Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles
Abstract
1. Introduction
2. Results
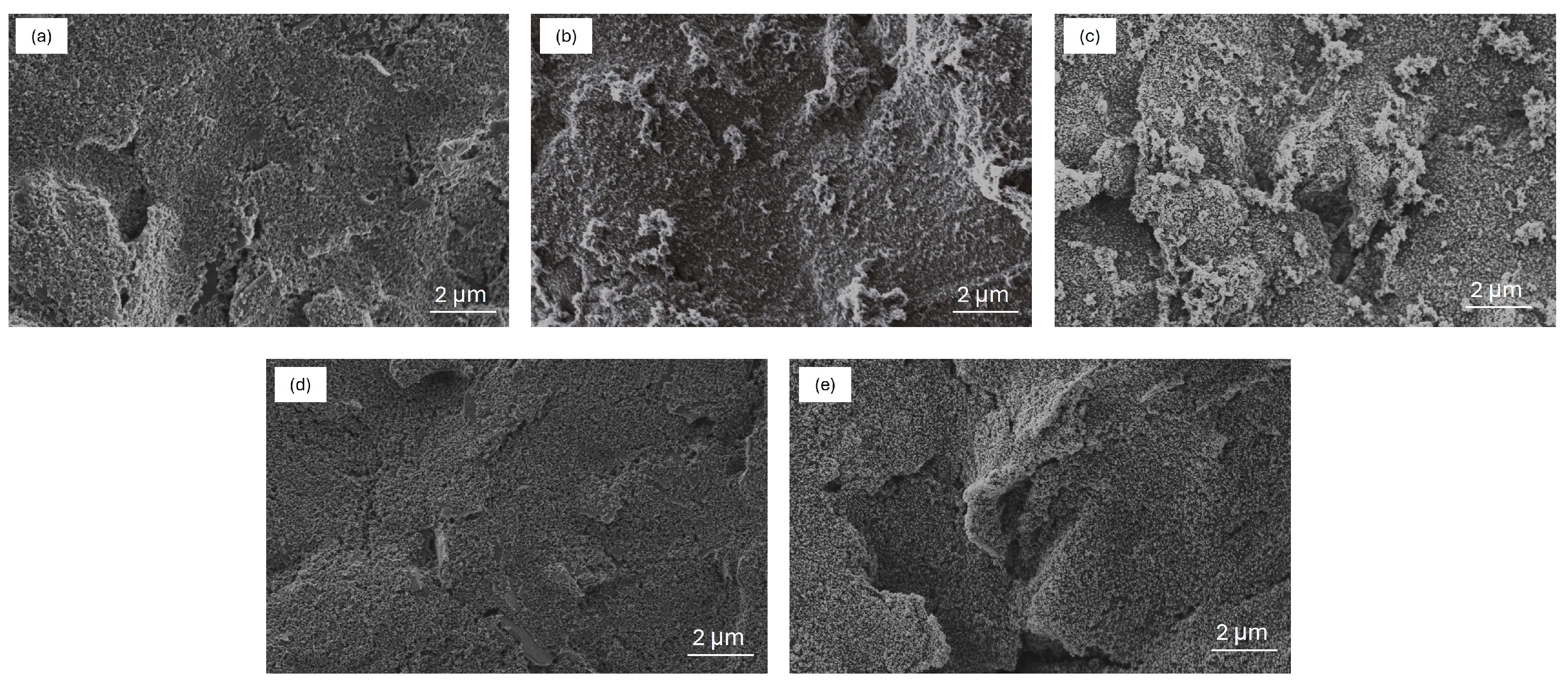
2.1. Morphological Characterization
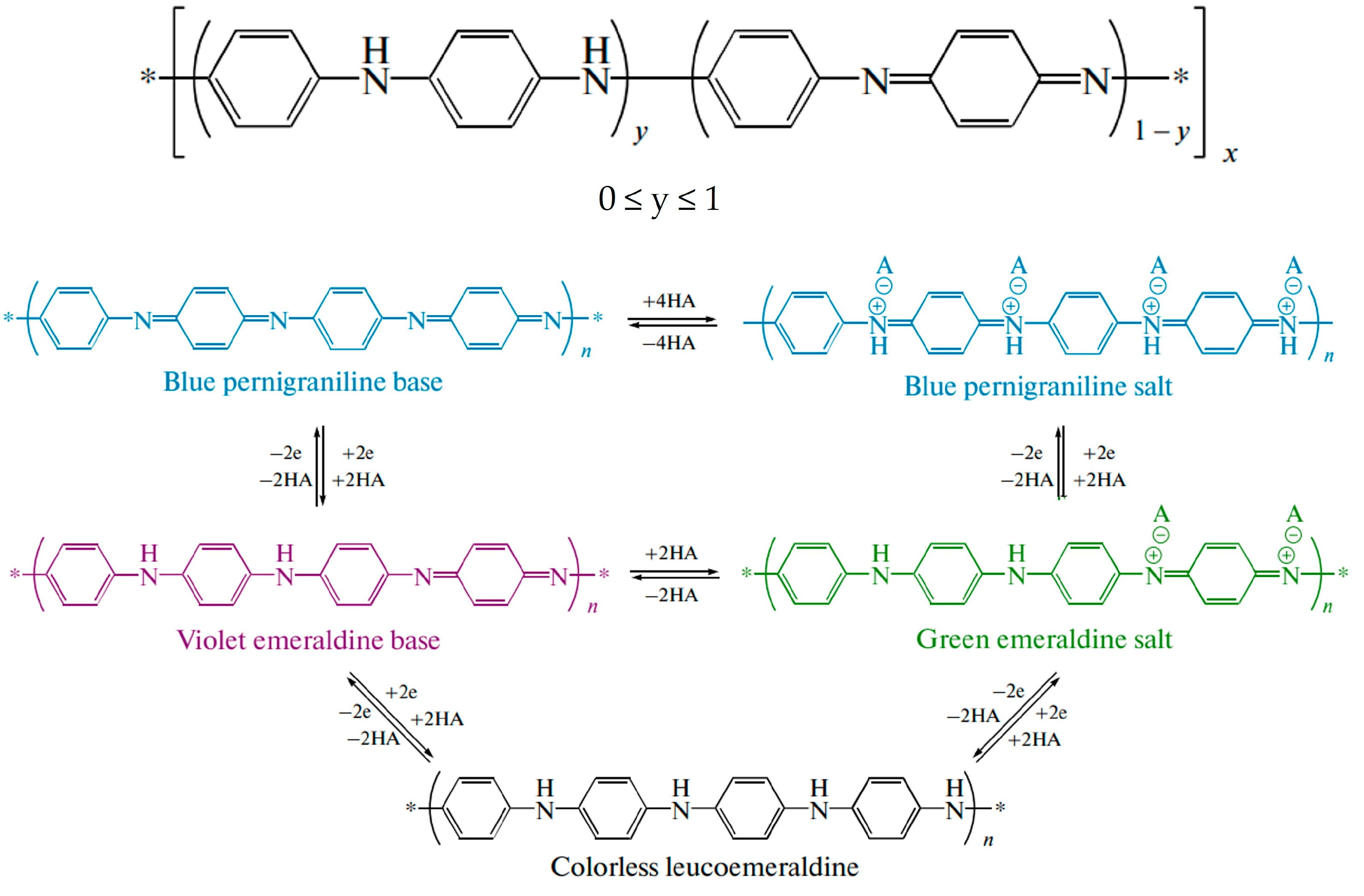
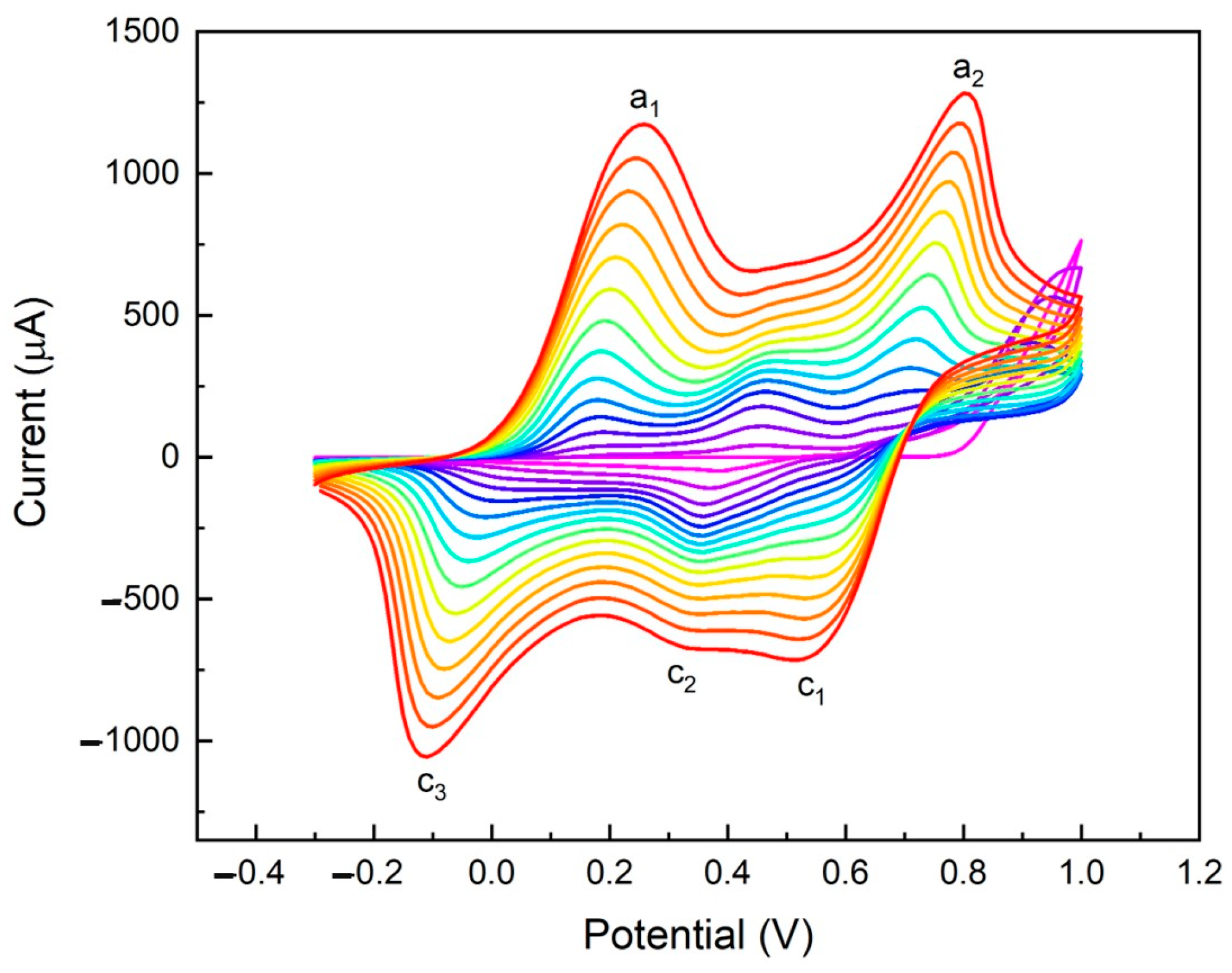
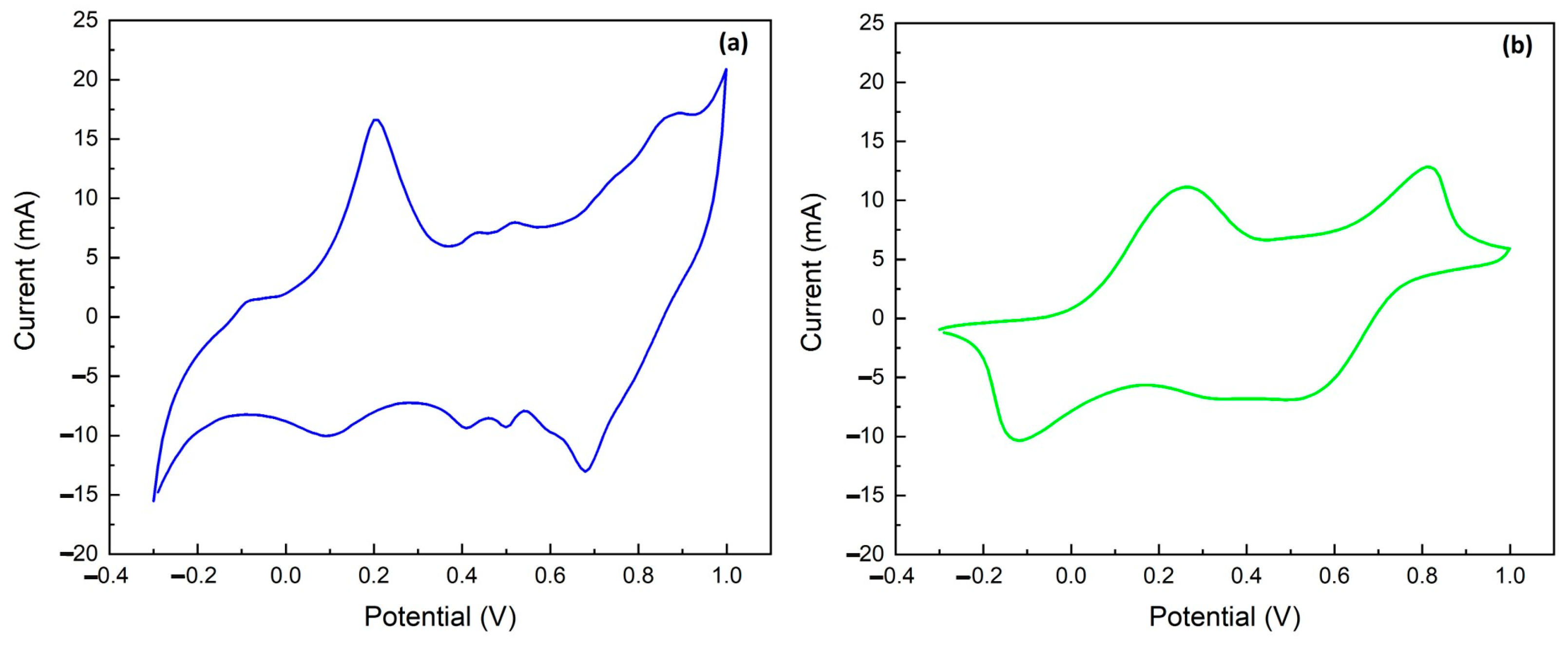
2.2. Electrochemical Characterization of Electropolymerized Polyaniline
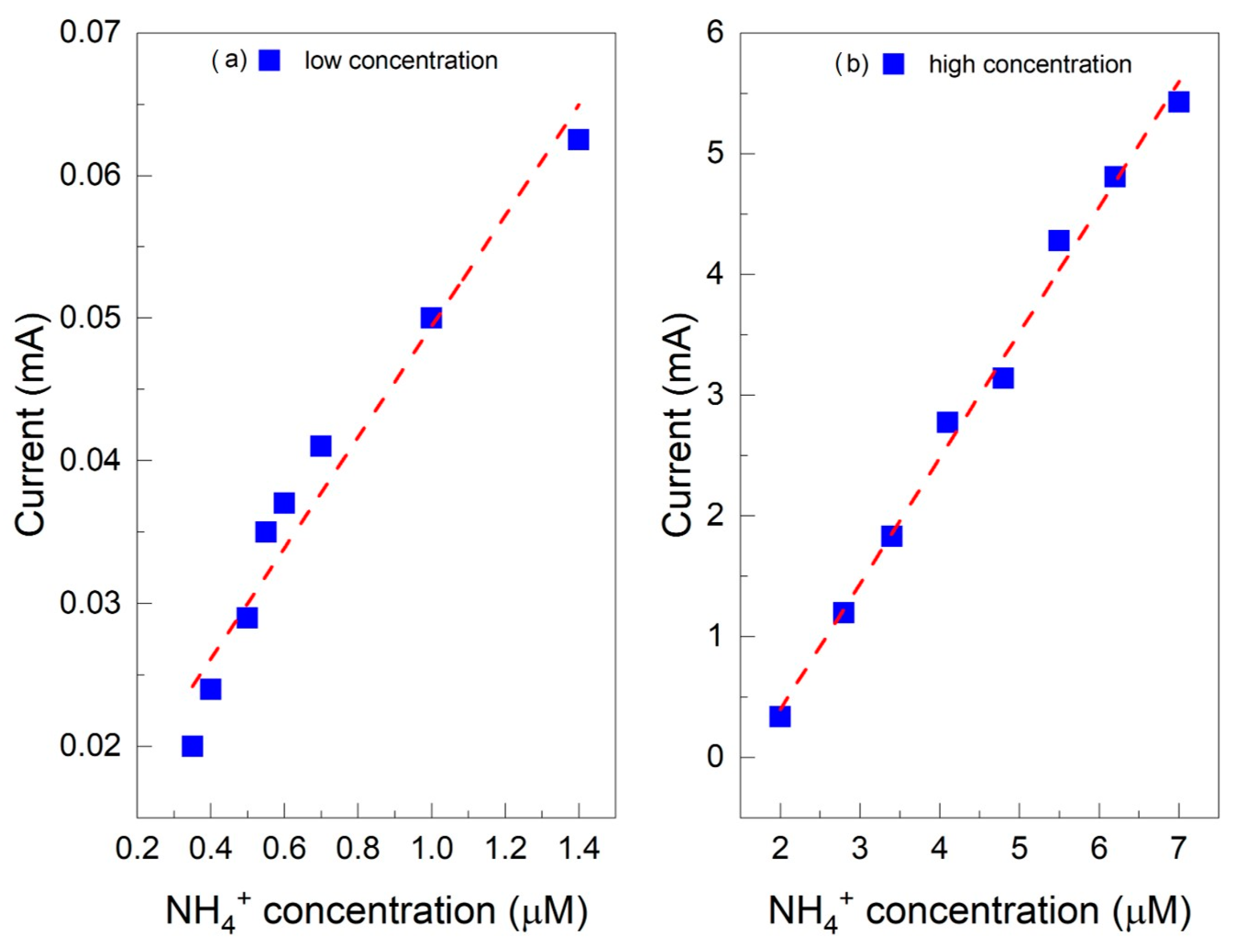
2.3. Chronoamperometric Ammonium Ion Detection in Water
2.4. Repeatability Test
3. Materials and Methods
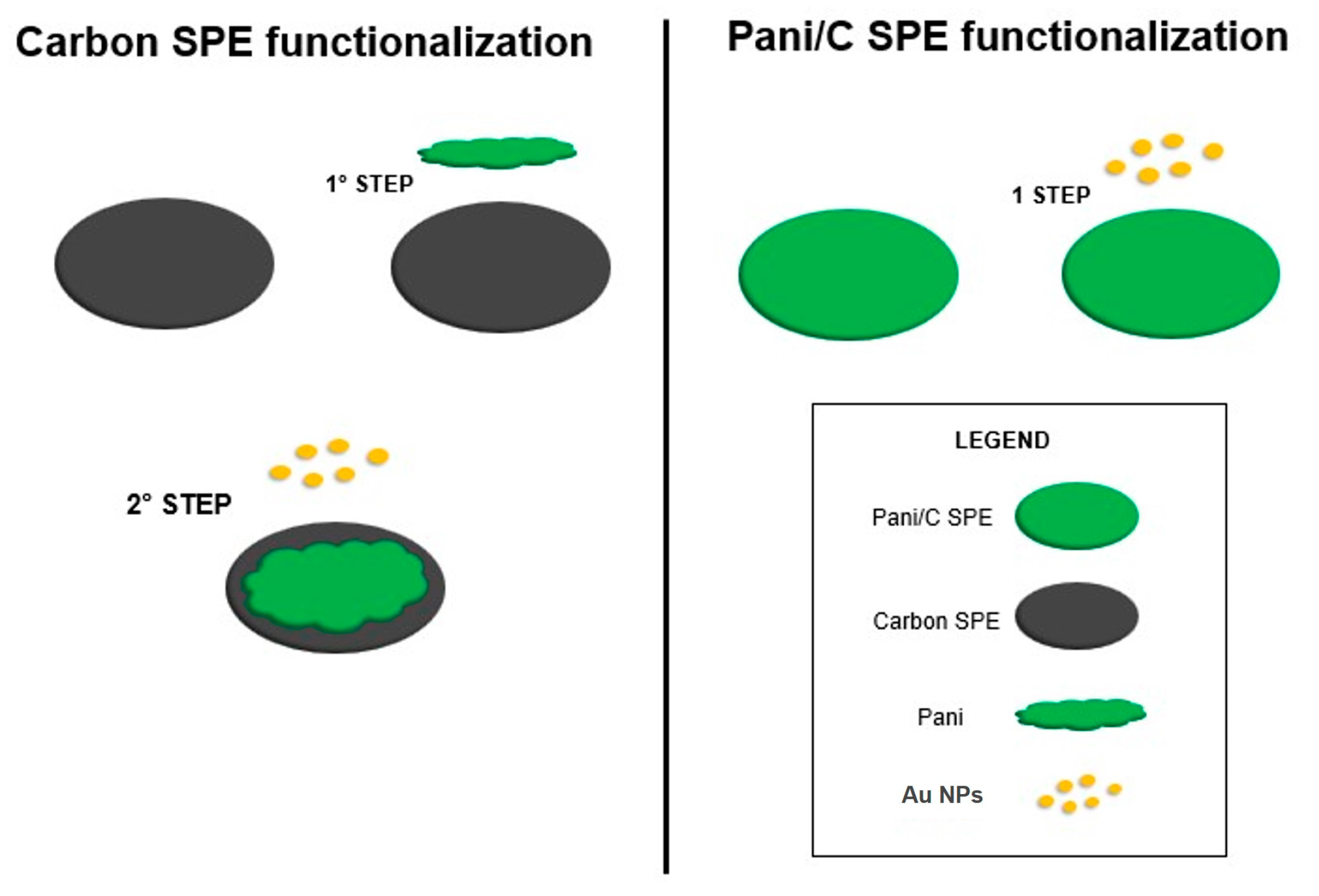
Electrode Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems—A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC 2020, 127, 115890. [Google Scholar] [CrossRef]
- Goldshleger, N.; Grinberg, A.; Harpaz, S.; Shulzinger, A.; Abramovich, A. Real-time advanced spectroscopic monitoring of ammonia concentration in water. Aquacult. Eng. 2018, 83, 103–108. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, D.; Huang, Y.; Ma, J.; Feng, S.; Lin, K. A modified method for on-line determination of trace ammonium in seawater with a long-path liquid waveguide capillary cell and spectrophotometric detection. Mar. Chem. 2014, 162, 114–121. [Google Scholar] [CrossRef]
- Amali, A.J.; Awwad, N.H.; Rana, R.K.; Patra, D. Nanoparticle assembled microcapsules for application as pH and ammonia sensor. Anal. Chim. Acta 2011, 708, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Siribunbandal, P.; Yong-Hoon, K.; Osotchan, T.; Zhu, Z.; Jaisutti, R. Quantitative Colorimetric Detection of Dissolved Ammonia Using Polydiacetylene Sensors Enabled by Machine Learning Classifiers. ACS Omega 2022, 7, 18714–18721. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, A.J.; Khan, A.S.; Hu, J.; Nawaz, A.; Zhu, J. E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture. Sustainability 2023, 15, 11601. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, L.; Tang, Y.; Han, T.; Liu, S.; Sun, Z.; Bao, Y.; Wang, H.; He, Y.; Wang, W.; et al. Beyond Nonactin: Potentiometric Ammonium Ion Sensing Based on Ion-selective Membrane-free Prussian Blue Analogue Transducers. Anal. Chem. 2022, 94, 10487–10496. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, Y.; Chen, Y.; Sonkusale, S.R. Dissolved ammonia sensing in complex mixtures using metalloporphyrin-based optoelectronic sensor and spectroscopic detection. Sens. Actuators B 2014, 202, 976–983. [Google Scholar] [CrossRef]
- Liu, Y.; Asset, T.; Chen, Y.; Murphy, E.; Potma, E.O.; Matanovic, I.; Fishman, D.A.; Atanassov, P. Facile All-Optical Method for In Situ Detection of Low Amounts of Ammonia. iScience 2020, 23, 101757. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. TrAC 2019, 24, e00073. [Google Scholar] [CrossRef]
- Debasis, M.; Mathankumar, M.; Kumar, R.; Thangavelu, R. Development of the PANI/MWCNT Nanocomposite-Based Fluorescent Sensor for Selective Detection of Aqueous Ammonia. ACS Omega 2020, 5, 8414–8422. [Google Scholar]
