Optimizing the Extraction of the Polyphenolic Fraction from Defatted Strawberry Seeds for Tiliroside Isolation Using Accelerated Solvent Extraction Combined with a Box–Behnken Design
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Conditions for Accelerated Solvent Extraction (ASE)
2.1.1. Extraction Yield
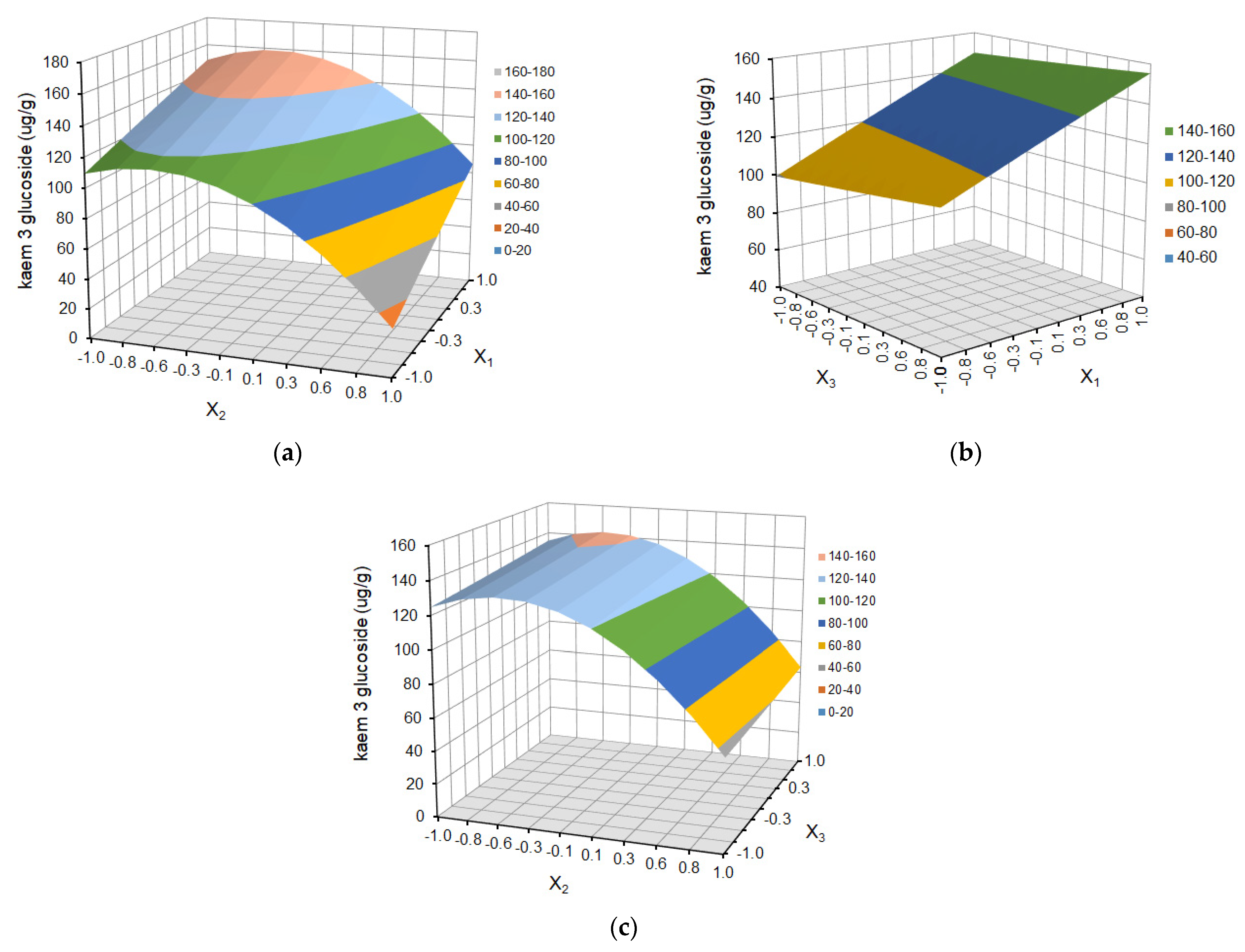
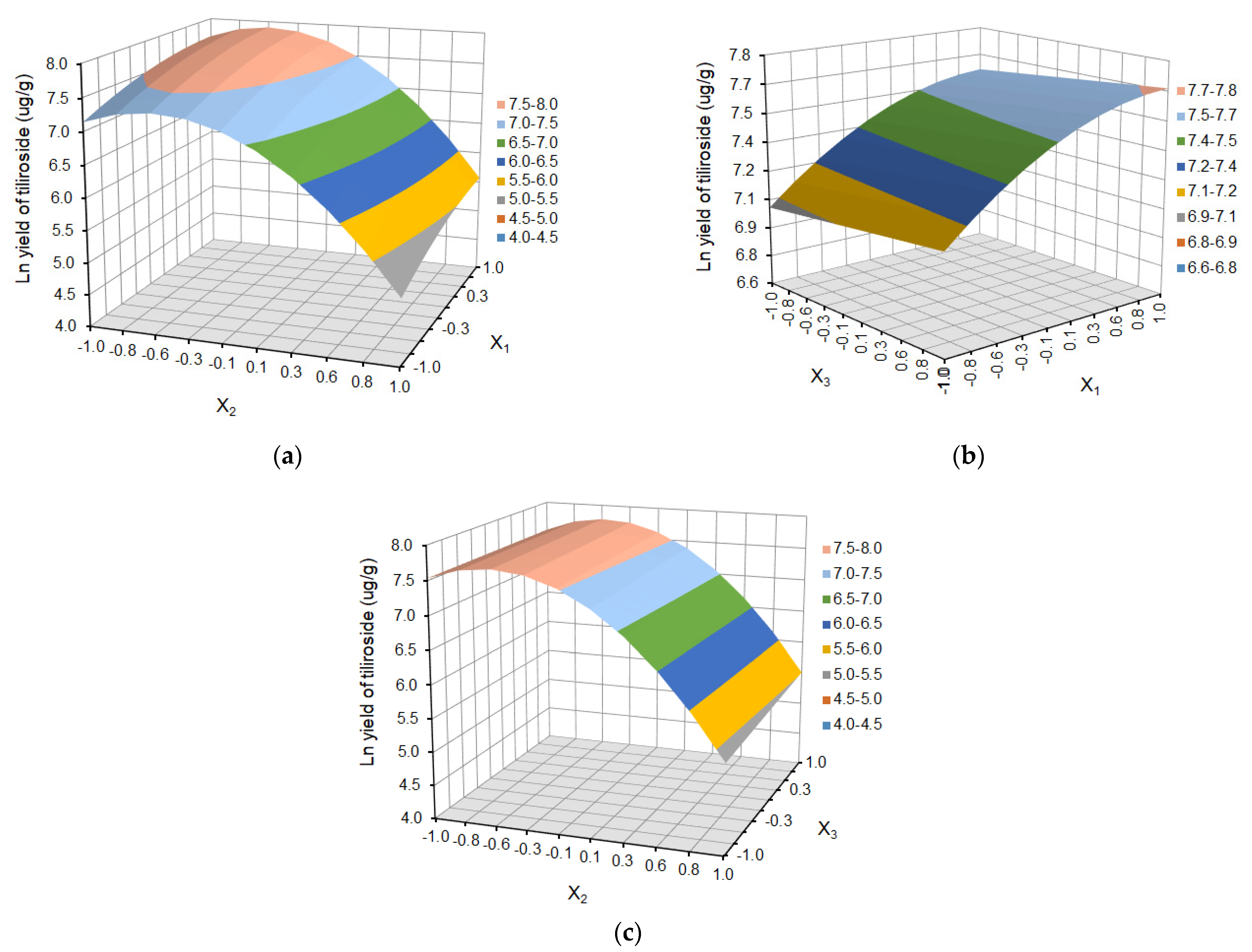
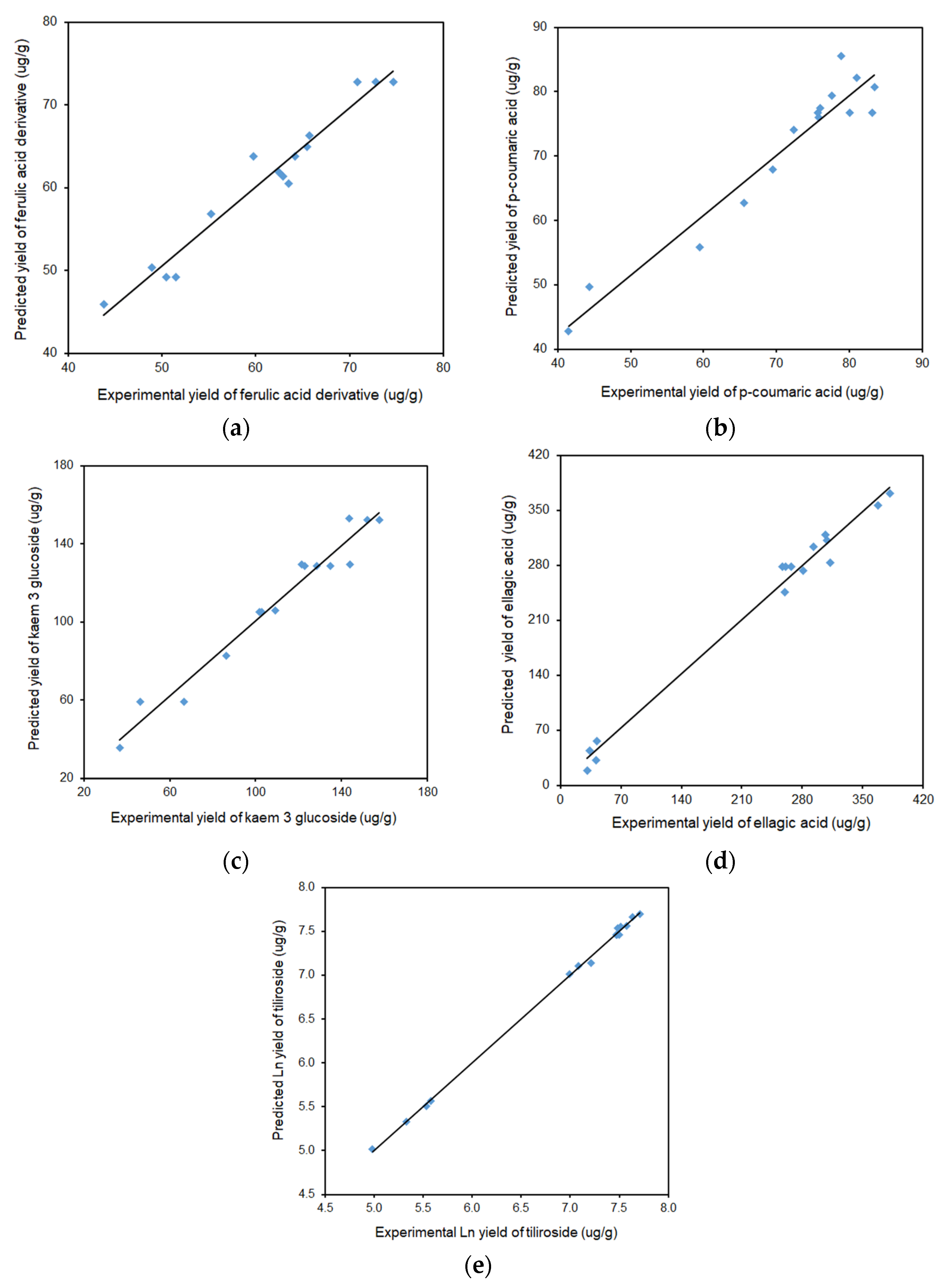
2.1.2. Individual Polyphenol Yield of Defatted Strawberry Seeds
2.2. Chromatographic Analysis and Isolation
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.3. Chromatography
3.3.1. Ultra-High-Performance Liquid Chromatography (UPLC)
3.3.2. Preparative Chromatography (pLC)
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, G.; Pereira, A.; Rezende, B.; Da Silva, J.; Cruz, J.; De Souza, M.; Gomes, R.; Teles, Y.; Cortes, S.; Lemos, V. Mechanism of the Antihypertensive and Vasorelaxant Effects of the Flavonoid Tiliroside in Resistance Arteries. Planta Med. 2013, 79, 1003–1008. [Google Scholar] [CrossRef]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a Dietary Glycosidic Flavonoid, Inhibits TRAF-6/NF-κB/P38-Mediated Neuroinflammation in Activated BV2 Microglia. Biochim. Biophys. Acta BBA-Gen. Subj. 2014, 1840, 3311–3319. [Google Scholar] [CrossRef]
- Goto, T.; Horita, M.; Nagai, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Nagaoka, S. Tiliroside, a Glycosidic Flavonoid, Inhibits Carbohydrate Digestion and Glucose Absorption in the Gastrointestinal Tract. Mol. Nutr. Food Res. 2012, 56, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Zhao, C.; Qin, N.; Zhai, H.Y.; Duan, H.Q. Identification of Trans-Tiliroside as Active Principle with Anti-Hyperglycemic, Anti-Hyperlipidemic and Antioxidant Effects from Potentilla Chinesis. J. Ethnopharmacol. 2011, 135, 515–521. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Liu, Y.; Chu, H.; Duan, H. Synthesis and Biological Activity of Trans-Tiliroside Derivatives as Potent Anti-Diabetic Agents. Molecules 2010, 15, 9174–9183. [Google Scholar] [CrossRef]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the Major Component of Agrimonia Pilosa Ledeb Ethanol Extract, Inhibits MAPK/JNK/P38-Mediated Inflammation in Lipopolysaccharide-Activated RAW 264.7 Macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.