Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens
Abstract
1. Introduction
2. Results and Discussion
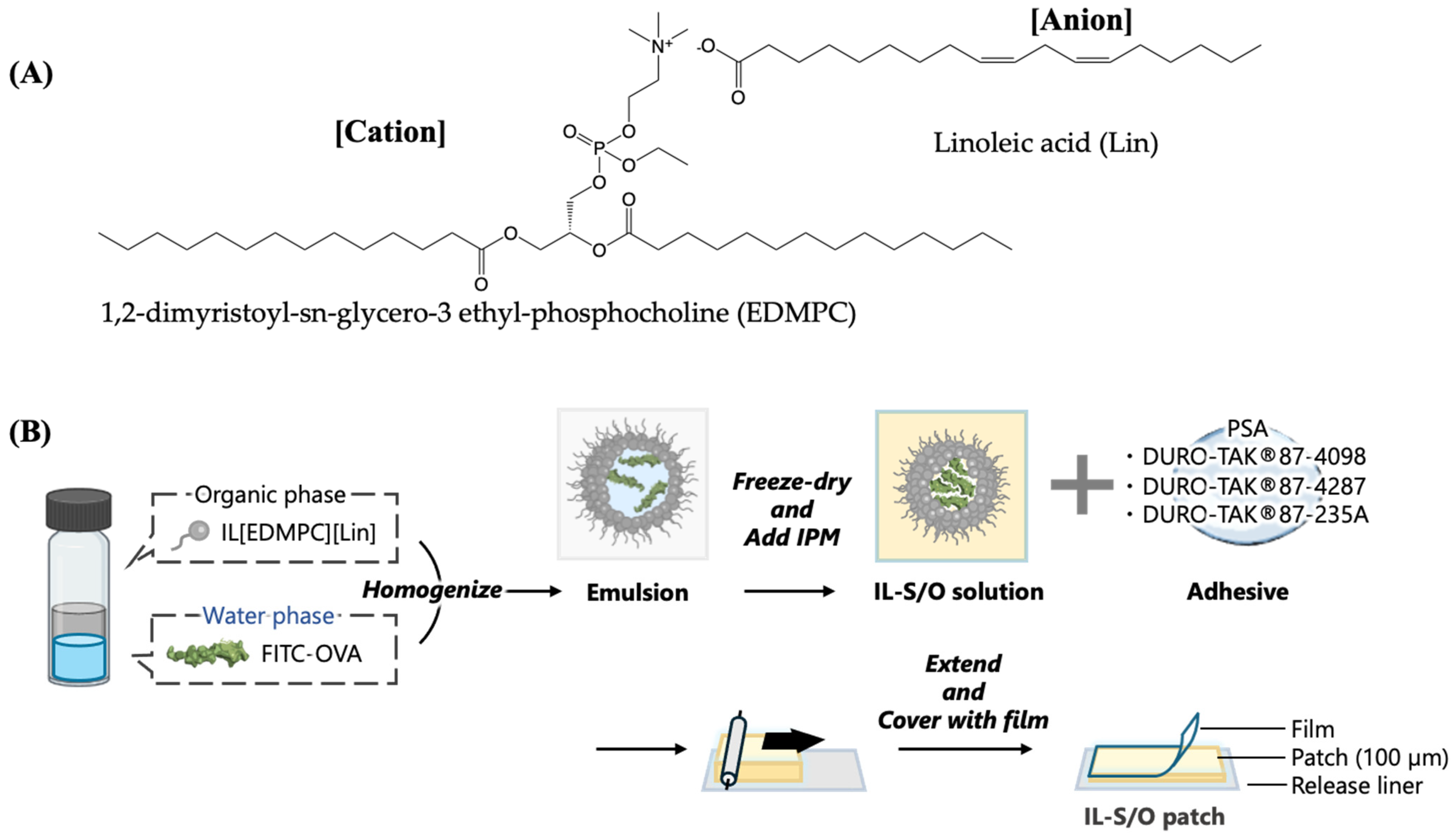
2.1. Preparation of the IL-S/O Patches
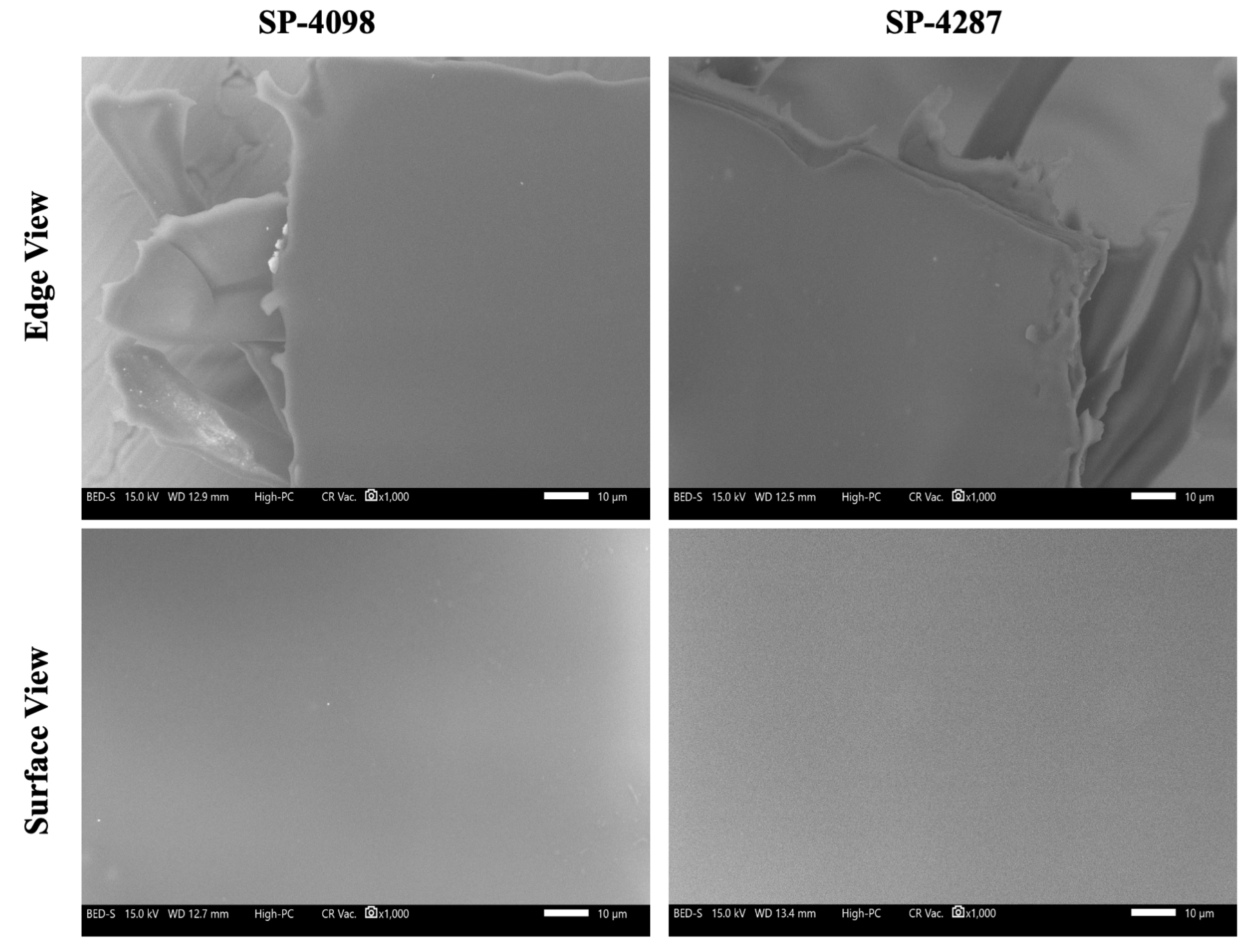
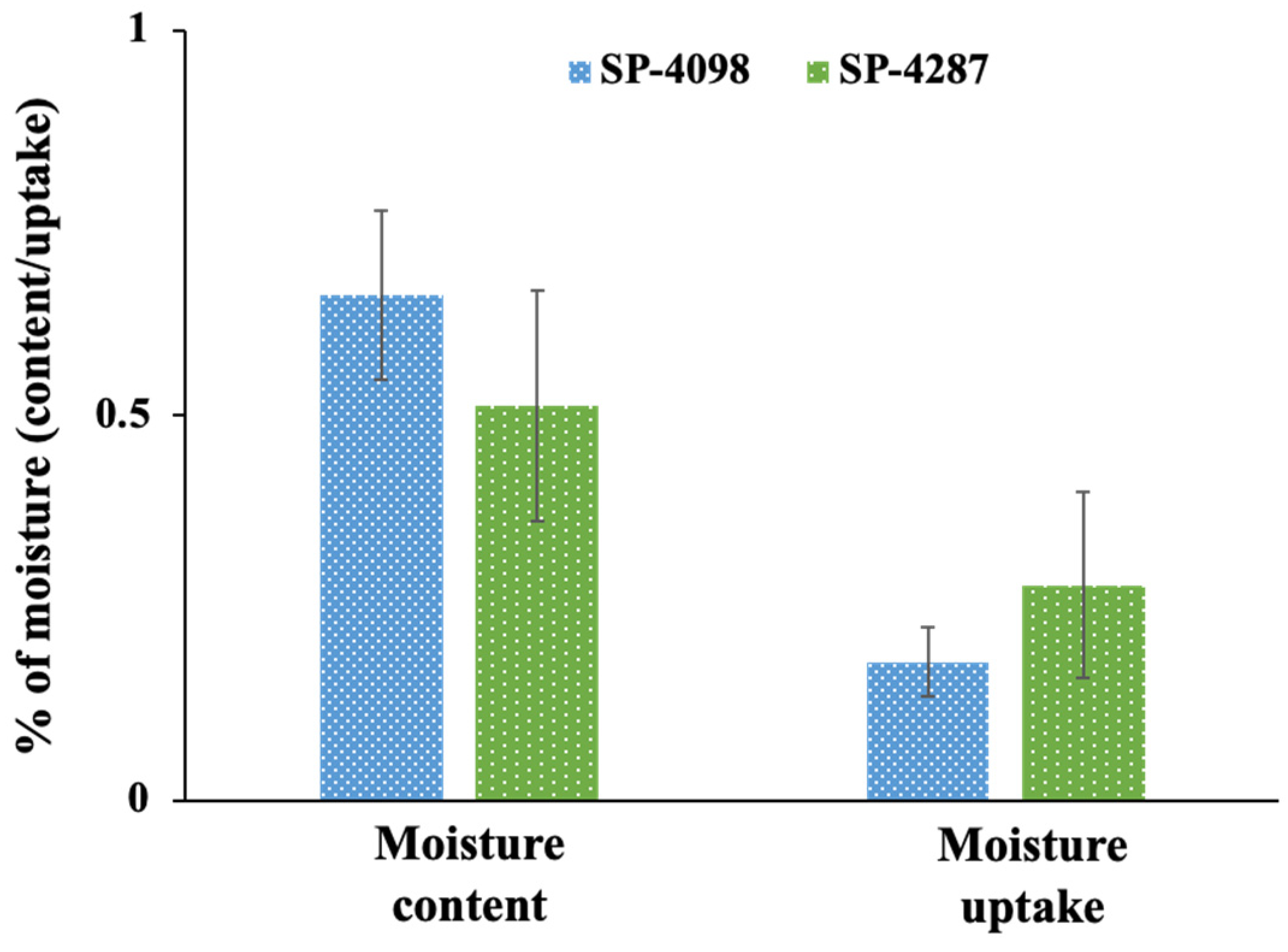
2.2. Characterization of the IL-S/O Patches
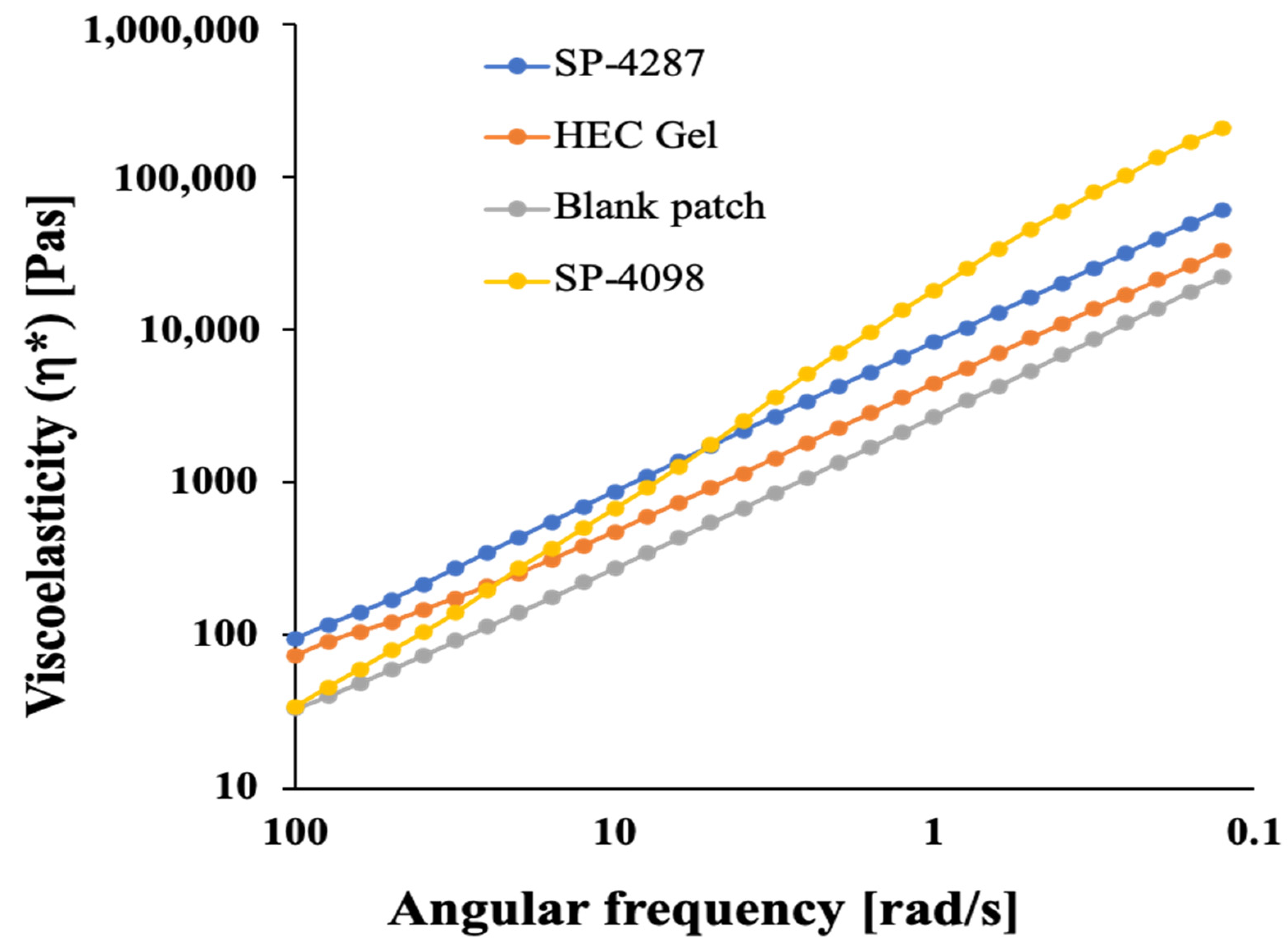
2.3. Rheological Properties of the IL-S/O Patches
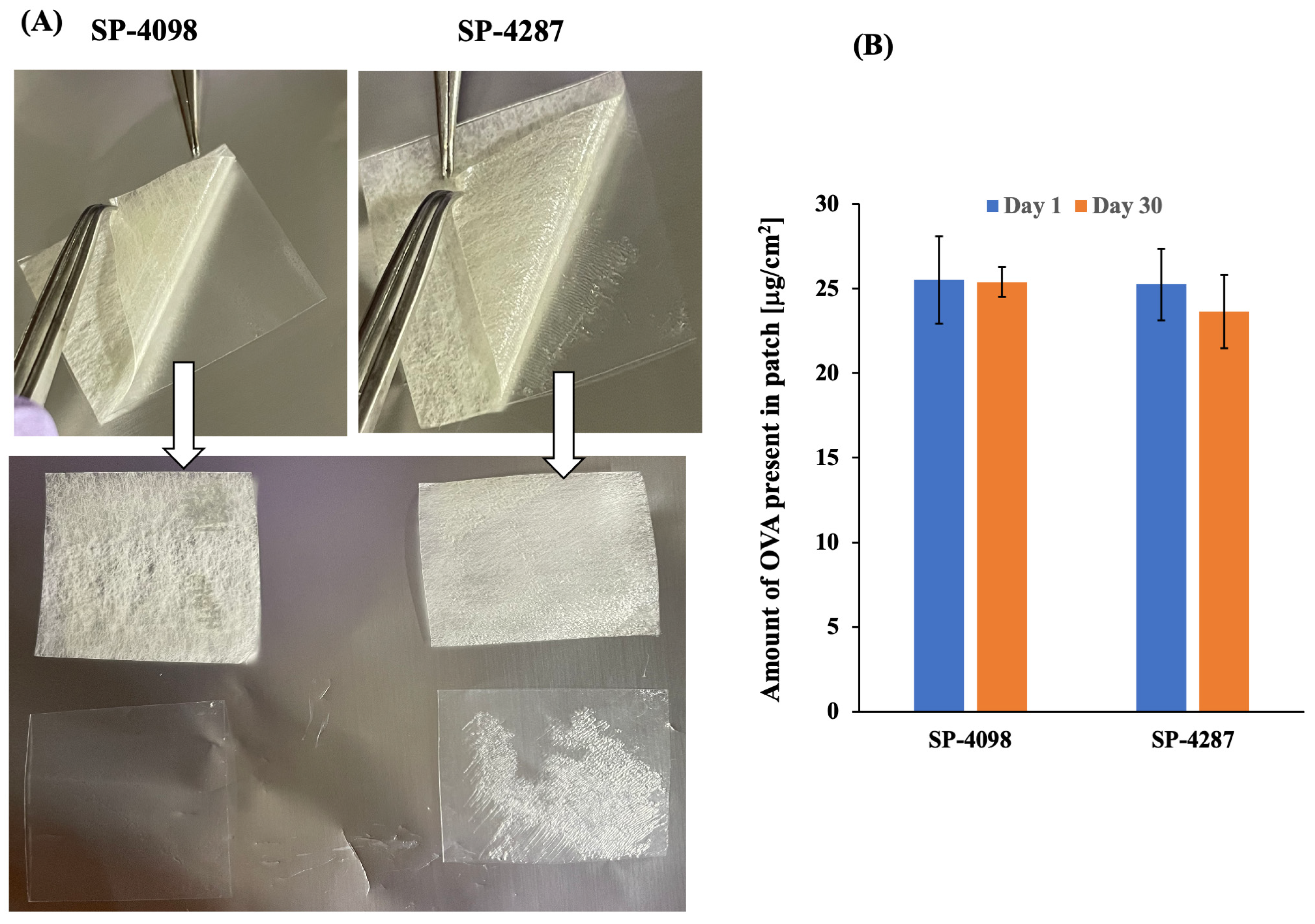
2.4. Stability of the IL-S/O Patches
2.5. In Vitro Skin Permeation Study
2.6. Effect of IL-S/O Patches on the SC Layer
2.7. In Vitro Skin Irritation Study
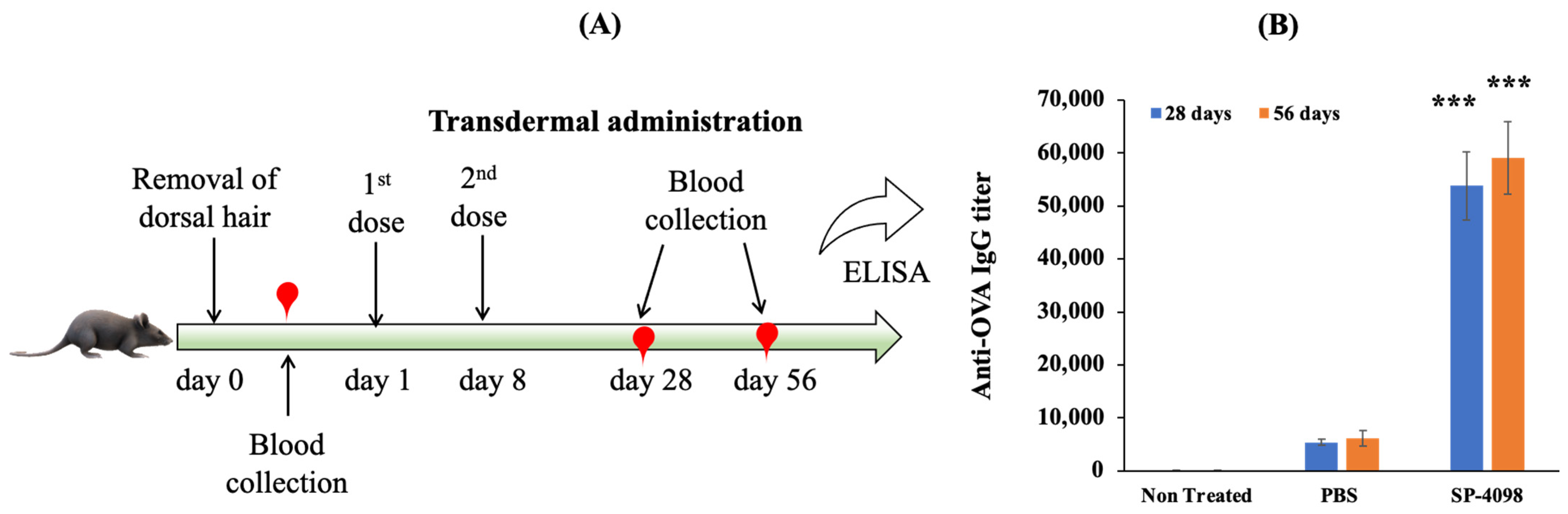
2.8. In Vivo Immunization Study
3. Materials and Methods
3.1. Materials
3.2. Methodology
3.2.1. Synthesis of IL
3.2.2. Preparation of IL-S/O Nanodispersion
3.2.3. Preparation of Transdermal IL-S/O Patches
3.2.4. Characterization of the IL-S/O Patches
3.2.5. Determination of Entrapped Drug in IL-S/O patches
3.2.6. Stability Study of the IL-S/O patches
3.2.7. In Vitro Skin Permeation Study
3.2.8. Effect of IL-S/O Patches on the SC Layer
3.2.9. In Vitro Skin Irritation Study
3.2.10. In Vivo Immunization and Antibody Measurement
3.2.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sáfadi, M.A.P. The Importance of Immunization as a Public Health Instrument. J. Pediatr. 2023, 99, S1–S3. [Google Scholar] [CrossRef]
- World Health Ortanization. Immunization Agenda 2030; WHO: Geneva, Switzerland, 2022; pp. 1–58. [Google Scholar]
- Caudill, C.; Perry, J.L.; Iliadis, K.; Tessema, A.T.; Lee, B.J.; Mecham, B.S.; Tian, S.; DeSimone, J.M. Transdermal Vaccination via 3D-Printed Microneedles Induces Potent Humoral and Cellular Immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2102595118. [Google Scholar] [CrossRef]
