The Chromenopyridine Scaffold: A Privileged Platform in Drug Design
Abstract
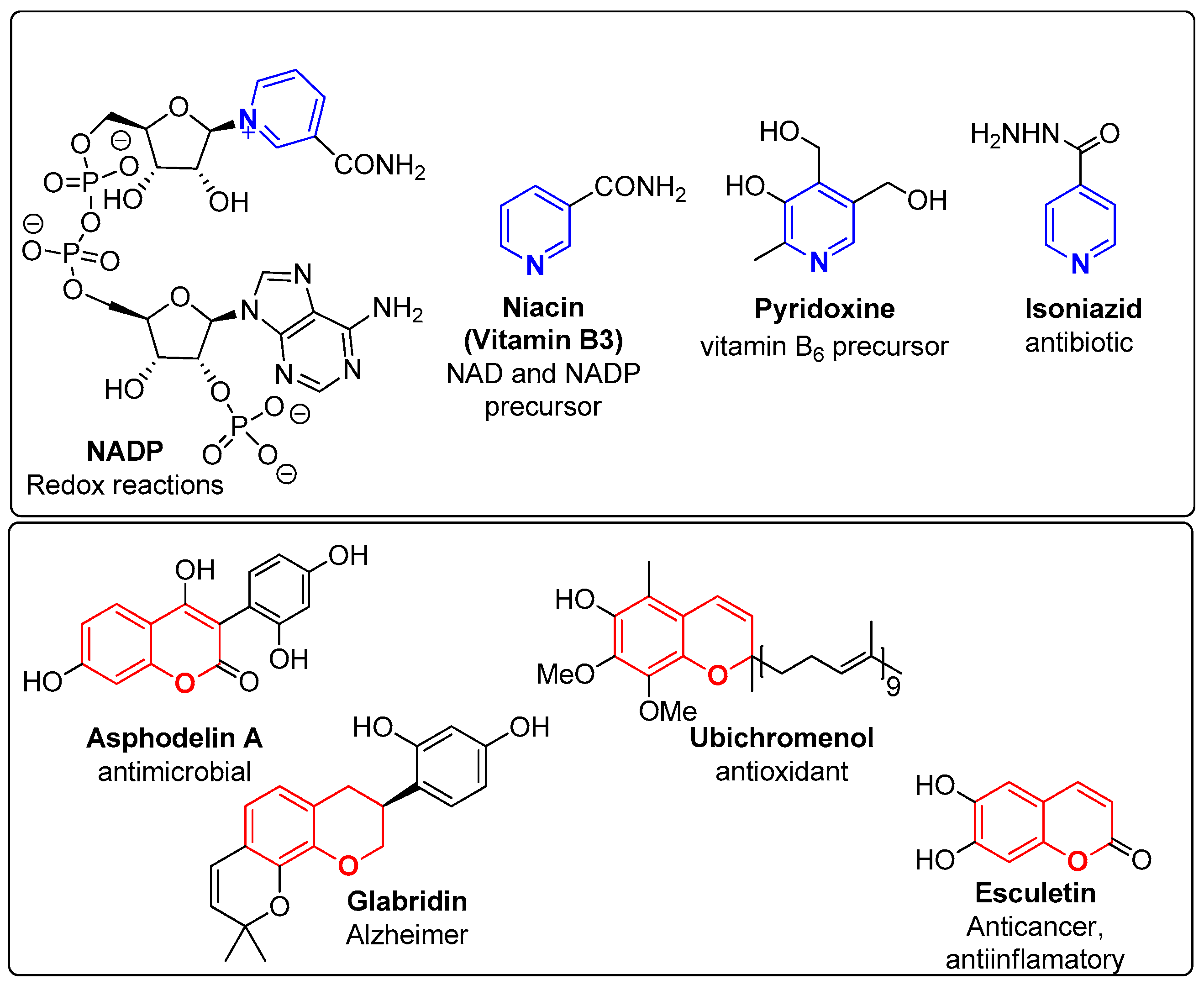
1. Introduction
2. Synthesis and Biological Activity of Chromenopyridines
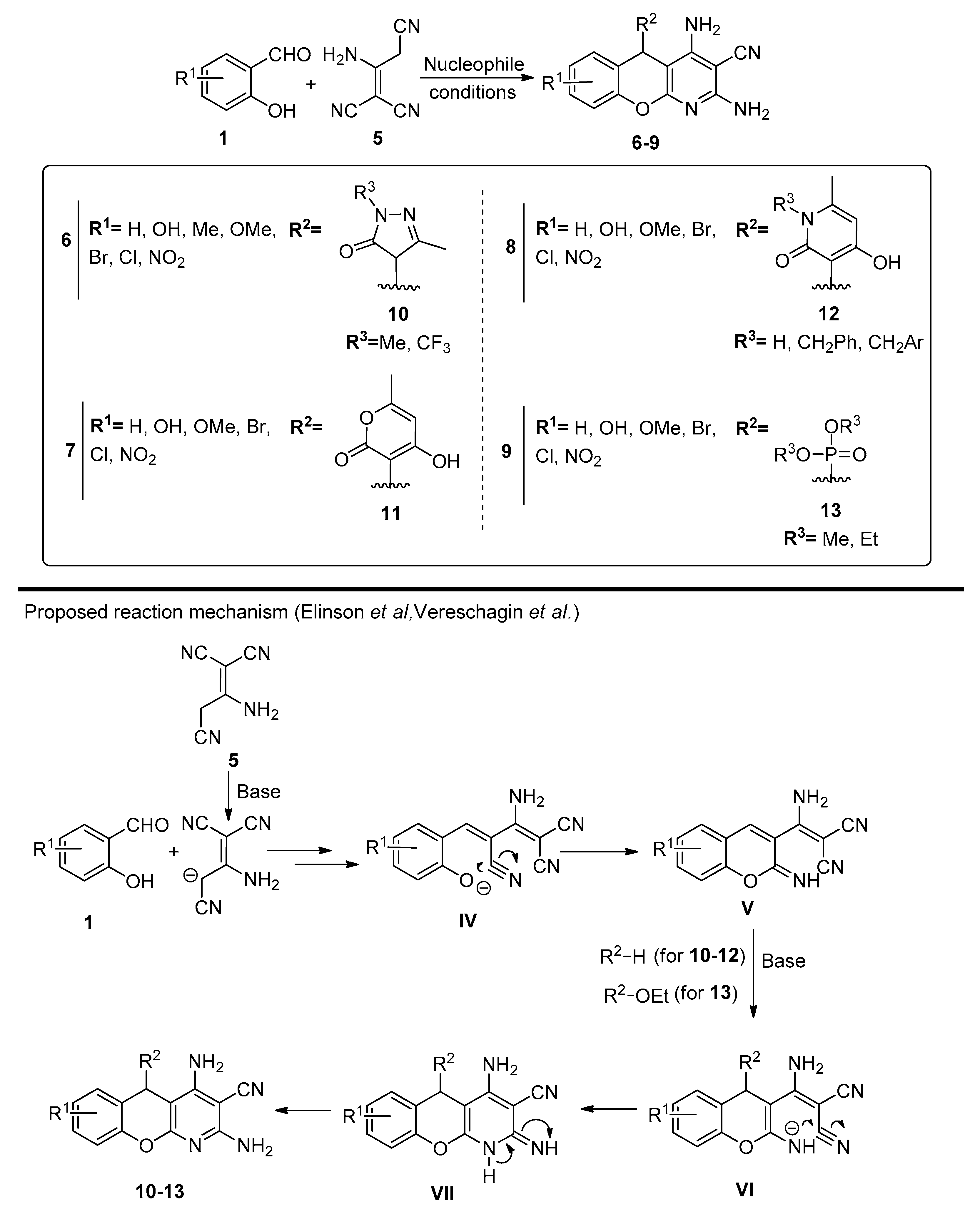
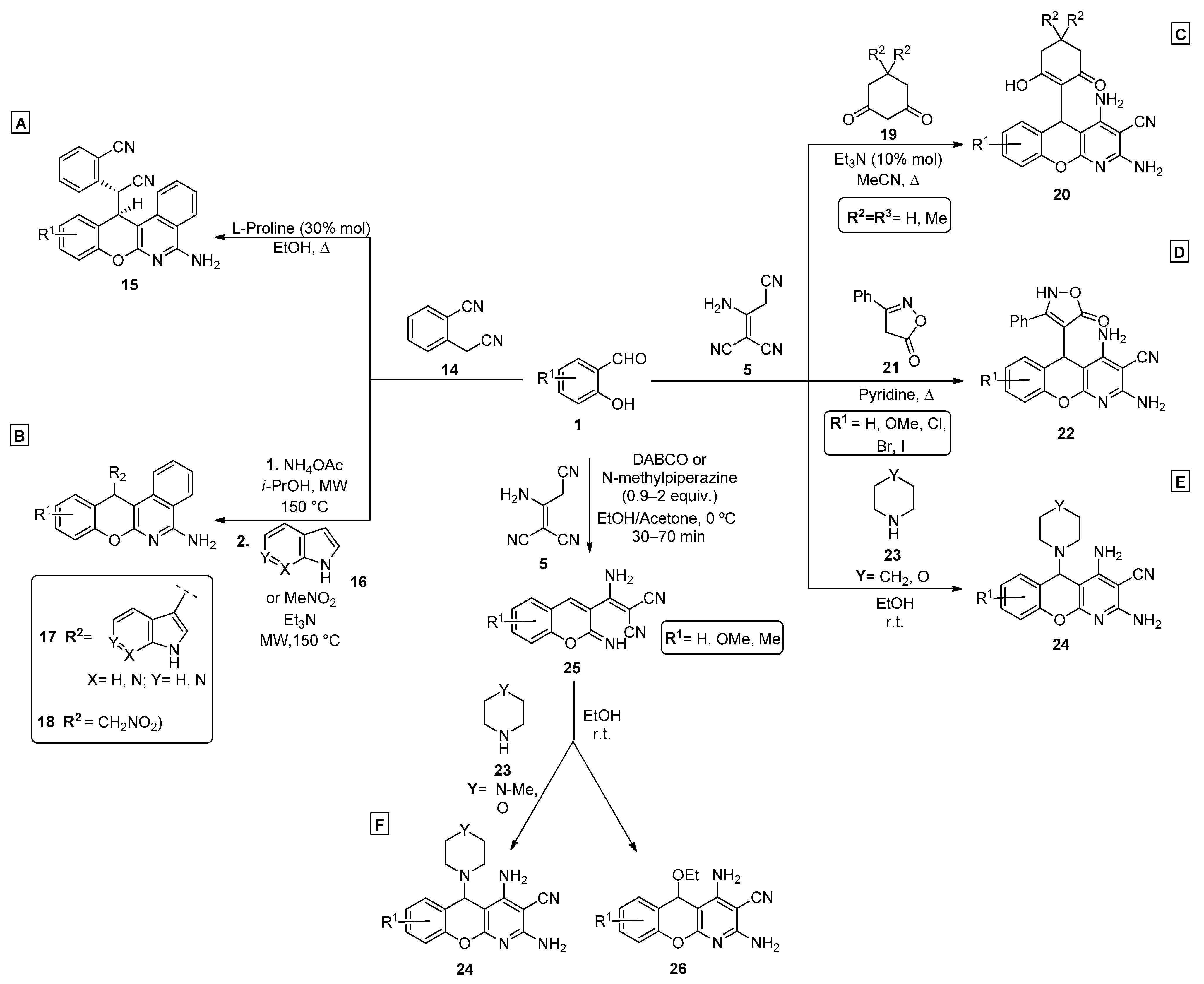
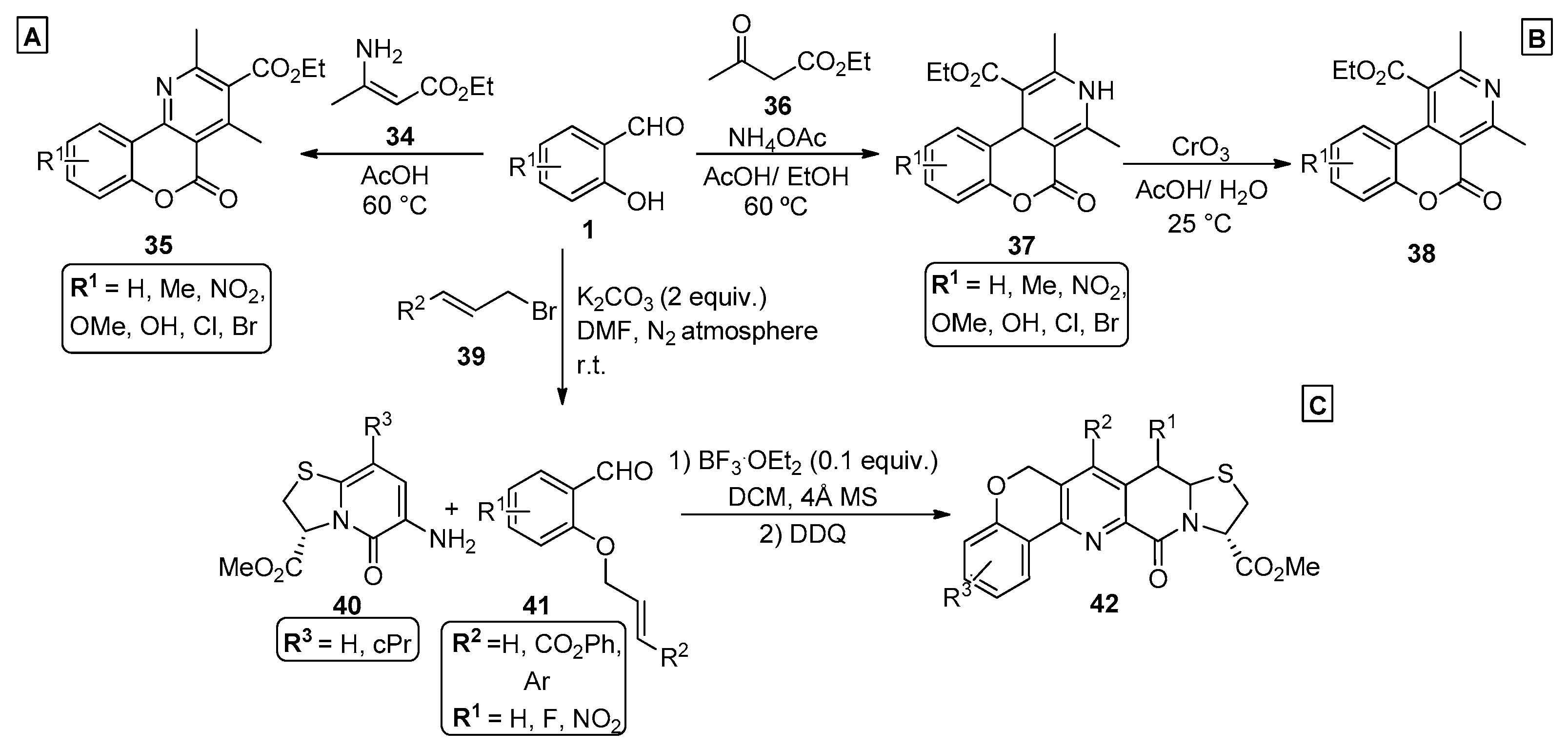
2.1. Salicylaldehydes
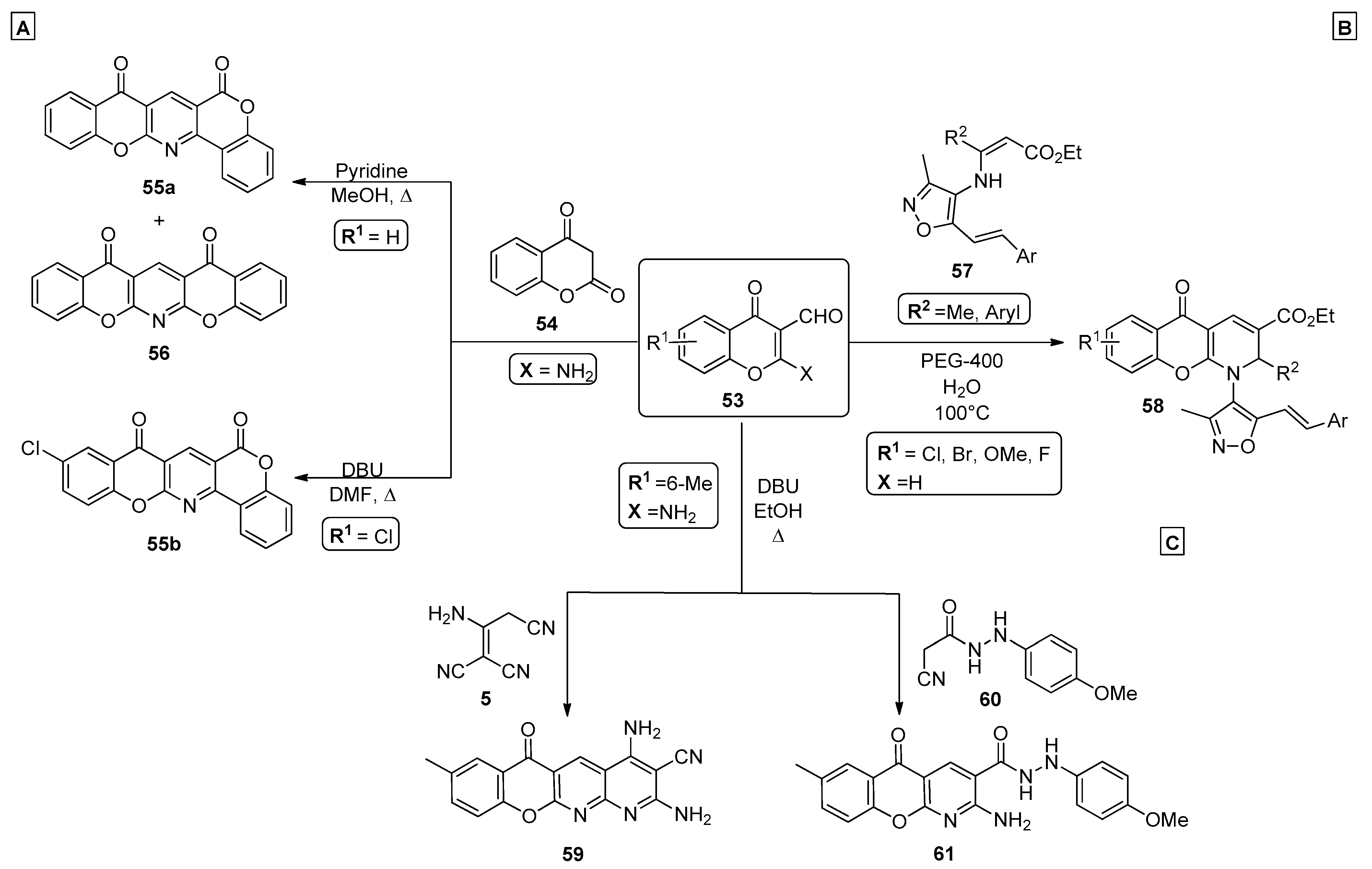
2.2. Chromones
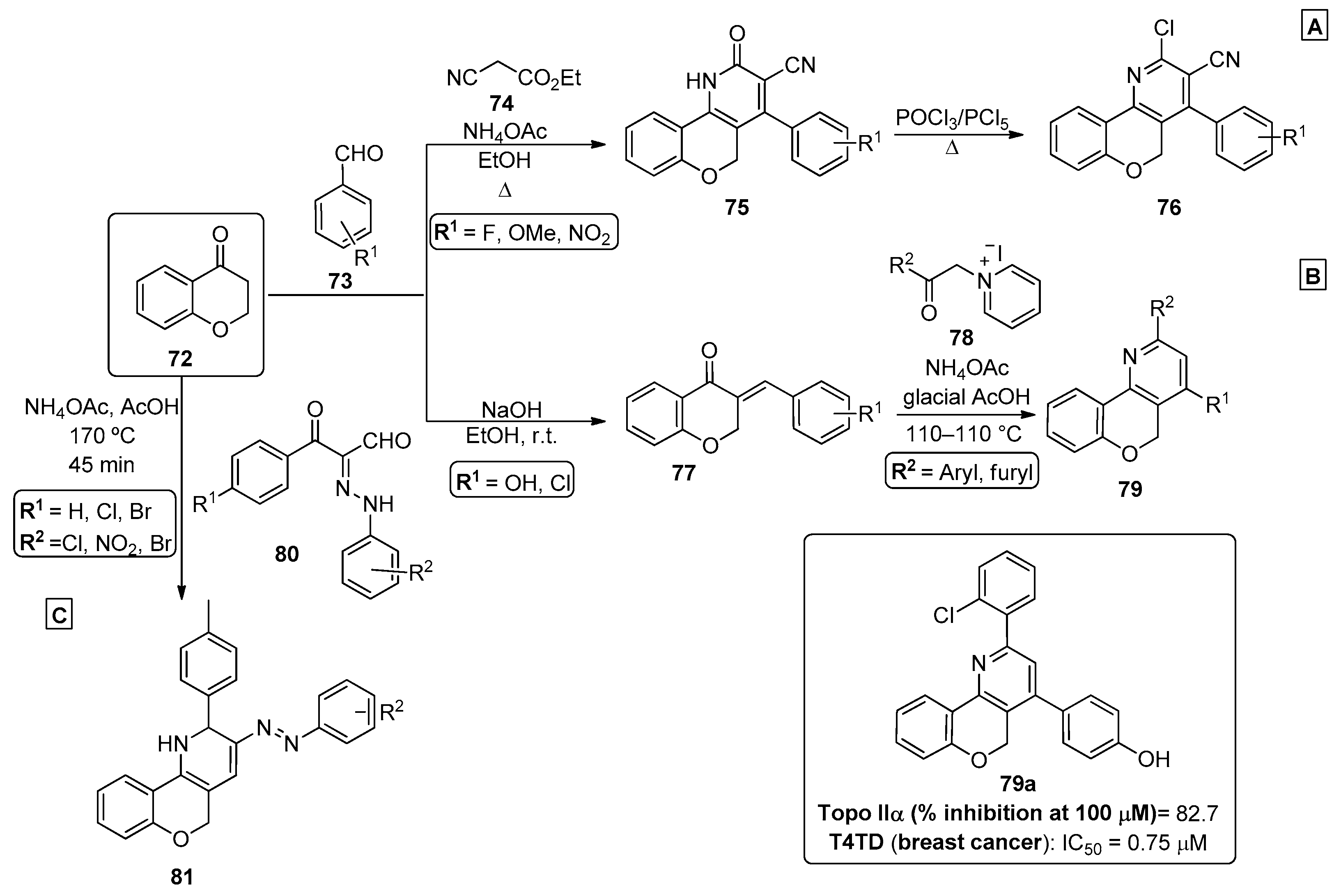
2.3. Chromanones
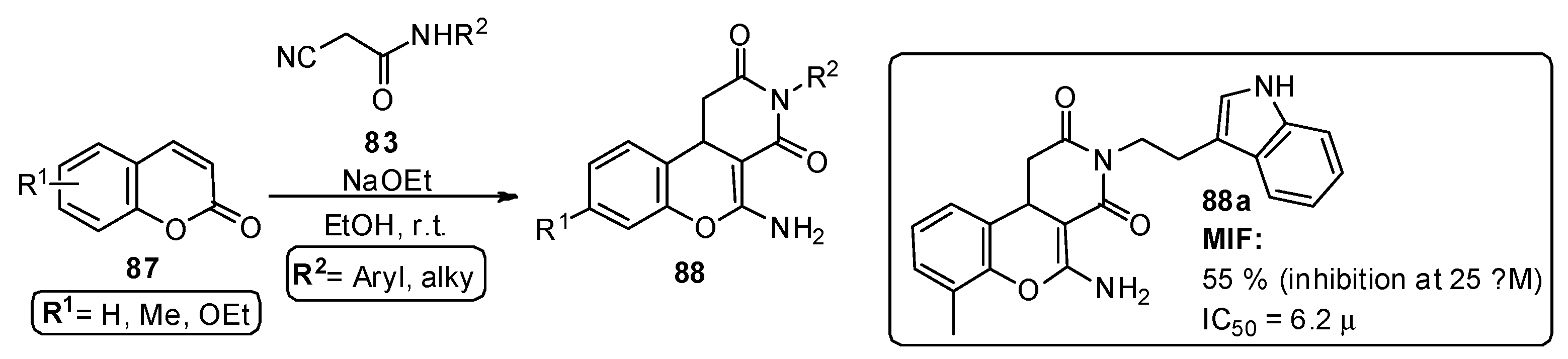
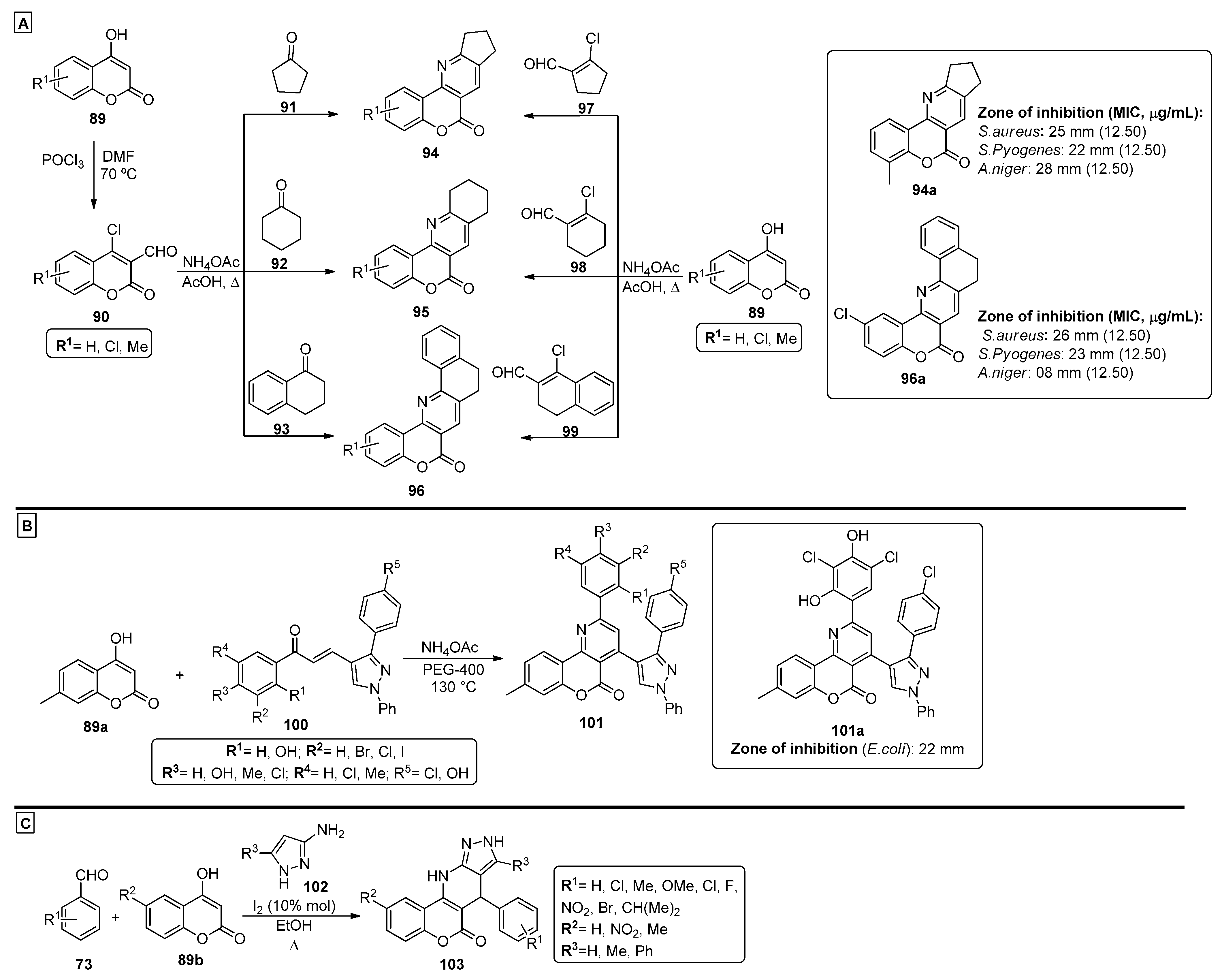
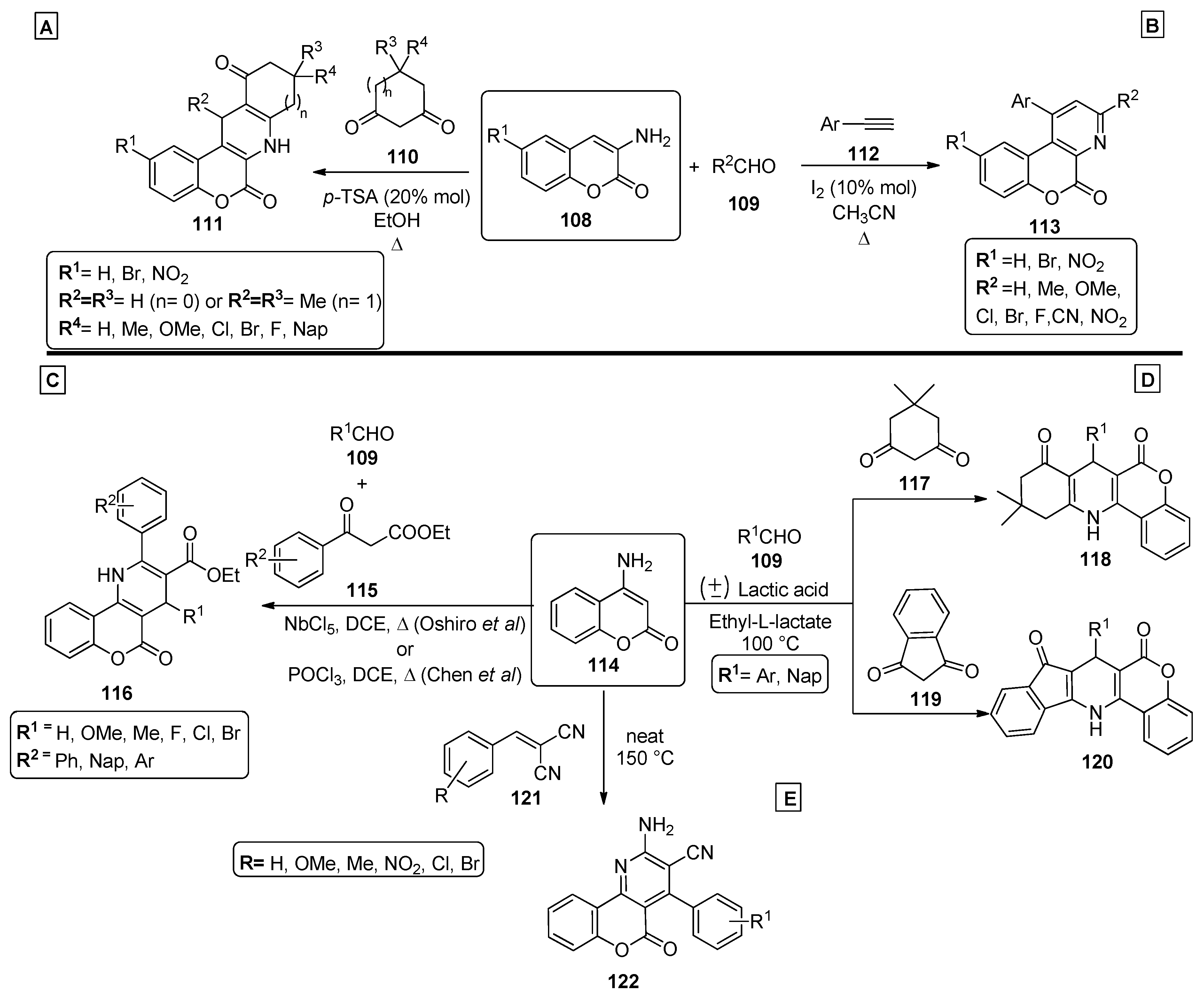
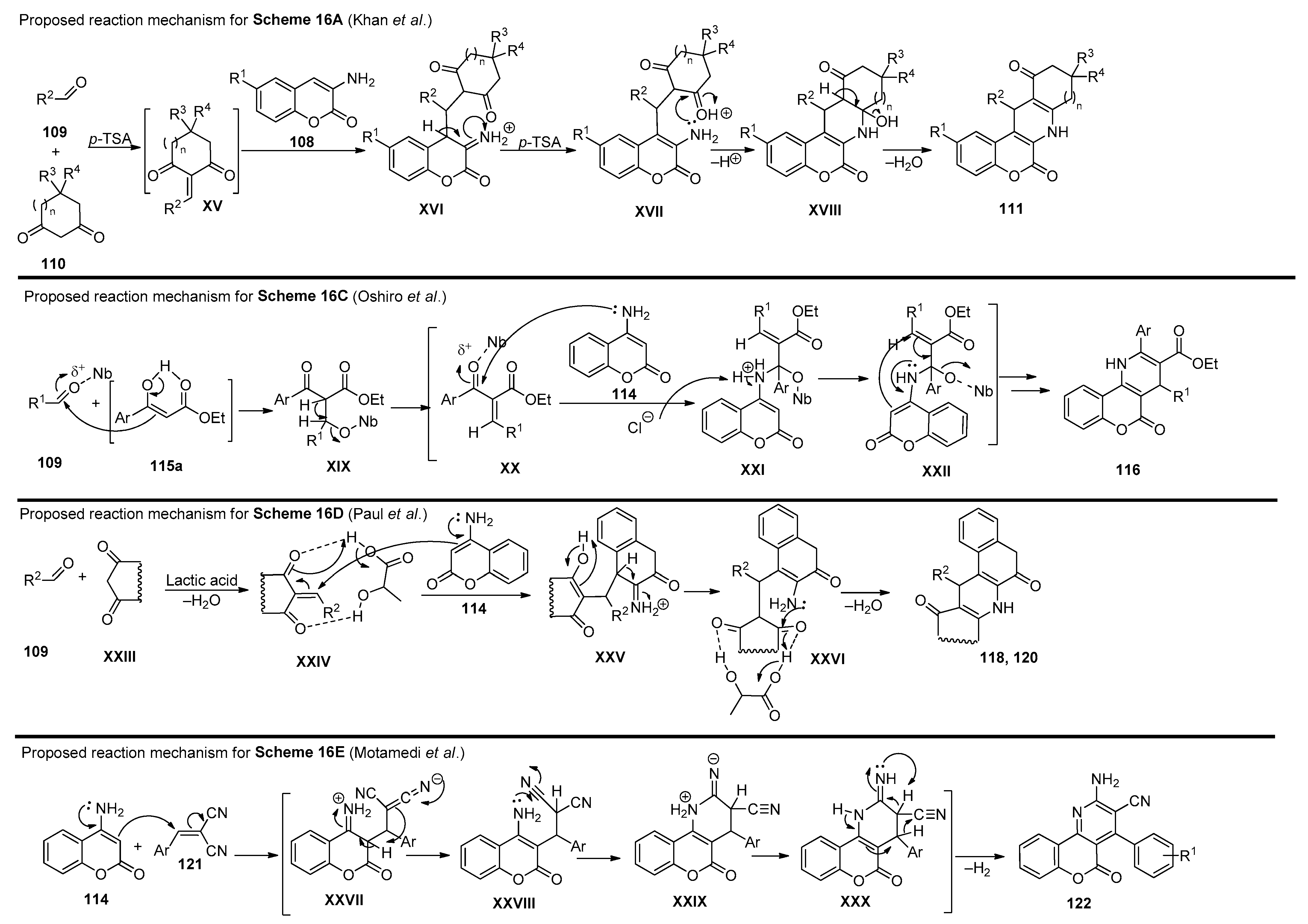
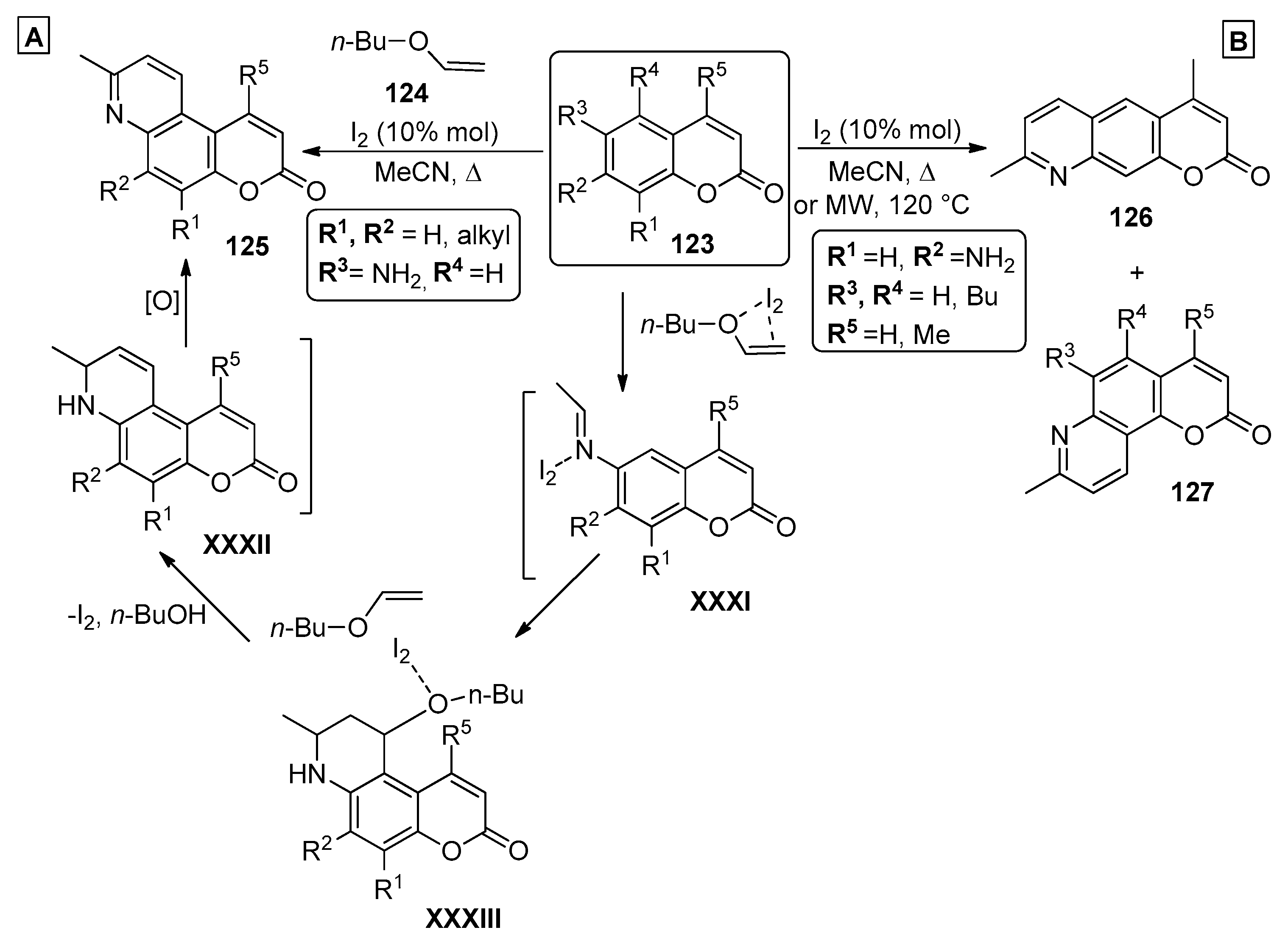
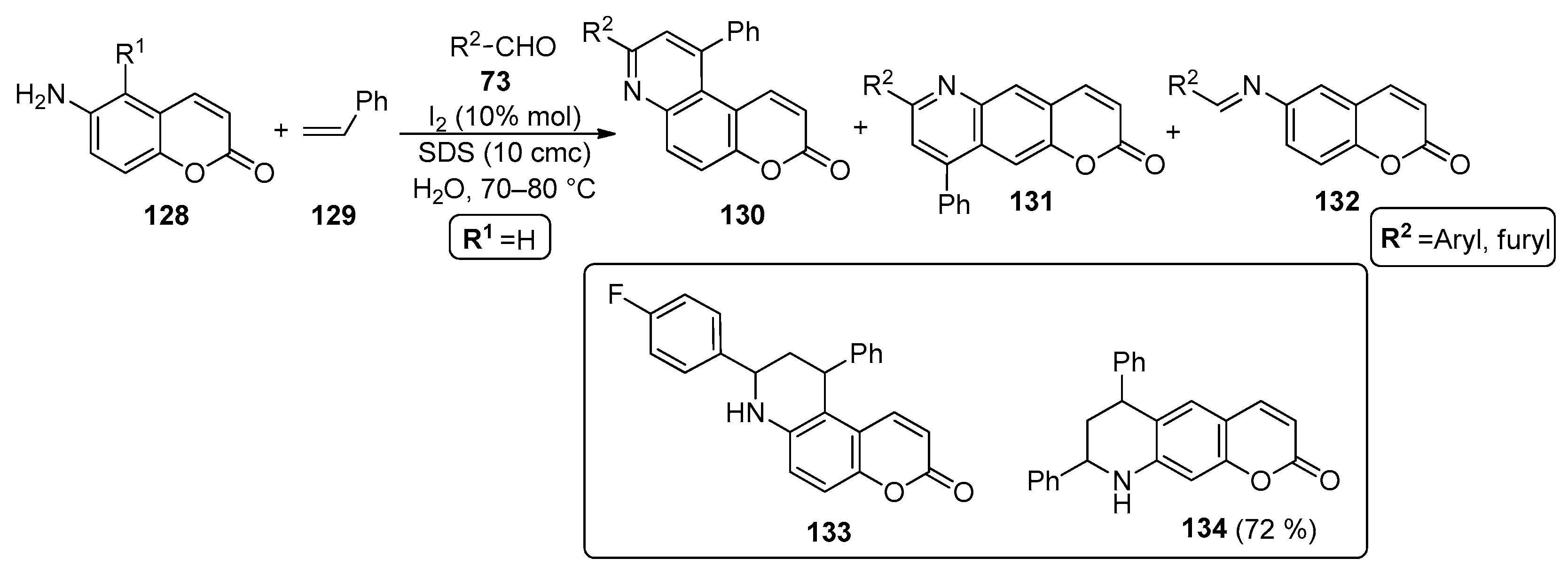
2.4. Coumarins
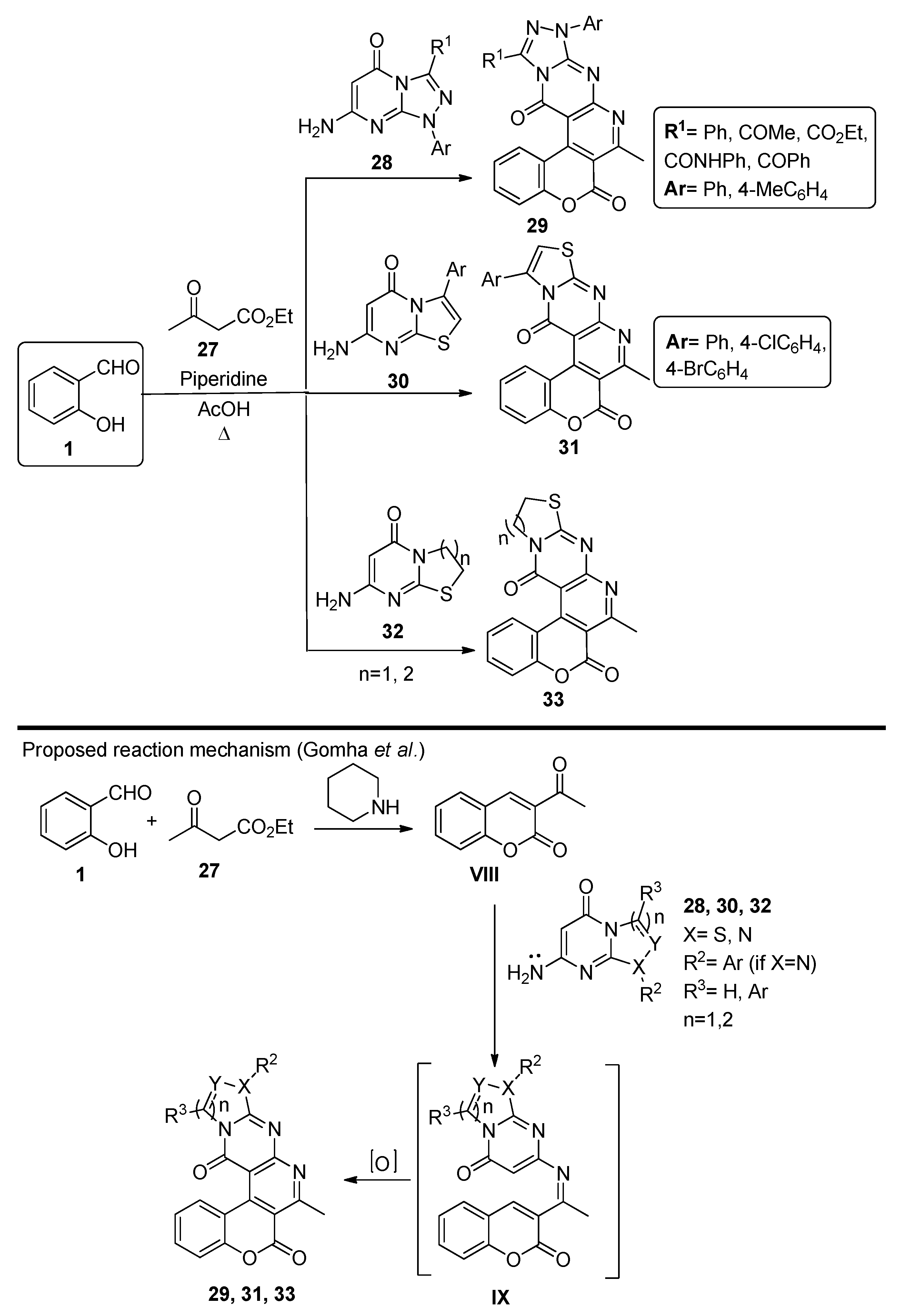
2.5. Miscellaneous
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and Drug Discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Park, S.B. Privileged Structures: Efficient Chemical “Navigators” toward Unexplored Biologically Relevant Chemical Spaces. J. Am. Chem. Soc. 2014, 136, 14629–14638. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Roles of Pyridine and Pyrimidine Derivatives as Privileged Scaffolds in Anticancer Agents. Mini-Rev. Med. Chem. 2017, 17, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagoniststs. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, C.; Sitkoff, D.; Cheadle, N.L.; Xu, S.; Muckelbauer, J.K.; Adam, L.P.; Wexler, R.R.; Quan, M.L. Identification of 5H-chromeno [3,4-c]pyridine and 6H-isochromeno[3,4-c]pyridine derivatives as potent and selective dual ROCK inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127474. [Google Scholar] [CrossRef] [PubMed]
- Marson, C.M. Targeting the histamine H4 receptor. Chem. Rev. 2011, 111, 7121–7156. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Tabrizi, M.A.; Gessi, S.; Borea, P.A. Adenosine receptor antagonists: Translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev. 2008, 108, 238–263. [Google Scholar] [CrossRef]
- Martinez-Gualda, B.; Pu, S.Y.; Froeyen, M.; Herdewijn, P.; Einav, S.; De Jonghe, S. Structure-activity relationship study of the pyridine moiety of isothiazolo[4,3-b]pyridines as antiviral agents Targeting cyclin G-associated kinase. Bioorg. Med. Chem. 2020, 28, 115188. [Google Scholar] [CrossRef]
- Barreiro, E.J. Chapter 1: Privileged Scaffolds in Medicinal Chemistry: An Introduction. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; The Royal Society of Chemistry: London, UK, 2016; pp. 1–15. ISBN 978-1-78262-030-3. [Google Scholar]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. One-Step Synthesis of Heterocyclic Privileged Medicinal Scaffolds by a Multicomponent Reaction of Malononitrile with Aldehydes and Thiols. J. Org. Chem. 2007, 72, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.D. De Novo Synthesis of Substituted Pyridines. Tetrahedron 2004, 60, 6043–6061. [Google Scholar] [CrossRef]
- Edwards, J.P.; Kindrachuk, D.E.; Venable, J.D. Benzo-Imidazolyl Pyridines as Modulators of the Histamine H4 Receptor. Hong. Kong Patent HK1124767A1, 24 July 2009. [Google Scholar]