-L. Assessment of the Anti-Inflammatory Activity and Free Radical Scavenger Activity of Tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Wikantyasning, E.; Wahyuni, A.; Kusumawati, I.; Saifudin, A.; Suhendi, A. Antiproliferative Properties of Tiliroside from Guazuma Ulmifolia Lamk on T47D and MCF7 Cancer Cell Lines. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 627. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the P-Coumaroyl Moiety in the Antioxidant and Cytoprotective Effects of Flavonoid Glycosides: Comparison of Astragalin and Tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef]
- Carola, C.; Pfluecker, F.; Graf, R.; Epstein, H.; Wirth, C. Tiliroside and Dihydroxy Methylchromone: From Nature to Cosmetic Applications. J. Appl. Cosmetol. 2010, 28, 109–123. [Google Scholar]
- Takeda, S.; Shimoda, H.; Takarada, T.; Imokawa, G. Strawberry Seed Extract and Its Major Component, Tiliroside, Promote Ceramide Synthesis in the Stratum Corneum of Human Epidermal Equivalents. PLoS ONE 2018, 13, e0205061. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Wei, D.; Wang, Z.; Tao, X. Tyrosinase Inhibitory Effect and Inhibitory Mechanism of Tiliroside from Raspberry. J. Enzyme Inhib. Med. Chem. 2009, 24, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A Review on the Dietary Flavonoid Tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Bazylko, A.; Staszewska, A. Determination of Polyphenolics in Extracts of Potentilla Species by High-performance Thin-layer Chromatography Photodensitometry Method. Phytochem. Anal. 2010, 21, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lin, J.; Yang, Y.; Abdulla, R.; Chen, J.; Aisa, H.A. Preparative Isolation of Three Flavonoids from Flos Gossypii by High-Speed Counter-Current Chromatography. Sep. Purif. Technol. 2009, 66, 295–298. [Google Scholar] [CrossRef]
