A Phytotoxin with Selective Herbicidal Activity and Related Metabolites from the Phytopathogenic Fungus Bipolaris cookei SYBL03
Abstract
1. Introduction
2. Results
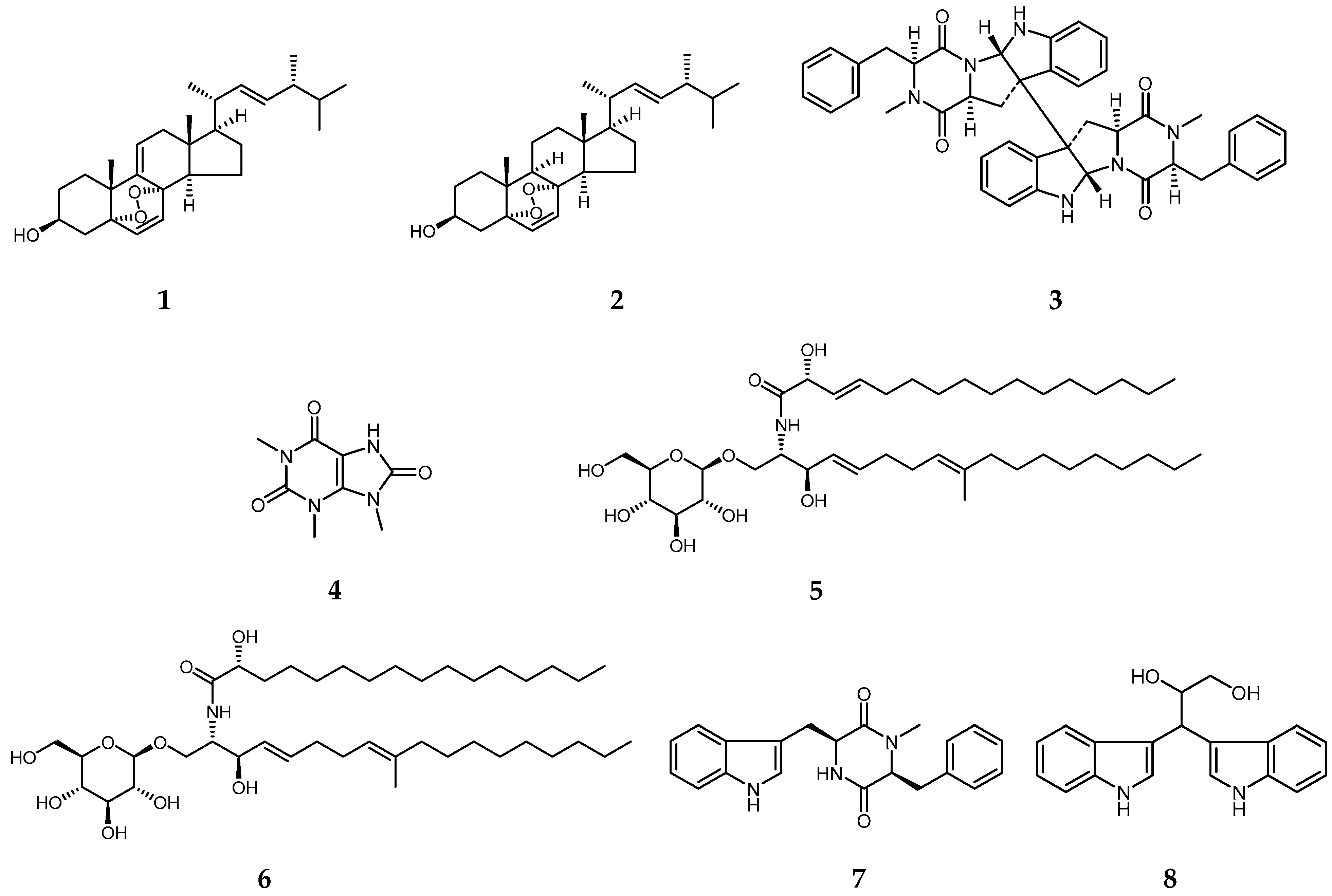
2.1. Isolation, Purification, and Structure Elucidation
2.2. Phytotoxic Activity of Compounds 1–8
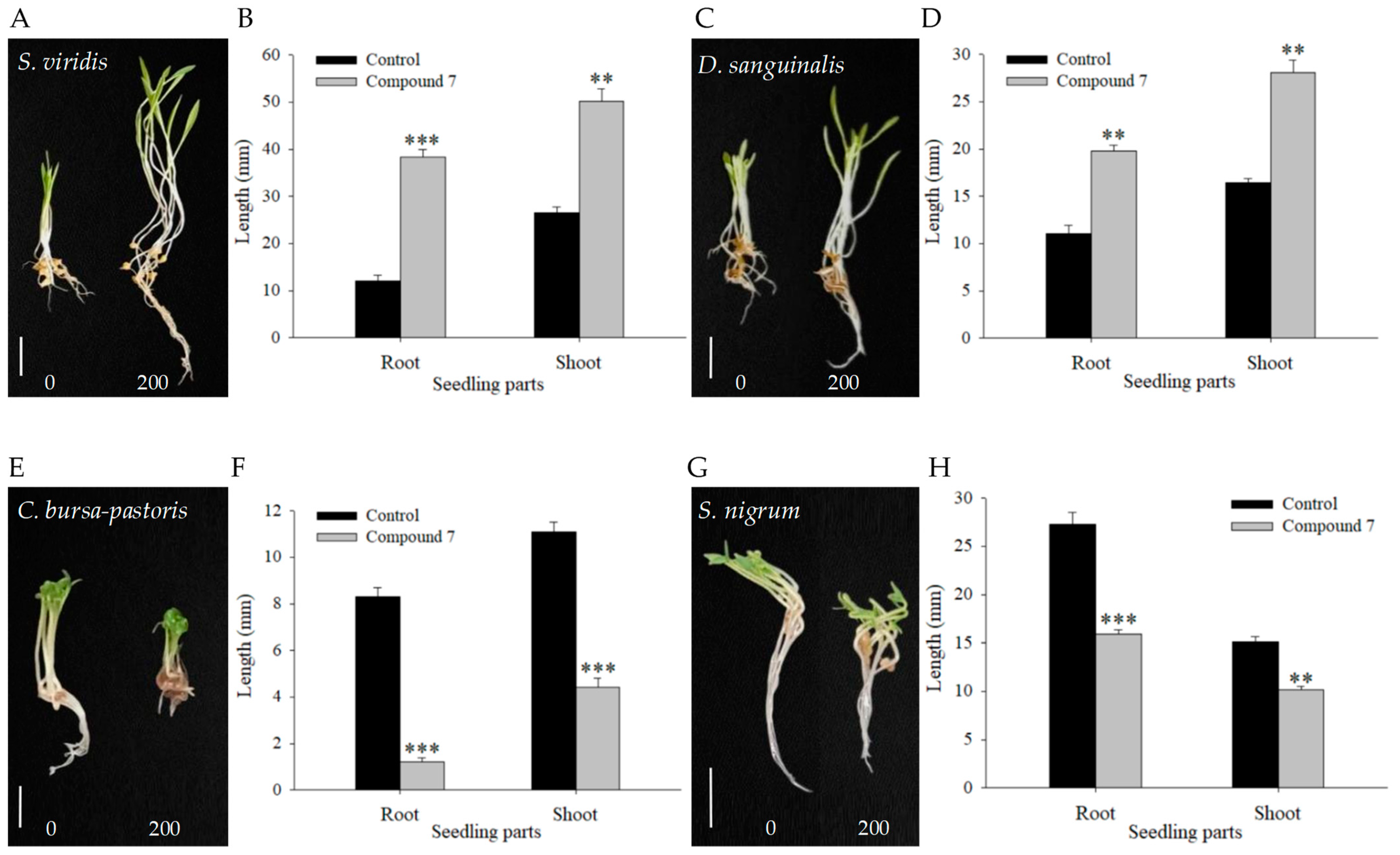
2.3. Effect of Compound 7 on the seedling growth of Monocots and Dicots
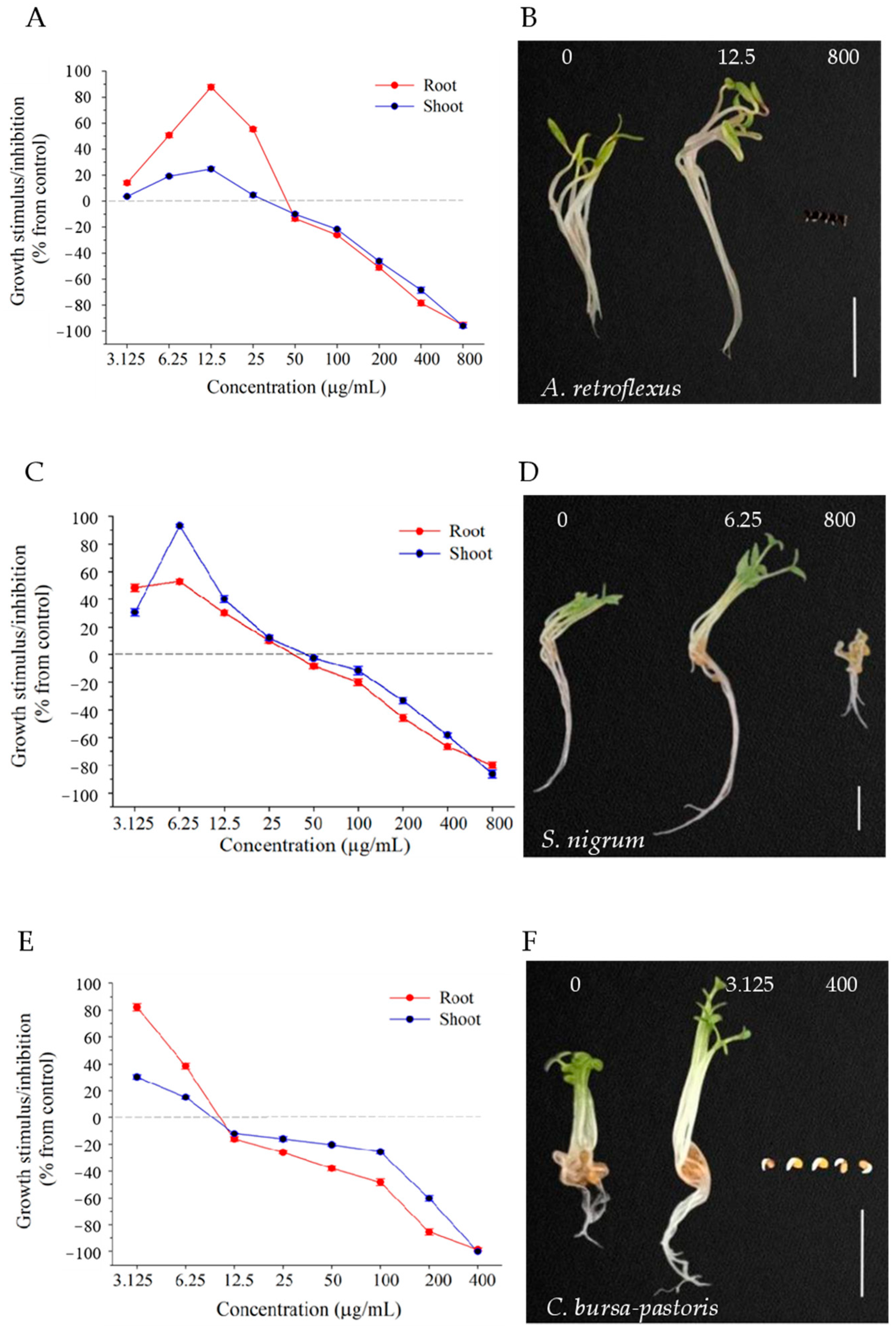
2.4. Effect of Compound 7 at Different Concentrations on Dicotyledons
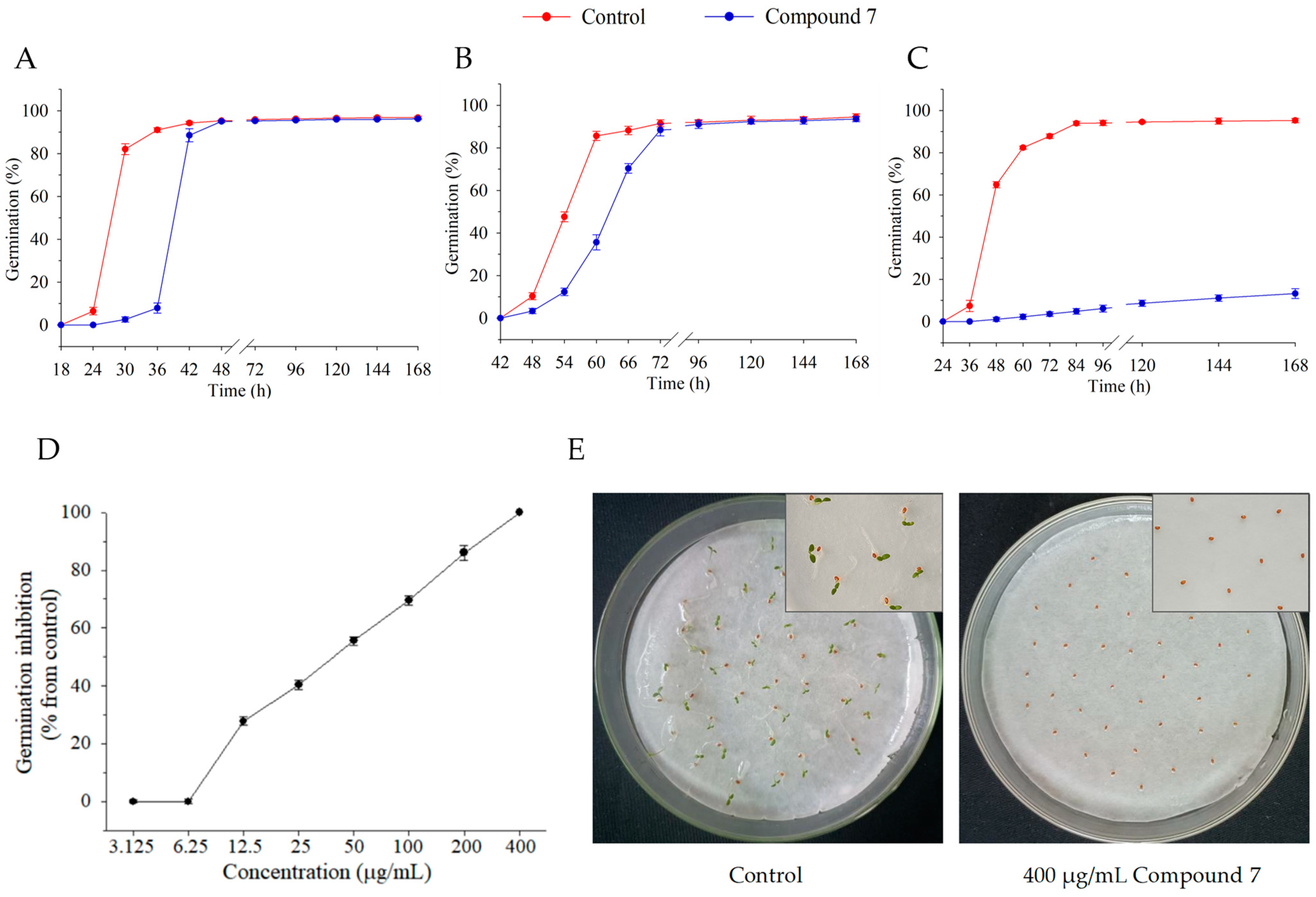
2.5. Effect of Compound 7 on Seed Germination
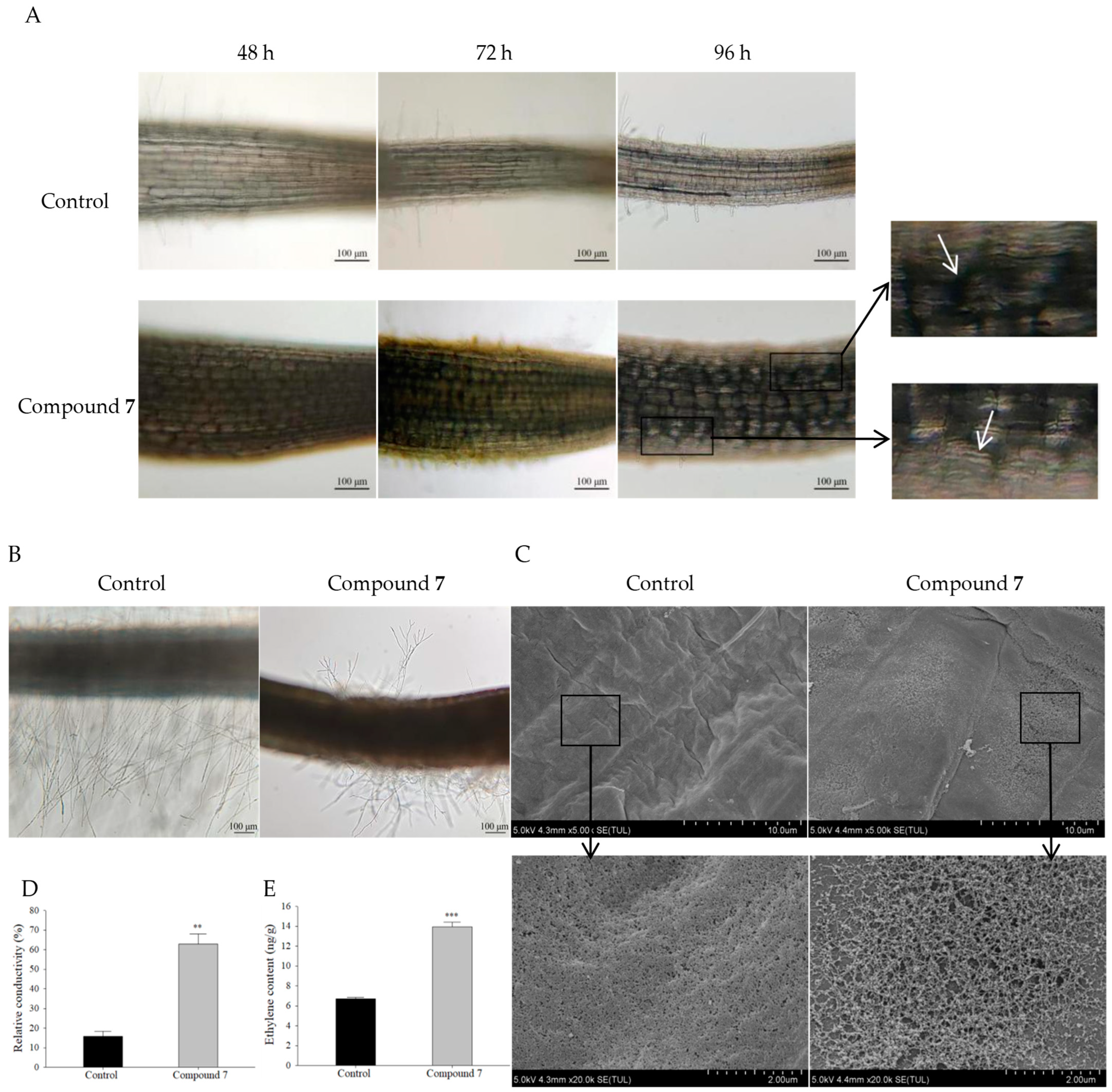
2.6. Inhibitory Mechanism of Compound 7 on A. retroflexus Root Growth
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Fermentation and Extraction
4.4. Fractionation and Purification of Compounds
4.4.1. 5α,8α-Epidioxyergosta-6,9(11),22-trien-3β-ol (1)
4.4.2. Ergosterol Peroxide (2)
4.4.3. (–)-Ditryptophenaline (3)
4.4.4. 1,3,9-Trimethyluric Acid (4)
4.4.5. Cerebroside A (5)
4.4.6. Cerebroside B (6)
4.4.7. Cyclo-N-methylphenylalanyltryptophenyl (7)
4.4.8. (2S)-3,3-di-1H-indol-3-yl-1,2-propanediol (8)
4.5. Herbicidal Activity Assay
4.6. Light Microscopy
4.7. Scanning Electron Microscopy (SEM)
4.8. Relative Conductivity Assay
4.9. Ethylene Content Assay
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects: 2019: Highlights; United Nations: New York, NY, USA, 2019; No. 423. [Google Scholar]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–43. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Council for Agricultural Science and Technology (CAST). The Contributions of Pesticides to Pest Management in Meeting the Global Need for Food Production by 2050; Issue Paper #55; CAST: Ames, IA, USA, 2014. [Google Scholar]
- Sun, Y.; Han, J.; Chen, X.; Guo, H. Advances in measures of reducing chemical pesticides to control plant diseases. Plant Dis. Pests 2021, 12, 1–6. [Google Scholar] [CrossRef]