- Radwan, M.A.A.; Alshubramy, M.A.; Abdel-Motaal, M.; Hemdan, B.A.; El-Kady, D.S. Synthesis, molecular docking and antimicrobial activity of new fused pyrimidine and pyridine derivatives. Bioorg. Chem. 2020, 96, 103516. [Google Scholar] [CrossRef]
- Jian, X.E.; Yang, F.; Jiang, C.S.; You, W.W.; Zhao, P.L. Synthesis and biological evaluation of novel pyrazolo[3,4-b]pyridines as cis-restricted combretastatin A-4 analogues. Bioorg. Med. Chem. Lett. 2020, 30, 127025. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Pontes, O.; Costa, M.; Santos, F.; Sampaio-Marques, B.; Dias, T.; Ludovico, P.; Proença, F.; Baltazar, F. Exploitation of new chalcones and 4H-chromenes as agents for cancer treatment. Eur. J. Med. Chem. 2018, 157, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Azm, F.S.M.; El-Shahawi, M.M.; Elgubbi, A.S.; Madkour, H.M.F. Design, synthesis, anti-proliferative activity, and molecular docking studies of novel benzo[f]chromene, chromeno[2,3-d]pyrimidines and chromenotriazolo[1,5-c]pyrimidines. Synth. Commun. 2020, 50, 669–683. [Google Scholar] [CrossRef]
- Alblewi, F.F.; Okasha, R.M.; Hritani, Z.M.; Mohamed, H.M.; El-Nassag, M.A.A.; Halawa, A.H.; Mora, A.; Fouda, A.M.; Assiri, M.A.; Al-Dies, A.A.M.; et al. Antiproliferative effect, cell cycle arrest and Aaoptosis generation of novel synthesized anticancer heterocyclic derivatives based 4H-benzo[h]chromene. Bioorg. Chem. 2019, 87, 560–571. [Google Scholar] [CrossRef]
- Halawa, A.H.; Elaasser, M.M.; El Kerdawy, A.M.; Abd El-Hady, A.M.A.I.; Emam, H.A.; El-Agrody, A.M. Anticancer activities, molecular docking and structure–activity relationship of novel synthesized 4H-chromene, and 5H-chromeno[2,3-d]pyrimidine candidates. Med. Chem. Res. 2017, 26, 2624–2638. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Haiba, M.E.; Al-Abdullah, E.S.; Ahmed, N.S.; Ghabbour, H.A.; Awad, H.M. Efficient and easy synthesis of new Benzo[h]chromene and Benzo[h]quinoline derivatives as a new class of cytotoxic agents. J. Mol. Struct. 2019, 1195, 702–711. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Albarrán-Velo, J.; Light, M.E.; Padrón, J.M.; Román, E.; Serrano, J.A.; Gil, M.V. Synthesis and antiproliferative activity of new 2-glyco-3-nitro-2H-chromenes. Bioorg. Chem. 2019, 87, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Okasha, R.M.; Alsehli, M.; Ihmaid, S.; Althagfan, S.S.; El-Gaby, M.S.A.; Ahmed, H.E.A.; Afifi, T.H. First example of Azo-Sulfa conjugated chromene moieties: Synthesis, characterization, antimicrobial assessment, docking simulation as potent class I histone deacetylase inhibitors and antitumor agents. Bioorg. Chem. 2019, 92, 103262. [Google Scholar] [CrossRef] [PubMed]
- Sabry, N.M.; Mohamed, H.M.; Khattab, E.S.A.E.H.; Motlaq, S.S.; El-Agrody, A.M. Synthesis of 4H-Chromene, Coumarin, 12H-Chromeno[2,3-d]Pyrimidine Derivatives and Some of Their Antimicrobial and Cytotoxicity Activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Zachariah, S.M. Pharmacological Activities of Chromene Derivatives: An Overview. Asian J. Pharm. Clin. Res. 2013, 6, 11–15. [Google Scholar]
- Li, M.; Zhao, X.; Yang, W.; Zhong, F.; Yuan, L.; Ren, Q. Asymmetric synthesis and biological evaluation of 3-nitro-2H-chromenes as potential antibacterial agents. Tetrahedron Lett. 2018, 59, 3511–3515. [Google Scholar] [CrossRef]
