Precursor-Driven Catalytic Performances of Al2O3-Supported Earth-Abundant Ni Catalysts in the Hydrogenation of Levulinic Acid and Hydroxymethylfurfural into Added-Value Chemicals
Abstract
1. Introduction
2. Results
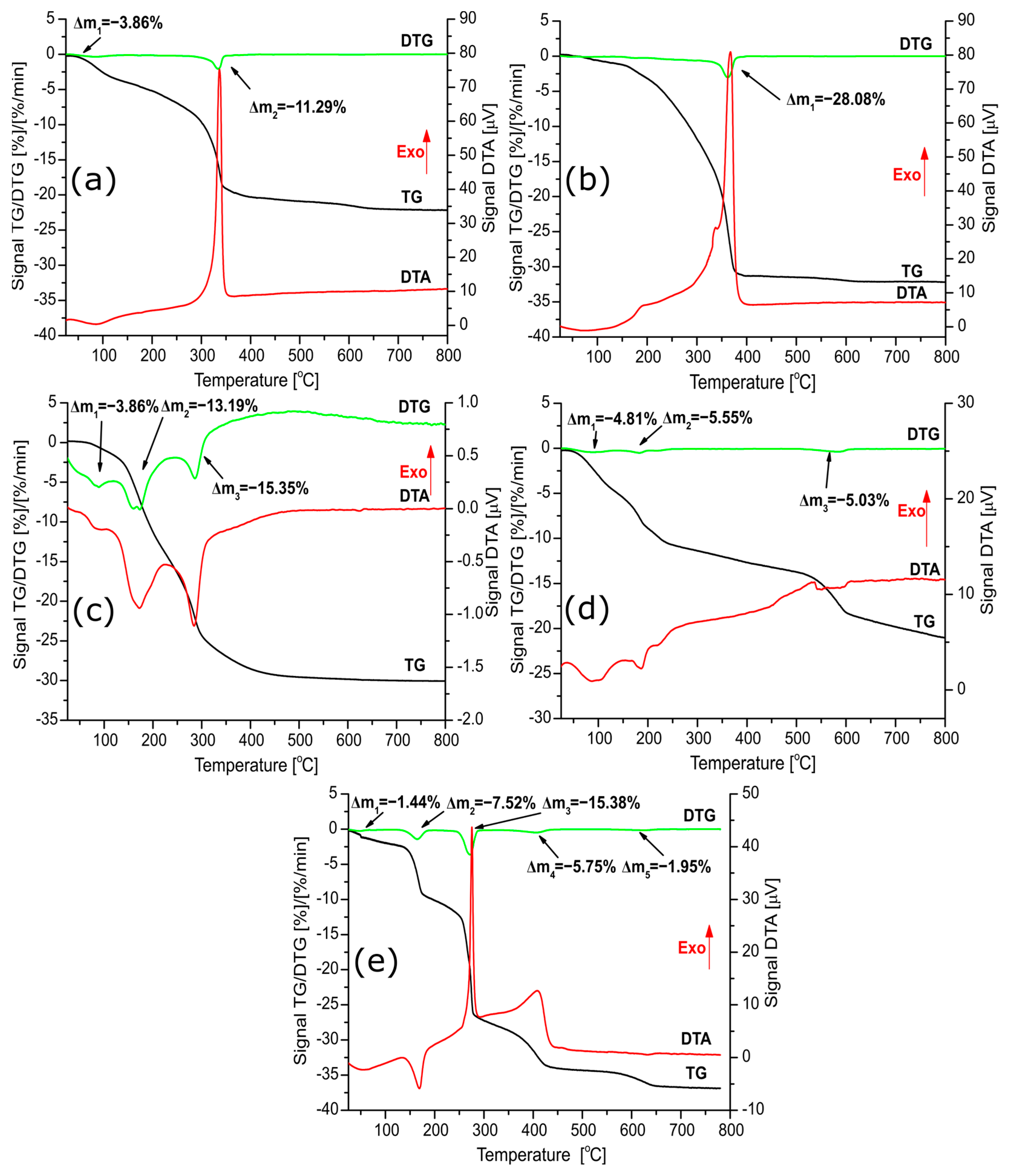
2.1. Thermal Decomposition of Catalysts Precursors
2.2. Temperature-Programmed Reduction (TPR-H2) of Ni Catalysts
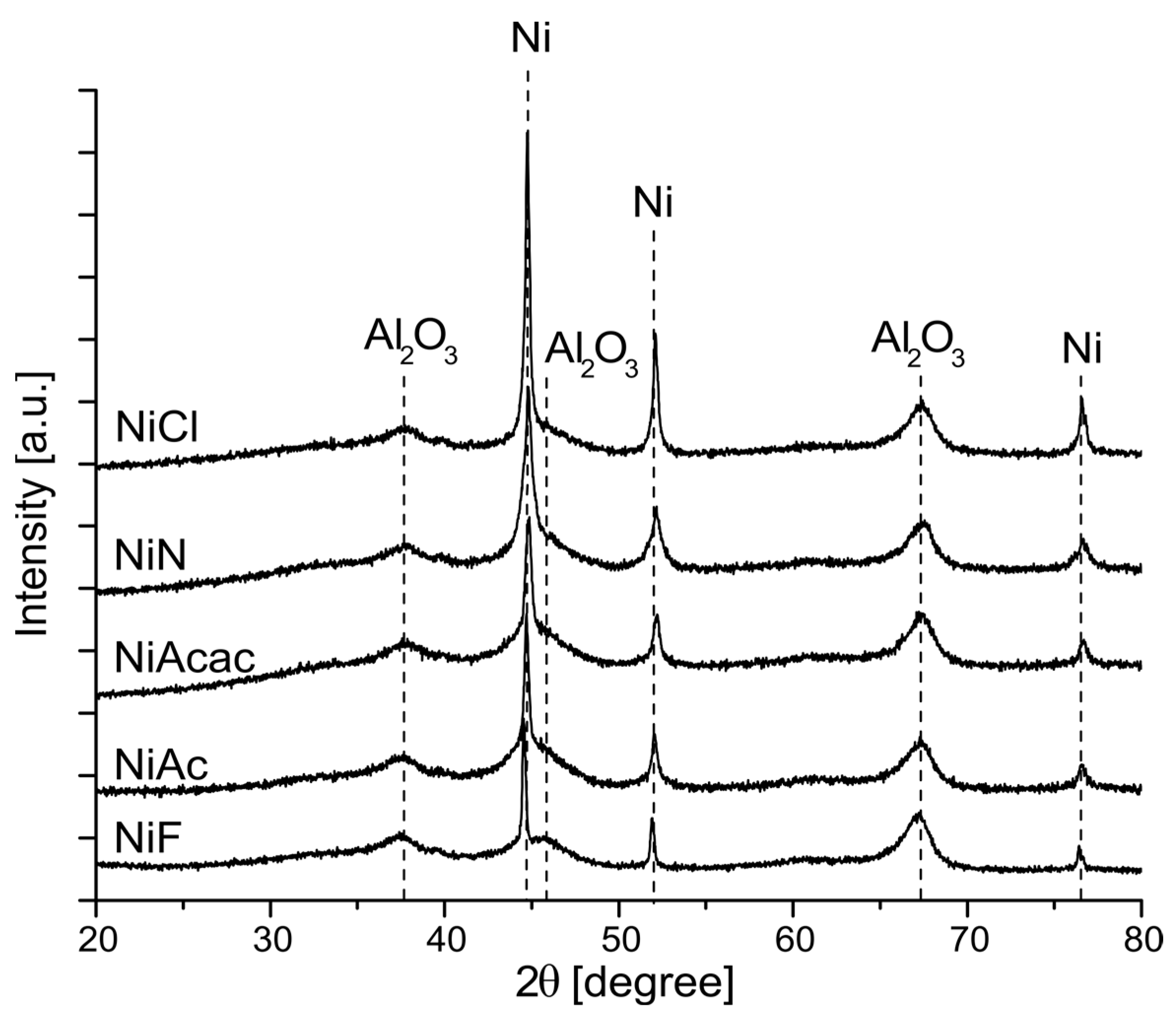
2.3. X-ray Diffraction (XRD) of Ni Catalysts
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Temperature-Programmed Desorption of Ammonia (NH3-TPD)
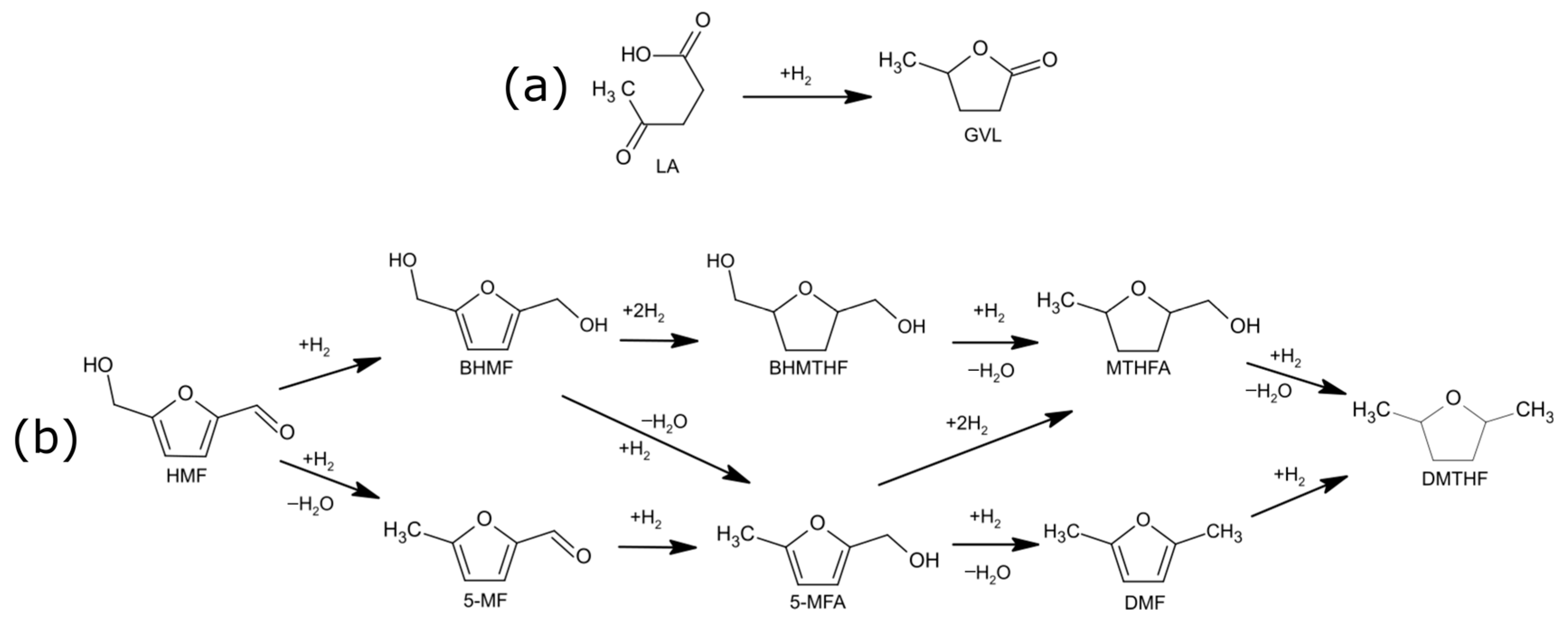
2.6. Catalytic Tests
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Catalysts Characterization
4.3. Catalytic Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikdar, S.K. Sustainability Perspective and Chemistry-Based Technologies. Ind. Eng. Chem. Res. 2007, 46, 4727–4733. [Google Scholar] [CrossRef]
- Song, B.; Wu, Z.; Yu, Y.; Wu, H. Hydrothermal Reactions of Biomass-Derived Platform Molecules: Distinct Effect of Aprotic and Protic Solvents on Primary Decomposition of Glucose and Fructose in Hot-Compressed Solvent/Water Mixtures. Ind. Eng. Chem. Res. 2020, 59, 7336–7345. [Google Scholar] [CrossRef]
- Garcés, D.; Díaz, E.; Ordóñez, S. Aqueous Phase Conversion of Hexoses into 5-Hydroxymethylfurfural and Levulinic Acid in the Presence of Hydrochloric Acid: Mechanism and Kinetics. Ind. Eng. Chem. Res. 2017, 56, 5221–5230. [Google Scholar] [CrossRef]
- Tan-Soetedjo, J.N.M.; Van De Bovenkamp, H.H.; Abdilla, R.M.; Rasrendra, C.B.; Van Ginkel, J.; Heeres, H.J. Experimental and Kinetic Modeling Studies on the Conversion of Sucrose to Levulinic Acid and 5-Hydroxymethylfurfural Using Sulfuric Acid in Water. Ind. Eng. Chem. Res. 2017, 56, 13228–13239. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective Hydrogenation of Levulinic Acid to γ-Valerolactone over Carbon-Supported Noble Metal Catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Priecel, P.; Endot, N.A.; Carà, P.D.; Lopez-Sanchez, J.A. Fast Catalytic Hydrogenation of 2,5-Hydroxymethylfurfural to 2,5-Dimethylfuran with Ruthenium on Carbon Nanotubes. Ind. Eng. Chem. Res. 2018, 57, 1991–2002. [Google Scholar] [CrossRef]
