C60 Fullerene Reduces the Level of Liver Damage in Chronic Alcohol Intoxication of Rats
Abstract
1. Introduction
2. Results and Discussion
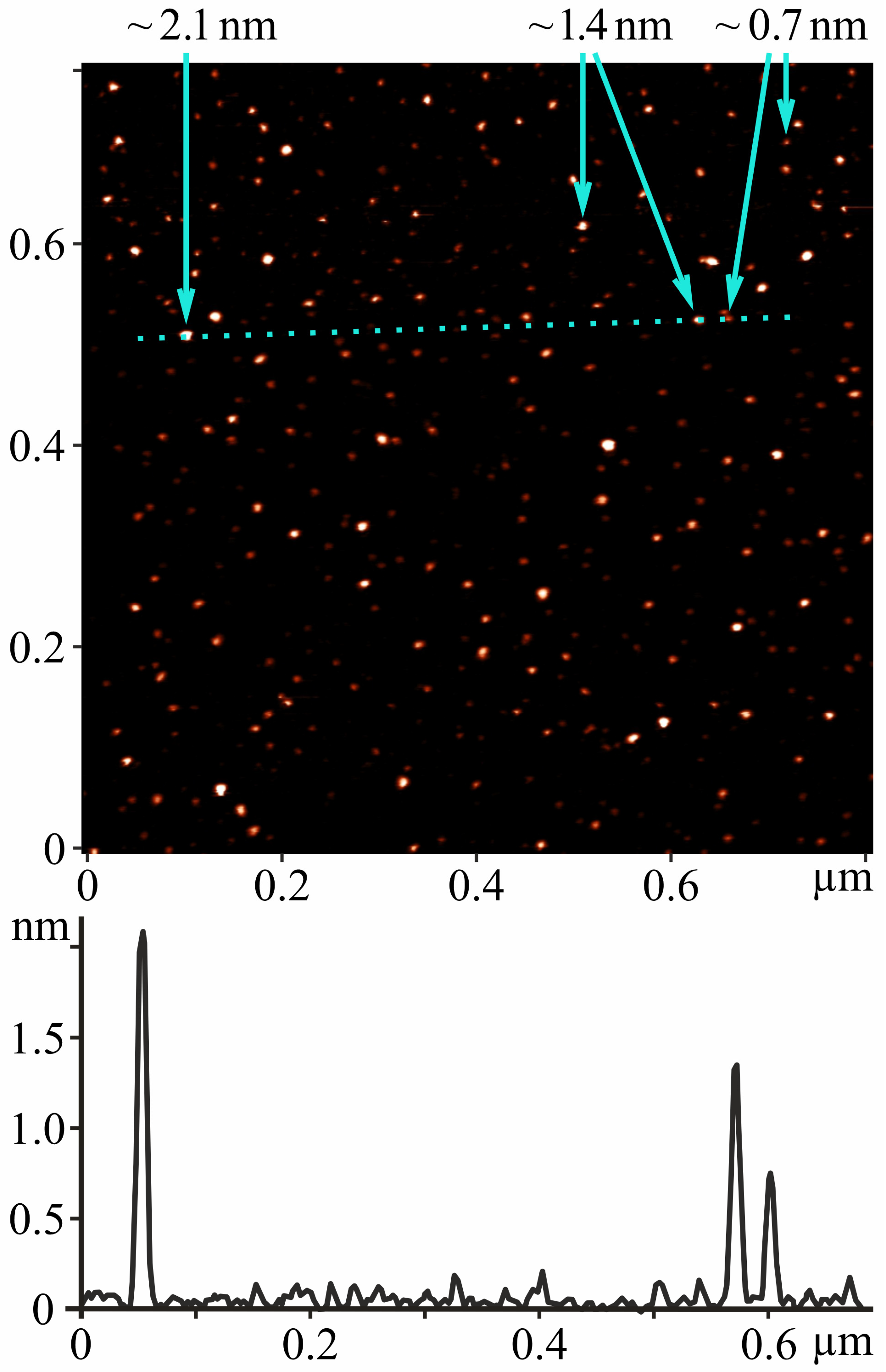
2.1. AFM Study
2.2. BAC Value
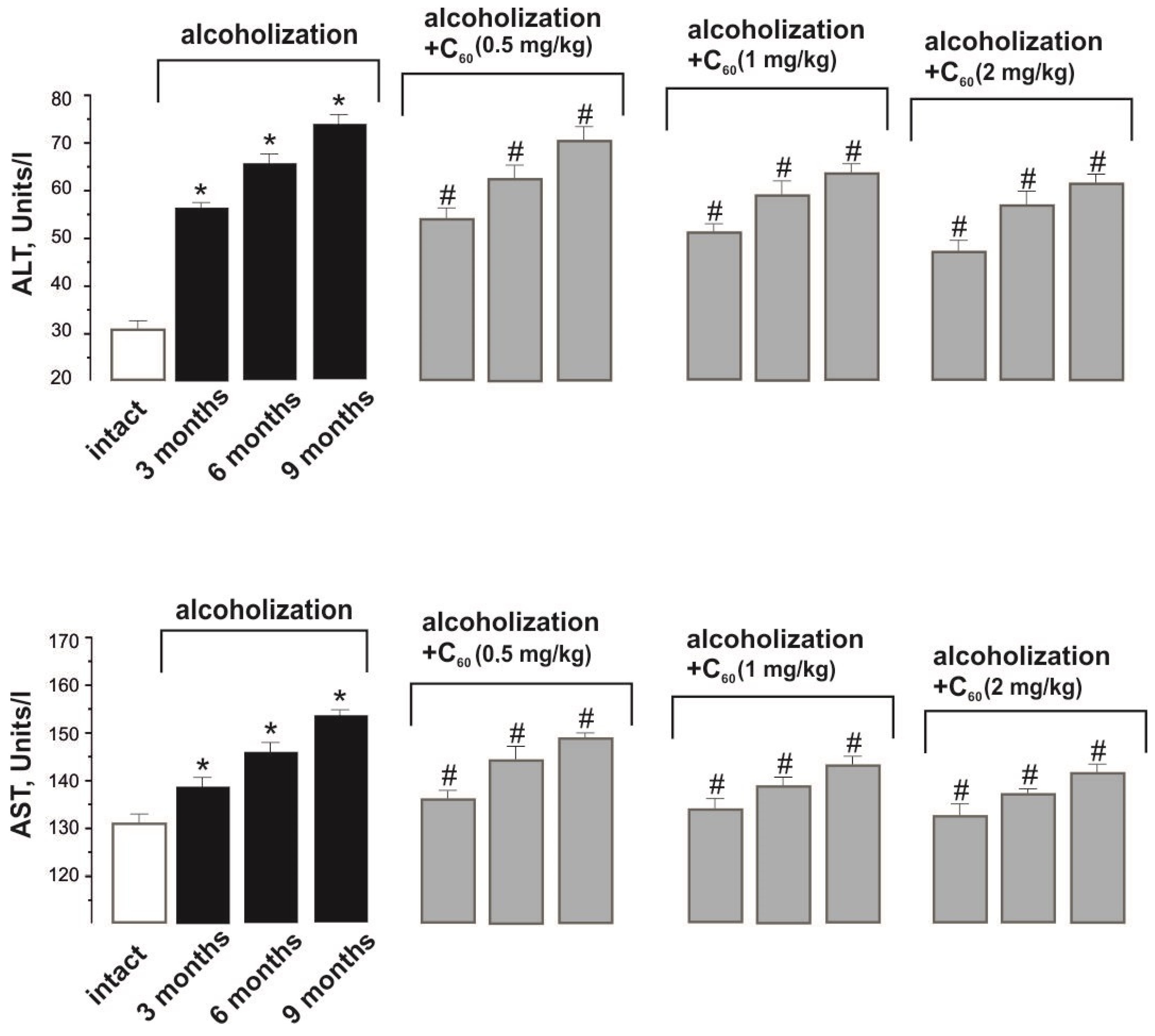
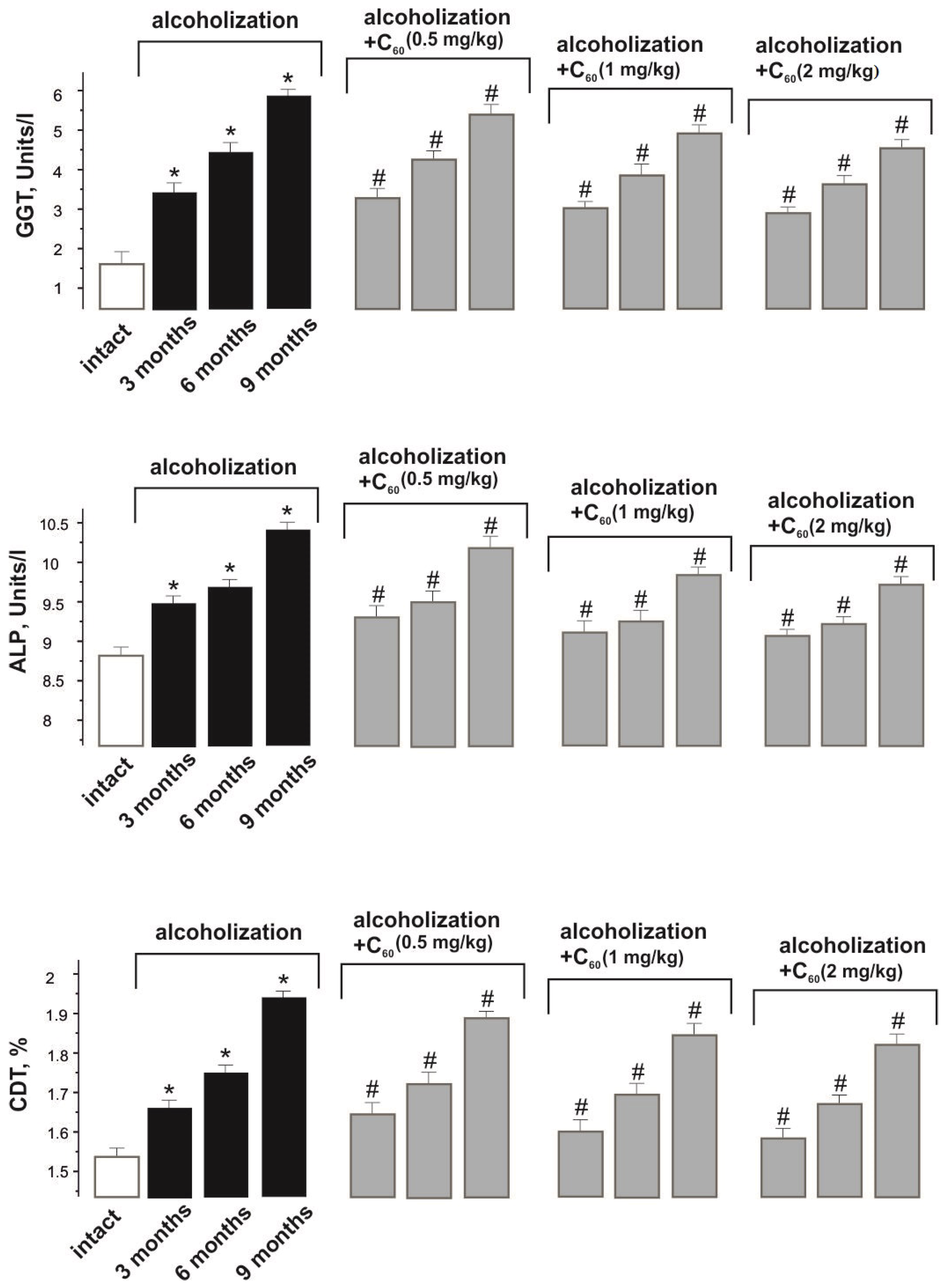
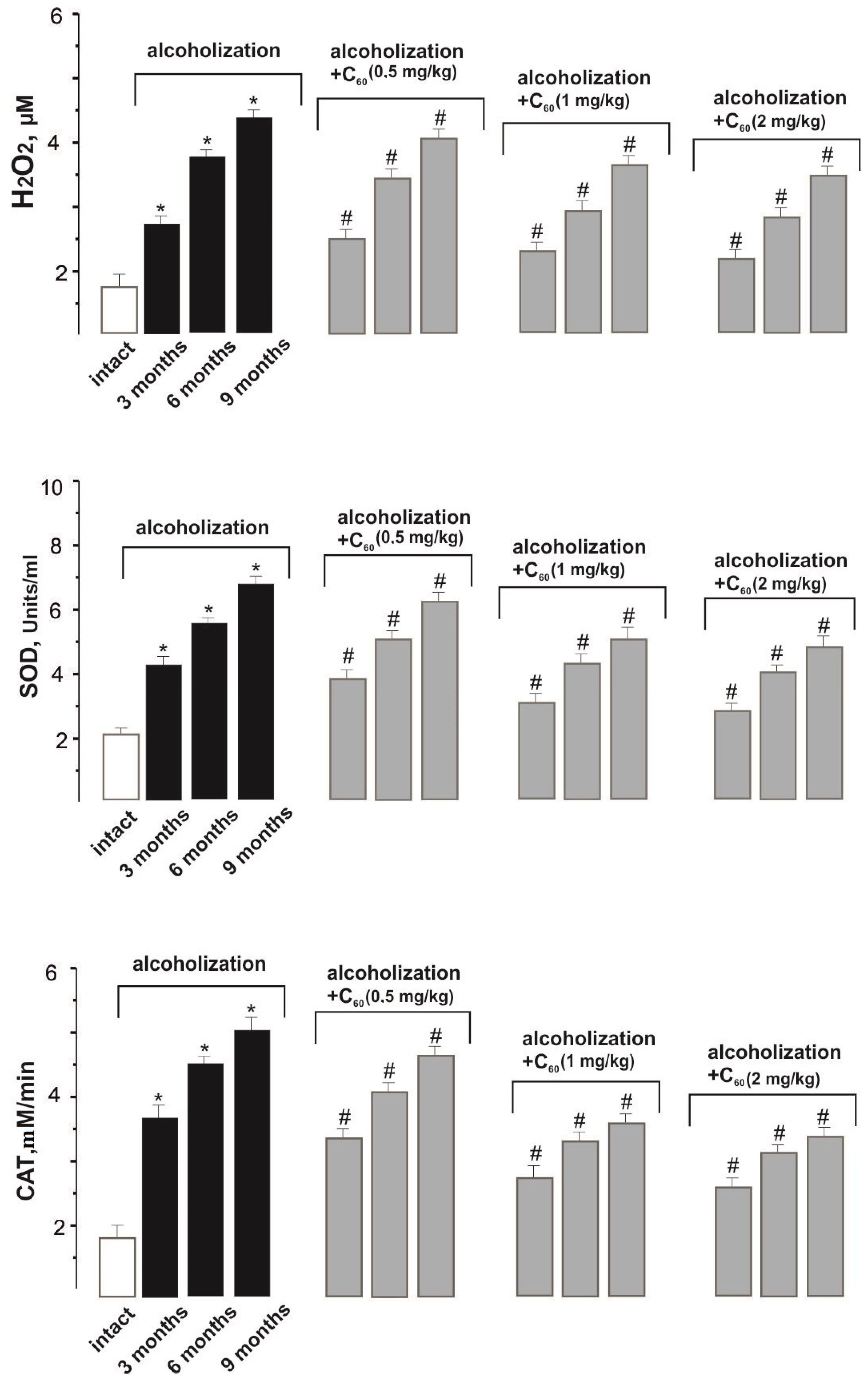
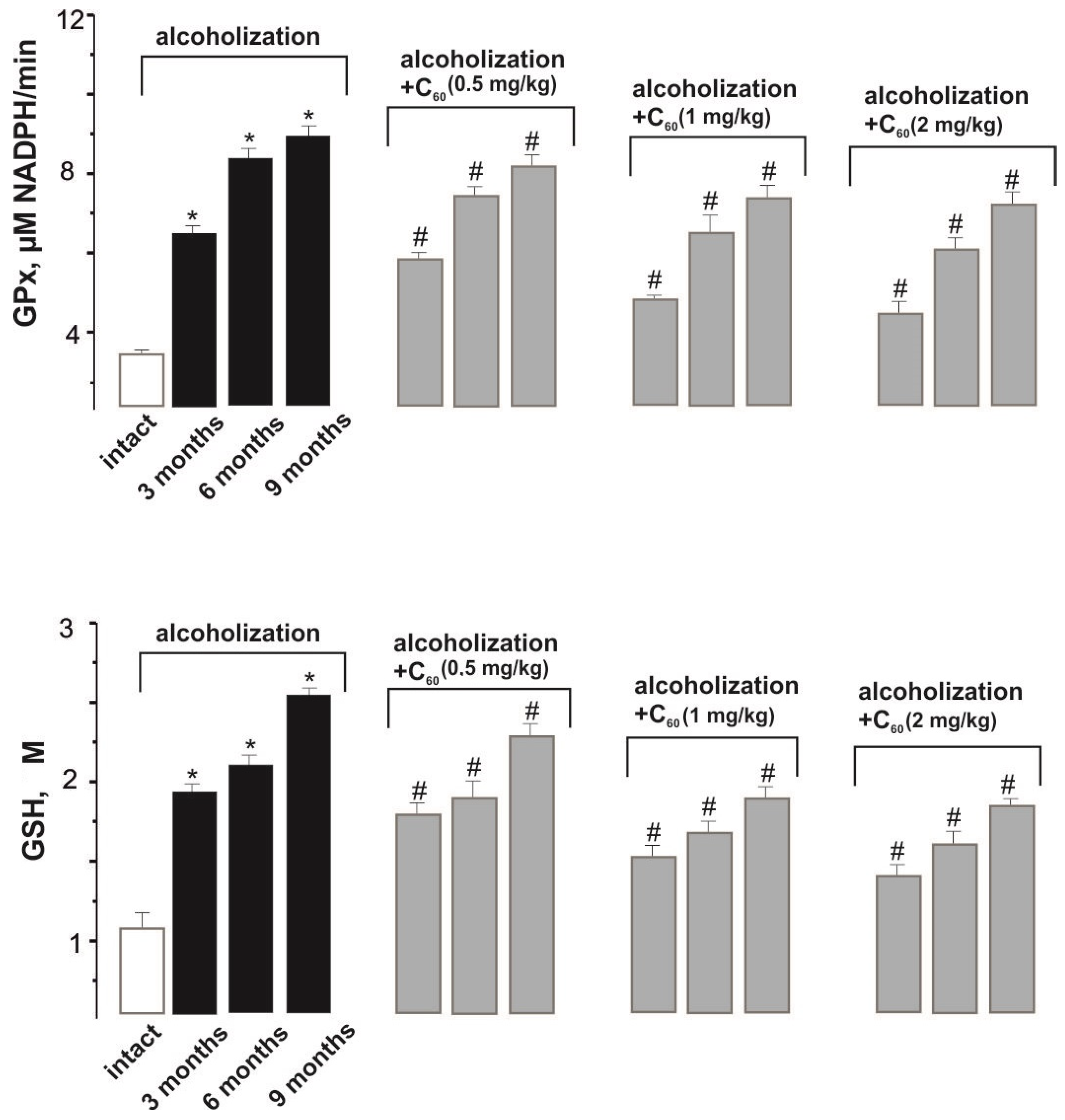
2.3. Biochemical Analysis
2.4. Histological Analysis
3. Materials and Methods
3.1. Preparation and Characterization of C60FAS
3.2. In Vivo Experiments
- -
- The intact group of rats (n = 10) received 100% drinking water;
- -
- Rats in the “alcoholization” group (control; n = 30; 10 animals in each group) were randomly selected. Each rat was placed in a separate cage to receive 40% ethanol in drinking water [34], which meant that the animals did not have access to 100% water until they had consumed the dosed portion of ethanol [18]. The amount of ethanol consumed was calculated with respect to 0.5% of the animal’s body weight. Recalculation of the ethanol dose was performed every 24 h throughout the experiment [35]. The duration of alcoholization was 3, 6, and 9 months. The target value of 40% ethanol in drinking water was chosen because it reflects the blood alcohol concentrations reported in chronic alcoholics [32];
- -
- Rats of the “alcoholization+C60” groups (n = 90; 10 animals in each group) together with ethanol were orally administered C60FAS at daily doses of 0.5, 1, and 2 mg/kg of rat weight. Recalculation of the C60FAS dose was performed every 24 h throughout the experiment. Control of the amount of C60FAS ingested was performed by denying the animals access to drinking 100% water until they fully consumed the applied drug.