- Thanh, N.D.; Hai, D.S.; Ngoc Bich, V.T.; Thu Hien, P.T.; Ky Duyen, N.T.; Mai, N.T.; Dung, T.T.; Toan, V.N.; Kim Van, H.T.; Dang, L.H.; et al. Efficient click chemistry towards novel 1H-1,2,3-triazole-tethered 4H-chromene−D-glucose conjugates: Design, synthesis and evaluation of in vitro antibacterial, MRSA and antifungal activities. Eur. J. Med. Chem. 2019, 167, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Subbareddy, C.V.; Subashini, R.; Sumathi, S. Synthesis of substituted 2H-chromenes by a three-component reaction as potential antioxidants. Mol. Divers. 2017, 21, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Yahagi, H.; Uesawa, Y.; Sugita, Y. 3-(E)-Styryl-2H-chromene derivatives as potent and selective monoamine oxidase B inhibitors. Bioorg. Chem. 2018, 77, 436–442. [Google Scholar] [CrossRef]
- Razdan, R.K.; Pars, H.G.; Granchelli, F.E.; Harris, L.S. Steroidal Analog of a Tetrahydrocannabinol. J. Med. Chem. 1968, 11, 377–378. [Google Scholar] [CrossRef]
- Pars, H.G.; Granchelli, F.E.; Keller, J.K.; Razdan, R.K. Physiologically Active Nitrogen Analogs of Tetrahydrocannabinols. Tetrahydrobenzopyrano[3,4-d]Pyridines. J. Am. Chem. Soc. 1966, 88, 3664–3665. [Google Scholar] [CrossRef]
- Pars, H.G.; Granchelli, F.E.; Razdan, R.K.; Keller, J.K.; Teiger, D.G.; Rosenberg, F.J.; Harris, L.S. Drugs derived from cannabinoids. 1. Nitrogen analogs, benzopyranopyridines and benzopyranopyrroles. J. Med. Chem. 1976, 19, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Altenbach, R.J.; Basha, F.Z.; Carroll, W.A.; Drizin, I.; Kerwin, J.F., Jr.; Wendt, M.D.; Haight, A.R.; Zhang, W. Benzopyranopyrrole and Benzopyranopyridine Alpha-1 Adrenergic Compounds. WO Patent WO9824791A1, 11 June 1998. [Google Scholar]
- Brown, R.E.; Puchalski, C.; Shavel, J., Jr. Novel Substituted Benzopyranopyridine. U.S. Patent 3,962,266, 8 June 1976. [Google Scholar]
- Connor, D.T.; Unangst, P.C.; Schwender, C.F.; Sorenson, R.J.; Carethers, M.E.; Puchalski, C.; Brown, R.E. Synthesis of 1,2,3,4-tetrahydro-5H-[1]benzopyrano[3,4-c]pyridin-5-ones. II. Substitution at the 3-position with 2-aminoethyl and 2-aminopropyl side chains. J. Heterocycl. Chem. 1984, 21, 1561–1564. [Google Scholar] [CrossRef]
- Connor, D.T.; Unangst, P.C.; Schwender, C.F.; Sorenson, R.J.; Carethers, M.E.; Brown, R.E.; Puchalski, C. Synthesis of 1,2,3,4-tetrahydro-5H-[1]benzopyrano[3,4-c]pyridin-5-ones. I. 3-unsubstituted compounds. J. Heterocycl. Chem. 1984, 21, 1557–1559. [Google Scholar] [CrossRef]
- Radulovic, N.; Stojanovic, G.; Vukicevic, R.; Dekic, V.; Dekic, B.; Palic, R. New 3,4-Annelated Coumarin Derivatives: Synthesis, Antimicrobial Activity, Antioxidant Capacity, and Molecular Modeling. Monatshefte Chem./Chem. Mon. 2006, 137, 1477–1486. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Bodade, R.G.; Bhosale, R.B. An efficient one-pot synthesis of some new 2,4-diaryl pyrido[3,2-c]coumarins as potent antimicrobial agents. J. Heterocycl. Chem. 2010, 47, 237–241. [Google Scholar] [CrossRef]
- Nunez-Vergara, L.J.; Squella, J.A.; Navarrete-Encina, P.A.; Vicente-Garcia, E.; Preciado, S.; Lavilla, R. Chromenopyridines: Promising Scaffolds for Medicinal and Biological Chemistry. Curr. Med. Chem. 2011, 18, 4761–4785. [Google Scholar] [CrossRef] [PubMed]
