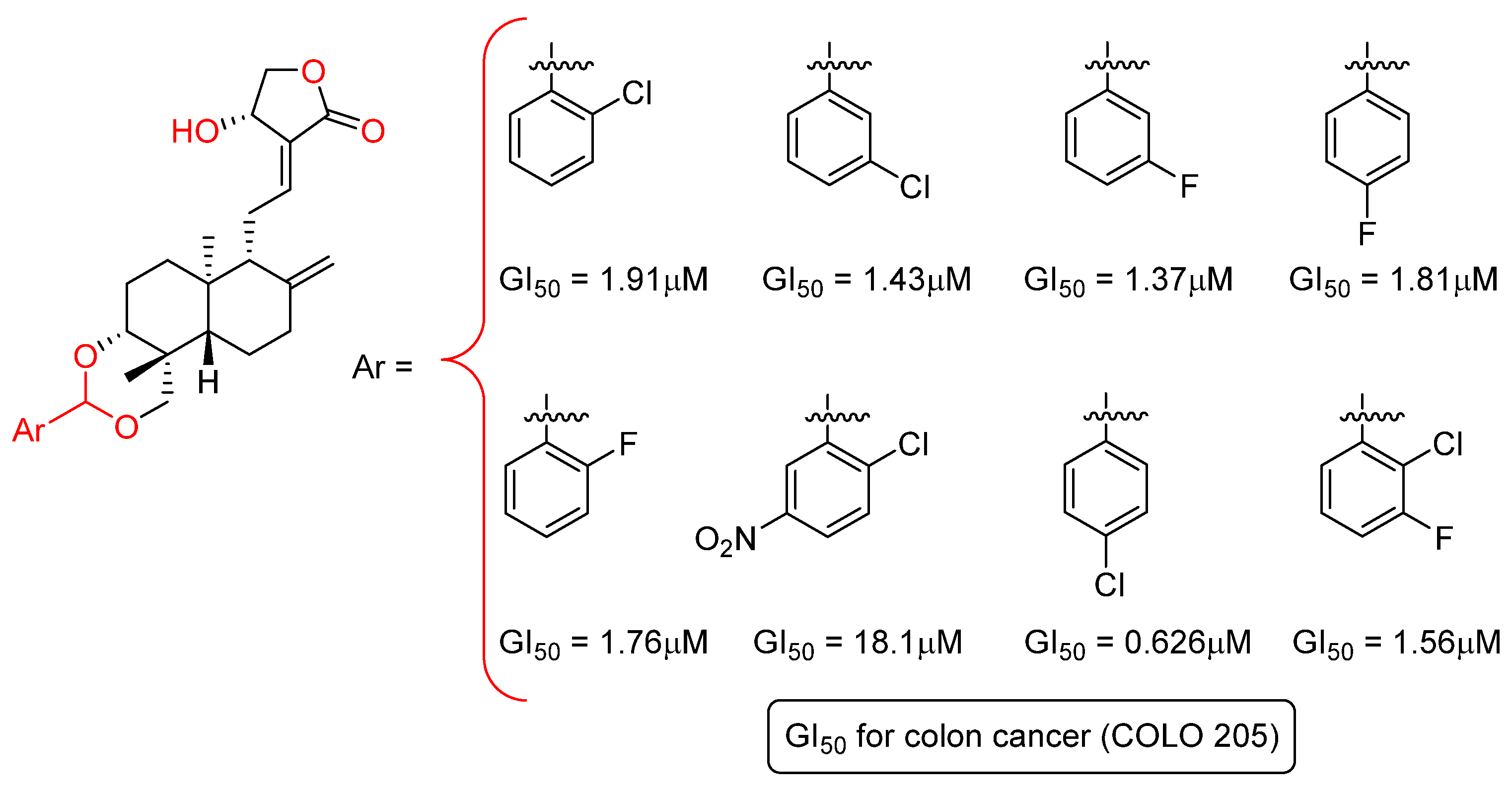
2.3. Modification at Positions C3 and C19
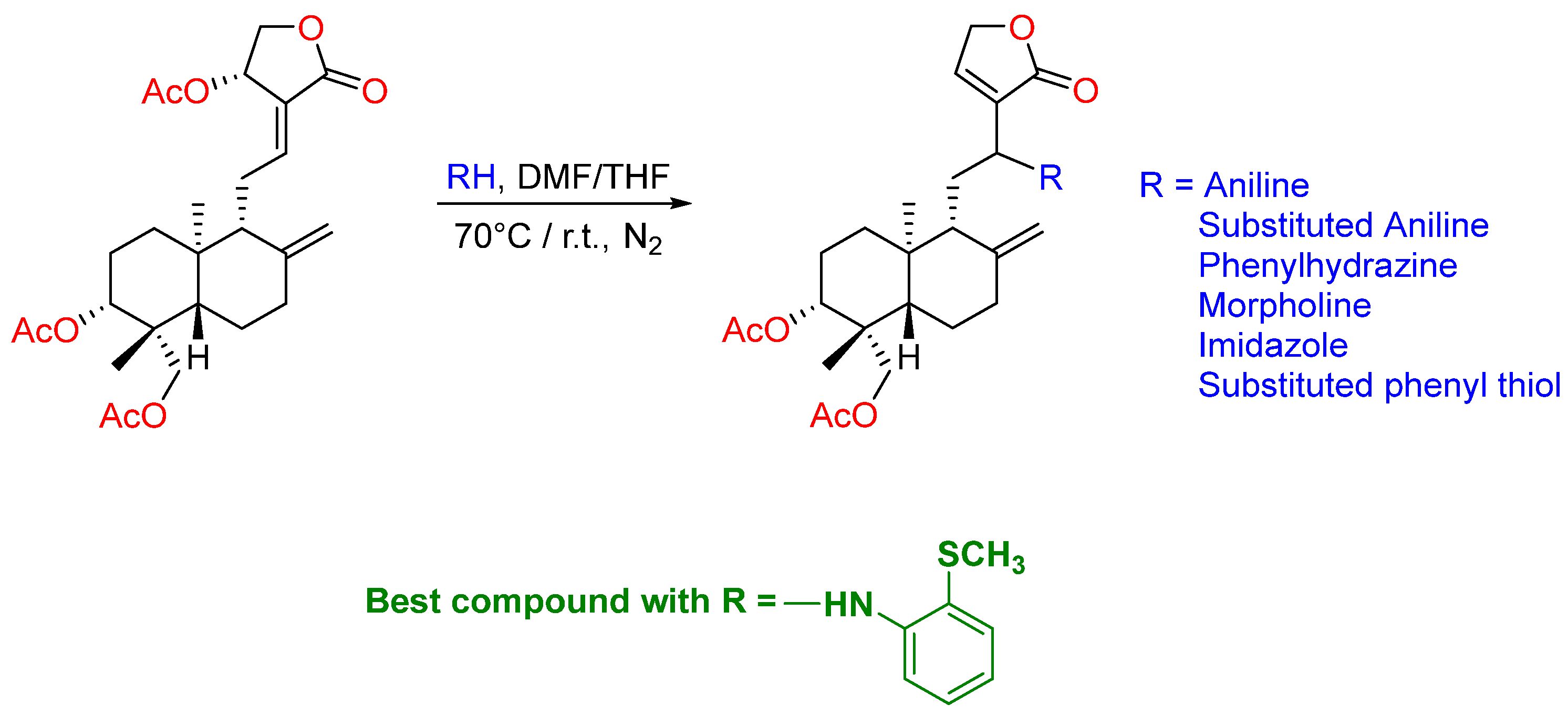
2.4. Modification at Positions C8–C17
2.5. Other Modifications
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Shim, H.J.; Shin, H.M.; Lee, Y.J.; Nam, H.; Kim, S.Y.; Youn, H.S. Andrographolide suppresses TRIF-dependent signaling of toll-like receptors by targeting TBK1. Int. Immunopharmacol. 2018, 57, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Villedieu-Percheron, E.; Ferreira, V.; Campos, J.F.; Destandau, E.; Pichon, C.; Berteina-Raboin, S. Quantitative determination of andrographolide and related compounds in Andrographis paniculata extracts and biological evaluation of their anti-inflammatory activity. Foods 2019, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a natural antioxidant: An update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and its bioactive diterpenoids against inflammation and oxidative stress in keratinocytes. Antioxidants 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and its bioactive diterpenoids protect dermal fibroblasts against inflammation and oxidative stress. Antioxidants 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Levita, J.; Nawawi, A.; Mutalib, A.; Ibrahim, S. Andrographolide: A review of its anti-inflammatory activity via inhibition of NF-kappaB activation from computational chemistry aspects. Int. J. Pharm. 2010, 6, 569–576. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Suthisisang, C.; Dhepakson, P.; Herunsalee, A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int. Immunopharmacol. 2010, 10, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Alarcón, P.; Jara, E.; Solis, L.; Hancke, J.L.; Concha, I.I.; Hidalgo, M.A.; Burgos, R.A. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. Eur. J. Pharmacol. 2009, 602, 413–421. [Google Scholar] [CrossRef]
- Li, X.; Yuan, W.; Wu, J.; Zhen, J.; Sun, Q.; Yu, M. Andrographolide, a natural anti-inflammatory agent: An update. Front. Pharmacol. 2022, 13, 920435. [Google Scholar] [CrossRef]
- Khan, I.; Mahfooz, S.; Ansari, I.A. Antiproliferative and Apoptotic Properties of Andrographolide Against Human Colon Cancer DLD1 Cell Line. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Kuttan, G. Andrographis paniculata downregulates proinflammatory cytokine production and augments cell mediated immune response in metastatic tumor-bearing mice. Asian Pac. J. Cancer Prev. 2010, 11, 723–729. [Google Scholar] [PubMed]