- Chen, Q.; Li, T.; Zhou, Y.; Bi, Y.; Guo, S.; Liu, X.; Kang, H.; Wang, M.; Liu, L.; Xing, E.; et al. Selective Hydrogenation of 5-Hydroxymethylfurfural via Zeolite Encapsulation to Avoid Further Hydrodehydroxylation. Ind. Eng. Chem. Res. 2020, 59, 12004–12012. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Feng, J.; Lu, X. A Review about GVL Production from Lignocellulose: Focusing on the Full Components Utilization. Ind. Crops Prod. 2020, 144, 112031. [Google Scholar] [CrossRef]
- Turkin, A.A.; Makshina, E.V.; Sels, B.F. Catalytic Hydroconversion of 5-HMF to Value-Added Chemicals: Insights into the Role of Catalyst Properties and Feedstock Purity. ChemSusChem 2022, 15, e202200412. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, C.; Liu, Q.; Zhang, J.; Tang, X.; Zeng, X.; Liu, S.; Lin, L. Catalytic Transfer Hydrogenolysis/Hydrogenation of Biomass-Derived 5-Formyloxymethylfurfural to 2, 5-Dimethylfuran over Ni-Cu Bimetallic Catalyst with Formic Acid As a Hydrogen Donor. Ind. Eng. Chem. Res. 2019, 58, 5414–5422. [Google Scholar] [CrossRef]
- Kadu, B.S.; Hengne, A.M.; Biradar, N.S.; Rode, C.V.; Chikate, R.C. Reductive Cyclization of Levulinic Acid to γ-Valerolactone over Non-Noble Bimetallic Nanocomposite. Ind. Eng. Chem. Res. 2016, 55, 13032–13039. [Google Scholar] [CrossRef]
- Jiang, K.; Sheng, D.; Zhang, Z.; Fu, J.; Hou, Z.; Lu, X. Hydrogenation of Levulinic Acid to γ-Valerolactone in Dioxane over Mixed MgO–Al2O3 Supported Ni Catalyst. Catal. Today 2016, 274, 55–59. [Google Scholar] [CrossRef]
- Przydacz, M.; Jędrzejczyk, M.; Brzezińska, M.; Rogowski, J.; Keller, N.; Ruppert, A.M. Solvothermal hydrodeoxygenation of hydroxymethylfurfural derived from biomass towards added value chemicals on Ni/TiO2 catalysts. J. Supercrit. Fluids 2020, 163, 104827. [Google Scholar] [CrossRef]