- Ding, L.; Ding, J.; Ding, B.; Qin, W. Solid-contact Potentiometric Sensor for the Determination of Total Ammonia Nitrogen in Seawater. Int. J. Electrochem. Sci. 2017, 12, 3296–3308. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Gonzalez, J.; Baraket, A.; Boudjaoui, S.; Metzner, T.; Hauser, F.; Rößler, T.; Krause, S.; Zine, N.; Streklas, A.; Alcácer, A.; et al. A fully integrated passive microfluidic Lab-on-a-Chip for real-time electrochemical detection of ammonium: Sewage applications. Sci. Total Environ. 2019, 653, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Trommer, K.; Mertig, M. Solid-contact ion-selective electrodes based on graphite paste for potentiometric nitrate and ammonium determinations. Am. J. Anal. Chem. 2019, 9, 591–601. [Google Scholar] [CrossRef]
- Cuartero, M.; Ruiz, A.; Oliva, D.J.; Ortuño, J.A. Multianalyte detection using potentiometric ionophore-based ion-selective electrodes. Sens. Actuators B Chem. 2017, 243, 144–151. [Google Scholar] [CrossRef]
- Bollmann, A.; Revsbech, N.P. An NH4+ biosensor based on ammonia-oxidizing bacteria for use under anoxic conditions. Sens. Actuators B Chem. 2005, 105, 412–418. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, J.; Kou, L.; Qin, W. A Potentiometric Flow Biosensor Based on Ammonia-Oxidizing Bacteria for the Detection of Toxicity in Water. Sensors 2013, 13, 6936–6945. [Google Scholar] [CrossRef]
- Pflüger, T.; Hernández, C.F.; Lewe, P.; Frank, F.; Mertens, H.; Svergun, D.; Baumstark, M.W.; Lunin, V.Y.; Jetten, M.S.M.; Andrade, S.L.A. Signaling ammonium across membranes through an ammonium sensor histidine kinase. Nat. Commun. 2018, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bai, Z.; Wang, T. Portable device for on-site detection of ammonia nitrogen. Inf. Process. Agric. 2022, 9, 475–484. [Google Scholar] [CrossRef]
