Research Progress on Ti3C2Tx-Based Composite Materials in Antibacterial Field
Abstract
:1. Introduction
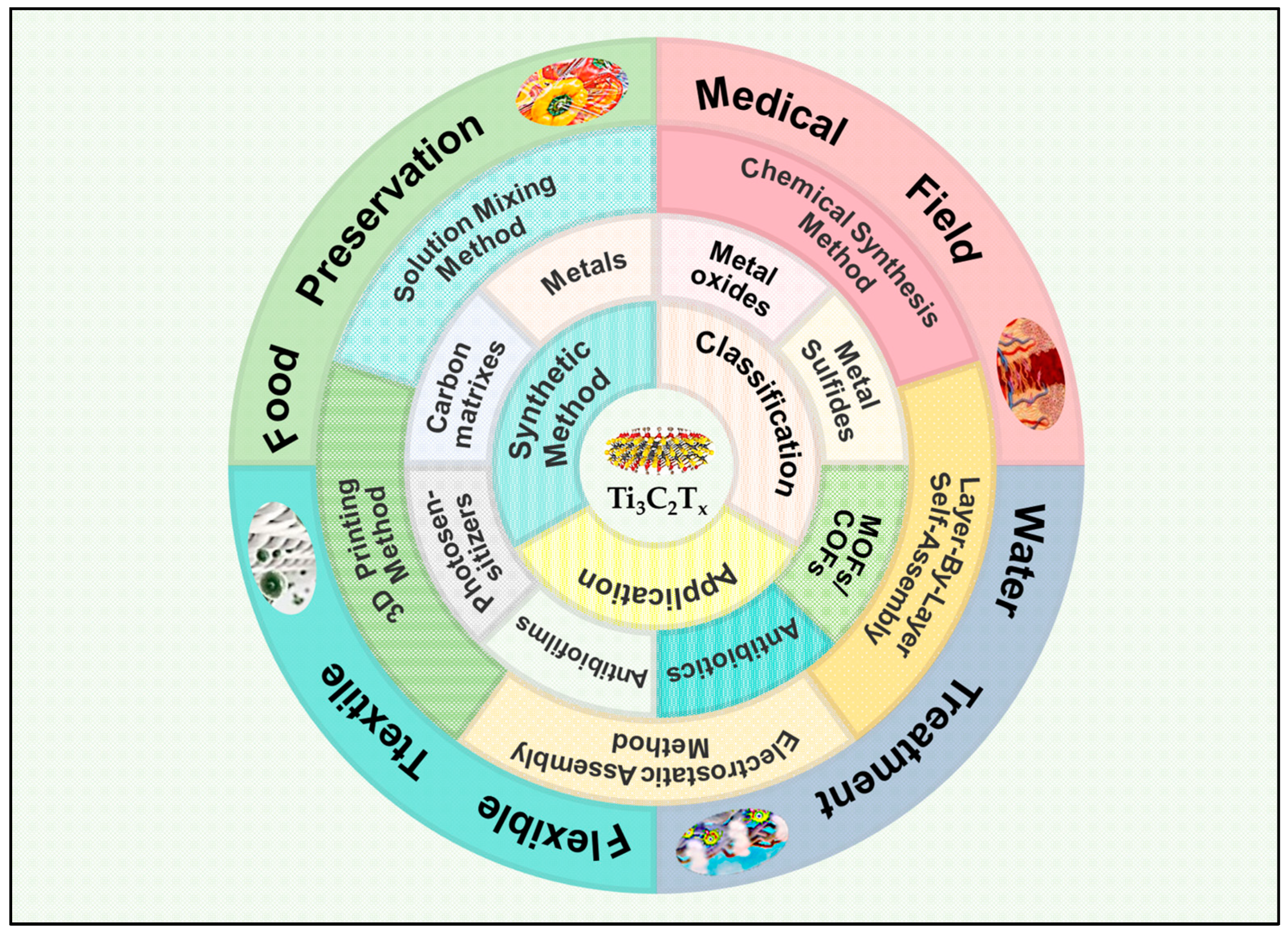
2. Classification of the Ti3C2Tx Composite Antimicrobial Materials
| Classification | Constituent | Cytocompatibility | Sterilization | Application | References |
|---|---|---|---|---|---|
| Metal | Au | The bacteria with materials in the dark showed essentially normal bacterial morphology | It effectively kills Staphylococcus aureus and prevents the development of biofilm. | Wound antibacterial healing auxiliary material | [35] |
| Au | Not mentioned | The antibacterial efficiency is as high as 99.9%. | Multifunctional flexible pressure sensor | [36] | |
| Metal oxide | V2O5 | Not mentioned | It is an effective antibacterial agent against Gram-positive bacteria and Gram-negative bacteria. | Waste disposal | [37] |
| Metal sulfide | CuS | Not mentioned | It has good antibacterial activity against Escherichia coli and Staphylococcus aureus, with sterilization rates of 99.6% and 99.1%, respectively. | Disinfecting and antibacterial materials | [38] |
| Organic framework | MOFs | In the cytotoxicity test, the L929 cell survival rates were all above 75% after 12 h of incubation | After two rounds of bacterial inactivation and six months of indoor environment preservation, 99.9999% of Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus can be eliminated. | Antibacterial material | [39] |
| Antibiotic | DOX | Not mentioned | It has an obvious inhibitory effect on Gram-negative Escherichia coli and Gram-positive Bacillus subtilis within 5 h. | Drug delivery | [40] |
| Antibiofilm | PVA | The relative viability changes of L929 cells after the co-incubation of the PVA hydrogel, Ag@M-PVA hydrogel, and Ag@M-H-PVA hydrogel extracts with L929 cells for 1 d and 3 d were above 85% | The inhibition rate of Escherichia coli under 808 nm near infrared radiation increased from 51.78% to 97.5%. | Tissue engineering repair material | [41] |
| Carbon matrix | Graphene | Only pure functionalized graphene shows some noteworthy cytotoxic activity after 24 h at a high concentration of 200 μg mL−1; however, after 48 h, the cytotoxicity of functionalized graphene and 50% Ti3C2Tx—50% FG and 25% Ti3C2Tx—75% FG increase to 80%, 55%, and 60%, respectively | For Escherichia coli, at the dosage of 200 mg/mL, Ti3C2 functionalized graphene nanocomposites can completely kill bacteria. | Biomedical application | [42] |
| GO-PEI | Not mentioned | GO-PEI/MXene folded microspheres have strong adsorption capacity, and the antibacterial effect can prolong the service life. | Molecular imprinting technique | [43] | |
| Natural antibacterial material | Chitosan | Not mentioned | The sterilization effect is close to 100%. | Health monitoring | [44] |
2.1. Single Ti3C2Tx
2.2. Ti3C2Tx/Metal Composites
2.3. Ti3C2Tx/Metal Oxide Composites
2.4. Ti3C2Tx/Metal Sulfide Composites
2.5. Ti3C2Tx/Organic Frameworks Composites
2.6. Ti3C2Tx/Antibiotic Composites
2.7. Ti3C2Tx/Antibiofilm Composites
2.8. Ti3C2Tx/Photosensitizer Composites
3. Preparation Methods
| Classification | Constituent | Sterilization | Application | Reference |
|---|---|---|---|---|
| Solution mixing | Cationic polymer poly-L-lysine, Ti3C2 | At the concentration of 200 mg L−1, the number of living cells of E. coli. decreased by two orders of magnitude. | Biotechnology or nanomedicine | [114] |
| Amidoxime group, Ti3C2, grafted polyamide | Excellent anti-biological pollution performance (92.9% antibacterial rate). | Extraction of uranium from seawater | [115] | |
| Ti3C2, Al2O3, Ag, Cu | A total of 99.6% bacteria can be collected in the filter. | Sewage disposal | [116] | |
| Ti3C2, tannin | Remarkable antibacterial properties against E. coli and S. aureus. | Intelligent wearable device | [117] | |
| Chemical synthesis | BiOI, CeO2, Ti3C2 | The photocatalytic bacteriostatic efficiency of E. coli and S. aureus were 99.76% and 99.89% respectively. | Double static electricity and antifouling of seawater | [118] |
| Ti3C2, Al2O3, Ag, SiO2, Pd | Antibacterial properties. | Ecotoxicology | [119] | |
| Ag, Ti3C2, Cu2O | Excellent antibacterial activity against P. aeruginosa and S.aureus. | Antifouling | [120] | |
| TiO2, W18O49Z, Ti3C2 | The photocatalytic sterilization rate of E. coil under illumination is 93.7% (λ ≥ 365 nm, 6 h). | Teleportation | [121] | |
| Layer-by-layer self-assembly | Ti3C2, dimethyl siloxane | A strong antibacterial effect on S. aureus and E. coli (≥99% adhered bacterial cells). | Medical implantation | [122] |
| Electrostatic assembly | Graphite carbonitride, Ti3C2 | In complex water matrix, 7 × 107 CFU/mL of S. aureus can be completely inactivated. | Natural water disinfection | [123] |
| Fe2O3, Ti3C2, glucose oxidase | Simultaneously promote Fe2+/Fe3+ to penetrate into bacteria and causes planktonic bacteria to die. | Fight stubborn infection | [124] | |
| 3D printing | PDA, Ti3C2, CFPEEK | Effectively kill bacteria after 10 min of NIR irradiation at 808 nm. The antibacterial rate reached 100%. | Orthopedic implant | [125] |
3.1. Solution Mixing Method
3.2. Chemical Synthesis Method
3.3. Layer-by-Layer Self-Assembly Method
3.4. Electrostatic Assembly Method
3.5. Three-Dimensional Printing
4. Application of Ti3C2Tx Composite in Antibacterial Field
4.1. Medical Field
4.1.1. Wound Healing
4.1.2. Medical Sensor
4.1.3. Medical Implant Field
4.2. Water Treatment
4.2.1. Seawater Desalination
4.2.2. Sewage Disposal
4.3. Textile Field
4.4. Food Filed
4.5. Other Filed
5. Conclusions
6. Challenges and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Huo, J.; Jia, Q.; Huang, H.; Zhang, J.; Li, P.; Dong, X.; Huang, W. Emerging Photothermal-Derived Multimodal Synergistic Therapy in Combating Bacterial Infections. Chem. Soc. Rev. 2021, 50, 8762–8789. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, M.; Jafari, S.M.; Van De Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 73. [Google Scholar] [CrossRef]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Haebich, D. Antibacterial Natural Products in Medicinal Chemistry—Exodus or Revival? Angew. Chem. Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef]
- Rutledge-Taylor, K.; Matlow, A.; Gravel, D.; Embree, J.; Le Saux, N.; Johnston, L.; Suh, K.; Embil, J.; Henderson, E.; John, M.; et al. A Point Prevalence Survey of Health Care-Associated Infections in Canadian Pediatric Inpatients. Am. J. Infect. Control 2012, 40, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bafail, A.S.; Alamri, A.M.; Spivakovsky, S. Effect of Antibiotics on Implant Failure and Postoperative Infection: Question: Among Patients Receiving Dental Implants, Does the Use of Antibiotics, When Compared with a Control Group, Reduce the Frequency of Implant Failure and Postoperative Infection? Evid. Based Dent. 2014, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wen, J.; Yu, P.; Liu, J.; Li, J.; Chu, H. Chemically Grafted Nanozyme Composite Cryogels to Enhance Antibacterial and Biocompatible Performance for Bioliquid Regulation and Adaptive Bacteria Trapping. ACS Nano 2021, 15, 19672–19683. [Google Scholar] [CrossRef]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Goddard, F.G.B.; Chang, H.H.; Clasen, T.F.; Sarnat, J.A. Exposure Measurement Error and the Characterization of Child Exposure to Fecal Contamination in Drinking Water. Npj Clean Water 2020, 3, 19. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; Wu, X.; Zhao, L.; Wang, N.; Mao, D.; Ren, H.; Luo, Y. Antibiotic Contamination Amplifies the Impact of Foreign Antibiotic-Resistant Bacteria on Soil Bacterial Community. Sci. Total Environ. 2021, 758, 143693. [Google Scholar] [CrossRef]