- Hengnea, A.M.; Kadub, B.S.; Biradara, N.S.; Chikateb, R.C.; Rodea, C.V. Transfer Hydrogenation of Biomass-Derived Levulinic Acid to γ-Valerolactone over Supported Ni Catalysts. RSC Adv. 2016, 6, 59753–59761. [Google Scholar] [CrossRef]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Anjaneyulu, C.; Bhargava, S.K.; Tardio, J.; Reddy, V.K.; Padmasri, A.H.; Venugopal, A. An Investigation on the Influence of Support Type for Ni Catalysed Vapour Phase Hydrogenation of Aqueous Levulinic Acid to γ-Valerolactone. RSC Adv. 2016, 6, 9872–9879. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.W. Rational Design of Ni-Based Catalysts Derived from Hydrotalcite for Selective Hydrogenation of 5-Hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Pomeroy, B.; Grilc, M.; Gyergyek, S.; Likozar, B. Kinetics and Mechanistic Insights into the Acidic-Basic Active Sites for Water-Containing Catalytic Hydrogenation of Hydroxymethylfurfural over Ceria-Doped Ni/Al2O3. Appl. Catal. B Environ. 2023, 334, 122868. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Carvalho, H.W.P.; Lu, C.; Kleist, W.; Grunwaldt, J.-D. Synthesis of V-Valerolactone by Hydrogenation of Levulinic Acid over Supported Nickel Catalysts. Appl. Catal. A Gen. 2015, 502, 18–26. [Google Scholar] [CrossRef]
- Soszka, E.; Jędrzejczyk, M.; Kocemba, I.; Keller, N.; Ruppert, A.M. Ni-Pd/γ-Al2O3 Catalysts in the Hydrogenation of Levulinic Acid and Hydroxymethylfurfural towards Value Added Chemicals. Catalysts 2020, 10, 1026. [Google Scholar] [CrossRef]
- Pomeroy, B.; Grilc, M.; Likozar, B. Process Condition-Based Tuneable Selective Catalysis of Hydroxymethylfurfural (HMF) Hydrogenation Reactions to Aromatic, Saturated Cyclic and Linear Poly-Functional Alcohols over Ni–Ce/Al2O3. Green Chem. 2021, 23, 7996–8002. [Google Scholar] [CrossRef]
- Kim, P.; Kim, H.; Joo, J.B.; Kim, W.; Song, I.K.; Yi, J. Effect of Nickel Precursor on the Catalytic Performance of Ni/Al2O3 Catalysts in the Hydrodechlorination of 1,1,2-Trichloroethane. J. Mol. Catal. A Chem. 2006, 256, 178–183. [Google Scholar] [CrossRef]