- Delost, M.D.; Smith, D.T.; Anderson, B.J.; Njardarson, J.T. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018, 61, 10996–11020. [Google Scholar] [CrossRef]
- Mishra, S.; Ghosh, R. K2CO3-Mediated, One-Pot, Multicomponent Synthesis of Medicinally Potent Pyridine and Chromeno[2,3-b]Pyridine Scaffolds. Synth. Commun. 2012, 42, 2229–2244. [Google Scholar] [CrossRef]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. Convenient one-step synthesis of a medicinally relevant benzopyranopyridine system. Tetrahedron Lett. 2006, 47, 9309–9312. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, J.; Pfeffer, S.; Ma, D.; Pfeffer, L.M.; Patil, S.A.; Li, W.; Miller, D.D. Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents. Molecules 2015, 20, 17152–17165. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Ranjan, S.; Rao, M.S.; Dar, A.H.; Shyam, M.; Jayaprakash, V.; Hussain, S. Borax Catalysed Domino Synthesis of Highly Functionalised Spirooxindole and Chromenopyridine Derivatives: X-Ray Structure, Hirshfeld Surface Analysis and Molecular Docking Studies. ChemistrySelect 2018, 3, 8669–8677. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Novikov, R.A.; Egorov, M.P. PASE Pseudo-Four-Component Synthesis and Docking Studies of New 5-C-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]Pyridine-3-Carbonitriles. ChemistrySelect 2017, 2, 4593–4597. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Goloveshkin, A.S.; Ushakov, I.E.; Egorov, M.P. PASE facile and efficient multicomponent approach to the new type of 5-C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2018, 28, 372–374. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Goloveshkin, A.S.; Ushakov, I.E.; Egorov, M.P. Multicomponent transformation of salicylaldehydes, 2-aminoprop-1-ene-1,1,3-tricarbonitrile, and pyrazolin-5-ones into substituted 2,4-diamino-5-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitriles. Russ. Chem. Bull. 2018, 67, 1695–1703. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Selective multicomponent ‘one-pot’ approach to the new 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)chromeno[2,3-b]pyridine scaffold in pyridine–ethanol catalyst/solvent system. Monatshefte Chem./Chem. Mon. 2019, 150, 1073–1078. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent Approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]Pyridine Scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot, atom and step economic (PASE) assembly of salicylaldehydes, malononitrile dimer and 4-hydroxypyridine-2(1H)-ones into medicinally relevant 5H-chromeno[2,3-b]pyridine scaffold. Mol. Divers. 2019, 24, 617–626. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Dorofeeva, E.O.; Goloveshkin, A.S.; Egorov, M.P. Pseudo six-component stereoselective synthesis of 2,4,6-triaryl-3,3,5,5-tetracyanopiperidines. Mendeleev Commun. 2018, 28, 384–386. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. A facile and efficient multicomponent approach to 5-[5-hydroxy-3-(trifluoromethyl)-1H-pyrazol-4-yl]-5H-chromeno[2,3-b]pyridines. J. Fluor. Chem. 2018, 213, 31–36. [Google Scholar] [CrossRef]