- Deng, S.; Doherty, W.; Mcauliffe, M.; Salaj-Kosla, U.; Lewis, L.; Huyet, G. A low-cost, portable optical sensing system with wireless communication compatible of real-time and remote detection of dissolved ammonia. Photonic Sens. 2016, 6, 107–114. [Google Scholar] [CrossRef]
- Zhou, M.; Li, T.M.T.; Fan, K.; Shu, Y.; Liu, P.; Zhao, H. Portable Conductometric Sensing Probe for Real-Time Monitoring Ammonia Profile in Coastal Waters. ACS Sens. 2023, 8, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Azmi, N.S.; Kafi, A.K.M. Recent advances in gold nanoparticles modified electrodes in electrochemical nonenzymatic sensing of chemical and biological compounds. Inorg. Chem. Commun. 2023, 153, 110767. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in electrochemical detection methods for measuring contaminants of emerging concerns. Electrochem. Sci. Adv. 2022, 2, e2100184. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. 2014, 56, 144–153. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Manohar, S.K.; Masters, J.G.; Sun, Y.; Weiss, H.; Epstein, A.J. Polyaniline: Synthesis and properties of pernigraniline base. Synth. Met. 1991, 41, 621–626. [Google Scholar] [CrossRef]
- Korent, A.; Trafela, Š.; Soderžnik, K.Ž.; Samardžija, Z.; Šturm, S.; Rožman, K.Ž. Au-decorated electrochemically synthesised polyaniline-based sensory platform for amperometric detection of aqueous ammonia in biological fluids. Electrochim. Acta 2022, 430, 141034. [Google Scholar] [CrossRef]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Schab-Balcerzak, E.; Libera, M. Effect of polyaniline content and protonating dopants on electroconductive composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylov, S.; Ogurtsov, N.; Noskov, Y.; Redon, N.; Coddeville, P.; Wojkiewicz, J.-L.; Pud, A. Ammonia/amine electronic gas sensors based on hybrid polyaniline–TiO2 nanocomposites. The effects of titania and the surface active doping acid. RSC Adv. 2015, 5, 20218–20226. [Google Scholar] [CrossRef]
- Kebiche, H.; Debarnot, D.; Merzouki, A.; Poncin-Epaillard, F.; Haddaoui, N. Relationship between ammonia sensing properties of polyaniline nanostructures and their deposition and synthesis methods. Anal. Chim. Acta 2012, 737, 64–71. [Google Scholar] [CrossRef]
- Duan, X.; Duan, Z.; Zhang, Y.; Liu, B.; Li, X.; Zhao, Q.; Yuan, Z.; Jiang, Y.; Tai, H. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Fitriyana, F.K. Polyaniline-invertase-gold nanoparticles modified gold electrode for sucrose detection. Indones. J. Chem. 2015, 15, 226–233. [Google Scholar]
- Gvozdenovic, M.; Jugovic, B.; Stevanovic, J.; Grgur, B. Electrochemical synthesis of electroconducting polymers. Hem. Ind. 2014, 68, 673–684. [Google Scholar] [CrossRef]