- Sheeja, K.; Kuttan, G. Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr. Cancer Ther. 2007, 6, 66–73. [Google Scholar] [CrossRef]
- Ciampi, E.; Uribe-San-Martin, R.; Cárcamo, C.; Cruz, J.P.; Reyes, A.; Reyes, D.; Pinto, C.; Vasquez, M.; Burgos, R.A.; Hancke, J. Efficacy of andrographolide in not active progressive multiple sclerosis: A prospective exploratory double-blind, parallel-group, randomized, placebo-controlled trial. BMC Neurol. 2020, 20, 173. [Google Scholar] [CrossRef]
- Das, S.; Mishra, K.P.; Ganju, L.; Singh, S.B. Andrographolide—A promising therapeutic agent, negatively regulates glial cell derived neurodegeneration of prefrontal cortex, hippocampus and working memory impairment. J. Neuroimmunol. 2017, 313, 161–175. [Google Scholar] [CrossRef]
- Chen, M.; Xie, C.; Liu, L. Solubility of andrographolide in various solvents from (288.2 to 323.2) K. J. Chem. Eng. Data 2010, 55, 5297–5298. [Google Scholar] [CrossRef]
- Pandey, G.; Rao, C.H. Andrographolide: Its pharmacology, natural bioavailability and current approaches to increase its content in Andrographis paniculata. Int. J. Complement. Alt. Med. 2018, 11, 355–360. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Islam, M.A.; Shaw, S.; Khan, I.N.; Saravi, S.S.S.; Ahmad, S.; Rehman, S.; Gupta, V.K.; et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018, 420, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mishra, K.; Ganju, L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017, 162, 611–623. [Google Scholar] [CrossRef]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Si, Y.; Xu, H. Andrographolide and its derivatives: Current achievements and future perspectives. Eur. J. Med. Chem. 2021, 224, 113710. [Google Scholar] [CrossRef]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.W.; Sagineedu, S.R.; Stanlas, J.; Wong, W.S.F. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Preet, R.; Chkraborty, B.; Siddharth, S.; Mohapatra, P.; Das, D.; Satapathy, S.R.; Das, S.; Maiti, N.C.; Maulik, P.R.; Kundu, C.N.; et al. Synthesis and biological evaluation of andrographolide analogues as anti-cancer agents. Eur. J. Med. Chem. 2014, 85, 95–106. [Google Scholar] [CrossRef]