- Nowak, R. Separation and Quantification of Tiliroside from Plant Extracts by SPE/RP-HPLC. Pharm. Biol. 2003, 41, 627–630. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Podgórski, R. Influence of Different Extraction Methods on the Quantification of Selected Flavonoids and Phenolic Acids from Tilia Cordata Inflorescence. Ind. Crops Prod. 2015, 76, 509–514. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Nowak, R.; Pielczyk, J.; Łamacz, K. Optimization of Extraction Conditions for Determination of Tiliroside in Tilia L. Flowers Using an LC-ESI-MS/MS Method. J. Anal. Methods Chem. 2019, 2019, 9052425. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Q.; Song, Z.-J.; Xu, H.-H. A New Antifungal and Cytotoxic C-Methylated Flavone Glycoside from Picea Neoveitchii. Bioorg. Med. Chem. Lett. 2012, 22, 5819–5822. [Google Scholar] [CrossRef]
- Gevrenova, R.; Badjakov, I.; Nikolova, M.; Doichinova, I. Phenolic Derivatives in Raspberry (Rubus L.) Germplasm Collection in Bulgaria. Biochem. Syst. Ecol. 2013, 50, 419–427. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Profile of Potentilla Thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Wójciak, W.; Żuk, M.; Sowa, I.; Mazurek, B.; Tyśkiewicz, K.; Wójciak, M. Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential. Appl. Sci. 2024, 14, 783. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Lv, G.; Xu, Y.; Xu, L.; Qi, Y.; Zheng, L.; Peng, J. Multiple Compounds Determination and Fingerprint Analysis of Agrimonia Pilosa Ledeb by High-Performance Liquid Chromatography. Acta Chromatogr. 2014, 26, 137–156. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Christaki, S.; Biliaderis, C.G.; Chatzopoulou, P. Sustainable Recovery of Phenolic Compounds from Distilled Rosemary By-Product Using Green Extraction Methods: Optimization, Comparison, and Antioxidant Activity. Molecules 2023, 28, 6669. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, M.; Chadni, M.; M’hiri, N.; Brunissen, F.; Rokbeni, N.; Allaf, K.; Besombes, C.; Ioannou, I.; Boudhrioua, N. Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules 2023, 28, 1858. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Náthia-Neves, G.; Tarone, A.G.; Tosi, M.M.; Maróstica Júnior, M.R.; Meireles, M.A.A. Extraction of Bioactive Compounds from Genipap (Genipa Americana L.) by Pressurized Ethanol: Iridoids, Phenolic Content and Antioxidant Activity. Food Res. Int. 2017, 102, 595–604. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed Fluids for the Extraction of Bioactive Compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Álvarez-Romero, M.; Ruíz-Rodríguez, A.; Barbero, G.F.; Vázquez-Espinosa, M.; El-Mansouri, F.; Brigui, J.; Palma, M. Comparison between Ultrasound- and Microwave-Assisted Extraction Methods to Determine Phenolic Compounds in Barley (Hordeum vulgare L.). Foods 2023, 12, 2638. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Karlińska, E.; Grzęda, K.; Rój, E.; Kołodziejczyk, K. Defatted Strawberry Seeds as a Source of Phenolics, Dietary Fiber and Minerals. LWT 2017, 84, 18–22. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General Method for Extraction of Blueberry Anthocyanins and Identification Using High Performance Liquid Chromatography–Electrospray Ionization-Ion Trap-Time of Flight-Mass Spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin Extraction from Plant Tissues: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-J.; Gao, Y.; Liu, Y.; Zheng, X.-Q.; Ye, J.-H.; Liang, Y.-R.; Lu, J.-L. Studies on the Mechanism of Efficient Extraction of Tea Components by Aqueous Ethanol. Food Chem. 2016, 194, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Kim, M.-T.; Truong, Q.-C.; Nguyen, M.-T.; Cao-Thi, B.-H.; Tong, T.-D.; Dao, T.P.; Tran, T.H.; Van Tan, L.; Le, X.-T. Optimized Extraction of Polyphenols from Leaves of Rosemary (Rosmarinus officinalis L.) Grown in Lam Dong Province, Vietnam, and Evaluation of Their Antioxidant Capacity. Open Chem. 2021, 19, 1043–1051. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal Degradation of Grape Marc Polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Darra, N.E.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure High-temperature Extraction of Phenolic Compounds from Grape Skins. Int. J. Food Sci. Technol. 2012, 47, 399–405. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Portugal, L.A.; Dos Anjos, J.P.; De Jesus, O.N.; De Oliveira, E.J.; David, J.P.; David, J.M. Accelerated Solvent Extraction of Phenolic Compounds Exploiting a Box-Behnken Design and Quantification of Five Flavonoids by HPLC-DAD in Passiflora Species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of Drying Process and Pressurized Liquid Extraction for Recovery of Bioactive Compounds from Avocado Peel By-product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized Liquid Extraction of Bioactive Compounds from Blackberry (Rubus fruticosus L.) Residues: A Comparison with Conventional Methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Owolabi, R.U.; Usman, M.A.; Kehinde, A.J. Modelling and Optimization of Process Variables for the Solution Polymerization of Styrene Using Response Surface Methodology. J. King Saud Univ.-Eng. Sci. 2018, 30, 22–30. [Google Scholar] [CrossRef]
- Klongdee, S.; Klinkesorn, U. Optimization of Accelerated Aqueous Ethanol Extraction to Obtain a Polyphenol-Rich Crude Extract from Rambutan (Nephelium lappaceum L.) Peel as Natural Antioxidant. Sci. Rep. 2022, 12, 21153. [Google Scholar] [CrossRef] [PubMed]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.E.; Mazza, G. Optimization of Extraction of Anthocyanins from Black Currants with Aqueous Ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Extraction Techniques for the Determination of Phenolic Compounds in Food. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 159–180. [Google Scholar] [CrossRef]
- Bruno, T.; Oscar, N.; Eacute, S.; Ernestine, Z.; Marie, T.; Lazare, S.; Fabrice, F.; Schneide, S. Chemical Constituents of Dissotis Perkinsiae (Melastomaceae) and Their Antimicrobial Activity. J. Appl. Pharm. Sci. 2016, 6, 096–101. [Google Scholar] [CrossRef]
- Wójciak, M.; Mazurek, B.; Tyśkiewicz, K.; Kondracka, M.; Wójcicka, G.; Blicharski, T.; Sowa, I. Blackcurrant (Ribes nigrum L.) Seeds—A Valuable Byproduct for Further Processing. Molecules 2022, 27, 8679. [Google Scholar] [CrossRef]
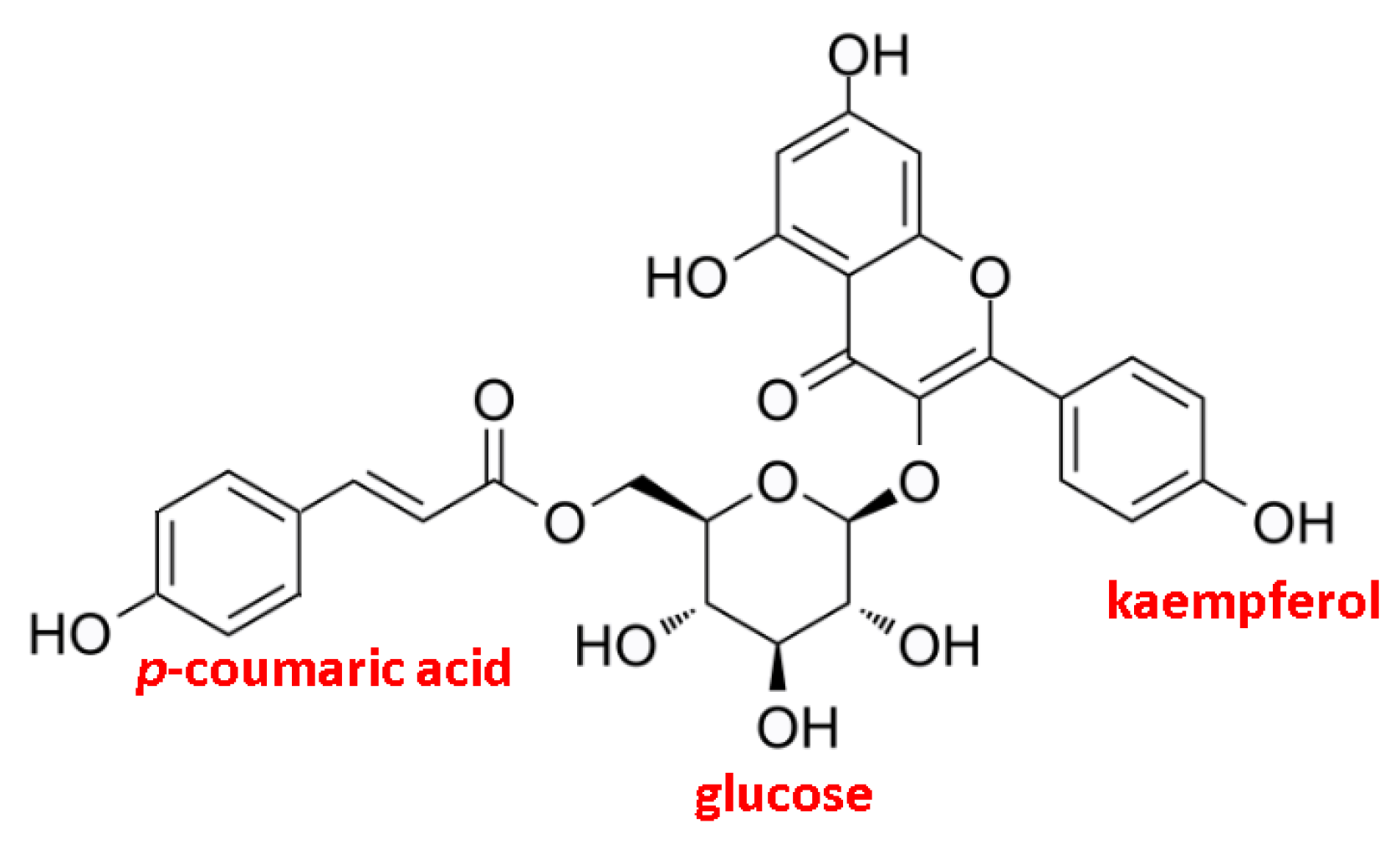

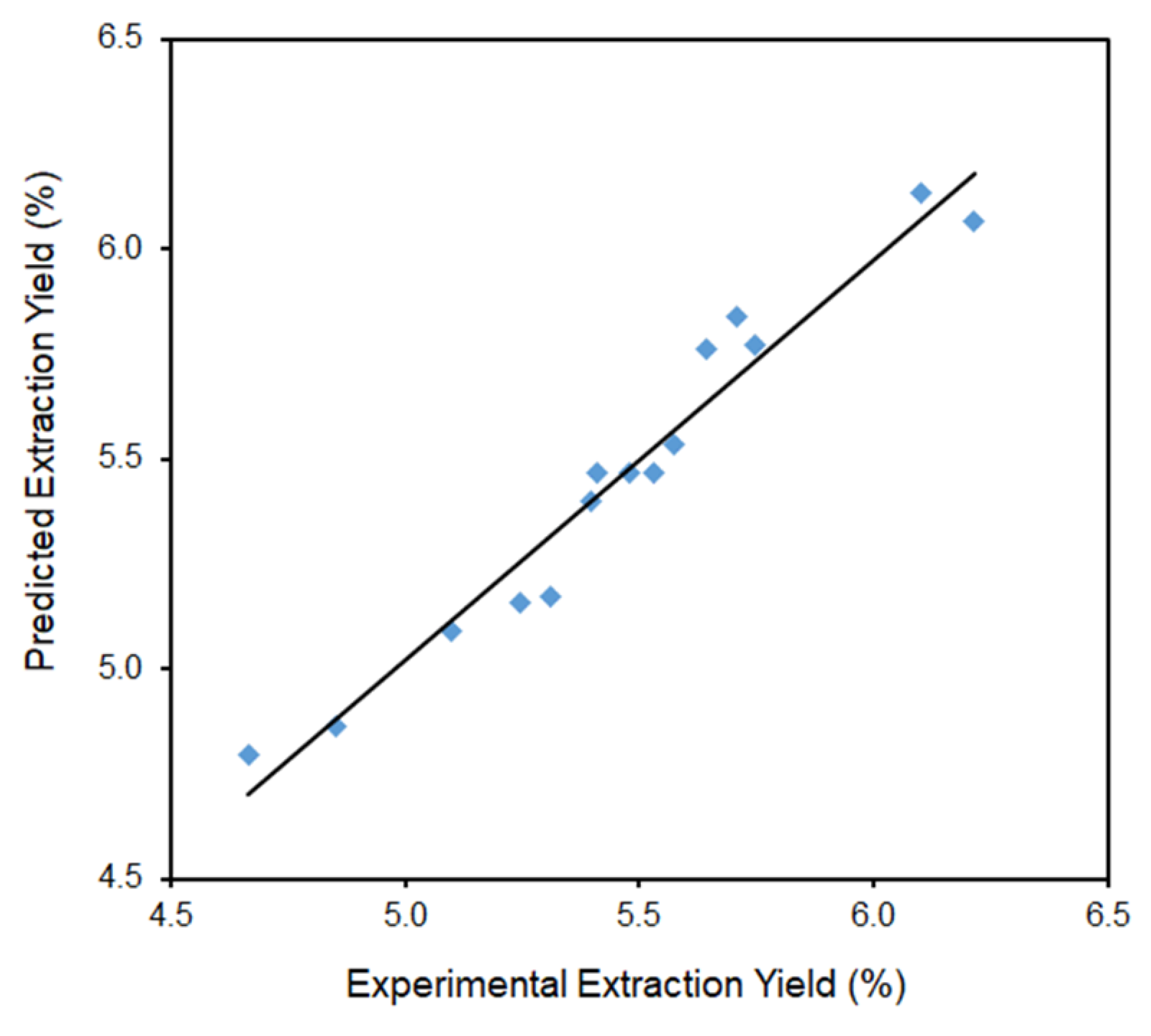
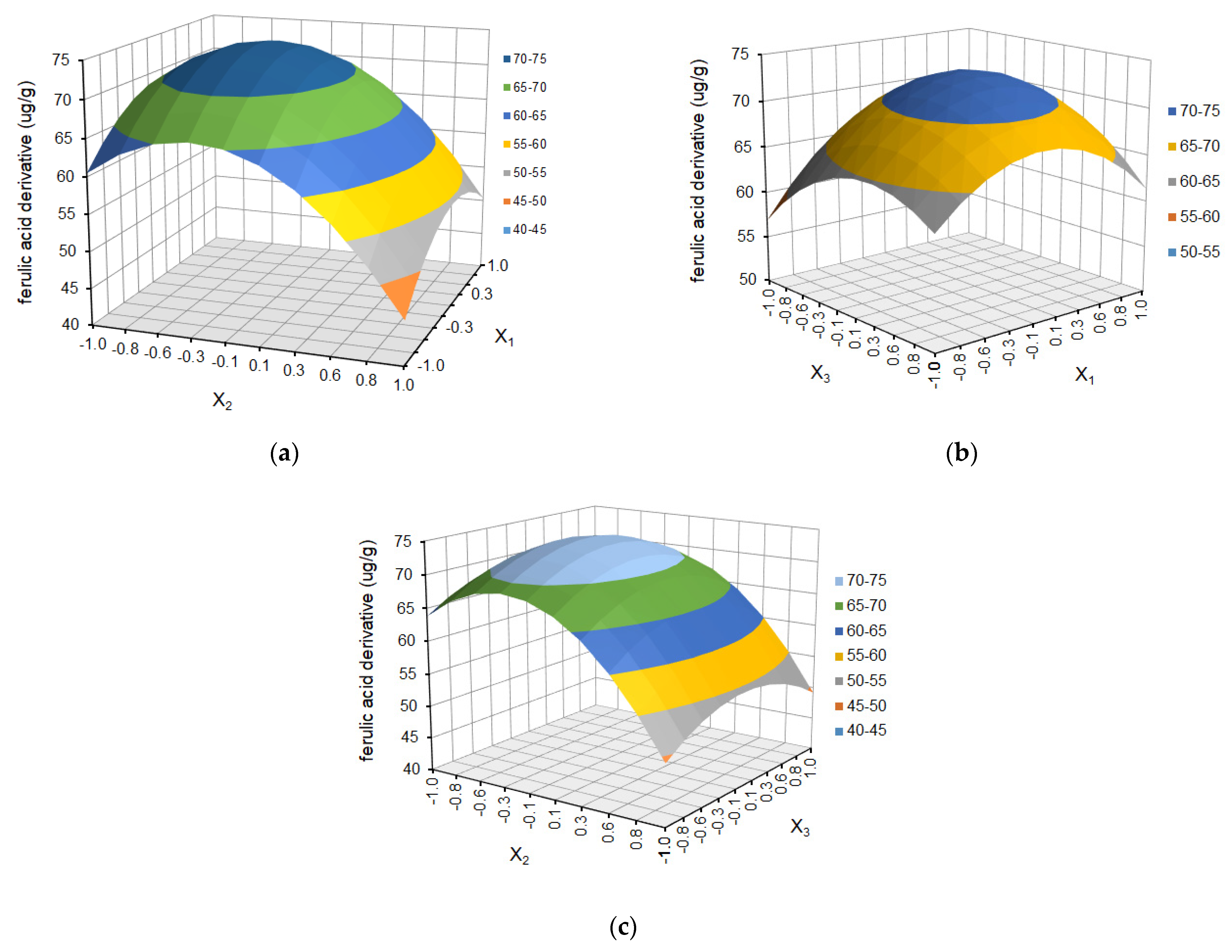