- Russell, A.D. Mechanisms of Antimicrobial Action of Antiseptics and Disinfectants: An Increasingly Important Area of Investigation. J. Antimicrob. Chemother. 2002, 49, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Zheng, J.; Li, J.; Zhang, L.; Chen, X.; Yu, Y.; Huang, H. Post-Graphene 2D Materials-Based Antimicrobial Agents: Focus on Fabrication Strategies and Biosafety Assessments. J. Mater. Sci. 2020, 55, 7226–7246. [Google Scholar] [CrossRef]
- Miao, H.; Teng, Z.; Wang, C.; Chong, H.; Wang, G. Recent Progress in Two-Dimensional Antimicrobial Nanomaterials. Chem. Eur. J. 2019, 25, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Yuan, H.; Lu, W.; Li, Z.; Wang, L.; Gao, L.-H. Conjugated Polymer and Triphenylamine Derivative Codoped Nanoparticles for Photothermal and Photodynamic Antimicrobial Therapy. ACS Appl. Bio Mater. 2020, 3, 3494–3499. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, Y.; Huang, J.; Fan, X.; Cheng, C.; Nie, C.; Ma, L.; Zhao, W.; Zhao, C. Size-Transformable Metal–Organic Framework–Derived Nanocarbons for Localized Chemo-Photothermal Bacterial Ablation and Wound Disinfection. Adv. Funct. Mater. 2019, 29, 1900143. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Chan, Y.K.; Yang, Y.; Jiao, Z.; Li, L.; Li, J.; Liang, K.; Deng, Y. Infection Micromilieu-Activated Nanocatalytic Membrane for Orchestrating Rapid Sterilization and Stalled Chronic Wound Regeneration. Adv. Funct. Mater. 2022, 32, 2109469. [Google Scholar] [CrossRef]
- Mathis, T.S.; Maleski, K.; Goad, A.; Sarycheva, A.; Anayee, M.; Foucher, A.C.; Hantanasirisakul, K.; Shuck, C.E.; Stach, E.A.; Gogotsi, Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. [Google Scholar] [CrossRef]
- Cui, L.; Wen, J.; Deng, Q.; Du, X.; Tang, T.; Li, M.; Xiao, J.; Jiang, L.; Hu, G.; Cao, X.; et al. Improving the Photocatalytic Activity of Ti3C2 MXene by Surface Modification of N Doped. Materials 2023, 16, 2836. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of Synthesis, Characteristics, and Environmental Applications of MXene: A Comprehensive Review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Solangi, N.H.; Mazari, S.A.; Mubarak, N.M.; Karri, R.R.; Rajamohan, N.; Vo, D.-V.N. Recent Trends in MXene-Based Material for Biomedical Applications. Environ. Res. 2023, 222, 115337. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, Y.; Deng, Q.; Jeong, S.-H.; Jang, T.-S.; Du, S.; Kim, H.-E.; Huang, Q.; Han, C.-M. Strong and Biocompatible Poly(Lactic Acid) Membrane Enhanced by Ti3C2Tz (MXene) Nanosheets for Guided Bone Regeneration. Mater. Lett. 2018, 229, 114–117. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Han, J.; Chen, Y.; Lin, H.; Yang, T. Surface Nanopore Engineering of 2D MXenes for Targeted and Synergistic Multitherapies of Hepatocellular Carcinoma. Adv. Mater. 2019, 31, 1902282. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, e1701394. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, X.; Yang, Z.; Yang, P.; Ma, J. Environmental Applications of Two-Dimensional Transition Metal Carbides and Nitrides for Water Purification: A Review. Environ. Chem. Lett. 2022, 20, 633–660. [Google Scholar] [CrossRef]
- Mahar, I.; Memon, F.H.; Lee, J.-W.; Kim, K.H.; Ahmed, R.; Soomro, F.; Rehman, F.; Memon, A.A.; Thebo, K.H.; Choi, K.H. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications. Membranes 2021, 11, 869. [Google Scholar] [CrossRef]
- Seidi, F.; Arabi Shamsabadi, A.; Dadashi Firouzjaei, M.; Elliott, M.; Saeb, M.R.; Huang, Y.; Li, C.; Xiao, H.; Anasori, B. MXenes Antibacterial Properties and Applications: A Review and Perspective. Small 2023, 19, 2206716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, Z.; Kong, W.; Zhang, R.; Yao, C. Applications of MXene and Its Modified Materials in Skin Wound Repair. Front. Bioeng. Biotechnol. 2023, 11, 1154301. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, D.; Murali, N.; K. C., D.; Karki, B.; Samatha, K.; Kim, A.A.; Park, M.; Pant, B. Advancements in MXene-Polymer Nanocomposites in Energy Storage and Biomedical Applications. Polymers 2022, 14, 3433. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Han, H.; Yang, Z.; Chen, M.; Jiang, Y.; Lu, G.; Dong, L.; Wen, H.; Li, H.; Liu, J.; et al. Recent Advancements on Photothermal Conversion and Antibacterial Applications over MXenes-Based Materials. Nano-Micro Lett. 2022, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Endo, Y.; Liu, G.; Wang, N.; Shao, Z. Natural Photothermal-Responsive MXene-Based Antimicrobial Nanomaterials Ti3C2/Au NPs for the Treatment of Deep Staphylococcus Aureus-Infected Wound. Mater. Des. 2023, 232, 112138. [Google Scholar] [CrossRef]
- Yu, Q.; Pan, J.; Li, J.; Su, C.; Huang, Y.; Bi, S.; Jiang, J.; Chen, N. A General Strategy to Immobilize Metal Nanoparticles on MXene Composite Fabrics for Enhanced Sensing Performance and Endowed Multifunctionality. J. Mater. Chem. C 2022, 10, 13143–13156. [Google Scholar] [CrossRef]
- Tahir, T.; Chaudhary, K.; Warsi, M.F.; Saif, M.S.; Alsafari, I.A.; Shakir, I.; Agboola, P.O.; Haider, S.; Zulfiqar, S. Synthesis of Sponge like Gd3+ Doped Vanadium Oxide/2D MXene Composites for Improved Degradation of Industrial Effluents and Pathogens. Ceram. Int. 2022, 48, 1969–1980. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Feng, H.; Cao, L.; Wang, H.; Wang, D.; Chen, S. NIR-Triggered Photocatalytic and Photothermal Performance for Sterilization Based on Copper Sulfide Nanoparticles Anchored on Ti3C2T MXene. J. Colloid Interface Sci. 2021, 604, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, S.; Huang, F.; Li, W.; Qiao, H.; Wang, Q.; Wei, Q. “Dew-of-Leaf” Structure Multiple Synergetic Antimicrobial Modality Hybrid: A Rapid and Long Lasting Bactericidal Material. Chem. Eng. J. 2021, 416, 129072. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Li, S.; Vijayan, V.; Lee, J.; Park, S.; Cui, X.; Chung, I.; Lee, J.; Ahn, S.; Kim, J.; et al. ROS- and pH-Responsive Polydopamine Functionalized Ti3C2Tx MXene-Based Nanoparticles as Drug Delivery Nanocarriers with High Antibacterial Activity. Nanomaterials 2022, 12, 4392. [Google Scholar] [CrossRef]