- Korent, A.; Soderžnik, K.Ž.; Šturm, S.; Rožman, K.Ž. A correlative study of polyaniline electropolymerization and its electrochromic behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline Nanofibers: Facile Synthesis and Chemical Sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Ramadin, Y.; Ahmad, M.; Zihlif, A.; Al-Haddad, R.; Makadsi, M.; Ragosta, G.; Martuscelli, E. Determination of the type of charge carriers in carbon fiber/polymer composite. Polym. Test. 1998, 17, 257–264. [Google Scholar] [CrossRef]
- Singh, R.C.; Bilash, K.R.; Kandulna, R. Robust optical and electrical properties of TiO2-sensitized polymeric (PANI-TiO2) nanocomposites for hybrid solar cell application. Bull. Mater. Sci. 2019, 42, 202. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Zhang, G. Functional gold nanoparticles for sensing applications. Nanotechnol. Rev. 2013, 2, 269–288. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, R. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Iost, R.M.; Travain, S.A.; Oliveira, O.N.; Zucolotto, V. Enzyme immobilization on Ag nanoparticles/polyaniline nanocomposites. Biosens. Bioelectron. 2009, 24, 3073–3077. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Thompson, D.; Huang, Y.; Li, B.; Lei, Y. Electrochemical sensors for nitrogen species: A review. Sens. Actuators Rep. 2020, 2, 100022. [Google Scholar] [CrossRef]
- Kuchena, S.F.; Wang, Y. Superior polyaniline cathode material with enhanced capacity for ammonium ion storage. ACS Appl. Energy Mater. 2020, 3, 11690–11698. [Google Scholar] [CrossRef]