- Festa, A.A.; Storozhenko, O.A.; Golantsov, N.E.; Subramani, K.; Novikov, R.A.; Zaitseva, S.O.; Baranov, M.S.; Varlamov, A.V.; Voskressensky, L.G. Homophtalonitrile for Multicomponent Reactions: Syntheses and Optical Properties of o-Cyanophenyl- or Indol-3-Yl-SubstitutedChromeno[2,3-c]Isoquinolin-5-Amines. ChemistryOpen 2019, 8, 23–30. [Google Scholar] [CrossRef]
- Festa, A.A.; Storozhenko, O.A.; Bella Ndoutoume, D.R.; Varlamov, A.V.; Voskressensky, L.G. Sequential three-component reaction of homophthalonitrile, salicylaldehydes and nitromethane. Mendeleev Commun. 2017, 27, 451–453. [Google Scholar] [CrossRef]
- Shaabani, A.; Hajishaabanha, F.; Mofakham, H.; Maleki, A. A new one-pot three-component synthesis of 2,4-diamino-5H-chromeno[2,3-b] pyridine-3-carbonitrile derivatives. Mol. Divers. 2010, 14, 179–182. [Google Scholar] [CrossRef]
- Lopes, D.; Oliveira-Pinto, S.; Pontes, O.; Sampaio-Marques, B.; Costa, M.D.; Carvalho, L.; Gonçalves, C.S.; Costa, B.M.; Maciel, P.; Ludovico, P.; et al. Unravelling the anticancer potential of functionalized chromeno[2,3-b]pyridines for breast cancer treatment. Bioorg. Chem. 2020, 100, 103942. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Multicomponent Synthesis of Novel Penta-Heterocyclic Ring Systems Incorporating a Benzopyranopyridine Scaffold. Synthesis 2014, 46, 258–262. [Google Scholar] [CrossRef]
- Navarrete-Encina, P.A.; Salazar, R.; Vega-Retter, C.; Pérez, K.; Squella, J.A.; Nuñez-Vergara, L.J. On the one pot syntheses of chromeno[4,3-b]pyridine-3-carboxylate and chromeno[3,4-c]pyridine-3-carboxylate and dihydropyridines. J. Braz. Chem. Soc. 2010, 21, 413–418. [Google Scholar] [CrossRef]
- Povarov, L.S.; Grigos, V.I.; Mikhailov, B.M. Reaction of benzylideneaniline with some unsaturated compounds. Russ. Chem. Bull. 1963, 12, 1878–1880. [Google Scholar] [CrossRef]
- Adolfsson, D.E.; Tyagi, M.; Singh, P.; Deuschmann, A.; Ådén, J.; Gharibyan, A.L.; Jayaweera, S.W.; Lindgren, A.E.G.; Olofsson, A.; Almqvist, F. Intramolecular Povarov Reactions for the Synthesis of Chromenopyridine Fused 2-Pyridone Polyheterocycles Binding to α-Synuclein and Amyloid-β Fibrils. J. Org. Chem. 2020, 85, 14174–14189. [Google Scholar] [CrossRef]
- Dimitriadou, E.; Raftopoulou, M.; Kasapidou, P.M.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J.; Hadjipavlou-Litina, D.J.; Kontogiorgis, C.; Pritsa, A.; Papadopoulos, A. Ultrasound promoted synthesis of chromeno[2,3-b]pyridines and their evaluation as lipid peroxidation inhibitors. Arkivoc 2014, 2014, 372–384. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M.; Ibrahim, S.S.; Said, S. Synthesis of Some Novel Heteroannelated Chromones by Basic Rearrangement of 6-Methylchromone-3-Carbonitrile. Chem. Heterocycl. Compd. 2015, 50, 1624–1633. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M. Studies on the Chemical Transformations of 6-Methylchromone-3-Carbonitrile under Nucleophilic Conditions. J. Heterocycl. Chem. 2016, 53, 859–864. [Google Scholar] [CrossRef]