- Deng, X. A mini review on natural safeners: Chemistry, uses, modes of action, and limitations. Plants 2022, 11, 3509. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Wang, X.; Dong, L. Herbicidal activity of curvulinic acid isolated from Nimbya alternantherae. Nat. Prod. Commun. 2012, 7, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front. Plant Sci. 2022, 13, 1044896. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Liu, J.Q.; Yang, Y.J.; Wang, C.Y.; Zhu, L.; Li, C.Z.; Cai, L.; Ding, Z.T. Phytotoxic meroterpenoids with herbicidal activities from the phytopathogenic fungus Pseudopestalotiopsis theae. Phytochemistry 2023, 206, 113522. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Khiralla, A.; Spin, R.; Saliba, S.; Laurain-Mattar, D. Diversity of natural products of the genera Curvularia and Bipolaris. Fungal Biol. Rev. 2019, 33, 101–122. [Google Scholar] [CrossRef]
- Lim, C.H.; Miyagawa, H.; Akamatsu, M.; Nakagawa, Y.; Ueno, T. Structures and biological activities of phytotoxins produced by the plant pathogenic fungus Bipolaris cynodontis cynA. J. Pestic. Sci. 1998, 23, 281–288. [Google Scholar] [CrossRef][Green Version]
- Lim, C.H.; Miyagawa, H.; Tsurushima, T.; Ueno, T.; Sato, M. Cochlioquinol: A new cochlioquinone derivative produced by the plant pathogenic fungus Bipolaris cynodontis. Biosci. Biotechnol. Biochem. 1996, 60, 724–725. [Google Scholar] [CrossRef][Green Version]
- Pena-Rodriguez, L.M.; Chilton, W.S. Victoxinine and prehelminthosporolactone, two minor phytotoxic metabolites produced by Bipolaris sp., a pathogen of Johnson grass. J. Nat. Prod. 1989, 52, 899–901. [Google Scholar] [CrossRef]
- Arnold, D.; Yazawa, T.; Kawahigashi, H.; Matsumoto, T.; Mizuno, H. Simultaneous transcriptome analysis of sorghum and Bipolaris sorghicola by using RNA-seq in combination with De Novo transcriptome assembly. PLoS ONE 2013, 8, e62460. [Google Scholar] [CrossRef]
- Peng, C.; Ge, T.T.; He, X.L.; Huang, Y.H.; Xu, Z.L.; Zhang, D.Y.; Shao, H.B.; Guo, S.W. Process of Bipolaris sorghicola invasion of host cells. Genet. Mol. Res. 2016, 15, 15016781. [Google Scholar] [CrossRef] [PubMed]