- Guo, L.; Hu, K.; Wang, H. Antimicrobial and Mechanical Properties of Ag@Ti3C2Tx-Modified PVA Composite Hydrogels Enhanced with Quaternary Ammonium Chitosan. Polymers 2023, 15, 2352. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.S.; Ahmed, U.; Aslfattahi, N.; Rahman, S.; Hardy, J.G.; Anwar, A. Potent Antibacterial Activity of MXene–Functionalized Graphene Nanocomposites. RSC Adv. 2022, 12, 33142–33155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lyu, Y.; Yang, Z.; Wang, T.; Zhang, Q.; Zhang, B. Preparation and Specific Adsorption of Dual Antibacterial BSA Surface Imprinted GO-PEI/MXene Wrinkled Microspheres. AIChE J. 2023, 69, e18157. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Wei, Q.; Zhao, Y.; Zhang, W.; Yang, Y.; Li, D. Multifunctional Biomass Composite Aerogel Co-Modified by MXene and Ag Nanowires for Health Monitoring and Synergistic Antibacterial Applications. Appl. Surf. Sci. 2022, 598, 153783. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, Y.; Lin, L.; He, Q.; Hu, H.; Luo, R.; Xian, D.; Wu, J.; Shi, Y.; Zeng, F.; et al. Titanium Carbide MXene-Based Hybrid Hydrogel for Chemo-Photothermal Combinational Treatment of Localized Bacterial Infection. Acta Biomater. 2022, 142, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, M.; Liao, X.; Wang, P.; Yu, Y.; Yuan, J.; Wang, Q. Developing a Multifunctional Silk Fabric with Dual-Driven Heating and Rapid Photothermal Antibacterial Abilities Using High-Yield MXene Dispersions. ACS Appl. Mater. Interfaces 2021, 13, 43414–43425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Sun, J.; Xu, N.; Tang, Q.; Ren, J.; Chen, C.; Lei, W.; Zhang, C.; Liu, D. Large-Scale Production of MXenes as Nanoknives for Antibacterial Application. Nanoscale Adv. 2023, 5, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xuan, J.; Liu, Y.; Li, Z.; Han, Y.; Wang, Z. NIR-Dependent Photothermal-Photodynamic Synergistic Antibacterial Mechanism for Titanium Carbide Nanosheets Intercalated and Delaminated by Tetramethylammonium Hydroxide. Biomater. Adv. 2023, 152, 213492. [Google Scholar] [CrossRef]
- Qu, X.; Guo, Y.; Xie, C.; Li, S.; Liu, Z.; Lei, B. Photoactivated MXene Nanosheets for Integrated Bone–Soft Tissue Therapy: Effect and Potential Mechanism. ACS Nano 2023, 17, 7229–7240. [Google Scholar] [CrossRef]
- Talreja, N.; Ashfaq, M.; Chauhan, D.; Mangalaraja, R.V. Cu-MXene: A Potential Biocide for the next-Generation Biomedical Application. Mater. Chem. Phys. 2023, 294, 127029. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, L.; Liu, R.; Zhao, W.; Yang, Z.; Zhang, J.; Jin, S. Versatile Self-Assembled MXene-Au Nanocomposites for SERS Detection of Bacteria, Antibacterial and Photothermal Sterilization. Chem. Eng. J. 2021, 426, 131914. [Google Scholar] [CrossRef]
- Li, L.; Ji, X.; Chen, K. Conductive, Self-Healing, and Antibacterial Ag/MXene-PVA Hydrogel as Wearable Skin-like Sensors. J. Biomater. Appl. 2023, 37, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, D.; Deng, Z.; Yin, J.; Qian, Y.; Hou, J.-T.; Ding, X.; Shen, J.; He, X. Single-Atom-Catalyzed MXene-Based Nanoplatform with Photo-Enhanced Peroxidase-Like Activity Nanotherapeutics for Staphylococcus Aureus Infection. Chem. Eng. J. 2023, 452, 139587. [Google Scholar] [CrossRef]
- Qu, W.; Zhao, H.; Zhang, Q.; Xia, D.; Tang, Z.; Chen, Q.; He, C.; Shu, D. Multifunctional Au/Ti3C2 Photothermal Membrane with Antibacterial Ability for Stable and Efficient Solar Water Purification under the Full Spectrum. ACS Sustain. Chem. Eng. 2021, 9, 11372–11387. [Google Scholar] [CrossRef]
- Wen, S.; Xiong, Y.; Cai, S.; Li, H.; Zhang, X.; Sun, Q.; Yang, R. Plasmon-Enhanced Photothermal Properties of Au@Ti3C2Tx Nanosheets for Antibacterial Applications. Nanoscale 2022, 14, 16572–16580. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yu, Z.; Zhao, W.; Yang, Z.; Peng, Y.; Zhou, Y.; Lin, X.; Jin, S. Self-Assembled MXene-Au Multifunctional Nanomaterials with Various Shapes for Label-Free SERS Detection of Pathogenic Bacteria and Photothermal Sterilization. Anal. Chem. 2023, 95, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dong, Z.; Zhang, R.; Yi, X.; Yang, K.; Jin, M.; Yuan, C.; Xiao, Z.; Liu, Z.; Cheng, L. Multifunctional Two-Dimensional Core–Shell MXene@Gold Nanocomposites for Enhanced Photo–Radio Combined Therapy in the Second Biological Window. ACS Nano 2019, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, Y.; Jia, K.; Abraha, B.S.; Li, Y.; Peng, W.; Zhang, F.; Fan, X.; Zhang, L. A Near-Infrared Light-Mediated Antimicrobial Based on Ag/Ti3C2Tx for Effective Synergetic Antibacterial Applications. Nanoscale 2020, 12, 19129–19141. [Google Scholar] [CrossRef] [PubMed]
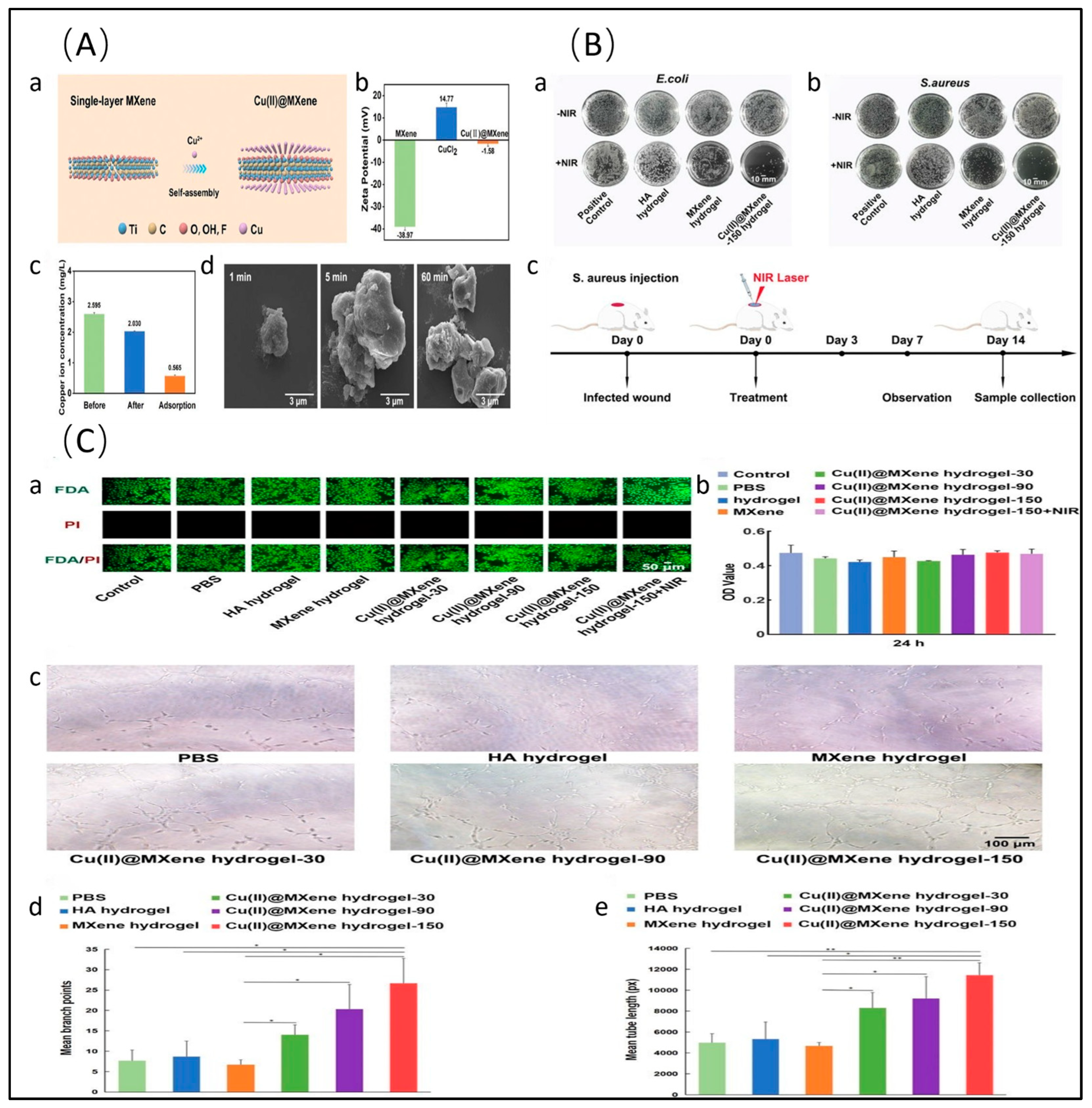
- Liu, M.; Zheng, L.; Zha, K.; Yang, Y.; Hu, Y.; Chen, K.; Wang, F.; Zhang, K.; Liu, W.; Mi, B.; et al. Cu(II)@MXene Based Photothermal Hydrogel with Antioxidative and Antibacterial Properties for the Infected Wounds. Front. Bioeng. Biotechnol. 2023, 11, 1308184. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Zhang, M.; Zhang, C.; Cao, L.; Zhang, C.; Liu, T.; Kong, D.; Li, W.; Chen, S. In Situ Synthesis of 2D/2D MXene-COF Heterostructure Anchored with Ag Nanoparticles for Enhancing Schottky Photocatalytic Antibacterial Efficiency under Visible Light. J. Colloid Interface Sci. 2022, 608, 735–748. [Google Scholar] [CrossRef]
- Jin, J.; Wu, S.; Wang, J.; Xu, Y.; Xuan, S.; Fang, Q. AgPd Nanocages Sandwiched between a MXene Nanosheet and PDA Layer for Photothermally Improved Catalytic Activity and Antibacterial Properties. Dalton Trans. 2023, 52, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, H.; Xu, C.; Fang, Q.; Wang, H.; Xu, Y.; Sang, M.; Xuan, S.; Hao, L. A MXene@AgAu@PDA Nanoplatform Loaded with AgAu Nanocages for Enhancing Catalytic Activity and Antibacterial Performance. J. Mater. Chem. B 2023, 11, 10678–10691. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Sun, D.; Zheng, Y.; Wang, R.; Bai, Y. Further Surface Modification by Carbon Coating for In-Situ Growth of Fe3O4 Nanoparticles on MXene Ti3C2 Multilayers for Advanced Li-Ion Storage. Electrochim. Acta 2018, 289, 228–237. [Google Scholar] [CrossRef]
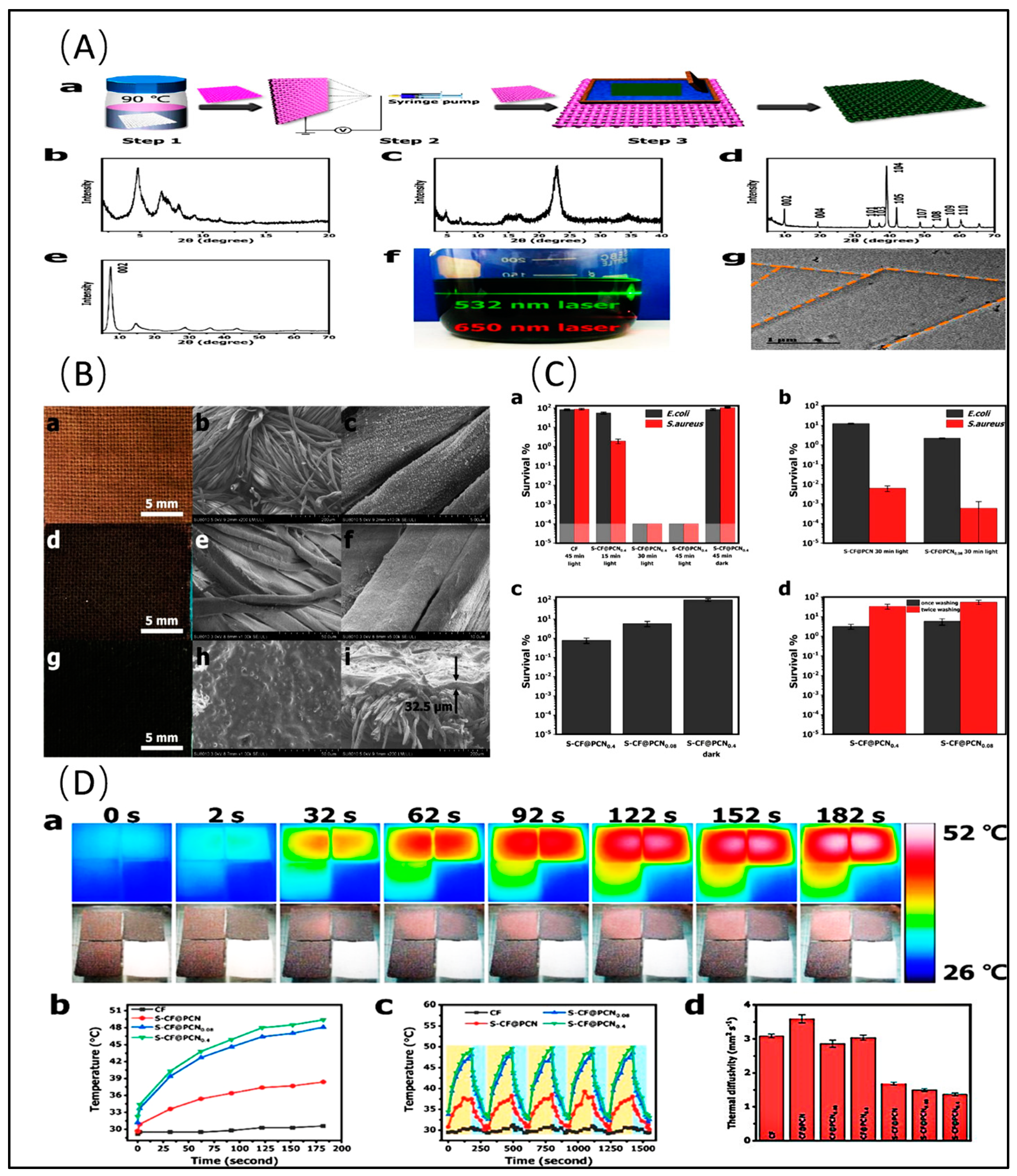
- Alsafari, I.A. Synthesis of CuO/MXene Nanocomposite to Study Its Photocatalytic and Antibacterial Properties. Ceram. Int. 2022, 48, 10960–10968. [Google Scholar] [CrossRef]
- Wang, W.; Feng, H.; Liu, J.; Zhang, M.; Liu, S.; Feng, C.; Chen, S. A Photo Catalyst of Cuprous Oxide Anchored MXene Nanosheet for Dramatic Enhancement of Synergistic Antibacterial Ability. Chem. Eng. J. 2020, 386, 124116. [Google Scholar] [CrossRef]
- Warsi, A.-Z.; Aziz, F.; Zulfiqar, S.; Haider, S.; Shakir, I.; Agboola, P.O. Synthesis, Characterization, Photocatalysis, and Antibacterial Study of WO3, MXene and WO3/MXene Nanocomposite. Nanomaterials 2022, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Chen, X.; Xu, Y.; Shah, K.J.; You, Z. One-Step Hydrothermal Generation of Oxygen-Deficient N-Doped Blue TiO2–Ti3C2 for Degradation of Pollutants and Antibacterial Properties. Environ. Res. 2023, 235, 116657. [Google Scholar] [CrossRef]
- Sukidpaneenid, S.; Chawengkijwanich, C.; Pokhum, C.; Isobe, T.; Opaprakasit, P.; Sreearunothai, P. Multi-Function Adsorbent-Photocatalyst MXene-TiO2 Composites for Removal of Enrofloxacin Antibiotic from Water. J. Environ. Sci. 2023, 124, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhao, C.; Li, G.; Shi, Z.; Liu, B. In Situ Oxidized TiO2/MXene Ultrafiltration Membrane with Photocatalytic Self-Cleaning and Antibacterial Properties. RSC Adv. 2023, 13, 15843–15855. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, H.; Xia, Y.; Sun, L.; Li, F.; Dai, H. 3D Printed Photothermal Scaffold Sandwiching Bacteria Inside and Outside Improves the Infected Microenvironment and Repairs Bone Defects. Adv. Healthc. Mater. 2024, 13, 2302879. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, X.; Xie, Z.; Huang, X.; Lin, L.; Jiao, Y.; Li, H. High-Efficiency Near-Infrared Light Responsive Antibacterial System for Synergistic Ablation of Bacteria and Biofilm. ACS Appl. Mater. Interfaces 2022, 14, 36947–36956. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhang, L.; Shuai, Y.; Wu, X.; Zeng, Z.; Peng, S.; Shuai, C. 3D-Printed CuFe2O4-MXene/PLLA Antibacterial Tracheal Scaffold against Implantation-Associated Infection. Appl. Surf. Sci. 2023, 614, 156108. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Q.; Xu, Y.; Fang, Q.; Leung, K.C.-F.; Sang, M.; Xuan, S.; Hao, L. Magnetically Separable MXene@Fe3O4/Au/PDA Nanosheets with Photothermal-Magnetolytic Coupling Antibacterial Performance. Appl. Surf. Sci. 2022, 590, 153125. [Google Scholar] [CrossRef]