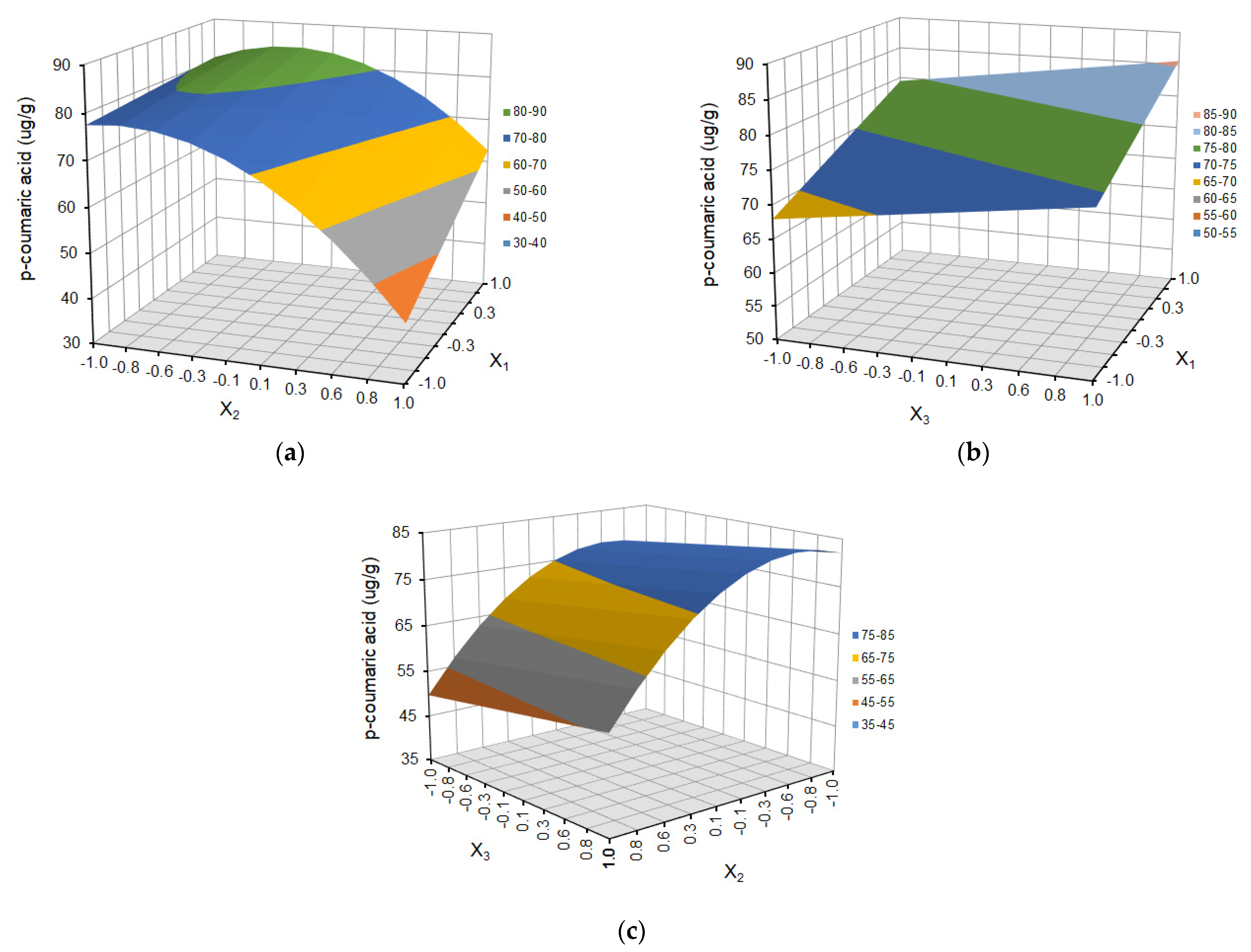

| Run Order | Independent Variables | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | FAd | p-CA | 3-gluK | EA | Tiliroside | Yield (%) | |
| 1 | 0 | 0 | 0 | 74.67 ± 1.70 | 83.10 ± 2.22 | 134.80 ± 2.71 | 257.44 ± 1.20 | 1757.4 ± 32.1 | 5.41 ± 0.07 |
| 2 | −1 | 0 | 1 | 62.49 ± 0.75 | 72.33 ± 1.13 | 101.88 ± 2.88 | 280.55 ± 1.52 | 1196.0 ± 45.6 | 5.40 ± 0.02 |
| 3 | 1 | 0 | −1 | 65.70 ± 0.69 | 77.57 ± 1.50 | 157.52 ± 3.60 | 312.61 ± 2.38 | 1948.1 ± 8.8 | 5.58 ± 0.05 |
| 4 | −1 | 0 | −1 | 55.28 ± 1.68 | 69.49 ± 1.16 | 102.84 ± 0.29 | 260.21 ± 1.75 | 1085.3 ± 24.6 | 5.31 ± 0.10 |
| 5 | 0 | 1 | 1 | 50.44 ± 0.79 | 59.47 ± 0.82 | 66.67 ± 1.25 | 41.28 ± 2.26 | 252.32 ± 4.87 | 6.22 ± 0.13 |
| 6 | 0 | 0 | 0 | 72.84 ± 1.41 | 75.65 ± 4.94 | 128.50 ± 1.69 | 267.20 ± 3.46 | 1766.1 ± 14.2 | 5.48 ± 0.02 |
| 7 | 1 | 0 | 1 | 62.88 ± 2.32 | 78.84 ± 0.98 | 151.83 ± 6.94 | 308.69 ± 3.32 | 2064.7 ± 36.3 | 5.65 ± 0.09 |
| 8 | 0 | 1 | −1 | 51.50 ± 1.69 | 44.36 ± 1.49 | 46.35 ± 3.42 | 34.29 ± 1.26 | 205.75 ± 17.3 | 5.71 ± 0.06 |
| 9 | 0 | −1 | 1 | 64.22 ± 0.51 | 81.03 ± 0.44 | 143.84 ± 1.56 | 381.02 ± 11.7 | 1840.5 ± 5.4 | 5.10 ± 0.07 |
| 10 | 0 | −1 | −1 | 59.76 ± 0.20 | 75.81 ± 1.37 | 121.50 ± 0.14 | 293.37 ± 2.76 | 1775.9 ± 32.3 | 4.85 ± 0.18 |
| 11 | 0 | 0 | 0 | 70.85 ± 2.39 | 80.09 ± 1.13 | 122.82 ± 4.01 | 261.01 ± 1.62 | 1805.3 ± 14.4 | 5.53 ± 0.04 |
| 12 | 1 | −1 | 0 | 65.45 ± 1.08 | 83.43 ± 1.24 | 143.37 ± 1.64 | 367.91 ± 3.20 | 2230.1 ± 19.3 | 5.25 ± 0.04 |
| 13 | 1 | 1 | 0 | 48.93 ± 6.07 | 65.57 ± 1.32 | 86.48 ± 3.40 | 42.36 ± 1.26 | 264.35 ± 6.91 | 6.10 ± 0.16 |
| 14 | −1 | 1 | 0 | 43.79 ± 1.81 | 41.48 ± 1.53 | 36.64 ± 3.69 | 31.13 ± 1.16 | 145.8 ± 5.29 | 5.75 ± 0.11 |
| 15 | −1 | −1 | 0 | 63.53 ± 2.63 | 76.02 ± 2.49 | 109.01 ± 11.9 | 307.05 ± 17.8 | 1353.4 ± 19.4 | 4.67 ± 0.13 |
| Source | FAd | p-CA | 3-gluK | EA | Tiliroside | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Model | 0.0026 | 0.0055 | 0.0018 | <0.0001 | 0.0001 | |||||
| Lack of Fit | 0.3003 | 0.4017 | 0.2095 | 0.0876 | 0.0275 | |||||
| β0 | 72.79 | 79.61 | 128.71 | 261.88 | 1776.29 | |||||