- Zaccaron, A.Z.; Bluhm, B.H. The genome sequence of Bipolaris cookei reveals mechanisms of pathogenesis underlying target leaf spot of sorghum. Sci. Rep. 2017, 7, 17217. [Google Scholar] [CrossRef]
- Moriwaki, A.; Kihara, J.; Kobayashi, T.; Tokunaga, T.; Arase, S.; Honda, Y. Insertional mutagenesis and characterization of a polyketide synthase gene (PKS1) required for melanin biosynthesis in Bipolaris oryzae. FEMS Microbiol. Lett. 2004, 238, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.; Verma, S.; Singh, K.; Aggarwal, R.; Srivastava, K.D.; Verma, P.K. High reliability transformation of the wheat pathogen Bipolaris sorokiniana using Agrobacterium tumefaciens. J. Microbiol. Methods 2012, 88, 386–392. [Google Scholar] [CrossRef]
- Gogoi, R.; Singh, S.; Singh, P.; Sakkaravarthi, K.; Rai, S. Genetic variability in the isolates of Bipolaris maydis causing maydis leaf blight of maize. Afr. J. Agric. Res. 2014, 9, 1906–1913. [Google Scholar] [CrossRef]
- Winder, R.S.; Van Dyke, C.G. The pathogenicity, virulence, and biocontrol potential of two Bipolaris species on John-songrass (Sorghum halepense). Weed Sci. 1990, 38, 89–94. [Google Scholar] [CrossRef]
- Sugawara, F.; Strobel, G.; Strange, R.N.; Siedow, J.N.; Van Duyne, G.D.; Clardy, J. Phytotoxins from the pathogenic fungi Drechslera maydis and Drechslera sorghicola. Proc. Natl. Acad. Sci. USA 1987, 84, 3081–3085. [Google Scholar] [CrossRef]
- Pena-Rodriguez, L.M.; Chilton, W.S. 3-Anhydroophiobolin A and 3-Anhydro-6-epi-ophiobolin A, phytotoxic metabolites of the Johnson grass pathogen Bipolaris sorghicola. J. Nat. Prod. 1989, 52, 1170–1172. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, J.; Shim, S.H. Chemical investigation on an endophytic fungus Gibberella moniliformis JS1055 derived from a halophyte Vitex rotundifolia. Nat. Prod. Sci. 2018, 24, 189–193. [Google Scholar] [CrossRef]
- Ma, J.; Lu, C.; Tang, Y.; Shen, Y. Phytotoxic metabolites isolated from Aspergillus sp., an endophytic fungus of Crassula arborescens. Molecules 2022, 27, 7710. [Google Scholar] [CrossRef] [PubMed]