- Banerjee, M.; Chattopadhyay, S.; Choudhuri, T.; Bera, R.; Kumar, S.; Chakraborty, B.; Mukherjee, S.K. Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J. Biomed. Sci. 2016, 23, 40. [Google Scholar] [CrossRef]
- Khan, I.; Khan, F.; Farooqui, A.; Ansari, I.A. Andrographolide Exhibits Anticancer Potential Against Human Colon Cancer Cells by Inducing Cell Cycle Arrest and Programmed Cell Death via Augmentation of Intracellular Reactive Oxygen Species Level. Nutr. Cancer 2018, 70, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Elancheran, R.; Maruthanibla, V.L.; Ramanathan, M.; Kabilan, S.; Devi, R.; Kunnumakara, A.; Kotoky, J. Recent discoveries and developments of androgen receptor based therapy for prostate cancer. Med. Chem. Commun. 2015, 6, 746–768. [Google Scholar] [CrossRef]
- Chen, W.; Feng, L.; Nie, H.; Zheng, X. Andrographolide induces autophagic cell death in human liver cancer cells through cyclophilin D-mediated mitochondrial permeability transition pore. Carcinogenesis 2012, 33, 2190–2198. [Google Scholar] [CrossRef]
- Bonomi, M.; Patsias, A.; Posner, M.; Sikora, A. The role of inflammation in head and neck cancer. Adv. Exp. Med. Biol. 2014, 816, 107–127. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, W.; Jiang, J. Prevention and treatment of cancer targeting chronic inflammation: Research progress, potential agents, clinical studies and mechanisms. Sci. China Life Sci. 2017, 60, 601–616. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Tang, F.; Zhang, J.; Li, R.; Sheng, D.; Lee, S.M.; Zhou, G.C.; Leung, G.P. AGS-30, an andrographolide derivative, suppresses tumor angiogenesis and growth in vitro and in vivo. Biochem. Pharmacol. 2020, 171, 113694. [Google Scholar] [CrossRef]
- Kandanur, S.G.S.; Kundu, S.; Cadena, C.; Juan, H.S.; Bajaj, A.; Guzman, J.D.; Nanduri, S.; Golakoti, N.R. Design, synthesis, and biological evaluation of new 12-substituted-14-deoxy-andrographolide derivatives as apoptosis inducers. Chem. Pap. 2019, 73, 1669–1675. [Google Scholar] [CrossRef]