- Gao, F.; Fang, M.; Zhang, S.; Ni, M.; Cai, Y.; Zhang, Y.; Tan, X.; Kong, M.; Xu, W.; Wang, X. Symmetry-Breaking Induced Piezocatalysis of Bi2S3 Nanorods and Boosted by Alternating Magnetic Field. Appl. Catal. B Environ. 2022, 316, 121664. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Liu, X.; Li, C.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Hu, W.; et al. Interfacial Engineering of Bi2S3/Ti3C2Tx MXene Based on Work Function for Rapid Photo-Excited Bacteria-Killing. Nat. Commun. 2021, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, W.; Wang, T.; Pu, Y.; Ma, C.; Chen, S. Interfacial Regulation of BiOI@Bi2S3/MXene Heterostructures for Enhanced Photothermal and Photodynamic Therapy in Antibacterial Applications. Acta Biomater. 2023, 171, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, G.; Li, Q.; Wang, Y.; Lu, X. Dual Optimized Ti3C2Tx MXene@ZnIn2S4 Heterostructure Based on Interface and Vacancy Engineering for Improving Electromagnetic Absorption. Chem. Eng. J. 2023, 453, 139488. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Fan, X.; Liu, H.; Bao, Y.; Hui, A.; Munir, H.A. Unveiling the Antibacterial Strategies and Mechanisms of MoS2: A Comprehensive Analysis and Future Directions. Biomater. Sci. 2024, 12, 596–620. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, X.; Ma, D.; Wang, Y.; Peng, L.; Shi, J.; Sun, J.; Gan, X.; Deng, Y.; Yang, W. Growth Factor-Decorated Ti3C2 MXene/MoS2 2D Bio-Heterojunctions with Quad-Channel Photonic Disinfection for Effective Regeneration of Bacteria-Invaded Cutaneous Tissue. Small 2021, 17, 2103993. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Sharifian Gh, M.; Attanayake, N.H.; Thenuwara, A.C.; Gogotsi, Y.; Anasori, B.; Strongin, D.R. Antimicrobial Properties of 2D MnO2 and MoS2 Nanomaterials Vertically Aligned on Graphene Materials and Ti3C2 MXene. Langmuir 2018, 34, 7192–7200. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, M.; Wang, P. Recent Advances in Small Copper Sulfide Nanoparticles for Molecular Imaging and Tumor Therapy. Mol. Pharm. 2019, 16, 3322–3332. [Google Scholar] [CrossRef]
- Wang, W.; Cao, L.; Li, Q.; Du, C.; Chen, S. Copper Sulfide Anchored MXene Improving Photo-Responsive Self-Healing Polyurethane with Enhanced Mechanical and Antibacterial Properties. J. Colloid Interface Sci. 2023, 630, 511–522. [Google Scholar] [CrossRef]
- Sun, G.; Jiang, X.; Liu, C.; Song, S.; Zhang, J.; Shen, J. FeS@LAB-35@Ti3C2 as a High-Efficiency Nanozyme for near Infrared Light Induced Photothermal Enhanced Chemodynamic Antibacterial Activity and Wound Healing. Nano Res. 2023, 16, 2840–2850. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Z.; Tao, K.; Han, L. A Heterostructure of a 2D Bimetallic Metal–Organic Framework Assembled on an MXene for High-Performance Supercapacitors. Dalton Trans. 2023, 52, 2455–2462. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Cao, P.; He, Y.; Zhao, Y.; Song, P.; Wang, R. Construction of UiO-NH2@TiC Schottky Junction and Their Effectively Photocatalytic and Antibacterial Performance. J. Clust. Sci. 2023, 34, 373–383. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Huang, F.; Wang, Q.; Wei, Q. Smart Textiles with Self-Disinfection and Photothermochromic Effects. ACS Appl. Mater. Interfaces 2021, 13, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Gao, M.-Y.; Sun, X.-J.; Tang, H.-L.; Dong, H.; Zhang, F.-M. Rational Combination of Covalent-Organic Framework and Nano TiO2 by Covalent Bonds to Realize Dramatically Enhanced Photocatalytic Activity. Appl. Catal. B Environ. 2020, 266, 118586. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Zhang, M.; Zhang, C.; Ma, C.; Cao, L.; Kong, D.; Feng, H.; Li, W.; Chen, S. Synthesis of MXene/COF/Cu2O Heterojunction for Photocatalytic Bactericidal Activity and Mechanism Evaluation. Chem. Eng. J. 2022, 430, 132663. [Google Scholar] [CrossRef]
- Sass, P. Antibiotics: Precious Goods in Changing Times. In Antibiotics; Sass, P., Ed.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2017; Volume 1520, pp. 3–22. ISBN 978-1-4939-6632-5. [Google Scholar]
- Durante-Mangoni, E.; Zarrilli, R. Global Spread of Drug-Resistant Acinetobacter Baumannii: Molecular Epidemiology and Management of Antimicrobial Resistance. Future Microbiol. 2011, 6, 407–422. [Google Scholar] [CrossRef]
- Ndowa, F.; Lusti-Narasimhan, M.; Unemo, M. The Serious Threat of Multidrug-Resistant and Untreatable Gonorrhoea: The Pressing Need for Global Action to Control the Spread of Antimicrobial Resistance, and Mitigate the Impact on Sexual and Reproductive Health. Sex. Transm. Infect. 2012, 88, 317–318. [Google Scholar] [CrossRef] [PubMed]
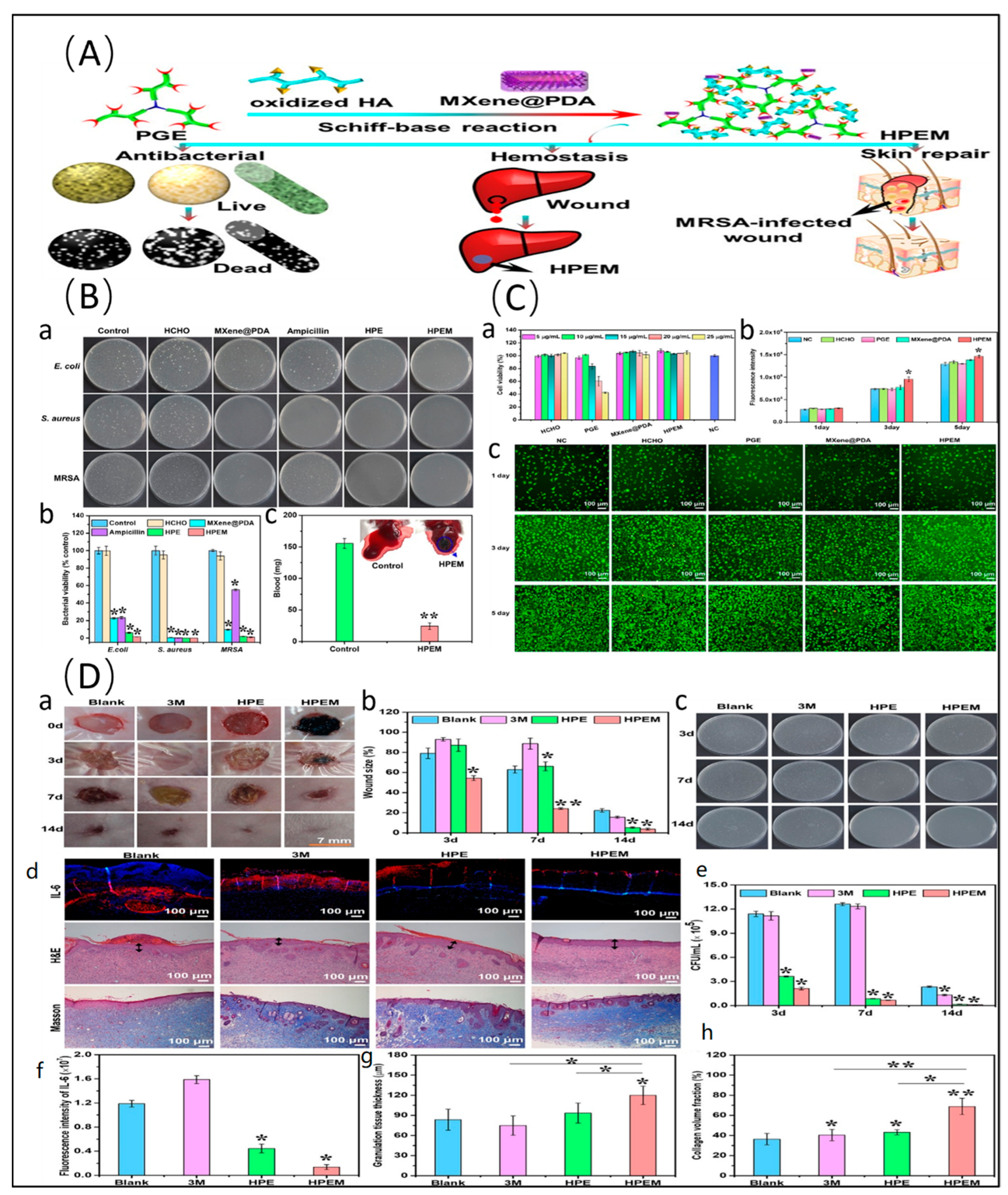
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A Multimodal Antimicrobial Platform Based on MXene for Treatment of Wound Infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, J.-U.; Kim, S.; Hwang, N.S.; Kim, H.D. Sprayable Ti3C2 MXene Hydrogel for Wound Healing and Drug Release System. Mater. Today Bio 2023, 23, 100881. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Han, Q.; Zhang, J.; Liu, Y.; Gan, X.; Xie, K.; Xie, L.; Deng, Y. MXene-Based Hydrogels Endow Polyetheretherketone with Effective Osteogenicity and Combined Treatment of Osteosarcoma and Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 45891–45903. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, H.; Lin, Z.; Zhang, C.; Liu, G.; Hu, Y. Augmented Wound Healing Potential of Photosensitive GelMA Hydrogel Incorporating Antimicrobial Peptides and MXene Nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1310349. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, W.; Zhang, M.; Guo, X.; Zhang, B.; Wang, D.; Jiang, X.; Liu, F.; Tang, J. Cryptotanshinone-Doped Photothermal Synergistic MXene@PDA Nanosheets with Antibacterial and Anti-Inflammatory Properties for Wound Healing. Adv. Healthc. Mater. 2023, 12, 2301060. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhou, Q.; Zhang, W.; Wang, J.; Liu, Y.; Yu, M.; Qiu, Y.; Ma, Z.; Liu, S. A Synergistic Antibacterial Study of Copper-Doped Polydopamine on Ti3C2Tx Nanosheets with Enhanced Photothermal and Fenton-like Activities. Materials 2023, 16, 7583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Song, P.; Li, J.; Cao, S.; Shi, J. Ti3C2 MXene/Gold Nanorods-Based Hybrid Nanoparticles with Photodynamic Antibacterial Activities. J. Mater. Sci. 2022, 57, 19957–19971. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Zhou, H.; Tan, Y.; Zhang, Z.; Yang, W.; Zhao, L.; Zhao, Z. A Ti3C2 MXene-Integrated near-Infrared-Responsive Multifunctional Porous Scaffold for Infected Bone Defect Repair. J. Mater. Chem. B 2024, 12, 79–96. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, X.; Wei, Y.; Gu, Y.; Xu, H.; Liao, Z.; Zhao, L.; Du, J.; Hu, Y.; Lian, X.; et al. Nanocatalytic Hydrogel with Rapid Photodisinfection and Robust Adhesion for Fortified Cutaneous Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 6354–6370. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, H.; Liu, Z.; Wang, S.; Liu, Z.; Chen, F.; Zhang, H.; Kong, J.; Zhou, F.; Zhang, Q. Conductive Antibacterial Hemostatic Multifunctional Scaffolds Based on Ti3C2Tx MXene Nanosheets for Promoting Multidrug-Resistant Bacteria-Infected Wound Healing. ACS Nano 2021, 15, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yang, L.; Pang, C.; Lian, L.; Hong, L. Bacteria-Targeted Photothermal Therapy for Combating Drug-Resistant Bacterial Infections. Biomater. Sci. 2023, 11, 5634–5640. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI Functionalized Sodium Alginate Aerogel and Its Excellent Adsorption Behavior for Cr(VI) and Congo Red from Aqueous Solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef] [PubMed]
- Diedkova, K.; Pogrebnjak, A.D.; Kyrylenko, S.; Smyrnova, K.; Buranich, V.V.; Horodek, P.; Zukowski, P.; Koltunowicz, T.N.; Galaszkiewicz, P.; Makashina, K.; et al. Polycaprolactone–MXene Nanofibrous Scaffolds for Tissue Engineering. ACS Appl. Mater. Interfaces 2023, 15, 14033–14047. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Cheng, N.; Liang, X.; Hu, H.; Luo, F.; Jin, J.; Li, Y. Electrospun Polycaprolactone Nanofibrous Membranes Loaded with Baicalin for Antibacterial Wound Dressing. Sci. Rep. 2022, 12, 10900. [Google Scholar] [CrossRef]
- Xu, S.; Du, C.; Zhang, M.; Wang, R.; Feng, W.; Wang, C.; Liu, Q.; Zhao, W. Electroactive and Antibacterial Wound Dressings Based on Ti3C2Tx MXene/Poly(ε-Caprolactone)/Gelatin Coaxial Electrospun Nanofibrous Membranes. Nano Res. 2023, 16, 9672–9687. [Google Scholar] [CrossRef]
- Yu, C.; Sui, S.; Yu, X.; Huang, W.; Wu, Y.; Zeng, X.; Chen, Q.; Wang, J.; Peng, Q. Ti3C2Tx MXene Loaded with Indocyanine Green for Synergistic Photothermal and Photodynamic Therapy for Drug-Resistant Bacterium. Colloids Surf. B Biointerfaces 2022, 217, 112663. [Google Scholar] [CrossRef]
- Liu, X.; Xie, H.; Zhuo, S.; Zhou, Y.; Selim, M.S.; Chen, X.; Hao, Z. Ru(II) Complex Grafted Ti3C2Tx MXene Nano Sheet with Photothermal/Photodynamic Synergistic Antibacterial Activity. Nanomaterials 2023, 13, 958. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Yang, Y.; Shi, H.; Shi, J.; Jiao, X.; Han, P.; Yao, X.; Chen, W.; Wei, X.; et al. Ti3C2TX MXene Modified with ZnTCPP with Bacteria Capturing Capability and Enhanced Visible Light Photocatalytic Antibacterial Activity. Small 2022, 18, 2200857. [Google Scholar] [CrossRef]
- Wang, H.; Mu, N.; He, Y.; Zhang, X.; Lei, J.; Yang, C.; Ma, L.; Gao, Y. Ultrasound-Controlled MXene-Based Schottky Heterojunction Improves Anti-Infection and Osteogenesis Properties. Theranostics 2023, 13, 1669–1683. [Google Scholar] [CrossRef]
- Jin, C.; Sun, D.; Sun, Z.; Rao, S.; Wu, Z.; Cheng, C.; Liu, L.; Liu, Q.; Yang, J. Interfacial Engineering of Ni-Phytate and Ti3C2Tx MXene-Sensitized TiO2 toward Enhanced Sterilization Efficacy under 808 Nm NIR Light Irradiation. Appl. Catal. B Environ. 2023, 330, 122613. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Mitrzak, J.; Szuplewska, A.; Chudy, M.; Woźniak, J.; Petrus, M.; Wojciechowski, T.; Vasilchenko, A.S.; Jastrzębska, A.M. Engineering of 2D Ti3C2 MXene Surface Charge and Its Influence on Biological Properties. Materials 2020, 13, 2347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Zhao, B.; Wang, X.; Pang, H.; Yu, S. Highly Efficient Extraction of Uranium from Seawater by Polyamide and Amidoxime Co-Functionalized MXene. Environ. Pollut. 2023, 317, 120826. [Google Scholar] [CrossRef] [PubMed]
- Jakubczak, M.; Karwowska, E.; Rozmysłowska-Wojciechowska, A.; Petrus, M.; Woźniak, J.; Mitrzak, J.; Jastrzębska, A. Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment. Materials 2021, 14, 182. [Google Scholar] [CrossRef]
- Wei, J.; Jia, S.; Wei, J.; Ma, C.; Shao, Z. Tough and Multifunctional Composite Film Actuators Based on Cellulose Nanofibers toward Smart Wearables. ACS Appl. Mater. Interfaces 2021, 13, 38700–38711. [Google Scholar] [CrossRef]
- Mao, Z.; Hao, W.; Wang, W.; Ma, F.; Ma, C.; Chen, S. BiOI@CeO2@Ti3C2 MXene Composite S-Scheme Photocatalyst with Excellent Bacteriostatic Properties. J. Colloid Interface Sci. 2023, 633, 836–850. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Poźniak, S.; Wojciechowski, T.; Chlubny, L.; Olszyna, A.; Ziemkowska, W.; Jastrzębska, A.M. Influence of Modification of Ti3C2 MXene with Ceramic Oxide and Noble Metal Nanoparticles on Its Antimicrobial Properties and Ecotoxicity towards Selected Algae and Higher Plants. RSC Adv. 2019, 9, 4092–4105. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, W.; Zhang, M.; Zhu, S.; Wang, Q.; Liu, J.; Chen, S. 2D Titanium Carbide-Based Nanocomposites for Photocatalytic Bacteriostatic Applications. Appl. Catal. B Environ. 2020, 266, 118609. [Google Scholar] [CrossRef]
- Jin, C.; Rao, S.; Xie, J.; Sun, Z.; Gao, J.; Li, Y.; Li, B.; Liu, S.; Liu, L.; Liu, Q.; et al. Enhanced Photocatalytic Antibacterial Performance by Hierarchical TiO2/W18O49 Z-Scheme Heterojunction with Ti3C2Tx-MXene Cocatalyst. Chem. Eng. J. 2022, 447, 137369. [Google Scholar] [CrossRef]
- Asadi Tokmedash, M.; Nagpal, N.; Chen, P.; VanEpps, J.S.; Min, J. Stretchable, Nano-Crumpled MXene Multilayers Impart Long-Term Antibacterial Surface Properties. Adv. Mater. Interfaces 2023, 10, 2202350. [Google Scholar] [CrossRef]
- Guo, H.; Niu, H.-Y.; Wang, W.-J.; Wu, Y.; Xiong, T.; Chen, Y.-R.; Su, C.-Q.; Niu, C.-G. Schottky Barrier Height Mediated Ti3C2 MXene Based Heterostructure for Rapid Photocatalytic Water Disinfection: Antibacterial Efficiency and Reaction Mechanism. Sep. Purif. Technol. 2023, 312, 123412. [Google Scholar] [CrossRef]
- Dai, W.; Shu, R.; Yang, F.; Li, B.; Johnson, H.M.; Yu, S.; Yang, H.; Chan, Y.K.; Yang, W.; Bai, D.; et al. Engineered Bio-Heterojunction Confers Extra- and Intracellular Bacterial Ferroptosis and Hunger-Triggered Cell Protection for Diabetic Wound Repair. Adv. Mater. 2024, 36, 2305277. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhao, S.; Dong, W.; Ma, W.; Zhou, X.; Wang, Y.; Zhang, M. Surface Modification of Carbon Fiber-Reinforced Polyetheretherketone with MXene Nanosheets for Enhanced Photothermal Antibacterial Activity and Osteogenicity. ACS Biomater. Sci. Eng. 2022, 8, 2375–2389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.; Xie, J. Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters. Adv. Healthc. Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, H.; Lu, Q.; Sun, J.; Zhang, P.; Zeng, L.; Vasilev, K.; Zhao, Y.; Chen, Y.; Liu, P. Spontaneous Formation of MXene-Oxidized Sono/Chemo-Dynamic Sonosensitizer/Nanocatalyst for Antibacteria and Bone-Tissue Regeneration. J. Nanobiotechnology 2023, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Huang, J.; Ma, S.; Li, Z.; Shi, Y.; Yang, X.; Fang, X.; Zeng, J.; Bi, P.; Qi, J.; et al. MXene-Hybridized Silane Films for Metal Anticorrosion and Antibacterial Applications. Appl. Surf. Sci. 2020, 527, 146915. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z.; Pu, J.; Hu, L.; Zi, Y.; Wang, M.; Feng, X.; Huang, W. 2D MXene Ti3C2Tx Nanosheets in the Development of a Mechanically Enhanced and Efficient Antibacterial Dental Resin Composite. Front. Chem. 2022, 10, 1090905. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Qin, X.; Su, Z.; Gou, X.; Yang, Z.; Wang, H. Research on the Antibacterial Properties of MXene-Based 2D–2D Composite Materials Membrane. Nanomaterials 2023, 13, 2121. [Google Scholar] [CrossRef]