- Saruwatari, T.; Yagishita, F.; Mino, T.; Noguchi, H.; Hotta, K.; Watanabe, K. Cytochrome P450 as dimerization catalyst in diketopiperazine alkaloid biosynthesis. ChemBioChem 2014, 15, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Koga, J.; Yamauchi, T.; Shimura, M.; Ogawa, N.; Oshima, K.; Umemura, K.; Kikuchi, M.; Ogasawara, N. Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J. Biol. Chem. 1998, 273, 31985–31991. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Choi, J.H.; Cho, W.K.; Park, J.C.; Choi, J.S. A sphingolipid and tyrosinase inhibitors from the fruiting body of Phellinus linteus. Arch. Pharmacal Res. 2004, 27, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sato, T. A mild and environmentally friendly scandium(III) trifluoromethanesulfonate-catalyzed synthesis of bis(3′-indolyl)alkanes and bis(3′-indolyl)-1-deoxyalditols. Carbohydr. Res. 2005, 340, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- She, G.M.; Chen, K.; Zhang, Y.-J.; Yang, C.R. The occurrence of 8-oxocaffeine and pyrimidine alkaloids in Pu-er ripe tea. Acta Bot. Yunnanica 2007, 29, 713–716. [Google Scholar] [CrossRef]
- Bobrownyzky, J. Production of branched root hairs under progressive drought stress in Arabidopsis thaliana. Cytol. Genet. 2016, 50, 324–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zhang, Z.; Li, Y.; Guo, J.; Liu, L.; Wang, C.; Fan, H.; Wang, B.; Han, G. Root hair development and adaptation to abiotic stress. J. Agric. Food Chem. 2023, 71, 9573–9598. [Google Scholar] [CrossRef]
- Iqbal, N.; Trivellini, A.; Masood, A.; Ferrante, A.; Khan, N.A. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol. Biochem. 2013, 73, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.Y.; He, B.; Yang, J.F.; Lin, H.Y.; Yang, W.C.; Wu, Q.Y.; Li, Q.X.; Yang, G.F. Where are the new herbicides? Pest Manag. Sci. 2021, 77, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Otomo, N.; Sato, H.; Sakamura, S. Novel phytotoxins produced by the causal fungus of the shoot blight of larches. Agric. Biol. Chem. 1983, 47, 1115–1119. [Google Scholar] [CrossRef]