- Ita, K. Transdermal Delivery of Vaccines—Recent Progress and Critical Issues. Biomed. Pharmacother. 2016, 83, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wei, T.; Xu, Y.; Jin, Q.; Chao, Y.; Lu, J.; Xu, J.; Zhu, J.; Yan, X.; Chen, M.; et al. Non-Invasive Transdermal Delivery of Biomacromolecules with Fluorocarbon-Modified Chitosan for Melanoma Immunotherapy and Viral Vaccines. Nat. Commun. 2024, 15, 820. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Hirobe, S.; Okada, N.; Nakagawa, S. Frontiers of Transcutaneous Vaccination Systems: Novel Technologies and Devices for Vaccine Delivery. Vaccine 2013, 31, 2403–2415. [Google Scholar] [CrossRef]
- Li, N.; Peng, L.H.; Chen, X.; Nakagawa, S.; Gao, J.Q. Transcutaneous Vaccines: Novel Advances in Technology and Delivery for Overcoming the Barriers. Vaccine 2011, 29, 6179–6190. [Google Scholar] [CrossRef]
- Nichols, I.R.; Hamadani, C.M.; Chism, C.M.; Hunter, A.N.; Ahmad, H.; Wontor, K.; Williams, A.E.; Vashisth, P.; Hammer, N.I.; Kundu, S.; et al. The Impact of Water on Choline-2-Octenoic Ionic Liquid-Facilitated Transdermal Transport. Adv. Ther. 2023, 6, 1–14. [Google Scholar] [CrossRef]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent Insights into Cutaneous Immunization: How to Vaccinate via the Skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Alex, M.; Alsawaftah, N.M.; Husseini, G.A. State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Appl. Sci. 2024, 14, 2926. [Google Scholar] [CrossRef]
- Harding, C.R. The Stratum Corneum: Structure and Function in Health and Disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.E.L.; Curreri, A.M.; Balkaran, J.P.R.; Selig-Wober, N.C.; Yang, A.B.; Kendig, C.; Fluhr, M.P.; Kim, N.; Mitragotri, S. Design Principles of Ionic Liquids for Transdermal Drug Delivery. Adv. Mater. 2019, 31, 1901103. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Yang, C.; Yang, D. Ionic Liquids as the Effective Technology for Enhancing Transdermal Drug Delivery: Design Principles, Roles, Mechanisms, and Future Challenges. Asian J. Pharm. Sci. 2024, 19, 100900. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Microneedles for Transdermal Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Mitragotri, S.; Blankschtein, D.; Langer, R. Ultrasound-Mediated Transdermal Protein Delivery. Science (1979) 1995, 269, 850–853. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Mitragotri, S. Therapeutic RNAi Robed with Ionic Liquid Moieties as a Simple, Scalable Prodrug Platform for Treating Skin Disease. J. Control. Release 2016, 242, 80–88. [Google Scholar] [CrossRef]
- Toyofuku, K.; Wakabayashi, R.; Kamiya, N.; Goto, M. Non-Invasive Transdermal Delivery of Antisense Oligonucleotides with Biocompatible Ionic Liquids. ACS Appl. Mater. Interfaces 2023, 15, 33299–33308. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Islam, M.R.; Md Moshikur, R.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Modification with Conventional Surfactants to Improve a Lipid-Based Ionic-Liquid-Associated Transcutaneous Anticancer Vaccine. Molecules 2023, 28, 2969. [Google Scholar] [CrossRef]
- Mandal, A.; Kumbhojkar, N.; Reilly, C.; Dharamdasani, V.; Ukidve, A.; Ingber, D.E.; Mitragotri, S. Treatment of Psoriasis with NFKBIZ SiRNA Using Topical Ionic Liquid Formulations. Sci. Adv. 2020, 6, eabb6049. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Zhao, X.; Zhang, L.; Wang, W.; Bai, D.; Liao, Y.; Wang, Z.; Wang, M.; Zhang, J. Thermodynamically Stable Ionic Liquid Microemulsions Pioneer Pathways for Topical Delivery and Peptide Application. Bioact. Mater. 2024, 32, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.; Bakhtawar; Naz, S.; Uroos, M.; Ali, B.; Sharif, F. Pharmaceutical Applications of Lidocaine-Based Ionic Liquids—A Remarkable Innovation in Drug Delivery. J. Mol. Liq. 2024, 397, 124052. [Google Scholar] [CrossRef]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Biocompatible Ionic Liquid-Mediated Micelles for Enhanced Transdermal Delivery of Paclitaxel. ACS Appl. Mater. Interfaces 2021, 13, 19745–19755. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-in-Oil Microemulsions Prepared with Biocompatible Choline Carboxylic Acids for Improving the Transdermal Delivery of a Sparingly Soluble Drug. Pharmaceutics 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Ukidve, A.; Cu, K.; Goetz, M.; Angsantikul, P.; Curreri, A.; Tanner, E.E.L.; Lahann, J.; Mitragotri, S. Ionic-Liquid-Based Safe Adjuvants. Adv. Mater. 2020, 32, 2002990. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.V.; Freitas, R.; Oliva, M.; Cuccaro, A.; Monni, G.; Mezzetta, A.; Guazzelli, L.; Pretti, C. Toxicity of Ionic Liquids in Marine and Freshwater Microorganisms and Invertebrates: State of the Art. Environ. Sci. Pollut. Res. 2023, 30, 39288–39318. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Bo, Y.; Yi, M.; Wang, Z.; Zhang, J.; Zhu, Z.; Zhao, Y.; Zhang, J. Enhancing the Solubility and Transdermal Delivery of Drugs Using Ionic Liquid-In-Oil Microemulsions. Adv. Funct. Mater. 2021, 31, 2102794. [Google Scholar] [CrossRef]
- Uddin, S.; Islam, M.R.; Md Moshikur, R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Transdermal Delivery of Antigenic Protein Using Ionic Liquid-Based Nanocarriers for Tumor Immunotherapy. ACS Appl. Bio Mater. 2022, 5, 2586–2597. [Google Scholar] [CrossRef]
- Nabila, F.H.; Islam, R.; Shimul, I.M.; Moniruzzaman, M.; Wakabayashi, R.; Kamiya, N.; Goto, M. Ionic Liquid-Mediated Ethosome for Transdermal Delivery of Insulin. Chem. Commun. 2024, 60, 4036–4039. [Google Scholar] [CrossRef]
- Hiranphinyophat, S.; Otaka, A.; Asaumi, Y.; Fujii, S.; Iwasaki, Y. Particle-Stabilized Oil-in-Water Emulsions as a Platform for Topical Lipophilic Drug Delivery. Colloids Surf. B Biointerfaces 2021, 197, 111423. [Google Scholar] [CrossRef]
- Islam, R.; Habib Nabila, F.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-Based Patch Formulation for Enhanced Transdermal Delivery of Sparingly Soluble Drug. J. Mol. Liq. 2024, 397, 124184. [Google Scholar] [CrossRef]
- Miura, R.; Tahara, Y.; Sawada, S.; Sasaki, Y.; Akiyoshi, K. Structural Effects and Lymphocyte Activation Properties of Self-Assembled Polysaccharide Nanogels for Effective Antigen Delivery. Sci. Rep. 2018, 8, 2–10. [Google Scholar] [CrossRef]