- Wu, Q.; Tan, L.; Liu, X.; Li, Z.; Zhang, Y.; Zheng, Y.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. The Enhanced Near-Infrared Photocatalytic and Photothermal Effects of MXene-Based Heterojunction for Rapid Bacteria-Killing. Appl. Catal. B Environ. 2021, 297, 120500. [Google Scholar] [CrossRef]
- Ku, M.; Mao, C.; Wu, S.; Zheng, Y.; Li, Z.; Cui, Z.; Zhu, S.; Shen, J.; Liu, X. Lattice Strain Engineering of Ti3C2 Narrows Band Gap for Realizing Extraordinary Sonocatalytic Bacterial Killing. ACS Nano 2023, 17, 14840–14851. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yuen, A.C.Y.; Oliver, S.; Liu, J.; Yu, B.; Yang, W.; Wu, S.; Yeoh, G.H.; Wang, C.H. Dual Functionalisation of Polyurethane Foam for Unprecedented Flame Retardancy and Antibacterial Properties Using Layer-by-Layer Assembly of MXene Chitosan with Antibacterial Metal Particles. Compos. Part B Eng. 2022, 244, 110147. [Google Scholar] [CrossRef]
- Wang, H.; Dong, A.; Hu, K.; Sun, W.; Wang, J.; Han, L.; Mo, L.; Li, L.; Zhang, W.; Guo, Y.; et al. LBL Assembly of Ag@Ti3C2TX and Chitosan on PLLA Substrate to Enhance Antibacterial and Biocompatibility. Biomed. Mater. 2022, 17, 035006. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wu, Z.; Feng, G.; Wei, L.; Weng, J.; Ruiz-Hitzky, E.; Wang, X. Multifunctional Sandwich-like Composite Film Based on Superhydrophobic MXene for Self-Cleaning, Photodynamic and Antimicrobial Applications. Chem. Eng. J. 2023, 454, 140457. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Chan, Y.K.; Wang, Z.; Li, L.; Li, J.; Liang, K.; Deng, Y. Photo-Activated Nanofibrous Membrane with Self-Rechargeable Antibacterial Function for Stubborn Infected Cutaneous Regeneration. Small 2022, 18, 2105988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, B.; Gao, X.; Huang, Y.; Li, M.; Yu, Z.; Li, J.; Shi, X.; Deng, Y.; Liang, K. Endogenous Oxygen-Evolving Bio-Catalytic Fabrics with Fortified Photonic Disinfection for Invasive Bacteria-Caused Refractory Cutaneous Regeneration. Nano Today 2022, 46, 101595. [Google Scholar] [CrossRef]
- He, J.; Li, A.; Wang, W.; Cui, C.; Jiang, S.; Chen, M.; Qin, W.; Tang, H.; Guo, R. Multifunctional Wearable Device Based on an Antibacterial and Hydrophobic Silver Nanoparticles/Ti3C2Tx MXene/Thermoplastic Polyurethane Fibrous Membrane for Electromagnetic Shielding and Strain Sensing. Ind. Eng. Chem. Res. 2023, 62, 9221–9232. [Google Scholar] [CrossRef]
- Feng, Q.; Zhan, Y.; Yang, W.; Dong, H.; Sun, A.; Li, L.; Chen, X.; Chen, Y. Ultra-High Flux and Synergistically Enhanced Anti-Fouling Ag@MXene Lamellar Membrane for the Fast Purification of Oily Wastewater through Nano-Intercalation, Photocatalytic Self-Cleaning and Antibacterial Effect. Sep. Purif. Technol. 2022, 298, 121635. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Wang, L.; Qin, X.; Yu, J. Nanofiber Based Origami Evaporator for Multifunctional and Omnidirectional Solar Steam Generation. Carbon 2021, 177, 199–206. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial Properties of Electrospun Ti3C2Tz (MXene)/Chitosan Nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Liu, X.; Wang, Y.; Li, C.; Fang, J.; Chang, B.; Qi, Y.; Li, Y.; Ning, G. Titanium Carbide/Zeolite Imidazole Framework-8/Polylactic Acid Electrospun Membrane for near-Infrared Regulated Photothermal/Photodynamic Therapy of Drug-Resistant Bacterial Infections. J. Colloid Interface Sci. 2021, 599, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lan, D.; Li, X.; Li, Z.; Dai, F. Conductive and Antibacterial Scaffold with Rapid Crimping Property for Application Prospect in Repair of Peripheral Nerve Injury. J. Appl. Polym. Sci. 2023, 140, e53426. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, D.; Wu, B.; Chen, X.; Li, X.; Li, Z.; Dai, F. Electrospun Piezoelectric Scaffold with External Mechanical Stimulation for Promoting Regeneration of Peripheral Nerve Injury. Biomacromolecules 2023, 24, 3268–3282. [Google Scholar] [CrossRef]
- Wang, L.; Du, L.; Wang, M.; Wang, X.; Tian, S.; Chen, Y.; Wang, X.; Zhang, J.; Nie, J.; Ma, G. Chitosan for Constructing Stable Polymer-Inorganic Suspensions and Multifunctional Membranes for Wound Healing. Carbohydr. Polym. 2022, 285, 119209. [Google Scholar] [CrossRef]
- Yan, B.; Bao, X.; Liao, X.; Wang, P.; Zhou, M.; Yu, Y.; Yuan, J.; Cui, L.; Wang, Q. Sensitive Micro-Breathing Sensing and Highly-Effective Photothermal Antibacterial Cinnamomum camphora Bark Micro-Structural Cotton Fabric via Electrostatic Self-Assembly of MXene/HACC. ACS Appl. Mater. Interfaces 2022, 14, 2132–2145. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, D.; Ding, Y.; Lv, X.; Huang, T.; Yuan, B.; Jiang, L.; Sun, X.; Yao, Y.; Tang, J. A Conductive Polyacrylamide Hydrogel Enabled by Dispersion-Enhanced MXene@chitosan Assembly for Highly Stretchable and Sensitive Wearable Skin. J. Mater. Chem. B 2021, 9, 8862–8870. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Li, Z.; Liu, X.; Zheng, Y.; Liang, Y.; Liang, C.; Cui, Z.; Zhu, S.; Wu, S. Oxygen Vacancies-Rich Heterojunction of Ti3C2/BiOBr for Photo-Excited Antibacterial Textiles. Small 2022, 18, 2104448. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Su, Z.; Fu, Y.; Li, S.; Mo, A. 3D Printing of Ti3C2-MXene-Incorporated Composite Scaffolds for Accelerated Bone Regeneration. Biomed. Mater. 2022, 17, 035002. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, H.; Lan, Y.; Khan, H.M.; Zhang, H.; Zhang, L.; Zhang, F.; Cui, Y.; Zhang, L.; Huang, D.; et al. Study on 3D Printed MXene-Berberine-Integrated Scaffold for Photo-Activated Antibacterial Activity and Bone Regeneration. J. Mater. Chem. B 2024, 12, 2158–2179. [Google Scholar] [CrossRef]
- Nie, R.; Sun, Y.; Lv, H.; Lu, M.; Huangfu, H.; Li, Y.; Zhang, Y.; Wang, D.; Wang, L.; Zhou, Y. 3D Printing of MXene Composite Hydrogel Scaffolds for Photothermal Antibacterial Activity and Bone Regeneration in Infected Bone Defect Models. Nanoscale 2022, 14, 8112–8129. [Google Scholar] [CrossRef]