- Parhira, S.; Zhu, G.Y.; Li, T.; Liu, L.; Bai, L.P.; Jiang, Z.H. Inhibition of IKK-β by epidioxysterols from the flowers of Calotropis gigantea (Niu jiao gua). Chin. Med. 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.P.; Bűchi, G.; Kobbe, B.; Demain, A.L.; Clardy, J. The structure of ditryptophenaline—A new metabolite of Aspergillus flavus. Tetrahedron Lett. 1977, 18, 2403–2406. [Google Scholar] [CrossRef]
- Sitrin, R.D.; Chan, G.; Dingerdissen, J.; DeBrosse, C.; Mehta, R.; Roberts, G.; Rottschaefer, S.; Staiger, D.; Valenta, J.; Snader, K.M.; et al. Isolation and structure determination of Pachybasium cerebrosides which potentiate the antifungal activity of aculeacin. J. Antibiot. 1988, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; El-Shazly, M.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory cerebrosides from cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hu, Q.B. Secondary metabolites of Purpureocillium lilacinum. Molecules 2021, 27, 18. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Ogawa, N.; Yamauchi, T.; Iwata, M.; Shimura, M.; Koga, J. Cerebroside elicitors found in diverse phytopathogens activate defense responses in rice plants. Plant Cell Physiol. 2000, 41, 676–683. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Yuan, W.P.; Xia, X.K.; Liu, X.; Meng, X.M.; Wang, X.J.; Zhang, M.S.; Liu, C.H. Isolation and identification of the nematicidal secondary metabolitesfrom one strain of entomogenous fungi. Chin. J. Pestic. Sci. 2010, 12, 225–228. [Google Scholar] [CrossRef]
- Kawai, G.; Ikeda, Y. Chemistry and functional moiety of a fruiting-inducing cerebroside in Schizophyllum commune. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1983, 754, 243–248. [Google Scholar] [CrossRef]