- Donphai, W.; Phichairatanaphong, O.; Klysubun, W.; Chareonpanich, M. Hydrogen and Carbon Allotrope Production through Methane Cracking over Ni/Bimodal Porous Silica Catalyst: Effect of Nickel Precursor. Int. J. Hydrogen Energy 2018, 43, 21798–21809. [Google Scholar] [CrossRef]
- He, S.; Wu, H.; Yu, W.; Mo, L.; Lou, H.; Zheng, X. Combination of CO2 Reforming and Partial Oxidation of Methane to Produce Syngas over Ni/SiO2 and Ni-Al2O3/SiO2 Catalysts with Different Precursors. Int. J. Hydrogen Energy 2009, 34, 839–843. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, X.; Jia, P.; Zhang, Z.; Dong, D.; Hu, G.; Hu, S.; Wang, Y.; Xiang, J. Steam Reforming of Acetic Acid over Nickel-Based Catalysts: The Intrinsic Effects of Nickel Precursors on Behaviors of Nickel Catalysts. Appl. Catal. B Environ. 2018, 237, 538–553. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, C.; Li, S.; Han, Z.; Wang, T.; Ma, X.; Gong, J. Hydrogen Production via Glycerol Steam Reforming over Ni/Al2O3: Influence of Nickel Precursors. ACS Sustain. Chem. Eng. 2013, 1, 1052–1062. [Google Scholar] [CrossRef]
- Lv, J.; Rong, Z.; Wang, Y.; Xiu, J.; Wanga, Y.; Qua, J. Highly Efficient Conversion of Biomass-Derived Levulinic Acid into γ-Valerolactone over Ni/MgO Catalyst. RSC Adv. 2015, 5, 72037–72045. [Google Scholar] [CrossRef]
- Ma, S.; Tan, Y.; Han, Y. Methanation of Syngas over Coral Reef-like Ni/Al2O3 Catalysts. J. Nat. Gas Chem. 2011, 20, 435–440. [Google Scholar] [CrossRef]
- Hong, J.; Guo, G.; Zhang, K. Kinetics and Mechanism of Non-Isothermal Dehydration of Nickel Acetate Tetrahydrate in Air. J. Anal. Appl. Pyrolysis 2006, 77, 111–115. [Google Scholar] [CrossRef]
- Goto, Y.; Taniguchi, K.; Omata, T.; Otsuka-Yao-Matsuo, S.; Ohashi, N.; Ueda, S.; Yoshikawa, H.; Yamashita, Y.; Oohashi, H.; Kobayashi, K. Formation of Ni3C Nanocrystals by Thermolysis of Nickel Acetylacetonate in Oleylamine: Characterization Using Hard X-ray Photoelectron Spectroscopy. Chem. Mater. 2008, 20, 4156–4160. [Google Scholar] [CrossRef]
- Kenfack, F.; Langbein, H. Influence of the Starting Powders on the Synthesis of Nickel Ferrite. Cryst. Res. Technol. 2006, 41, 748–758. [Google Scholar] [CrossRef]