| X1 | 2.23 | 0.0612 | 5.76 | 0.0140 | 23.60 | 0.0011 | 19.08 | 0.0084 | 340.85 | 0.0005 |
| X2 | −7.29 | 0.0005 | −13.18 | 0.0004 | −35.20 | 0.0002 | −150.04 | <0.0001 | −791.46 | <0.0001 |
| X3 | 0.97 | 0.3425 | 3.06 | 0.1067 | 4.50 | 0.2566 | 13.88 | 0.0281 | 42.31 | 0.3606 |
| X1X2 | 0.80 | 0.5676 | 4.17 | 0.1164 | 3.87 | 0.4716 | −12.41 | 0.1110 | −189.56 | 0.0244 |
| X1X3 | −2.51 | 0.1147 | −0.39 | 0.8648 | −1.18 | 0.8215 | −6.06 | 0.3880 | 1.47 | 0.9813 |
| X2X3 | −1.38 | 0.3405 | 2.47 | 0.3123 | −0.50 | 0.9232 | −20.17 | 0.0256 | −4.49 | 0.9428 |
| X12 | −6.13 | 0.0065 | −1.80 | 0.4680 | −0.45 | 0.9335 | 14.13 | 0.0880 | −111.48 | 0.1317 |
| X22 | −11.24 | 0.0004 | −11.19 | 0.0045 | −34.38 | 0.0012 | −88.90 | <0.0001 | −666.39 | 0.0001 |
| X32 | −5.07 | 0.0138 | −3.25 | 0.2145 | 0.26 | 0.9614 | 14.50 | 0.0819 | −91.28 | 0.2004 |
| R2 | 0.9704 | 0.9598 | 0.9747 | 0.9963 | 0.9910 | |||||
| Adjusted R2 | 0.9172 | 0.8874 | 0.9290 | 0.9895 | 0.9747 | |||||
| Predicted R2 | 0.6131 | 0.5167 | 0.6450 | 0.9432 | 0.8576 | |||||
| Source | FAd | p-CA | 3-gluK | EA | Tiliroside * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Model | 0.0002 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| Lack of Fit | 0.3598 | 0.4685 | 0.8357 | 0.0560 | 0.0842 | |||||
| β0 | 72.79 | 76.72 | 128.60 | 278.25 | 7.46 | |||||
| X1 | 2.23 | 0.0411 | 5.76 | 0.0046 | 23.60 | <0.0001 | 19.08 | 0.0160 | 0.28 | <0.0001 |
| X2 | −7.29 | 0.0001 | −13.18 | <0.0001 | −35.20 | <0.0001 | −150.04 | <0.0001 | −1.06 | <0.0001 |
| X3 | 0.97 | 0.3121 | 3.06 | 0.0779 | 13.88 | 0.0597 | 0.05 | 0.0106 | ||
| X1X2 | 4.17 | 0.0870 | ||||||||
| X1X3 | −2.51 | 0.0878 | ||||||||
| X2X3 | −20.17 | 0.0543 | 0.04 | 0.0807 | ||||||
| X12 | −6.13 | 0.0023 | −0.13 | 0.0004 | ||||||
| X22 | −11.24 | 0.0001 | −10.83 | 0.0010 | −34.37 | <0.0001 | −90.94 | <0.0001 | −0.98 | <0.0001 |
| X32 | −5.07 | 0.0052 | ||||||||
| R2 | 0.9617 | 0.9294 | 0.9629 | 0.9864 | 0.9989 | |||||
| Adjusted R2 | 0.9233 | 0.8902 | 0.9528 | 0.9788 | 0.9981 | |||||
| Predicted R2 | 0.8479 | 0.8044 | 0.9305 | 0.9611 | 0.9943 | |||||