- Gong, M.; Yue, L.; Kong, J.; Lin, X.; Zhang, L.; Wang, J.; Wang, D. Knittable and Sewable Spandex Yarn with Nacre-Mimetic Composite Coating for Wearable Health Monitoring and Thermo- and Antibacterial Therapies. ACS Appl. Mater. Interfaces 2021, 13, 9053–9063. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-J.; Zhao, X.; Pu, J.-H.; Tang, L.-S.; Ke, K.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Flexible Anti-Biofouling MXene/Cellulose Fibrous Membrane for Sustainable Solar-Driven Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 36589–36597. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kang, S.; Zhang, Q.; Chen, S.; Yang, Q.; Yan, B. Self-Assembly Fabrication of Chitosan-Tannic Acid/MXene Composite Film with Excellent Antibacterial and Antioxidant Properties for Fruit Preservation. Food Chem. 2023, 410, 135405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, M.; Cai, Y.; Jiang, B.; Zhang, Y.; Yuan, B.; Zhou, F.; Cao, C. Muscle-Inspired MXene/PVA Hydrogel with High Toughness and Photothermal Therapy for Promoting Bacteria-Infected Wound Healing. Biomater. Sci. 2022, 10, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q. Bio-Interface Engineering of MXene Nanosheets with Immobilized Lysozyme for Light-Enhanced Enzymatic Inactivation of Methicillin-Resistant Staphylococcus Aureus. Chem. Eng. J. 2023, 452, 139078. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, W.; Xiao, Y.; Du, B.; Zhang, X.; Wen, M.; Lai, C.; Huang, Y.; Sheng, L. Multifunctional, Robust, and Porous PHBV—GO/MXene Composite Membranes with Good Hydrophilicity, Antibacterial Activity, and Platelet Adsorption Performance. Polymers 2021, 13, 3748. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Cheng, G.; Guo, J.; Du, S.; Qiu, J.; Wang, C.; Li, C.; Yang, X.; Chen, T.; et al. Tailored Hydrogel Delivering Niobium Carbide Boosts ROS-Scavenging and Antimicrobial Activities for Diabetic Wound Healing. Small 2022, 18, 2201300. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, S.; Wang, J.; Ding, Y.; Ding, C. Ultrasensitive Ratiometric Electrochemiluminescence Sensor with an Efficient Antifouling and Antibacterial Interface of PSBMA@SiO2-MXene for Oxytetracycline Trace Detection in the Marine Environment. Anal. Chem. 2023, 95, 16327–16334. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Qin, Z.; Dassanayake, R.; Sun, Z.; Cao, M.; Fu, K.; Zhou, Y.; Liu, Y. Antimicrobial MXene-Based Conductive Alginate Hydrogels as Flexible Electronics. Chem. Eng. J. 2023, 455, 140546. [Google Scholar] [CrossRef]
- Fan, Y.; Kong, F.; Yang, J.; Xiong, X.; Gao, S.; Yuan, J.; Meng, S.; Chen, L. Flexible Wearable Sensor Based on SF/EEP/GR/MXene Nanocomposites. Appl. Phys. A 2023, 129, 551. [Google Scholar] [CrossRef]
- Li, C.; Zheng, A.; Zhou, J.; Huang, W.; Zhang, Y.; Han, J.; Cao, L.; Yang, D. A Self-Adhesive, Self-Healing and Antibacterial Hydrogel Based on PVA/MXene-Ag/Sucrose for Fast-Response, High-Sensitivity and Ultra-Durable Strain Sensors. New J. Chem. 2023, 47, 6621–6630. [Google Scholar] [CrossRef]
- He, J.; Zou, X.; Wang, W.; Chen, M.; Jiang, S.; Cui, C.; Tang, H.; Yang, L.; Guo, R. Highly Stretchable, Highly Sensitive, and Antibacterial Electrospun Nanofiber Strain Sensors with Low Detection Limit and Stable CNT/MXene/CNT Sandwich Conductive Layers for Human Motion Detection. Ind. Eng. Chem. Res. 2023, 62, 8327–8338. [Google Scholar] [CrossRef]
- Cao, Y.-M.; Li, Y.-F.; Dong, X.-X.; Chen, J.; Zhang, K.-Q.; Zhao, Y.-D.; Zhai, W.-Y.; Zheng, M.; Zheng, M.; Wang, Z.-S.; et al. Knitted Structural Design of MXene/Cu2O Based Strain Sensor for Smart Wear. Cellulose 2022, 29, 9453–9467. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Y.; Ji, Y.; Zhong, K.; Du, X.; Du, Z.; Cheng, X.; Wang, S. Tough and Extremely Temperature-Tolerance Nanocomposite Organohydrogels as Ultrasensitive Wearable Sensors for Wireless Human Motion Monitoring. Compos. Part Appl. Sci. Manuf. 2022, 157, 106905. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, J.; Chen, W.; He, Y.; Lin, T.; Zhao, L. Radiation Synthesis of Rapidly Self-Healing, Durable, and Flexible Poly(Ionic Liquid)/MXene Gels with Anti-Freezing Property for Multi-Functional Strain Sensors. Chem. Eng. J. 2023, 468, 143660. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ding, Y.; Zhang, M.; Lin, Z.; Hao, Y.; Li, Y.; Chang, J. Capacitive Pressure Sensor Combining Dual Dielectric Layers with Integrated Composite Electrode for Wearable Healthcare Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 12974–12985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Cao, T.; Wang, T.; Sun, L.; Wang, K.; Fan, X. Antiliquid-Interfering, Antibacteria, and Adhesive Wearable Strain Sensor Based on Superhydrophobic and Conductive Composite Hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 46022–46032. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pan, Z.; Zhang, W.; Fan, Q.; Li, W.; Liang, H. High-Sensitivity Microchannel-Structured Collagen Fiber-Based Sensors with Antibacterial and Hydrophobic Properties. ACS Sustain. Chem. Eng. 2022, 10, 16814–16824. [Google Scholar] [CrossRef]
- Fu, Y.; Li, C.; Cheng, Y.; He, Y.; Zhang, W.; Wei, Q.; Li, D. Biomass Aerogel Composite Containing BaTiO3 Nanoparticles and MXene for Highly Sensitive Self-Powered Sensor and Photothermal Antibacterial Applications. Compos. Part Appl. Sci. Manuf. 2023, 173, 107663. [Google Scholar] [CrossRef]
- Wang, X.; Tao, Y.; Pan, S.; Fang, X.; Lou, C.; Xu, Y.; Wu, J.; Sang, M.; Lu, L.; Gong, X.; et al. Biocompatible and Breathable Healthcare Electronics with Sensing Performances and Photothermal Antibacterial Effect for Motion-Detecting. Npj Flex. Electron. 2022, 6, 95. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, W.; Shi, X.; Sun, D.; Yang, J.; Qi, X.; Wang, Y. MXene/Ag Doped Hydrated-Salt Hydrogels with Excellent Thermal/Light Energy Storage, Strain Sensing and Photothermal Antibacterial Performances for Intelligent Human Healthcare. Compos. Part Appl. Sci. Manuf. 2023, 170, 107526. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Lian, L.; Liu, K.; Lu, M.; Chen, Y.; Zhang, L.; Zhang, X.; Wan, P. Flexible Accelerated-Wound-Healing Antibacterial MXene-Based Epidermic Sensor for Intelligent Wearable Human-Machine Interaction. Adv. Funct. Mater. 2022, 32, 2208141. [Google Scholar] [CrossRef]
- Wang, S.; Bi, S.; Zhang, L.; Liu, R.; Wang, H.; Gu, J. Skin-Inspired Antibacterial Conductive Hydrogels Customized for Wireless Flexible Sensor and Collaborative Wound Healing. J. Mater. Chem. A 2023, 11, 14096–14107. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Mo, A. Multilayered Titanium Carbide MXene Film for Guided Bone Regeneration. Int. J. Nanomed. 2019, 14, 10091–10103. [Google Scholar] [CrossRef]
- Mazinani, A.; Rastin, H.; Nine, M.J.; Lee, J.; Tikhomirova, A.; Tung, T.T.; Ghomashchi, R.; Kidd, S.; Vreugde, S.; Losic, D. Comparative Antibacterial Activity of 2D Materials Coated on Porous-Titania. J. Mater. Chem. B 2021, 9, 6412–6424. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fu, Y.; Mo, A. Electrophoretic-Deposited MXene Titanium Coatings in Regulating Bacteria and Cell Response for Peri-Implantitis. Front. Chem. 2022, 10, 991481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Han, Q.; Yin, J.; Zhang, J.; Yu, Y.; Yang, W.; Deng, Y. Synergism of 2D/1D MXene/Cobalt Nanowire Heterojunctions for Boosted Photo-Activated Antibacterial Application. Chem. Eng. J. 2021, 410, 128209. [Google Scholar] [CrossRef]
- Wang, Q.; Zang, C.; Chan, Y.K.; Wang, S.; Yang, W.; Deng, Y.; Zhu, T.; He, M. CoFe2O4/MXene Nanosheets Modified Hydrogel on PEEK with Phototherapeutic and GPx-Mimetic Activities for Anti-Pathogens in Infectious Bone Defect Repairment. Mater. Chem. Phys. 2023, 308, 128269. [Google Scholar] [CrossRef]
- Li, K.; Chang, T.; Li, Z.; Yang, H.; Fu, F.; Li, T.; Ho, J.S.; Chen, P. Biomimetic MXene Textures with Enhanced Light-to-Heat Conversion for Solar Steam Generation and Wearable Thermal Management. Adv. Energy Mater. 2019, 9, 1901687. [Google Scholar] [CrossRef]