- Qusti, A.; Samarkandy, A.; Al-Thabaiti, S.; Diefallah, E.-H. The Kinetics of Thermal Decomposition of Nickel Formate Dihydrate in Air. J. King Abdulaziz Univ. 1997, 9, 73–81. [Google Scholar] [CrossRef]
- Xiang, L.; Gong, Y.L.; Li, J.C.; Wang, Z.W. Influence of Hydrothermal Modification on the Properties of Ni/Al2O3 Catalyst. Appl. Surf. Sci. 2004, 239, 94–100. [Google Scholar] [CrossRef]
- Tomer, A.; Wyrwalski, F.; Przybylski, C.; Paul, J.F.; Monflier, E.; Pera-Titus, M.; Ponchel, A. Facile Preparation of Ni/Al2O3 Catalytic Formulations with the Aid of Cyclodextrin Complexes: Towards Highly Active and Robust Catalysts for the Direct Amination of Alcohols. J. Catal. 2017, 356, 111–124. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kanungo, S.B. Thermal Dehydration and Decomposition of Nickel Chloride Hydrate (NiCl2·xH2O). J. Therm. Anal. 1992, 38, 2417–2436. [Google Scholar] [CrossRef]
- Kanungo, S.B.; Mishra, S.K. Non-Isothermal Kinetics of Dehydration and Decomposition of Nickel Chloride Hydrate. Thermochim. Acta 1994, 241, 171–189. [Google Scholar] [CrossRef]
- Molina, R.; Centeno, M.A.; Poncelet, G. α-Alumina-Supported Nickel Catalysts Prepared with Nickel Acetylacetonate. 1. Adsorption in the Liquid Phase. J. Phys. Chem. B 1999, 103, 6036–6046. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.W. Temperature-Programmed-Reduction Studies of Nickel Oxide/Alumina Catalysts: Effects of the Preparation Method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Shafiee, P.; Alavi, S.M.; Rezaei, M. Mechanochemical Synthesis Method for the Preparation of Mesoporous Ni–Al2O3 Catalysts for Hydrogen Purification via CO2 Methanation. J. Energy Inst. 2021, 96, 1–10. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.G.; Joo, O.S.; Jung, K.D. Stabilization of Ni/Al2O3 Catalyst by Cu Addition for CO2 Reforming of Methane. Appl. Catal. A Gen. 2004, 269, 1–6. [Google Scholar] [CrossRef]