- Savych, I.; Ejaz, S.A.; Shah, S.J.A.; Iaroshenko, V.O.; Villinger, A.; Sosnovskikh, V.Y.; Iqbal, J.; Abbasi, A.; Langer, P. Reactions of 3-Acylchromones with Heterocyclic Ketene Aminals: One-Pot Synthesis and Phosphatase Inhibitory Activity of Fused Pyridine Derivatives. Eur. J. Org. Chem. 2017, 2017, 186–202. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Praveen, S.; Farooq, F. Novel benzopyranopyridine derivatives of 2-amino-3-formylchromone. Chem. Pap. 2010, 64, 818–824. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Farag, A.A.M.; Roushdy, N.; El-Gohary, N.M. Synthesis, optical and photoelectrical characterizations of the novel 10-chloro-6H,8H-dichromeno[2,3-b:3′,4′-e]pyridine-6,8-dione (CDPD) and its photodiode application. Opt. Mater. 2016, 51, 70–77. [Google Scholar] [CrossRef]
- Ponduri, R.; Kumar, P.; Vadali, L.R.A.O.; Modugu, N.R. Water-PEG-400 Mediated an Efficient One-Pot Eco-Friendly Synthesis of Functionalized Isoxazole Substituted Chromeno[2,3-b]pyridine-3-carboxylate Derivatives. ChemistrySelect 2018, 3, 7766–7770. [Google Scholar] [CrossRef]
- Dolatkhah, Z.; Nasiri-Aghdam, M.; Bazgir, A. A Three-Component Synthesis of Benzochromenodiazocines and Chromenopyridines. Tetrahedron Lett. 2013, 54, 1960–1962. [Google Scholar] [CrossRef]
- Zhang, C.H.; Huang, R.; Hu, X.M.; Lin, J.; Yan, S.J. Three-Component Site-Selective Synthesis of Highly Substituted 5H-Chromeno-[4,3-b]Pyridines. J. Org. Chem. 2018, 83, 4981–4989. [Google Scholar] [CrossRef]
- Lozinski, O.A.; Shokol, T.V.; Zubatyuk, R.I.; Shishkin, O.V.; Khilya, V.P. An alternative approach to the synthesis of 5H-chromeno[4,3-b]pyridin-5-one system using the cleavage of 5H,9H-pyrano[2′,3′:5,6]chromeno[4,3-b]pyridine-5,9-diones with binucleophiles. Chem. Heterocycl. Comp. 2018, 54, 96–99. [Google Scholar] [CrossRef]
- Ali, K.A.; Abdel Hafez, N.A.; Elsayed, M.A.; Ibrahim, A.A. Microwave-assisted synthesis and heterocyclic functionalization of chromenopyridines on calixarene scaffold. J. Heterocycl. Chem. 2020, 57, 1838–1844. [Google Scholar] [CrossRef]
- Thapa, U.; Thapa, P.; Karki, R.; Yun, M.; Choi, J.H.; Jahng, Y.; Lee, E.; Jeon, K.H.; Na, Y.; Ha, E.M.; et al. Synthesis of 2,4-diaryl chromenopyridines and evaluation of their topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship. Eur. J. Med. Chem. 2011, 46, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Lee, E.S. 2,4-Diaryl-5,6-Dihydro-1,10-Phenanthrolines with Furyl or Thienyl Moiety at 4-Position: Synthesis, Topoisomerase I and II Inhibitory Activity, and Cytotoxicity. Bull. Korean Chem. Soc. 2012, 33, 1769–1772. [Google Scholar] [CrossRef][Green Version]
- Thapa, P.; Jun, K.Y.; Kadayat, T.M.; Park, C.; Zheng, Z.; Thapa Magar, T.B.; Bist, G.; Shrestha, A.; Na, Y.; Kwon, Y.; et al. Design and synthesis of conformationally constrained hydroxylated 4-phenyl-2-aryl chromenopyridines as novel and selective topoisomerase II-targeted antiproliferative agents. Bioorg. Med. Chem. 2015, 23, 6454–6466. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.B.; Park, C.; Jeon, K.H.; Lee, E.; Park, S.E.; Jun, K.Y.; Kadayat, T.M.; Thapa, P.; Karki, R.; Na, Y.; et al. A Series of Novel Terpyridine-Skeleton Molecule Derivants Inhibit Tumor Growth and Metastasis by Targeting Topoisomerases. J. Med. Chem. 2015, 58, 1100–1122. [Google Scholar] [CrossRef] [PubMed]