- Kajal, K.; Panda, A.K.; Bhat, J.; Chakraborty, D.; Bose, S.; Bhattacharjee, P.; Sarkar, T.; Chatterjee, S.; Kar, S.K.; Sa, G. Andrographolide binds to ATP-binding pocket of VEGFR2 to impede VEGFA-mediated tumor-angiogenesis. Sci. Rep. 2019, 9, 4073. [Google Scholar] [CrossRef]
- Ji, L.; Zheng, Z.; Shi, L.; Huang, Y.; Lu, B.; Wang, Z. Andrographolide decreased VEGFD expression in hepatoma cancer cells by inducing ubiquitin/proteasome-mediated cFos protein degradation. Biochim. Biophys. Acta 2015, 1850, 750–758. [Google Scholar] [CrossRef]
- Shen, K.K.; Ji, L.L.; Lu, B.; Xu, C. Andrographolide inhibits tumor angiogenesis via blocking VEGFA/VEGFR2-M APKs signaling cascade. Chem. Biol. Interact. 2014, 218, 99–106. [Google Scholar] [CrossRef]
- Nateewattana, J.; Dutta, S.; Reabroi, S.; Saeeng, R.; Kasemsook, S.; Chairoungdua, A.; Weerachayaphorn, J.; Wongkham, S.; Piyachaturawat, P. Induction of apoptosis in cholangiocarcinoma by an andrographolide analogue is mediated through topoisomerase II alpha inhibition. Eur. J. Pharmacol. 2014, 723, 148–155. [Google Scholar] [CrossRef]
- Dai, G.F.; Xu, H.W.; Wang, J.F.; Liu, F.W.; Liu, H.M. Studies on the novel α-glucosidase inhibitory activity and structure–activity relationships for andrographolide analogues. Bioorg. Med. Chem. Lett. 2006, 16, 2710–2713. [Google Scholar] [CrossRef]
- Song, Y.; Xin, Z.; Wan, Y.; Li, J.; Ye, B.; Xue, X. Synthesis and anticancer activity of some novel indolo[3,2-b]andrographolide derivatives as apoptosis-inducing agents. Eur. J. Med. Chem. 2015, 90, 695–706. [Google Scholar] [CrossRef]
- Wei, S.; Tang, Y.B.; Hua, H.; Ohkoshi, E.; Goto, M.; Wang, L.T.; Lee, K.H.; Xiao, Z. Discovery of novel andrographolide derivatives as cytotoxic agents. Bioorg. Med. Chem. Lett. 2013, 23, 4056–4060. [Google Scholar] [CrossRef]
- Wu, Z.W.; Xu, H.W.; Dai, G.F.; Liu, M.J.; Zhu, L.P.; Wu, J.; Wang, Y.N.; Wu, F.J.; Zhao, D.; Gao, M.F.; et al. Improved inhibitory activities against tumor-cell migration and invasion by 15-benzylidene substitution derivatives of andrographolide. Bioorg. Med. Chem. Lett. 2013, 23, 6421–6426. [Google Scholar] [CrossRef]
- Yang, S.H.; Lin, J.; Lu, F.; Han, Z.H.; Fu, C.X.; Lv, P.; Liu, H.; Gao, D.M. Evaluation of antiangiogenic and antiproliferative effects of sorafenib by sequential histology and intravoxel incoherent motion diffusion-weighted imaging in an orthotopic hepatocellular carcinoma xenograft model. J. Magn. Reson. Imaging 2017, 45, 270–280. [Google Scholar] [CrossRef]