- Mihaylov, M.; Hadjiivanov, K.; Knözinger, H. Formation of Ni(CO)4 during the Interaction between CO and Silica-Supported Nickel Catalyst: An FTIR Spectroscopic Study. Catal. Lett. 2001, 76, 59–63. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Pannell, R.B. The Stoichiometry of Hydrogen and Carbon Monoxide Chemisorption on Alumina- and Silica-Supported Nickel. J. Catal. 1980, 65, 390–401. [Google Scholar] [CrossRef]
- Rossetti, I.; Gallo, A.; DalSanto, V.; Bianchi, C.L.; Nichele, V.; Signoretto, M.; Finocchio, E.; Ramis, G.; Di Michele, A. Nickel Catalysts Supported Over TiO2, SiO2 and ZrO2 for the Steam Reforming of Glycerol. ChemCatChem 2013, 5, 294–306. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.P.; Liu, C.J. CO Adsorbed Infrared Spectroscopy Study of Ni/Al2O3 Catalyst for CO2 Reforming of Methane. Catal. Lett. 2007, 118, 306–312. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Al-sagheer, F.A. In Situ FTIR Spectra of Pyridine Adsorbed on SiO2–Al2O3, TiO2, ZrO2 and CeO2: General Considerations for the Identification of Acid Sites on Surfaces of Finely Divided Metal Oxides. Colloids Surf. A Physicochem. Eng. 2001, 190, 261–274. [Google Scholar] [CrossRef]
- Stanislaus, A.; Absi-Halabi, M.; Absi-Halabi, K. Effect of Nickel on the Surface Acidity of Molybdenum Hydrotreating Catalysts. Appl. Catal. 1989, 50, 237–245. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Nowosielska, M.; Rynkowski, J. Reforming of Methane with Carbon Dioxide over Supported Bimetallic Catalysts Containing Ni and Noble Metal I. Characterization and Activity of SiO2 Supported Ni–Rh Catalysts. Appl. Catal. A Gen. 2005, 280, 233–244. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liu, A.F.; Zhang, Q.; Li, K.M.; Porterfield, W.B.; Li, L.C.; Wang, F. Mechanistic Insights into the Solvent-Driven Adsorptive Hydrodeoxygenation of Biomass Derived Levulinate Acid/Ester to 2-Methyltetrahydrofuran over Bimetallic Cu-Ni Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 11477–11490. [Google Scholar] [CrossRef]
- Soszka, E.; Jędrzejczyk, M.; Lefèvre, C.; Ihiawakrim, D.; Keller, N.; Ruppert, A.M. TiO2-Supported Co Catalysts for the Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran: Influence of the Support. Catal. Sci. Technol. 2022, 12, 5802–5813. [Google Scholar] [CrossRef]