- Li, L.; Yang, R.; Sun, K.; Bai, Y.; Zhang, Z.; Zhou, L.; Qi, Z.; Qi, J.; Chen, L. Cerebroside-A provides potent neuroprotection after cerebral ischaemia through reducing glutamate release and Ca2+ influx of NMDA receptors. Int. J. Neuropsychopharmacol. 2012, 15, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Tanino, S.; Nagatsuka, T.; Koga, J.; Iwata, M.; Nagashima, K.; Amemiya, Y. Cerebroside elicitor confers resistance to fusarium disease in various plant species. Phytopathology 2004, 94, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.K.; Bacon, C.W.; Robbins, J.D.; Himmelsbach, D.S.; Higman, H.C. Indole alkaloids from Balansia epichloe (Weese). J. Agric. Food Chem. 1977, 25, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Han, Y.; Wang, Y.; Zhang, X.H.; Zhu, W.M. Bis- and tris- indole alkaloids from Edwardsiella tarda. Microbiol. China 2010, 37, 1325–1330. [Google Scholar] [CrossRef]
- Wieland, P.; Prelog, V. On the isolation of ergosterol, ergosterol palmitate and ergosterol peroxide from the mycelium of Aspergillus fumigatus, mut. helvola, Yuill. Helv. Chim. Acta 1947, 30, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ge, X.; Wang, J.; Wei, Z.; Feng, W.H.; Wang, J. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-κB and p38/MAPK signaling pathways in vitro. Int. Immunopharmacol. 2021, 93, 107317. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.H.; Jeong, S.J.; Lee, H.J.; Lee, H.J.; Koh, W.; Jung, J.H.; Kim, S.H.; Sung-Hoon, K. Inhibition of STAT3 signaling and induction of SHP1 mediate antiangiogenic and antitumor activities of ergosterol peroxide in U266 multiple myeloma cells. BMC Cancer 2012, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef]
- Wang, F.W. Bioactive metabolites from Guignardia sp., an endophytic fungus residing in Undaria pinnatifida. Chin. J. Nat. Med. 2012, 10, 72–76. [Google Scholar] [CrossRef]
- Macías, F.A.; Chinchilla, N.; Varela, R.M.; Molinillo, J.M. Bioactive steroids from Oryza sativa L. Steroids 2006, 71, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.L.; Li, G.H.; Liu, F.F.; Lei, L.P.; Xia, Z.Y.; Zhang, K.Q. A new sesquiterpene from endophytic fungus Aspergillus sp. Nat. Prod. Res. 2012, 26, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Cobb, A.H.; Reade, J.P.H. (Eds.) Auxin-type herbicides. In Herbicides and Plant Physiology, 2nd ed.; Wiley-Blackwell: West Sussex, UK, 2010; pp. 133–155. [Google Scholar]