| Compound | Temperature, °C | Ratio H2O:EtOH | Extraction Cycles |
|---|---|---|---|
| ferulic acid deriv. | 57 | 42:58 | 3 |
| p-coumaric acid | 65 | 40:60 | 4 |
| kaempferol 3-glucoside | 65 | 37:63 | 4 |
| ellagic acid | 65 | 27:73 | 4 |
| tiliroside | 65 | 37:63 | 4 |
| Run Order | Coded Independent Variables | Uncoded Independent Variables | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Temperature (°C) | Solvent Ratio (H2O:EtOH) | Extraction Cycles | |
| 1 | 0 | 0 | 0 | 55 | 50:50 | 3 |
| 2 | −1 | 0 | 1 | 45 | 50:50 | 4 |
| 3 | 1 | 0 | −1 | 65 | 50:50 | 2 |
| 4 | −1 | 0 | −1 | 45 | 50:50 | 2 |
| 5 | 0 | 1 | 1 | 55 | 75:25 | 4 |
| 6 | 0 | 0 | 0 | 55 | 50:50 | 3 |
| 7 | 1 | 0 | 1 | 65 | 50:50 | 4 |
| 8 | 0 | 1 | −1 | 55 | 75:25 | 2 |
| 9 | 0 | −1 | 1 | 55 | 25:75 | 4 |
| 10 | 0 | −1 | −1 | 55 | 25:75 | 2 |
| 11 | 0 | 0 | 0 | 55 | 50:50 | 3 |
| 12 | 1 | −1 | 0 | 65 | 25:75 | 3 |
| 13 | 1 | 1 | 0 | 65 | 75:25 | 3 |
| 14 | −1 | 1 | 0 | 45 | 75:25 | 3 |
| 15 | −1 | −1 | 0 | 45 | 25:75 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, M.; Mazurek, B.; Wójciak, W.; Kostrzewa, D.; Żuk, M.; Chmiel, M.; Kubrak, T.; Sowa, I. Optimizing the Extraction of the Polyphenolic Fraction from Defatted Strawberry Seeds for Tiliroside Isolation Using Accelerated Solvent Extraction Combined with a Box–Behnken Design. Molecules 2024, 29, 3051. https://doi.org/10.3390/molecules29133051
Wójciak M, Mazurek B, Wójciak W, Kostrzewa D, Żuk M, Chmiel M, Kubrak T, Sowa I. Optimizing the Extraction of the Polyphenolic Fraction from Defatted Strawberry Seeds for Tiliroside Isolation Using Accelerated Solvent Extraction Combined with a Box–Behnken Design. Molecules. 2024; 29(13):3051. https://doi.org/10.3390/molecules29133051
Chicago/Turabian StyleWójciak, Magdalena, Barbara Mazurek, Weronika Wójciak, Dorota Kostrzewa, Magdalena Żuk, Mariusz Chmiel, Tomasz Kubrak, and Ireneusz Sowa. 2024. "Optimizing the Extraction of the Polyphenolic Fraction from Defatted Strawberry Seeds for Tiliroside Isolation Using Accelerated Solvent Extraction Combined with a Box–Behnken Design" Molecules 29, no. 13: 3051. https://doi.org/10.3390/molecules29133051