3.3. Biochemical Analysis
3.4. Histological Analysis
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.-Y.; Tsai, I.-T.; Hsu, Y.-C. Alcohol-Related Liver Disease: Basic Mechanisms and Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 5170. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.S.; González-Reimers, E.; Santolaria-Fernández, F.; de la Vega-Prieto, M.J.; Martínez-Riera, A.; González, P.A.; Rodríguez, E.R.; Durán-Castellón, M.C. Lipid peroxidation and serum cytokines in acute alcoholic hepatitis. Alcohol. Alcohol. 2006, 41, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef] [PubMed]
- Kashinakunti, S.V.; Kallaganada, G.S.; Rangappa, M.; Hiremath, K.; Bannale, S. Are we over estimating serum oxidative stress/lipid peroxidation in alcoholic liver diseases? A review. Free. Radic. Antioxid. 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Manzo-Aralos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, G.H.; Khidr, B.M.; Younes, H.A. Role of melatonin in reducing hypoxia-induced oxidative stress and morphological changes in the liver of male mice. Eur. J. Pharmacol. 2006, 50, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hines, I.N.; Wheeler, M.D. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G310–G314. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.D. Endotoxin and Kupffer Cell Activations in Alcoholic Liver Disease. Alcohol. Res. Health 2003, 27, 300–306. [Google Scholar] [PubMed]