- Crowley, K.; O’Malley, E.; Morrin, A.; Smyth, M.R.; Killard, A.J. An aqueous ammonia sensor based on an inkjet-printed polyaniline nanoparticle -modified electrode. Analyst 2008, 133, 391–399. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Yang, L.S.; Huang, W.S.; Humphrey, B.D. Polyaniline: Electrochemistry and application to rechargeable batteries. Synth. Met. 1987, 18, 393–398. [Google Scholar] [CrossRef]
- Teo, C.H.; Karode, N.S.; Abid, K.; Rahman, F. Interfacial behaviour of polyaniline as an organic electronic material. J. Phys. Chem. Solids 2011, 72, 886–890. [Google Scholar]
- Galiev, A.F.; Karamov, D.D.; Lachinov, A.A.; Zaynullina, L.I.; Sarkeeva, E.A.; Alexandrov, I.V.; Lachinov, A.N. Non-conjugated polymer films to monitoring strain deformation of metals and alloys. J. Mater. Sci. Mater. Electron. 2024, 35, 976. [Google Scholar] [CrossRef]
- Kahn, A. Fermi level, work function and vacuum level. Mater. Horiz. 2016, 3, 7–10. [Google Scholar] [CrossRef]
- Bhadra, S.; Chattopadhyay, S.; Singha, N.K.; Khastgir, D. Improvement of conductivity of electrochemically synthesized polyaniline. J. Appl. Polym. Sci. 2008, 108, 57–64. [Google Scholar] [CrossRef]
- Kissel, D.E.; Cabrera, M.L. AMMONIA. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 56–64. [Google Scholar]
- Zhang, L.; Liu, J.; Peng, X.; Cui, Q.; He, D.; Zhao, C.; Suo, H. Fabrication of a Ni foam-supported platinum nanoparticles-silver/polypyrrole electrode for aqueous ammonia sensing. Synth. Met. 2020, 259, 116257. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, J.; Li, J.; Cui, Q.; He, D.; Zhao, C.; Suo, H. Fabricating a self-supported electrode for detecting ammonia in water based on electrodepositing platinum-polypyrrole on Ni foam. J. Electrochem. Soc. 2020, 167, 027537. [Google Scholar] [CrossRef]