- Chen, J.; Li, B.; Hu, G.; Aleisa, R.; Lei, S.; Yang, F.; Liu, D.; Lyu, F.; Wang, M.; Ge, X.; et al. Integrated Evaporator for Efficient Solar-Driven Interfacial Steam Generation. Nano Lett. 2020, 20, 6051–6058. [Google Scholar] [CrossRef]
- Dixit, F.; Zimmermann, K.; Dutta, R.; Prakash, N.J.; Barbeau, B.; Mohseni, M.; Kandasubramanian, B. Application of MXenes for Water Treatment and Energy-Efficient Desalination: A Review. J. Hazard. Mater. 2022, 423, 127050. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Mi, Y.; Gao, S.; Wang, G.; Bai, J.; Ma, S.; Wang, B. High-Efficiency, Thermal Stable, and Self-Floating Silver Nanowires/Ti3C2Tx MXene/Aramid Nanofibers Composite Aerogel for Photothermal Water Evaporation and Antibacterial Application. Chem. Eng. J. 2023, 477, 147276. [Google Scholar] [CrossRef]
- Ma, X.; Tian, N.; Wang, G.; Wang, W.; Miao, J.; Fan, T. Biomimetic Vertically Aligned Aerogel with Synergistic Photothermal Effect Enables Efficient Solar-Driven Desalination. Desalination 2023, 550, 116397. [Google Scholar] [CrossRef]
- Li, Y.; Wu, T.; Shen, H.; Yang, S.; Qin, Y.; Zhu, Z.; Zheng, L.; Wen, X.; Xia, M.; Yin, X. Flexible MXene-Based Janus Porous Fibrous Membranes for Sustainable Solar-Driven Desalination and Emulsions Separation. J. Clean. Prod. 2022, 347, 131324. [Google Scholar] [CrossRef]
- Guan, H.-S.; Fan, T.-T.; Bai, H.-Y.; Su, Y.; Liu, Z.; Ning, X.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. A Waste Biomass-Derived Photothermic Material with High Salt-Resistance for Efficient Solar Evaporation. Carbon 2022, 188, 265–275. [Google Scholar] [CrossRef]
- Rasheed, T.; Rasheed, A.; Munir, S.; Ajmal, S.; Muhammad Shahzad, Z.; Alsafari, I.A.; Ragab, S.A.; Agboola, P.O.; Shakir, I. A Cost-Effective Approach to Synthesize NiFe2O4/MXene Heterostructures for Enhanced Photodegradation Performance and Anti-Bacterial Activity. Adv. Powder Technol. 2021, 32, 2248–2257. [Google Scholar] [CrossRef]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane Based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ding, C.; Tian, Y.; Dong, J.; Cheng, B. Multifunctional Ti3C2Tx MXene/Silver Nanowire Membranes with Excellent Catalytic, Antifouling, and Antibacterial Properties for Nitrophenol-Containing Water Purification. ACS Appl. Mater. Interfaces 2023, 15, 48154–48167. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-Flux and Fouling-Resistant Membranes Based on Layered Silver/MXene (Ti3C2Tx) Nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Hojjati–Najafabadi, A.; Pei, R. MXene–Laden Bacteriophage: A New Antibacterial Candidate to Control Bacterial Contamination in Water. Chemosphere 2022, 290, 133383. [Google Scholar] [CrossRef]
- Mao, Z.; Peng, X.; Chen, H. Sunlight Propelled Two-Dimensional Nanorobots with Enhanced Mechanical Damage of Bacterial Membrane. Water Res. 2023, 235, 119900. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, X.; Li, L.; Wang, J.; Yang, Y.; Cao, Y.; Wang, W. Air-Permeable, Multifunctional, Dual-Energy-Driven MXene-Decorated Polymeric Textile-Based Wearable Heaters with Exceptional Electrothermal and Photothermal Conversion Performance. J. Mater. Chem. A 2020, 8, 12526–12537. [Google Scholar] [CrossRef]
- Liu, X.; Du, X.; Li, L.; Cao, Y.; Yang, Y.; Wang, W.; Wang, J. Multifunctional AgNW@MXene Decorated Polymeric Textile for Highly-Efficient Electro-/Photothermal Conversion and Triboelectric Nanogenerator. Compos. Part Appl. Sci. Manuf. 2022, 156, 106883. [Google Scholar] [CrossRef]
- Tang, L.; Lyu, B.; Gao, D.; Jia, Z.; Fu, Y.; Ma, J. A Janus Textile with Tunable Heating Modes toward Precise Personal Thermal Management in Cold Conditions. Small 2024, 20, 2308194. [Google Scholar] [CrossRef] [PubMed]
- Purbayanto, M.A.K.; Jakubczak, M.; Bury, D.; Nair, V.G.; Birowska, M.; Moszczyńska, D.; Jastrzębska, A. Tunable Antibacterial Activity of a Polypropylene Fabric Coated with Bristling Ti3C2Tx MXene Flakes Coupling the Nanoblade Effect with ROS Generation. ACS Appl. Nano Mater. 2022, 5, 5373–5386. [Google Scholar] [CrossRef]
- Deng, C.; Yu, Z.; Liang, F.; Liu, Y.; Seidi, F.; Yong, Q.; Liu, C.; Zhang, Y.; Han, J.; Xiao, H. Surface Nano-Engineering of Cellulosic Textiles for Superior Biocidal Performance and Effective Bacterial Detection. Chem. Eng. J. 2023, 473, 145492. [Google Scholar] [CrossRef]
- Zhai, W.; Cao, Y.; Li, Y.; Zheng, M.; Wang, Z. MoO3–x QDs/MXene (Ti3C2Tx) Self-Assembled Heterostructure for Multifunctional Application with Antistatic, Smoke Suppression, and Antibacterial on Polyester Fabric. J. Mater. Sci. 2022, 57, 2597–2609. [Google Scholar] [CrossRef]
- Su, X.; Sha, Q.; Gao, X.; Li, J.; Wu, Y.; Li, W.; Wu, W.; Han, N.; Zhang, X. Lightweight, Multifunctional Smart MXene@PET Non-Woven with Electric/Photothermal Conversion, Antibacterial and Flame Retardant Properties. Appl. Surf. Sci. 2023, 639, 158205. [Google Scholar] [CrossRef]
- Santos, X.; Álvarez, M.; Videira-Quintela, D.; Mediero, A.; Rodríguez, J.; Guillén, F.; Pozuelo, J.; Martín, O. Antibacterial Capability of MXene (Ti3C2Tx) to Produce PLA Active Contact Surfaces for Food Packaging Applications. Membranes 2022, 12, 1146. [Google Scholar] [CrossRef]
- Cao, Y.; Chang, T.; Fang, C.; Zhang, Y.; Liu, H.; Zhao, G. Inhibition Effect of Ti3C2Tx MXene on Ice Crystals Combined with Laser-Mediated Heating Facilitates High-Performance Cryopreservation. ACS Nano 2022, 16, 8837–8850. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Li, S.; Yan, Y.-Z.; Park, S.S.; Mohan, A.; Chung, I.; Ahn, S.; Kim, J.R.; Ha, C.-S. Dual (pH- and ROS-) Responsive Antibacterial MXene-Based Nanocarrier for Drug Delivery. Int. J. Mol. Sci. 2022, 23, 14925. [Google Scholar] [CrossRef] [PubMed]
- Bose, N.; Danagody, B.; Rajappan, K.; Ramanujam, G.M.; Anilkumar, A.K. Sustainable Routed Mxene-Based Aminolyzed PU/PCL Film for Increased Oxidative Stress and a pH-Sensitive Drug Delivery System for Anticancer Therapy. ACS Appl. Bio Mater. 2024, 7, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wu, Z.; Fang, J.; Li, S.; Tang, S.; Wang, X. MXene-Intercalated Montmorillonite Nanocomposites for Long-Acting Antibacterial. Appl. Surf. Sci. 2023, 616, 156521. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.; Ma, Z.; Xu, X.; Mohesen Seraji, S.; Yu, B.; Sun, Z.; Wang, H.; Song, P. A Reactive Copper-Organophosphate-MXene Heterostructure Enabled Antibacterial, Self-Extinguishing and Mechanically Robust Polymer Nanocomposites. Chem. Eng. J. 2022, 430, 132712. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, Y.; Chen, X.; Wang, Z.; Wu, Y.; Dai, Q.; Zhao, W.; Wei, T.; Yang, Q.; Huang, B.; et al. Research Progress on Ti3C2Tx-Based Composite Materials in Antibacterial Field. Molecules 2024, 29, 2902. https://doi.org/10.3390/molecules29122902
Chen H, Wang Y, Chen X, Wang Z, Wu Y, Dai Q, Zhao W, Wei T, Yang Q, Huang B, et al. Research Progress on Ti3C2Tx-Based Composite Materials in Antibacterial Field. Molecules. 2024; 29(12):2902. https://doi.org/10.3390/molecules29122902
Chicago/Turabian StyleChen, Huangqin, Yilun Wang, Xuguang Chen, Zihan Wang, Yue Wu, Qiongqiao Dai, Wenjing Zhao, Tian Wei, Qingyuan Yang, Bin Huang, and et al. 2024. "Research Progress on Ti3C2Tx-Based Composite Materials in Antibacterial Field" Molecules 29, no. 12: 2902. https://doi.org/10.3390/molecules29122902