- Aksoy, H.; Koruk, M.; Akcay, F. The Relationship Between Serum Malondialdehyde and Ceruloplasmin in Chronic Liver Disease. Turk. J. Biochem. 2003, 28, 32–34. [Google Scholar]
- Yang, L.; Latchoumycandane, C.; McMullen, M.R.; Pratt, B.T.; Zhang, R.; Papouchado, B.G.; Nagy, L.E.; Feldstein, A.E.; McIntyre, T.M. Chronic alcohol exposure increases circulating bioactive oxidised phospholipids. J. Biol. Chem. 2010, 285, 22211–22220. [Google Scholar] [CrossRef]
- Hong-Brown, L.Q.; Frost, R.A.; Lang, C.H. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol. Clin. Exp. Res. 2001, 25, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, V.; Chakravarthi, S.; Jegasothy, R.; Seng, W.Y.; Fuloria, N.K.; Fuloria, S.; Hazarika, I.; Das, A. Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy. Toxicol. Rep. 2021, 8, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.V. Water Soluble Nanocarbon Materials: A Panacea for All? Curr. Sci. 2018, 114, 1846–1850. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef] [PubMed]
- Motuziuk, O.; Nozdrenko, D.; Prylutska, S.; Bogutska, K.; Mishchenko, I.; Abramchuk, O.; Khrapatyi, S.; Ritter, U.; Prylutskyy, Y. C60 fullerene reduces the level of fluctuations in the force response of muscle gastrocnemius in chronically alcoholized rats. Appl. Nanosci. 2023, 13, 7057–7067. [Google Scholar] [CrossRef]