- Grossmann, K. Mediation of herbicide effects by hormone interactions. J. Plant Growth Regul. 2003, 22, 109–122. [Google Scholar] [CrossRef]
- Sterling, T. Mechanism of action of natural auxins and the auxinic herbicides. Rev. Toxicol. 1997, 1, 111–141. [Google Scholar]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- DeBolt, S.; Gutierrez, R.; Ehrhardt, D.W.; Melo, C.V.; Ross, L.; Cutler, S.R.; Somerville, C.; Bonetta, D. Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc. Natl. Acad. Sci. USA 2007, 104, 5854–5859. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L. Root hair development in grasses and cereals (Poaceae). Curr. Opin. Genet. Dev. 2017, 45, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root Hairs. Arab. Book 2014, 12, e0172. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.; Zhang, Y.; Liu, W. Enantioselectivity in the phytotoxicity of herbicide imazethapyr. J. Agric. Food Chem. 2009, 57, 1624–1631. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Watson, S.B. Plant cell membrane as a marker for light-dependent and light-independent herbicide mechanisms of action. Pestic. Biochem. Physiol. 2011, 101, 182–190. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Ethylene in Plant Biology, 2nd ed.; Academic Press Inc.: San Diego, CA, USA, 1992. [Google Scholar]
- Wang, Y.; Ji, Y.; Fu, Y.; Guo, H. Ethylene-induced microtubule reorientation is essential for fast inhibition of root elongation in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Xu, X.D.; Xu, J.; Liu, K.J.; Jiang, Y.; Hu, L. Pathogen population diversity of target leaf spot in sorghum. J. Shenyang Agric. Univ. 2012, 43, 163–167. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Method, 2nd ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1995. [Google Scholar]