- Uddin, S.; Chowdhury, M.R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Lipid Based Biocompatible Ionic Liquids: Synthesis, Characterization and Biocompatibility Evaluation. Chem. Commun. 2020, 56, 13756–13759. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Gennari, C.G.M.; Minghetti, P. Adhesive Properties: A Critical Issue in Transdermal Patch Development. Expert Opin. Drug Deliv. 2012, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Pichayakorn, W.; Boontawee, H.; Taweepreda, W.; Suksaeree, J.; Boonme, P. Physicochemical and Drug Release Characterization of Lidocaine-Loaded Transdermal Patches Prepared from STR-5L Block Rubber. Ind. Eng. Chem. Res. 2014, 53, 1672–1677. [Google Scholar] [CrossRef]
- Mikolaszek, B.; Jamrógiewicz, M.; Mojsiewicz-Pieńkowska, K.; Sznitowska, M. Microscopic and Spectroscopic Imaging and Thermal Analysis of Acrylates, Silicones and Active Pharmaceutical Ingredients in Adhesive Transdermal Patches. Polymers 2022, 14, 2888. [Google Scholar] [CrossRef]
- Muñoz, M.D.; Castán, H.; Ruiz, M.A.; Morales, M.E. Design, Development and Characterization of Transdermal Patch of Methadone. J. Drug Deliv. Sci. Technol. 2017, 42, 255–260. [Google Scholar] [CrossRef]
- Liu, C.; Hui, M.; Quan, P.; Fang, L. Drug in Adhesive Patch of Palonosetron: Effect of Pressure Sensitive Adhesive on Drug Skin Permeation and in Vitro-in Vivo Correlation. Int. J. Pharm. 2016, 511, 1088–1097. [Google Scholar] [CrossRef]
- Sagare, A.; Yadav, R.; Gautam, V.; Yadav, S.; Malviya, J.; Sawale, J. Development, Characterization, and In-Vitro Evaluation of Niosomal Transdermal Patch of Lamotrigine Drug. Macromol. Symp. 2024, 413, 2300147. [Google Scholar] [CrossRef]
- Kong, B.J.; Kim, A.; Park, S.N. Properties and in Vitro Drug Release of Hyaluronic Acid-Hydroxyethyl Cellulose Hydrogels for Transdermal Delivery of Isoliquiritigenin. Carbohydr. Polym. 