- Motuziuk, O.; Nozdrenko, D.; Prylutska, S.; Vareniuk, I.; Bogutska, K.; Braniuk, S.; Korotkyi, O.; Prylutskyy, Y.; Ritter, U.; Piosik, J. The effect of C60 fullerene on the mechanokinetics of muscle gastrocnemius contraction in chronically alcoholized rats. Heliyon 2023, 9, e18745. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Grebinyk, A.G.; Lynchak, O.V.; Byelinska, I.V.; Cherepanov, V.V.; Tauscher, E.; Matyshevska, O.; Prylutskyy, Y.; Rybalchenko, V.; Ritter, U.; et al. In vitro and in vivo toxicity of pristine C60 fullerene aqueous colloid solution. Fuller. Nanotub. Carbon Nanostruct 2019, 27, 715–728. [Google Scholar] [CrossRef]
- Song, K.; Coleman, R.A.; Zhu, X.; Alber, C.; Ballas, Z.K.; Waldschmidt, T.J.; Cook, R.T. Chronic ethanol consumption by mice results in activated splenic T cells. J. Leukoc. Biol. 2002, 72, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.; Kandi, S.; Kumar, P.V.B.; Ramana, K.V.; Muddeshwar, M. Effect of Alcohol Consumption on Oxidative Stress Markers and its Role in the Pathogenesis and Progression of Liver Cirrhosis. Am. J. Med. Biol. Res. 2013, 1, 99–102. [Google Scholar] [CrossRef][Green Version]
- Nyblom, H.; Berggren, U.; Balldin, J.; Olsson, R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol. Alcohol. 2004, 39, 336–339. [Google Scholar] [CrossRef]
- Cho, E.J.; Jeong, S.-M.; Chung, G.E.; Yoo, J.-J.; Cho, Y.; Lee, K.-N.; Shin, D.W.; Kim, Y.J.; Yoon, J.-H.; Han, K.; et al. Gamma-glutamyl transferase and risk of all-cause and disease-specific mortality: A nationwide cohort study. Sci. Rep. 2023, 13, 1751. [Google Scholar] [CrossRef]