- Liu, W.W.; Qi, H.Y.; Xu, B.H.; Li, Y.; Tian, X.B.; Jiang, Y.Y.; Xu, X.F.; Lv, D.Q. Ethanol treatment inhibits internal ethylene concentrations and enhances ethyl ester production during storage of oriental sweet melons (Cucumis melo var. makuwa Makino). Postharvest Biol. Technol. 2012, 67, 75–83. [Google Scholar] [CrossRef]

| Compounds | A. Retroflexus | E. crus-galli | ||
|---|---|---|---|---|
| Shoot Length (mm) | Root Length (mm) | Shoot Length (mm) | Root Length (mm) | |
| 1 | 11.0 ± 0.3 b | 20.3 ± 0.3 b | 30.2 ± 2.1 b | 9.6 ± 0.3 b |
| 2 | 8.7 ± 0.3 c | 11.1 ± 0.3 c | 33.3 ± 2.5 b | 9.1 ± 0.7 b |
| 3 | 15.0 ± 0.6 a | 24.0 ± 0.4 a | 31.9 ± 1.6 b | 9.6 ± 0.9 b |
| 4 | 14.4 ± 0.4 a | 24.8 ± 0.5 a | 32.8 ± 2.2 b | 9.8 ± 1.1 b |
| 5 | 16.1 ± 0.3 a | 25.4 ± 0.2 a | 31.4 ± 2.1 b | 9.8 ± 1.6 b |
| 6 | 15.7 ± 0.4 a | 26.9 ± 0.3 a | 31.0 ± 2.9 b | 10.2 ± 1.0 b |
| 7 | 7.7 ± 0.3 c | 12.2 ± 0.3 c | 46.0 ± 2.7 a | 22.8 ± 1.7 a |
| 8 | 14.8 ± 0.5 a | 23.9 ± 0.3 a | 32.3 ± 2.4 b | 10.1 ± 1.6 b |
| Control | 14.3 ± 0.4 a | 25.2 ± 0.7 a | 31.4 ± 2.7 b | 9.7 ± 0.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hou, J.; Li, B.; Zhang, L.; Yu, Z. A Phytotoxin with Selective Herbicidal Activity and Related Metabolites from the Phytopathogenic Fungus Bipolaris cookei SYBL03. Molecules 2024, 29, 3040. https://doi.org/10.3390/molecules29133040
Li H, Hou J, Li B, Zhang L, Yu Z. A Phytotoxin with Selective Herbicidal Activity and Related Metabolites from the Phytopathogenic Fungus Bipolaris cookei SYBL03. Molecules. 2024; 29(13):3040. https://doi.org/10.3390/molecules29133040
Chicago/Turabian StyleLi, Haiyan, Jingzhuo Hou, Bing Li, Lizhong Zhang, and Zhiguo Yu. 2024. "A Phytotoxin with Selective Herbicidal Activity and Related Metabolites from the Phytopathogenic Fungus Bipolaris cookei SYBL03" Molecules 29, no. 13: 3040. https://doi.org/10.3390/molecules29133040
APA StyleLi, H., Hou, J., Li, B., Zhang, L., & Yu, Z. (2024). A Phytotoxin with Selective Herbicidal Activity and Related Metabolites from the Phytopathogenic Fungus Bipolaris cookei SYBL03. Molecules, 29(13), 3040. https://doi.org/10.3390/molecules29133040