- Trimmel, G.; Lembacher, C.; Kickelbick, G.; Schubert, U. Sol-Gel Processing of Alkoxysilyl-Substituted Nickel Complexes for the Preparation of Highly Dispersed Nickel in Silica. New J. Chem. 2002, 26, 759–765. [Google Scholar] [CrossRef]
- Chen, I.; Lin, S.-Y.; Shiue, D.-W. Calcination of Nickel/Alumina Catalysts. Ind. Eng. Chem. Res. 1988, 27, 926–929. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Reforming of Methane with Carbon Dioxide over Ni/Al2O3 Catalysts: Effect of Nickel Precursor. Appl. Catal. A Gen. 1998, 169, 271–280. [Google Scholar] [CrossRef]
- Ren, S.; Qiu, J.; Wang, C.; Xu, B.; Fan, Y.; Chen, Y. Influence of Nickel Salt Precursors on the Hydrogenation Activity of Ni/γ-Al2O3 Catalyst. Chin. J. Catal. 2007, 28, 651–656. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Liu, D.; Shang, Y.; Yin, X.; Zhang, P.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Hydrothermal Carbon-Supported Ni Catalysts for Selective Hydrogenation of 5-Hydroxymethylfurfural toward Tunable Products. J. Mater. Sci. 2020, 55, 14179–14196. [Google Scholar] [CrossRef]
- Chen, S.; Ciotonea, C.; De Oliveira Vigier, K.; Jérôme, F.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. Hydroconversion of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran and 2,5-Dimethyltetrahydrofuran over Non-Promoted Ni/SBA-15. ChemCatChem 2020, 12, 2050–2059. [Google Scholar] [CrossRef]
- Morales, M.V.; Conesa, J.M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Tunable Selectivity of Ni Catalysts in the Hydrogenation Reaction of 5-Hydroxymethylfurfural in Aqueous Media: Role of the Carbon Supports. Carbon 2021, 182, 265–275. [Google Scholar] [CrossRef]




| Catalyst | Ni Particle Size [nm] |
|---|---|
| NiAc | 26 |
| NiAcac | 20 |
| NiN | 12 |
| NiCl | 26 |
| NiF | 36 |
| Catalyst | Acidity [µmol/g] |
|---|---|
| NiAc | 865 |
| NiAcac | 980 |
| NiN | 925 |
| NiCl | 670 |
| NiF | 1090 |
| Catalyst | LA Conversion [%] | GVL Yield [%] |