- Niemelä, O.; Alatalo, P. Biomarkers of alcohol consumption and related liver disease. Scand. J. Clin. Lab. Investig. 2010, 70, 305–312. [Google Scholar] [CrossRef]
- Golka, K.; Wiese, A. Carbohydrate-deficient transferrin (CDT)-a biomarker for long-term alcohol consumption. J. Toxicol. Environ. Health Crit. Rev. 2004, 7, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Antioxidant and redox regulation of cellular signaling: Introduction. Med. Sci. Sports Exerc. 2001, 33, 368–370. [Google Scholar] [CrossRef]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Gonchar, O.O.; Maznychenko, A.V.; Bulgakova, N.V.; Vereschaka, I.V.; Tomiak, T.; Ritter, U.; Prylutskyy, Y.; Mankovska, I.M.; Kostyukov, A.I. C60 Fullerene Prevents Restraint Stress-Induced Oxidative Disorders in Rat Tissues: Possible Involvement of the Nrf2/ARE-Antioxidant Pathway. Oxid. Med. Cell Longev. 2018, 2018, 2518676. [Google Scholar] [CrossRef] [PubMed]
- Schukit, M.A. Ethanol and methanol. In Goodman & Gillman’s Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Ed.; MaGraw Hill: New Delhi, India, 2011; pp. 629–647. [Google Scholar]
- Kuznietsova, H.M.; Dziubenko, N.V.; Lynchak, O.V.; Herheliuk, T.S.; Zavalny, D.K.; Remeniak, O.V.; Prylutskyy, Y.; Ritter, U. Effects of Pristine C60 Fullerenes on Liver and Pancreas in α-Naphthylisothiocyanate-Induced Cholangitis. Dig. Diss. Sci. 2020, 65, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Patel, V.B.; Reilly, M.E.; Richardson, P.J.; Falkous, G.; Mantle, D. Oxidants, antioxidants and alcohol: Implications for skeletal and cardiac muscle. Front. Biosci. 1999, 4, e58–e66. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Adachi, J.; Asano, M.; Koll, M.; Mantle, D.; Niemela, O.; Parkkila, S.; Paice, A.G.; Peters, T.; Rajendram, R.; et al. Free radicals in alcoholic myopathy: Indices of damage and preventive studies. Free Radic. Biol. Med. 2002, 32, 683–687. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Gutiérrez-Reyes, G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. Mex. 2014, 79, 135–144. [Google Scholar] [CrossRef]
- Otis, J.S.; Guidot, D.M. Procysteine increases alcohol-depleted glutathione stores in rat plantaris following a period of abstinence. Alcohol. Alcohol. 2010, 45, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ritter, U.; Prylutskyy, Y.I.; Evstigneev, M.P.; Davidenko, N.A.; Cherepanov, V.V.; Senenko, A.I.; Marchenko, O.A.; Naumovets, A.G. Structural features of highly stable reproducible C60 fullerene aqueous colloid solution probed by various techniques. Fuller. Nanotub. Carbon Nanostruct 2015, 23, 530–534. [Google Scholar] [CrossRef]
- Collins, M.A.; Neafsey, E.J. Alcohol, Excitotoxicity and Adult Brain Damage: An Experimentally Unproven Chain-of-Events. Front. Mol. Neurosci. 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- D’Souza El-Guindy, N.B.; Kovacs, E.J.; De Witte, P.; Spies, C.; Littleton, J.M.; De Villiers, W.J.S.; Lott, A.J.; Plackett, T.P.; Lanzke, N.; Meadows, G.G. Laboratory models available to study alcohol-induced organ damage and immune variations: Choosing the appropriate model. Alcohol. Clin. Exp. Res. 2010, 34, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, N.; Vranjes-Ethuric, S.; Jankovic, D.; Ethokic, D.; Mirkovic, M.; Bibic, N.; Trajkovic, V. Preparation and biodistribution of radiolabeled fullerene C60 nanocrystals. Nanotechnology 2009, 20, 385102. [Google Scholar] [CrossRef] [PubMed]
- Motuziuk, O.; Nozdrenko, D.; Prylutska, S.; Bogutska, K.; Korotkyi, O.; Prylutskyy, Y. Biochemical parameters of blood and tissue of the muscle gastrocnemius in chronically alcoholized rats under oral administration of C60 fullerene aqueous solution. Ukr. Biochem. J. 2023, 95, 58–67. [Google Scholar] [CrossRef]
- Fentener van Vlissingen, J.M.; Borrens, M.; Girod, A.; Lelovas, P.; Morrison, F.; Torres, Y.S. The reporting of clinical signs in laboratory animals. FELASA Working Group Report. Lab. Anim. 2015, 49, 267–283. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Limited: Amsterdam, The Netherlands, 2019; 519p. [Google Scholar]
- Zhang, S.; Wang, C. Precise Analysis of Nanoparticle Size Distribution in TEM Image. Methods Protoc. 2023, 6, 63. [Google Scholar] [CrossRef]
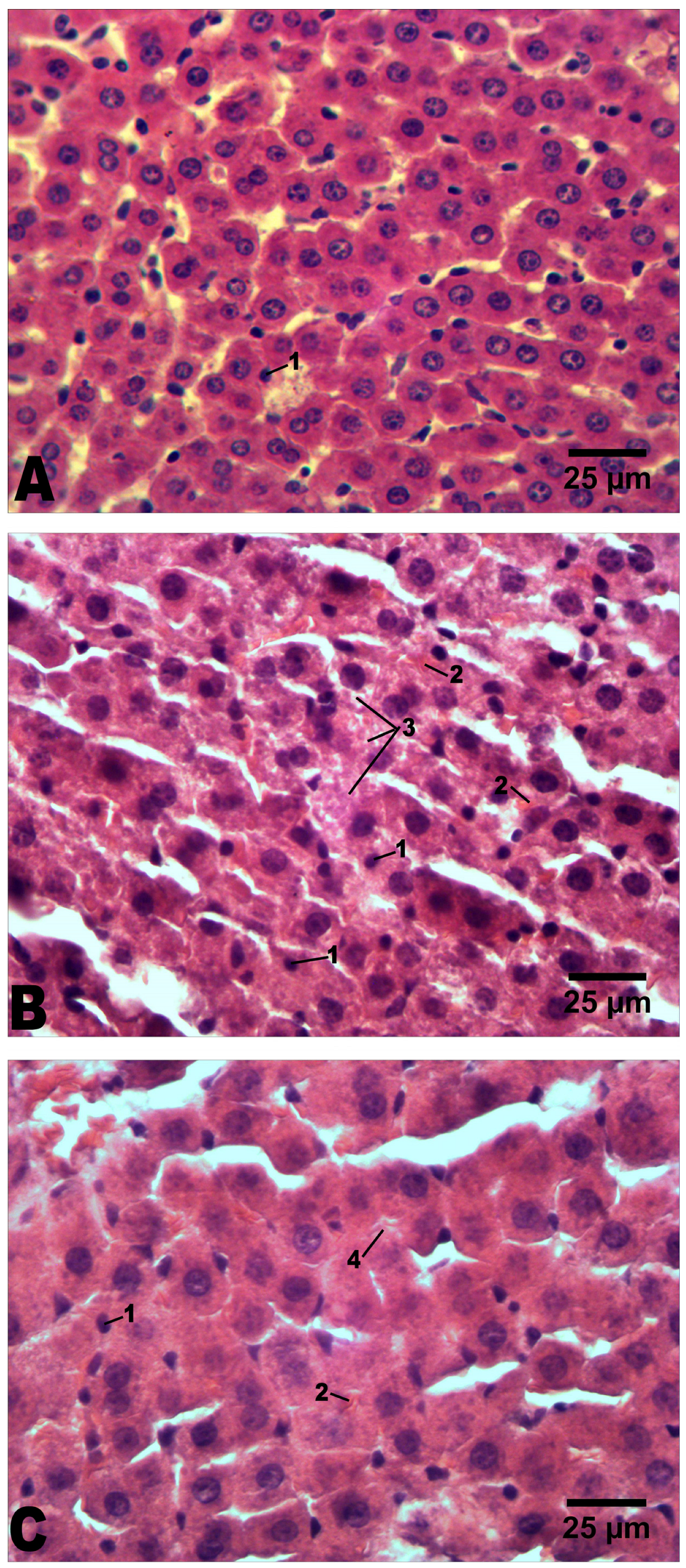
| Groups | Intact | Alcoholization | Alcoholization+C60 (1 mg/kg) | |
|---|---|---|---|---|
| Histopathological Features | ||||
| Volume of hepatocyte’s nucleus, µm3 | 138 ± 8 | 248 ± 11 * | 284 ± 19 # | |
| Cross-sectional area of hepatocyte, µm2 | 205 ± 11 | 273 ± 20 * | 244 ± 11 # | |
| Pyknotic nuclei in hepatocytes | + | ++ | + | |
| Mallory bodies | – | +++ | ++ | |
| Lipid dystrophy of hepatocytes | – | +++ | + | |
| Hydropic dystrophy of hepatocytes | – | +++ | ++ | |