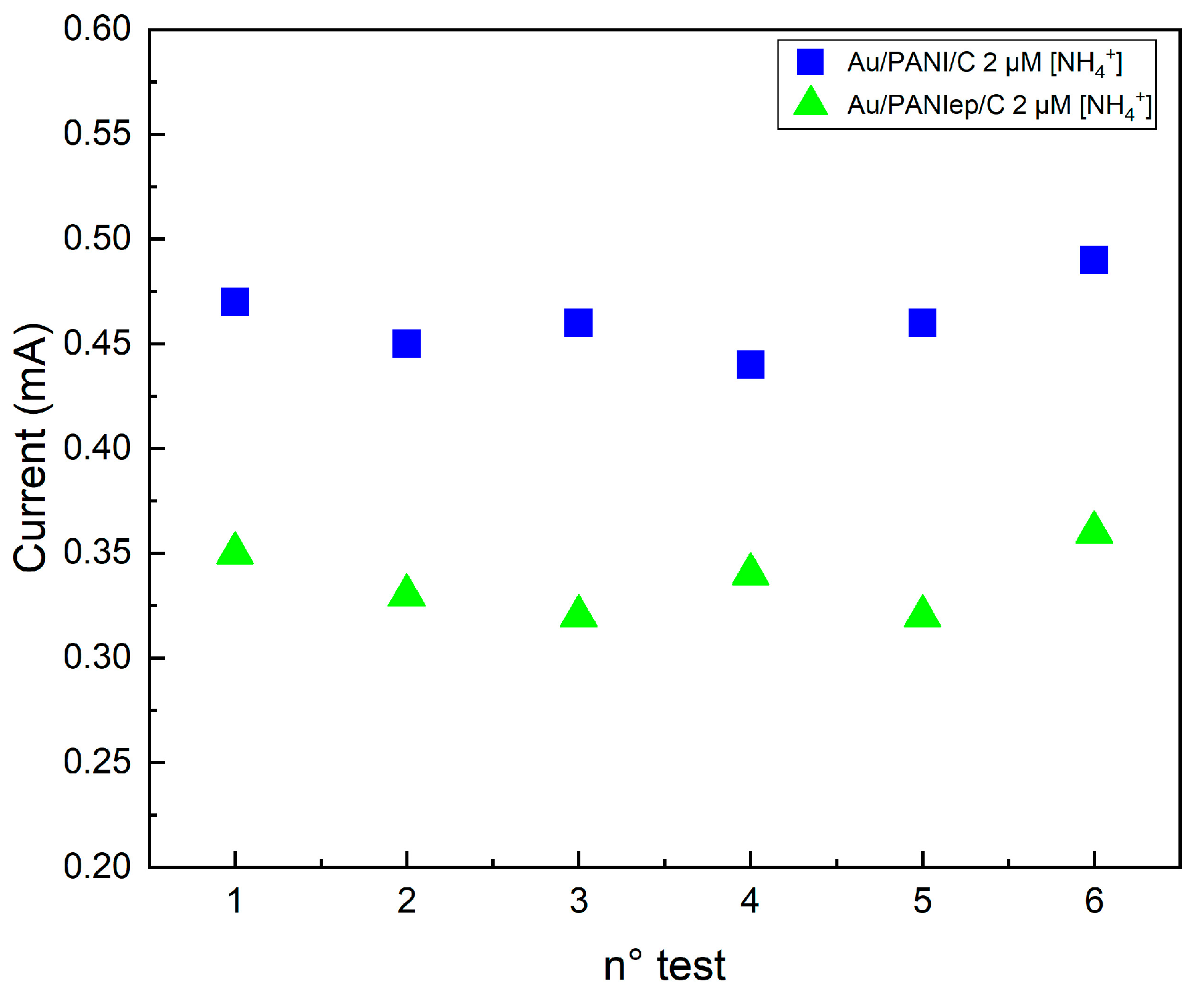
| Electrodes | SD |
|---|---|
| Au/PANIep/C | 0 µA |
| 14.14 µA | |
| 21 µA | |
| 7.10 µA | |
| 21.20 µA | |
| 7 µA | |
| Au/PANI/C | 0 µA |
| 14 µA | |
| 6.9 µA | |
| 20.1 µA | |
| 7 µA | |
| 14.1 µA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, R.; Scalese, S.; Corso, D.; Capuano, G.E.; Screpis, G.A.; Coniglio, M.A.; Condorelli, G.G.; Libertino, S. Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles. Molecules 2024, 29, 3028. https://doi.org/10.3390/molecules29133028
Farina R, Scalese S, Corso D, Capuano GE, Screpis GA, Coniglio MA, Condorelli GG, Libertino S. Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles. Molecules. 2024; 29(13):3028. https://doi.org/10.3390/molecules29133028
Chicago/Turabian StyleFarina, Roberta, Silvia Scalese, Domenico Corso, Giuseppe Emanuele Capuano, Giuseppe Andrea Screpis, Maria Anna Coniglio, Guglielmo Guido Condorelli, and Sebania Libertino. 2024. "Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles" Molecules 29, no. 13: 3028. https://doi.org/10.3390/molecules29133028
APA StyleFarina, R., Scalese, S., Corso, D., Capuano, G. E., Screpis, G. A., Coniglio, M. A., Condorelli, G. G., & Libertino, S. (2024). Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles. Molecules, 29(13), 3028. https://doi.org/10.3390/molecules29133028