|---|---|---|
| NiAc | 24 | 18 |
| NiAcac | 39 | 30 |
| NiN | 76 | 73 |
| NiCl | 9 | 2 |
| NiF | 8 | 0 |
| Catalyst | Conversion [%] | Product Yield [%] | |||||||
|---|---|---|---|---|---|---|---|---|---|
HMF | 5-MF | BHMF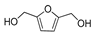 | 5-MFA | DMF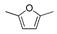 | BHMTHF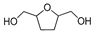 | MTHFA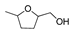 | DMTHF | Others | |
| NiAc | 97 | 0 | 1 | 0 | 0 | 43 | 32 | 18 | 3 |
| NiAcac | 97 | 0 | 0 | 0 | 0 | 42 | 39 | 14 | 2 |
| NiN | 97 | 0 | 1 | 0 | 0 | 56 | 26 | 7 | 7 |
| NiCl | 28 | 8 | 5 | 4 | 11 | 0 | 0 | 0 | 0 |
| NiF | 100 | 0 | 8 | 11 | 45 | 14 | 0 | 0 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejczyk, M.; Żyłka, E.; Chałupka-Śpiewak, K.; Ruppert, A.M. Precursor-Driven Catalytic Performances of Al2O3-Supported Earth-Abundant Ni Catalysts in the Hydrogenation of Levulinic Acid and Hydroxymethylfurfural into Added-Value Chemicals. Molecules 2024, 29, 2963. https://doi.org/10.3390/molecules29132963
Jędrzejczyk M, Żyłka E, Chałupka-Śpiewak K, Ruppert AM. Precursor-Driven Catalytic Performances of Al2O3-Supported Earth-Abundant Ni Catalysts in the Hydrogenation of Levulinic Acid and Hydroxymethylfurfural into Added-Value Chemicals. Molecules. 2024; 29(13):2963. https://doi.org/10.3390/molecules29132963
Chicago/Turabian StyleJędrzejczyk, Marcin, Emilia Żyłka, Karolina Chałupka-Śpiewak, and Agnieszka M. Ruppert. 2024. "Precursor-Driven Catalytic Performances of Al2O3-Supported Earth-Abundant Ni Catalysts in the Hydrogenation of Levulinic Acid and Hydroxymethylfurfural into Added-Value Chemicals" Molecules 29, no. 13: 2963. https://doi.org/10.3390/molecules29132963
APA StyleJędrzejczyk, M., Żyłka, E., Chałupka-Śpiewak, K., & Ruppert, A. M. (2024). Precursor-Driven Catalytic Performances of Al2O3-Supported Earth-Abundant Ni Catalysts in the Hydrogenation of Levulinic Acid and Hydroxymethylfurfural into Added-Value Chemicals. Molecules, 29(13), 2963. https://doi.org/10.3390/molecules29132963