2016, 147, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Junqueira Garcia, M.T.; Pedralino Gonçalves, T.; São Félix Martins, É.; Silva Martins, T.; Carvalho de Abreu Fantini, M.; Regazi Minarini, P.R.; Costa Fernandez, S.; Cassone Salata, G.; Biagini Lopes, L. Improvement of Cutaneous Delivery of Methylene Blue by Liquid Crystals. Int. J. Pharm. 2018, 548, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Philippi, F.; Rauber, D.; Eliasen, K.L.; Bouscharain, N.; Niss, K.; Kay, C.W.M.; Welton, T. Pressing Matter: Why Are Ionic Liquids so Viscous? Chem. Sci. 2022, 13, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, S.; Nurumbetov, G.; Ross, A.; Li, Y.; Haddleton, D.M. Moisture-Cured Solvent Free Silylated Poly(Ether-Urea) Pressure-Sensitive Adhesives (PSAs) for Use as Skin Adhesives for Application in Transdermal Drug Delivery (TDD). Mater. Adv. 2024, 5, 3396–3410. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Liu, Y.; Chen, G.; Zhao, N.; Liu, C.; Li, X.; Wang, M.; Song, J.; Luo, Z. A Semi-Interpenetrating Network Acrylic Pressure-Sensitive Adhesive for Efficient Transdermal Application with High Cohesion and Adhesion. Mater. Des. 2024, 241, 112970. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Song, Y.; Ruan, J.; Quan, P.; Fang, L. High Drug-Loading and Controlled-Release Hydroxyphenyl-Polyacrylate Adhesive for Transdermal Patch. J. Control. Release 2023, 353, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The Role of Viscosity on Skin Penetration from Cellulose Ether-Based Hydrogels. Skin. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Transdermal Drug Delivery Approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Lu, H.; Meng, R.; Feng, C.; Jia, H.; Yang, H.F.; Wang, F. Effect of Penetration Enhancer on the Structure of Stratum Corneum: On-Site Study by Confocal Polarized Raman Imaging. Mol. Pharm. 2024, 21, 1300–1308. [Google Scholar] [CrossRef]
- Yang, C.; Peng, X.; Shi, Y.; Zhang, Y.; Feng, M.F.; Tian, Y.; Zhang, J.; Cen, S.; Li, Z.; Dai, X.; et al. Umbilical Therapy for Promoting Transdermal Delivery of Topical Formulations: Enhanced Effect and Underlying Mechanism. Int. J. Pharm. 2024, 652, 123834. [Google Scholar] [CrossRef]