| Balloon dystrophy of hepatocytes | – | ++ | – | |
| Necrotic hepatocytes | – | ++ | + | |
| Blood vessel dilatation | – | ++ | + | |
| Inflammatory cell infiltration | – | ++ | ++ | |
| Expansion of connective tissue (fibrosis) | – | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motuziuk, O.; Nozdrenko, D.; Prylutska, S.; Vareniuk, I.; Cherepanov, V.; Bogutska, K.; Rudenko, S.; Prylutskyy, Y.; Piosik, J.; Ritter, U. C60 Fullerene Reduces the Level of Liver Damage in Chronic Alcohol Intoxication of Rats. Molecules 2024, 29, 2951. https://doi.org/10.3390/molecules29132951
Motuziuk O, Nozdrenko D, Prylutska S, Vareniuk I, Cherepanov V, Bogutska K, Rudenko S, Prylutskyy Y, Piosik J, Ritter U. C60 Fullerene Reduces the Level of Liver Damage in Chronic Alcohol Intoxication of Rats. Molecules. 2024; 29(13):2951. https://doi.org/10.3390/molecules29132951
Chicago/Turabian StyleMotuziuk, Olexandr, Dmytro Nozdrenko, Svitlana Prylutska, Igor Vareniuk, Vsevolod Cherepanov, Kateryna Bogutska, Sergii Rudenko, Yuriy Prylutskyy, Jacek Piosik, and Uwe Ritter. 2024. "C60 Fullerene Reduces the Level of Liver Damage in Chronic Alcohol Intoxication of Rats" Molecules 29, no. 13: 2951. https://doi.org/10.3390/molecules29132951
APA StyleMotuziuk, O., Nozdrenko, D., Prylutska, S., Vareniuk, I., Cherepanov, V., Bogutska, K., Rudenko, S., Prylutskyy, Y., Piosik, J., & Ritter, U. (2024). C60 Fullerene Reduces the Level of Liver Damage in Chronic Alcohol Intoxication of Rats. Molecules, 29(13), 2951. https://doi.org/10.3390/molecules29132951