- Magar, T.B.T.; Seo, S.H.; Kadayat, T.M.; Jo, H.; Shrestha, A.; Bist, G.; Katila, P.; Kwon, Y.; Lee, E.S. Synthesis and SAR study of new hydroxy and chloro-substituted 2,4-diphenyl 5H-chromeno[4,3-b]pyridines as selective topoisomerase IIα-targeting anticancer agents. Bioorg. Med. Chem. 2018, 26, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, H.; Dawood, K.M.; Aryan, F.A.; Ibrahim, H.M. Green Protocol for the Novel Synthesis of Thiochromeno[4,3-b]Pyridine and Chromeno[4,3-b]Pyridine Derivatives Utilizing a High-Pressure System. ACS Omega 2021, 6, 34065–34074. [Google Scholar] [CrossRef] [PubMed]
- El-Essawy, F.; El-Etrawy, A.S. Synthesis of New Chromeno[4,3-b]pyrazolo[4,3-e]pyridines Derivatives with Antimicrobial Evaluation. J. Heterocycl. Chem. 2013, 51, 191–195. [Google Scholar] [CrossRef]
- Rong, L.; Han, H.; Jiang, H.; Zhang, Q.; Tu, S. Efficient one-pot synthesis of 4-aryl-3-cyano-2,5-dihydro-1H-indeno[1,2-b]pyridin-2-one and 4-aryl-3-cyano-1,2,5,6-tetrahydrobenzo[h] quinolin-2-one derivatives under solvent-free conditions. Synth. Commun. 2009, 39, 1027–1034. [Google Scholar] [CrossRef][Green Version]
- Kok, T.; Wapenaar, H.; Wang, K.; Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Proietti, G.; Eleftheriadis, N.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Cool, R.H.; et al. Discovery of chromenes as inhibitors of macrophage migration inhibitory factor. Bioorg. Med. Chem. 2018, 26, 999–1005. [Google Scholar] [CrossRef]
- Patel, M.A.; Bhila, V.G.; Patel, N.H.; Patel, A.K.; Brahmbhatt, D.I. Synthesis, characterization and biological evaluation of some pyridine and quinoline fused chromenone derivatives. Med. Chem. Res. 2012, 21, 4381–4388. [Google Scholar] [CrossRef]
- Pal, S.; Khan, M.N.; Karamthulla, S.; Choudhury, L.H. Molecular iodine catalyzed one-pot multicomponent reactions for the synthesis of dihydrochromeno[4,3-b]pyrazolo[4,3-e]pyridin-6(7H)-ones. RSC Adv. 2013, 3, 15705–15711. [Google Scholar] [CrossRef]
- Patel, A.A.; Lad, H.B.; Pandya, K.R.; Patel, C.V.; Brahmbhatt, D.I. Synthesis of a new series of 2-(2-oxo-2H-chromen-3-yl)-5H-chromeno[4,3-b]pyridin-5-ones by two facile methods and evaluation of their antimicrobial activity. Med. Chem. Res. 2013, 22, 4745–4754. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K. Michael Initiated Ring Closure (MIRC) reaction on in situ generated benzylidenecyclohexane-1,3-diones for the construction of chromeno[3,4-b]quinoline derivatives. Tetrahedron Lett. 2012, 53, 2345–2351. [Google Scholar] [CrossRef]
- Khan, A.T.; Das, D.K.; Islam, K.; Das, P. A simple and expedient synthesis of functionalized pyrido[2,3-c] coumarin derivatives using molecular iodine catalyzed three-component reaction. Tetrahedron Lett. 2012, 53, 6418–6422. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, J.; Su, W. An Efficient Protocol for Multicomponent Synthesis of 1H-Chromeno[4,3-b]Pyridin-5(4H)-Ones Derivatives. J. Chem. Res. 2013, 37, 327–330. [Google Scholar] [CrossRef]
- Oshiro, P.B.; Bregadiolli, B.A.; da Silva-Filho, L.C. A facile one-step synthesis of chromeno[4,3-b]pyridine derivatives promoted by niobium pentachloride. J. Heterocycl. Chem. 2020, 57, 2795–2800. [Google Scholar] [CrossRef]