- Fernandes, E.; López-Sicilia, I.; Martín-Romero, M.T.; Giner-Casares, J.; Lúcio, M. A Stratum Corneum Lipid Model as a Platform for Biophysical Profiling of Bioactive Chemical Interactions at the Skin Level. J. Mol. Liq. 2024, 400, 124513. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal Patches: History, Development and Pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S.; Al-Dhubiab, B.E.; Alhumam, R.N. Influence of Skin Permeation Enhancers on the Transdermal Delivery of Palonosetron: An in Vitro Evaluation. J. Appl. Biomed. 2018, 16, 192–197. [Google Scholar] [CrossRef]
- Tahara, Y.; Namatsu, K.; Kamiya, N.; Hagimori, M.; Kamiya, S.; Arakawa, M.; Goto, M. Transcutaneous Immunization by a Solid-in-Oil Nanodispersion. Chem. Commun. 2010, 46, 9200–9202. [Google Scholar] [CrossRef] [PubMed]
- Kdimati, S.; Mullins, C.S.; Linnebacher, M. Cancer-Cell-Derived Igg and Its Potential Role in Tumor Development. Int. J. Mol. Sci. 2021, 22, 11597. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Huang, J.; Zhang, S.; Liu, Q.; Liao, Q.; Qiu, X. Immunoglobulin Expression in Cancer Cells and Its Critical Roles in Tumorigenesis. Front. Immunol. 2021, 12, 613530. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Zhang, J.; Huang, T.; Sun, Y.; Chen, Z.; Yi, W.; Korteweg, C.; Wang, J.; Gu, J. IgG Expression in Human Colorectal Cancer and Its Relationship to Cancer Cell Behaviors. PLoS ONE 2012, 7, e47362. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.T.T.; Phung, C.D.; Lim, C.M.H.; Jayasinghe, M.K.; Ang, J.; Tran, T.; Schwarz, H.; Le, M.T.N. Dendritic Cell-Targeted Delivery of Antigens Using Extracellular Vesicles for Anti-Cancer Immunotherapy. Cell Prolif. 2024, e13622. [Google Scholar] [CrossRef] [PubMed]