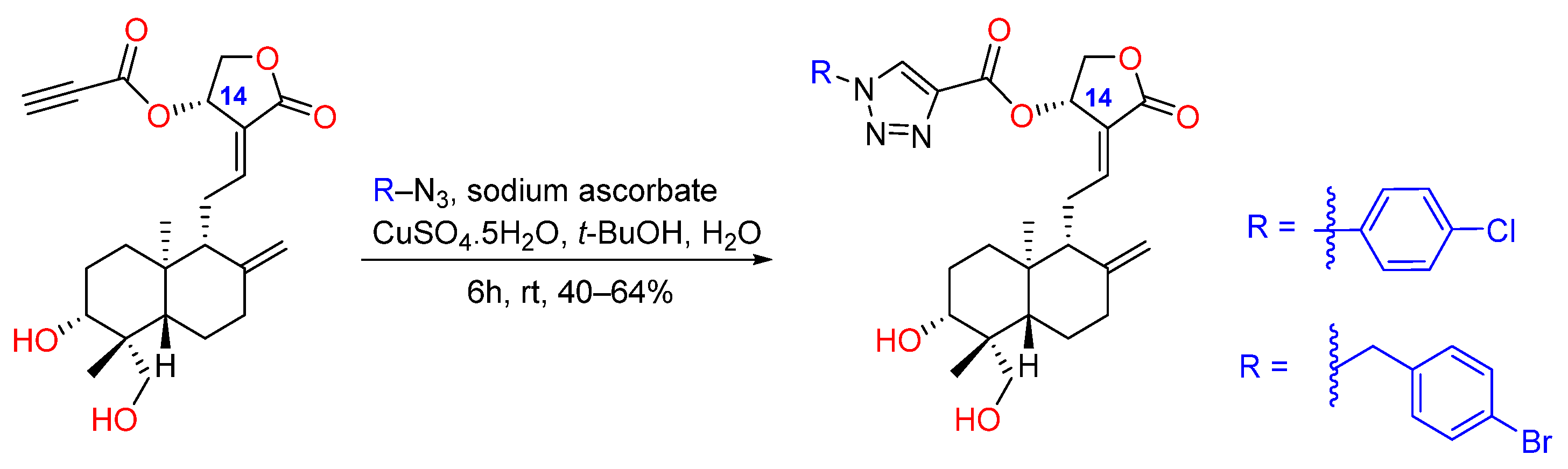
- Mokenapelli, S.; Gutam, M.; Vadiyaala, N.; Yerrabelli, J.R.; Banerjee, S.; Roy, P.; Kancha, R.K.; Kunduru, B.R.; Sagurthi, S.R.; Chitneni, P.R. Synthesis and cytotoxicity of novel 14α-O-(1,4-disubstituted-1,2,3-triazolyl) ester derivatives of andrographolide. Nat. Prod. Res. 2021, 35, 289–297. [Google Scholar] [CrossRef]
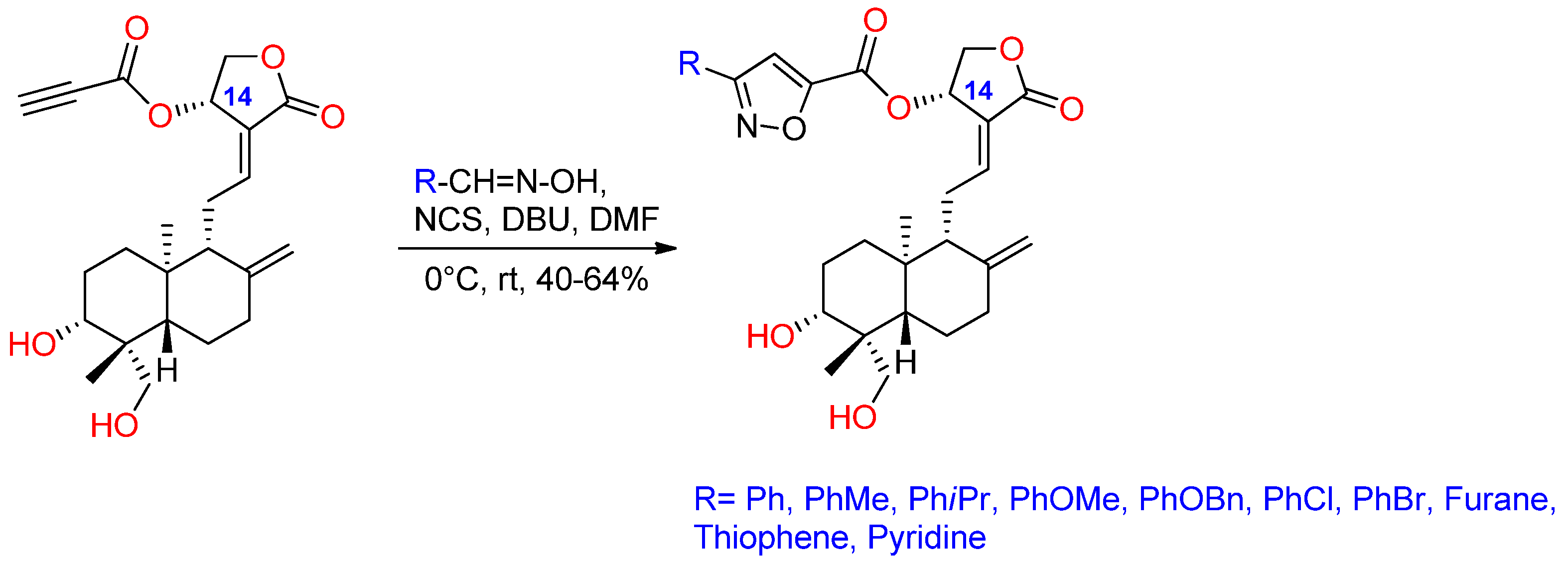
- Mokenapelli, S.; Yerrabelli, J.R.; Das, N.; Roy, P.; Chitneni, P.R. Synthesis and cytotoxicity of novel 14α-O-(andrographolide-3-substituted-isoxazole-5-carboxylate) derivatives. Nat. Prod. Res. 2021, 35, 3738–3744. [Google Scholar] [CrossRef]
- Das, B.; Chowdhury, C.; Kumar, D.; Sen, R.; Roy, R.; Das, P.; Chatterjee, M. Synthesis, cytotoxicity, and structure–activity rela-tionship (SAR) studies of andrographolide analogues as anti-cancer agent. Bioorg. Med. Chem. Lett. 2010, 20, 6947–6950. [Google Scholar] [CrossRef]
- Agarwal, D.; Singh, P. Synthesis, characterization and antibacterial activity of C (14)-sulfonyl ester-type andrographolide derivatives. J. Global. Biosci. 2015, 4, 2348–2354. [Google Scholar]
- Tian, K.; Li, H.; Zhao, B.; Su, Y.; Zou, Z.; Wang, W. Synthesis, Characterization and Bioactivity Evaluation of a Novel Nano Bagasse Xylan/Andrographolide Grafted and Esterified Derivative. Polymers 2022, 14, 3432. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, Q.; Yan, Y.; Peng, F.; Sun, R.; Ren, J. Hemicellulose from plant biomass in medical and pharmaceutical application: A critical review. Curr. Med. Chem. 2019, 26, 2430–2455. [Google Scholar] [CrossRef]
- El-Din, N.K.B.; Ali, D.A.; Othman, R.; French, S.W.; Ghoneum, M. Chemopreventive role of arabinoxylan rice bran, MGN 3/Biobran, on liver carcinogenesis in rats. Biomed. Pharmacother. 2020, 126, 110064. [Google Scholar] [CrossRef]
- Kumar, S.U.; Kumar, V.; Priyadarshi, R.; Gopinath, P.; Negi, Y.S. pH-responsive prodrug nanoparticles based on xylan-curcumin conjugate for the efficient delivery of curcumin in cancer therapy. Carbohydr. Polym. 2018, 188, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, B.; Gao, F.; Lan, M. Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm. Biol. 2016, 54, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylans, gut microbiota and immunity. Carbohydr. Polym. 2016, 139, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo-oligosaccharides and inulin impact inter-individual variation on microbial metabolism and composition, which immunomodulates human cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.S.; P, V.R.; Patil, V.; Patil, R. Design, synthesis of new C(14)-andrographolide analogues and their cytotoxic activity. Int. J. Appl. Biol. Pharm. Tech. 2014, 5, 141–145. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Nat. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, J.; Sun, Y.; Chan, J.Y.W.; Sheng, D.; Wang, K.; Wei, P.; Ouyang, P.; Wang, D.; Lee, S.M.Y.; et al. SAR studies of 3, 14, 19-derivatives of andrographolide on anti-proliferative activity to cancer cells and toxicity to zebrafish: An in vitro and in vivo study. RSC Adv. 2015, 5, 22510–22526. [Google Scholar] [CrossRef]
- Sombut, S.; Bunthawong, R.; Sirion, U.; Kasemsuk, T.; Piyachaturawat, P.; Suksen, K.; Suksamrarn, A.; Saeeng, R. Synthesis of 14-deoxy-11, 12-didehydroandrographolide analogues as potential cytotoxic agents for cholangiocarcinoma. Bioorg. Med. Chem. Lett. 2017, 27, 5139–5143. [Google Scholar] [CrossRef]
- Chinthala, Y.; Manjulatha, K.; Sharma, P.; Kvn, S.S.; Jonnala, K.; Arigari, N.K.; Khan, F.; Oh, S.-H. Synthesis and cytotoxicity evaluation of novel andrographolide-1, 2, 3-triazole derivatives. J. Heterocycl. Chem. 2016, 53, 1902–1910. [Google Scholar] [CrossRef]
- Chen, D.; Song, Y.; Lu, Y.; Xue, X. Synthesis and in vitro cytotoxicity of andrographolide-19-oic acid analogues as anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 3166–3169. [Google Scholar] [CrossRef] [PubMed]
- Sirion, U.; Kasemsook, S.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. New substituted C-19-andrographolide analogues with potent cytotoxic activities. Bioorg. Med. Chem. Lett. 2012, 22, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Devendar, P.; Kumar, A.N.; Bethu, M.; Zehra, A.; Pamanji, R.; Rao, J.V.; Tiwari, A.K.; Sridhar, B.; Srinivas, K.S.; Kumar, J.K. Highly selective one pot synthesis and biological evaluation of novel 3-(allyloxy)-propylidene acetals of some natural ter- penoids. RSC Adv. 2015, 5, 93122–93130. [Google Scholar] [CrossRef]