- Paul, S.; Das, A.R. An efficient green protocol for the synthesis of coumarin fused highly decorated indenodihydropyridyl and dihydropyridyl derivatives. Tetrahedron Lett. 2012, 53, 2206–2210. [Google Scholar] [CrossRef]
- Motamedi, R. Solvent-free synthesis of novel 5-oxo-5H-chromeno [4,3-b]pyridine derivatives. Chem. Heterocyc. Compd. 2013, 48, 1839–1843. [Google Scholar] [CrossRef]
- Symeonidis, T.S.; Litinas, K.E. Synthesis of methyl substituted [5,6]- and [7,8]-fused pyridocoumarins via the iodine-catalyzed reaction of aminocoumarins with n-butyl vinyl ether. Tetrahedron Lett. 2013, 54, 6517–6519. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Chandra, S. One-pot access to pyridocoumarins via Povarov-hydrogen transfer cascade under auto-tandem catalysis of iodine in aqueous micelles. Tetrahedron Lett. 2014, 55, 1564–1568. [Google Scholar] [CrossRef]
- Epstein, O.; Bryan, M.C.; Cheng, A.C.; Derakhchan, K.; Dineen, T.A.; Hickman, D.; Hua, Z.; Human, J.B.; Kreiman, C.; Marx, I.E.; et al. Lead Optimization and Modulation of HERG Activity in a Series of Aminooxazoline Xanthene β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE1) Inhibitors. J. Med. Chem. 2014, 57, 9796–9810. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Brown, J.; Judd, T.C.; Lopez, P.; Qian, W.; Powers, T.S.; Chen, J.J.; Bartberger, M.D.; Chen, K.; Dunn, R.T.; et al. An Orally Available BACE1 Inhibitor That Affords Robust CNS Aβ Reduction without Cardiovascular Liabilities. ACS Med. Chem. Lett. 2015, 6, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; La, D.S.; Cheng, A.C.; Whittington, D.A.; Patel, V.F.; Chen, K.; Dineen, T.A.; Epstein, O.; Graceffa, R.; Hickman, D.; et al. Structure- and Property-Based Design of Aminooxazoline Xanthenes as Selective, Orally Efficacious, and Cns Penetrable BACE Inhibitors for the Treatment of Alzheimers Disease. J. Med. Chem. 2012, 55, 9156–9169. [Google Scholar] [CrossRef]
- Dineen, T.A.; Chen, K.; Cheng, A.C.; Derakhchan, K.; Epstein, O.; Esmay, J.; Hickman, D.; Kreiman, C.E.; Marx, I.E.; Wahl, R.C.; et al. Inhibitors of β-Site Amyloid Precursor Protein Cleaving Enzyme (BACE1): Identification of (S)-7-(2-Fluoropyridin-3-yl)-3-((3-methyloxetan-3-yl)ethynyl)-5′H-Spiro[Chromeno[2,3-b]pyridine-5,4′-oxazol]-2′-amine (AMG-8718). J. Med. Chem. 2014, 57, 9811–9831. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroso de Lima, F.; Costa, M.; Sousa, A.; Proença, M.F. The Chromenopyridine Scaffold: A Privileged Platform in Drug Design. Molecules 2024, 29, 3004. https://doi.org/10.3390/molecules29133004
Pedroso de Lima F, Costa M, Sousa A, Proença MF. The Chromenopyridine Scaffold: A Privileged Platform in Drug Design. Molecules. 2024; 29(13):3004. https://doi.org/10.3390/molecules29133004
Chicago/Turabian StylePedroso de Lima, Fábio, Marta Costa, Ana Sousa, and Maria Fernanda Proença. 2024. "The Chromenopyridine Scaffold: A Privileged Platform in Drug Design" Molecules 29, no. 13: 3004. https://doi.org/10.3390/molecules29133004
APA StylePedroso de Lima, F., Costa, M., Sousa, A., & Proença, M. F. (2024). The Chromenopyridine Scaffold: A Privileged Platform in Drug Design. Molecules, 29(13), 3004. https://doi.org/10.3390/molecules29133004