- Gadag, S.; Narayan, R.; Nayak, Y.; Garg, S.; Nayak, U.Y. Design, Development and Evaluation of Resveratrol Transdermal Patches for Breast Cancer Therapy. Int. J. Pharm. 2023, 632, 122558. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with in Vivo-in Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Vovesná, A.; Zhigunov, A.; Balouch, M.; Zbytovská, J. Ceramide Liposomes for Skin Barrier Recovery: A Novel Formulation Based on Natural Skin Lipids. Int. J. Pharm. 2021, 596, 120264. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.M.; Mitragotri, S. Mechanistic Study of Transdermal Delivery of Macromolecules Assisted by Ionic Liquids. J. Control. Release 2019, 311–312, 162–169. [Google Scholar] [CrossRef] [PubMed]



| Properties of PSAs | Patch Properties | ||||||
|---|---|---|---|---|---|---|---|
| Name of PSA | Polymer Name | Vinyl Acetate | Functional Group | Crosslinker | IL-S/O: PSA | Patch Name | Comment |
| DURO-TAK 87-4098 | Acrylate copolymers | Yes | None | N/A | 1:1 | SP-4098 | * Suitable |
| DURO-TAK 87-4287 | Acrylate copolymers | Yes | -OH | No | 1:1 | SP-4287 | * Suitable |
| DURO-TAK 87-235A | Acrylate copolymers | No | -COOH | No | 1:1 | SP-235A | ** Not suitable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, R.; Nabila, F.H.; Wakabayashi, R.; Kawaguchi, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens. Molecules 2024, 29, 2995. https://doi.org/10.3390/molecules29132995
Islam R, Nabila FH, Wakabayashi R, Kawaguchi Y, Kamiya N, Moniruzzaman M, Goto M. Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens. Molecules. 2024; 29(13):2995. https://doi.org/10.3390/molecules29132995
Chicago/Turabian StyleIslam, Rashedul, Fahmida Habib Nabila, Rie Wakabayashi, Yoshirou Kawaguchi, Noriho Kamiya, Muhammad Moniruzzaman, and Masahiro Goto. 2024. "Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens" Molecules 29, no. 13: 2995. https://doi.org/10.3390/molecules29132995
APA StyleIslam, R., Nabila, F. H., Wakabayashi, R., Kawaguchi, Y., Kamiya, N., Moniruzzaman, M., & Goto, M. (2024). Ionic Liquid-Based Immunization Patch for the Transdermal Delivery of Antigens. Molecules, 29(13), 2995. https://doi.org/10.3390/molecules29132995