- Kasemsuk, S.; Sirion, U.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. 12-Amino-andrographolide analogues: Synthesis and cytotoxic activity. Arch. Pharm. Res. 2013, 36, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Kandanur, S.G.S.; Nanduri, S.; Golakoti, N.R. Synthesis and biological evaluation of new C-12(α/β)-(N-)sulfamoyl-phenylamino-14-deoxy-andrographolide derivatives as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Kandanur, S.G.S.; Nageswara Rao, G.; Nanduri, S. Synthesis and in vitro cytotoxicity of novel C-12 substituted-14-deoxy-andro- grapholide derivatives as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 5781–5786. [Google Scholar] [CrossRef] [PubMed]
- Arsakhant, P.; Sirion, U.; Chairoungdua, A.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. Design and synthesis of C-12 dithiocarbamate andrographolide analogues as an anticancer agent. Bioorg. Med. Chem. Lett. 2020, 30, 127263. [Google Scholar] [CrossRef]
- Xie, R.; Li, Y.; Tang, P.; Yuan, Q. Design, synthesis and biological evaluation of novel 2- aminobenzamides containing dithiocar-bamate moiety as histone deacetylase inhibitors and potent antitumor agents. Eur. J. Med. Chem. 2018, 143, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Sirion, U.; Kasemsuk, T.; Piyachaturawat, P.; Suksen, K.; Suksamrarn, A.; Saeeng, R. Synthesis and cytotoxic activity of 14-deoxy-12- hydroxyandrographolide analogs. Med. Chem. Res. 2017, 26, 1653–1663. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Piyachaturawat, P.; Bunthawong, R.; Sirion, U.; Suksen, K.; Suksamrarn, A.; Saeeng, R. One-pot three steps cascade synthesis of novel isoandrographolide analogues and their cytotoxic activity. Eur. J. Med. Chem. 2017, 138, 952–963. [Google Scholar] [CrossRef]
- Xin, Z.; Lu, Y.; Song, Z.; He, Y.; Li, J.; Lin, K.; Xue, X. Stereoselective synthesis and biological evaluation of ent-asperolide C and its analogues. Eur. J. Org. Chem. 2018, 2018, 477–484. [Google Scholar] [CrossRef]
- Jada, S.R.; Hamzah, A.S.; Lajis, N.H.; Saad, M.S.; Stevens, M.F.G.; Stanslas, J. Semisynthesis and cytotoxic activities of andrographolide analogues. J. Enzym. Inhib. Med. Chem. 2006, 21, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.W.; Zhang, J.; Liu, H.M.; Wang, J.F. Synthesis of andrographolide cyclophosphate derivatives and their antitumor activities. Synth. Commun. 2006, 36, 407–414. [Google Scholar] [CrossRef]
- Menon, V.; Bhat, S. Anticancer Activity of Andrographolide Semisynthetic Derivatives. Nat. Prod. Commun. 2010, 5, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Nyavanandi, V.K.; Thunuguntla, S.S.R.; Kasu, S.; Pallerla, M.K.; Ram, P.S.; Rajagopal, S.; Kumar, R.A.; Ramanujam, R.; Babu, J.M.; et al. Synthesis and structure-activity relationship of andrographolide analogues as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 2004, 14, 4711–4717. [Google Scholar] [CrossRef]
- Kumar, G.; Thapa, S.; Tali, J.A.; Singh, D.; Sharma, B.K.; Panda, K.N.; Singh, S.K.; Shankar, R. Site-Selective Synthesis of C-17 Ester Derivatives of Natural Andrographolide for Evaluation as a Potential Anticancer Agent. ACS Omega 2023, 8, 6099–6123. [Google Scholar] [CrossRef]





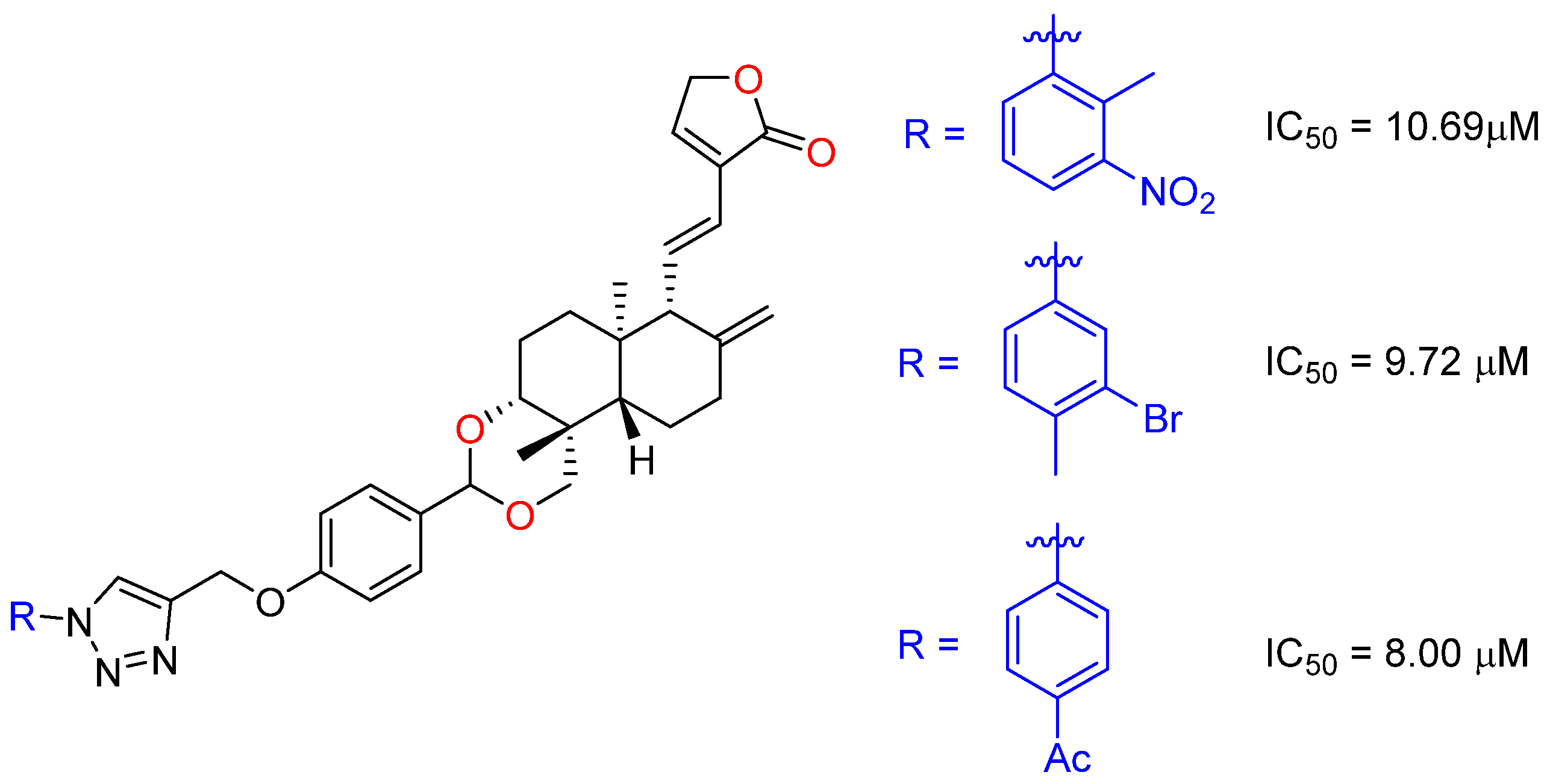
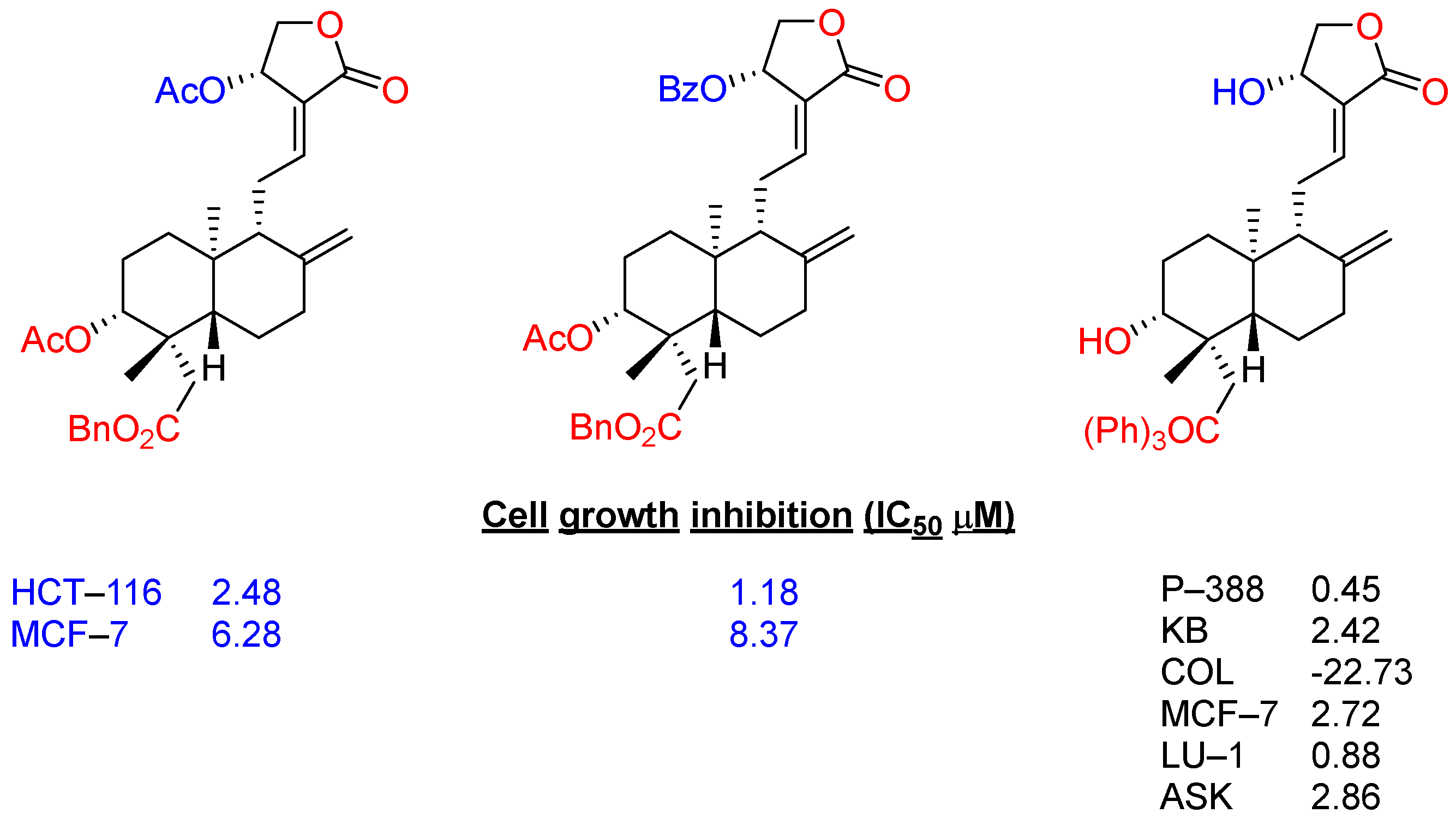
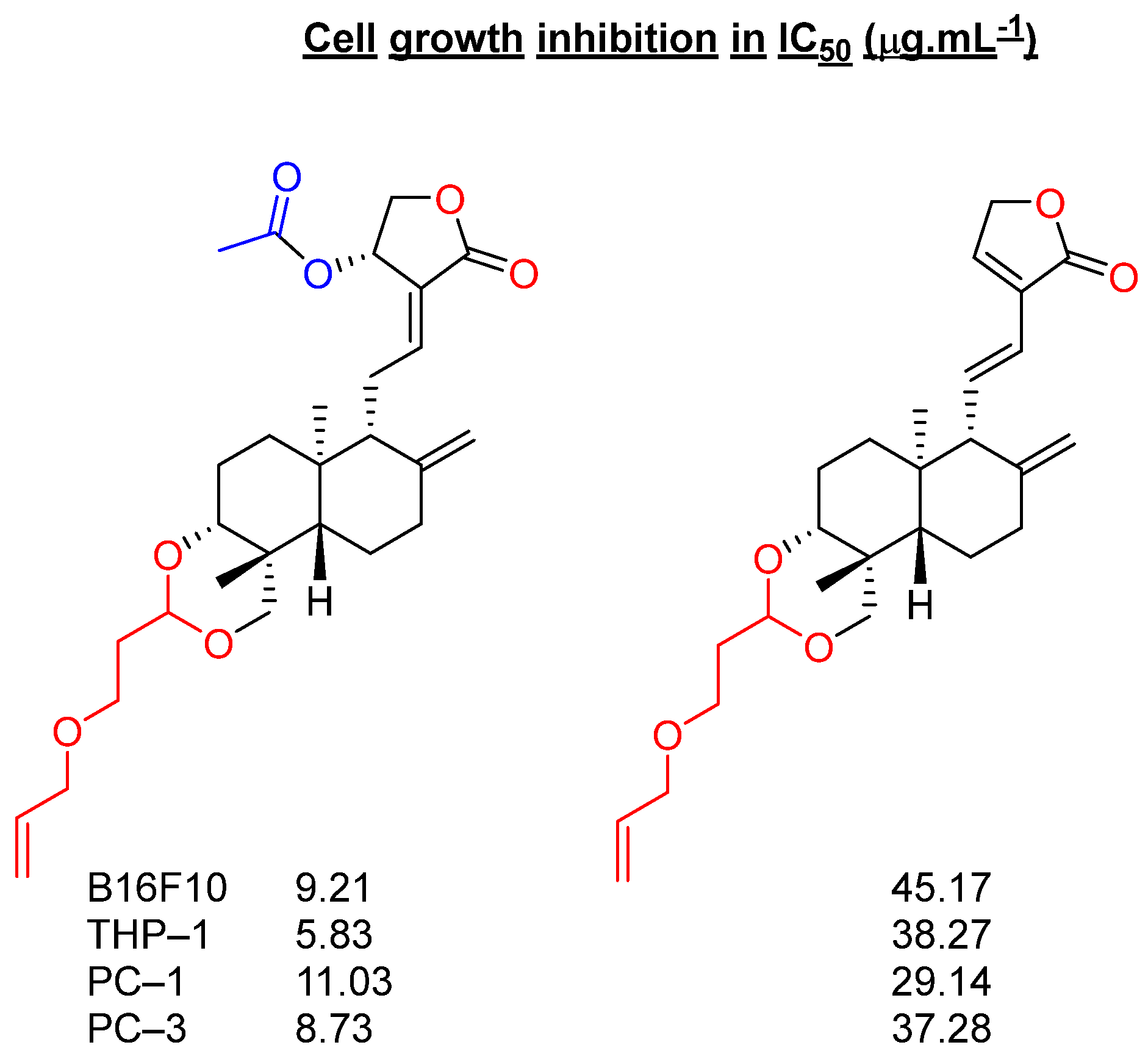
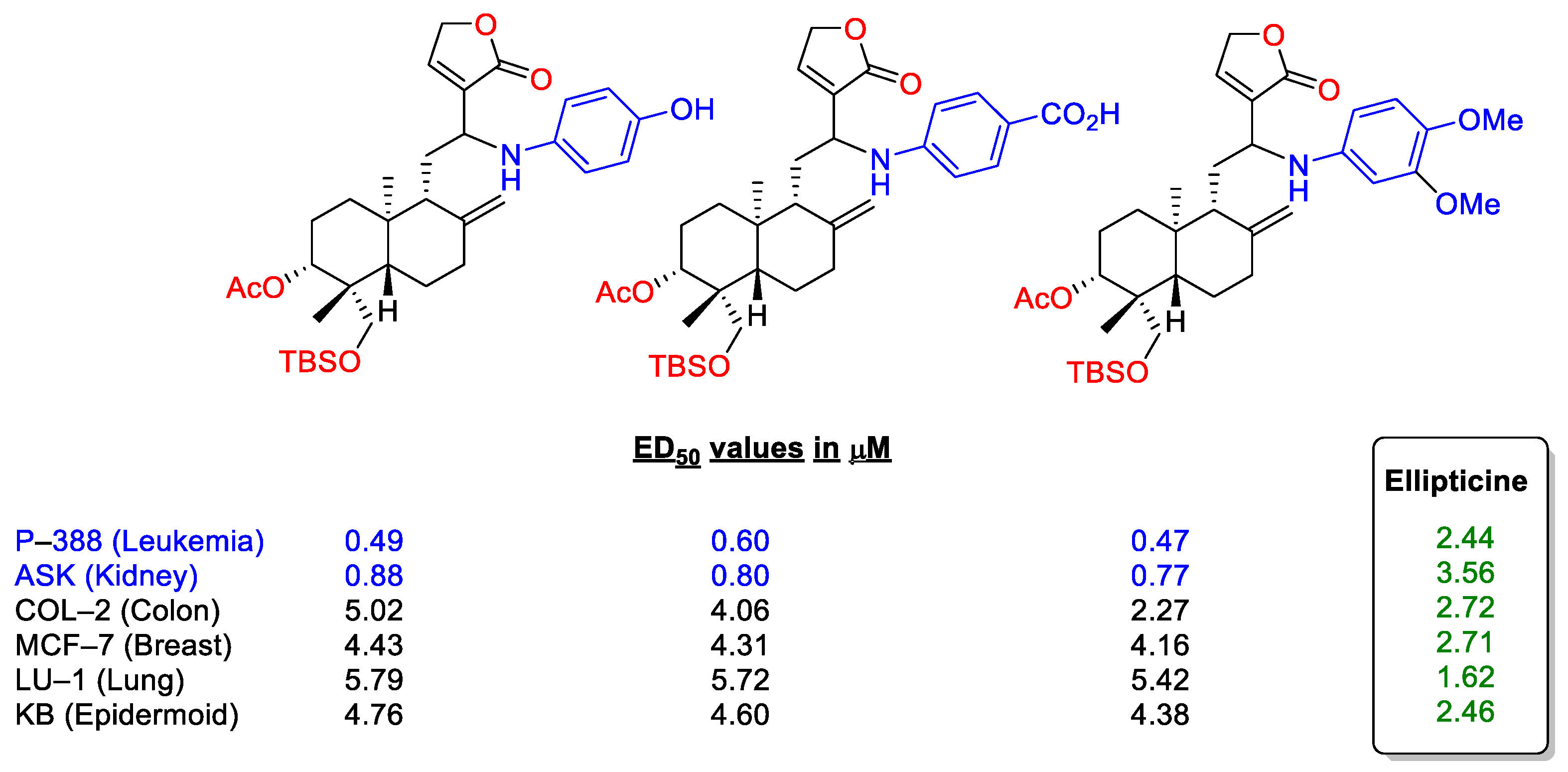




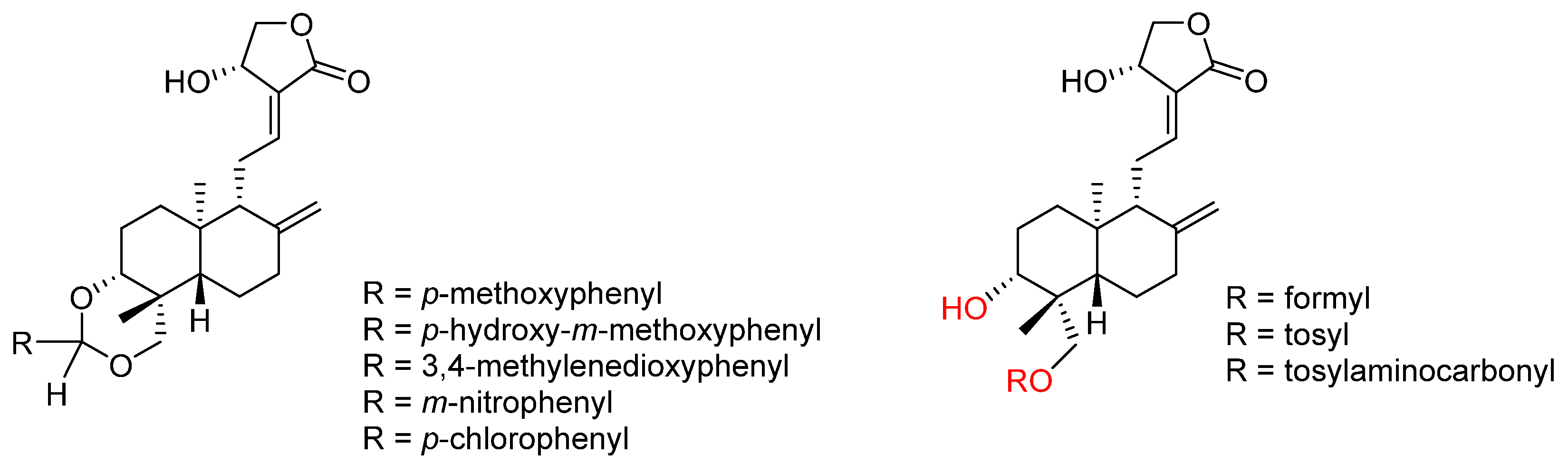


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messire, G.; Rollin, P.; Gillaizeau, I.; Berteina-Raboin, S. Synthetic Modifications of Andrographolide Targeting New Potential Anticancer Drug Candidates: A Comprehensive Overview. Molecules 2024, 29, 2884. https://doi.org/10.3390/molecules29122884
Messire G, Rollin P, Gillaizeau I, Berteina-Raboin S. Synthetic Modifications of Andrographolide Targeting New Potential Anticancer Drug Candidates: A Comprehensive Overview. Molecules. 2024; 29(12):2884. https://doi.org/10.3390/molecules29122884
Chicago/Turabian StyleMessire, Gatien, Patrick Rollin, Isabelle Gillaizeau, and Sabine Berteina-Raboin. 2024. "Synthetic Modifications of Andrographolide Targeting New Potential Anticancer Drug Candidates: A Comprehensive Overview" Molecules 29, no. 12: 2884. https://doi.org/10.3390/molecules29122884
APA StyleMessire, G., Rollin, P., Gillaizeau, I., & Berteina-Raboin, S. (2024). Synthetic Modifications of Andrographolide Targeting New Potential Anticancer Drug Candidates: A Comprehensive Overview. Molecules, 29(12), 2884. https://doi.org/10.3390/molecules29122884









