Antioxidant and Anti-Inflammatory Profiles of Two Mexican Heteropterys Species and Their Relevance for the Treatment of Mental Diseases: H. brachiata (L.) DC. and H. cotinifolia A. Juss. (Malpighiaceae)
Abstract
:1. Introduction
2. Results and Discussion
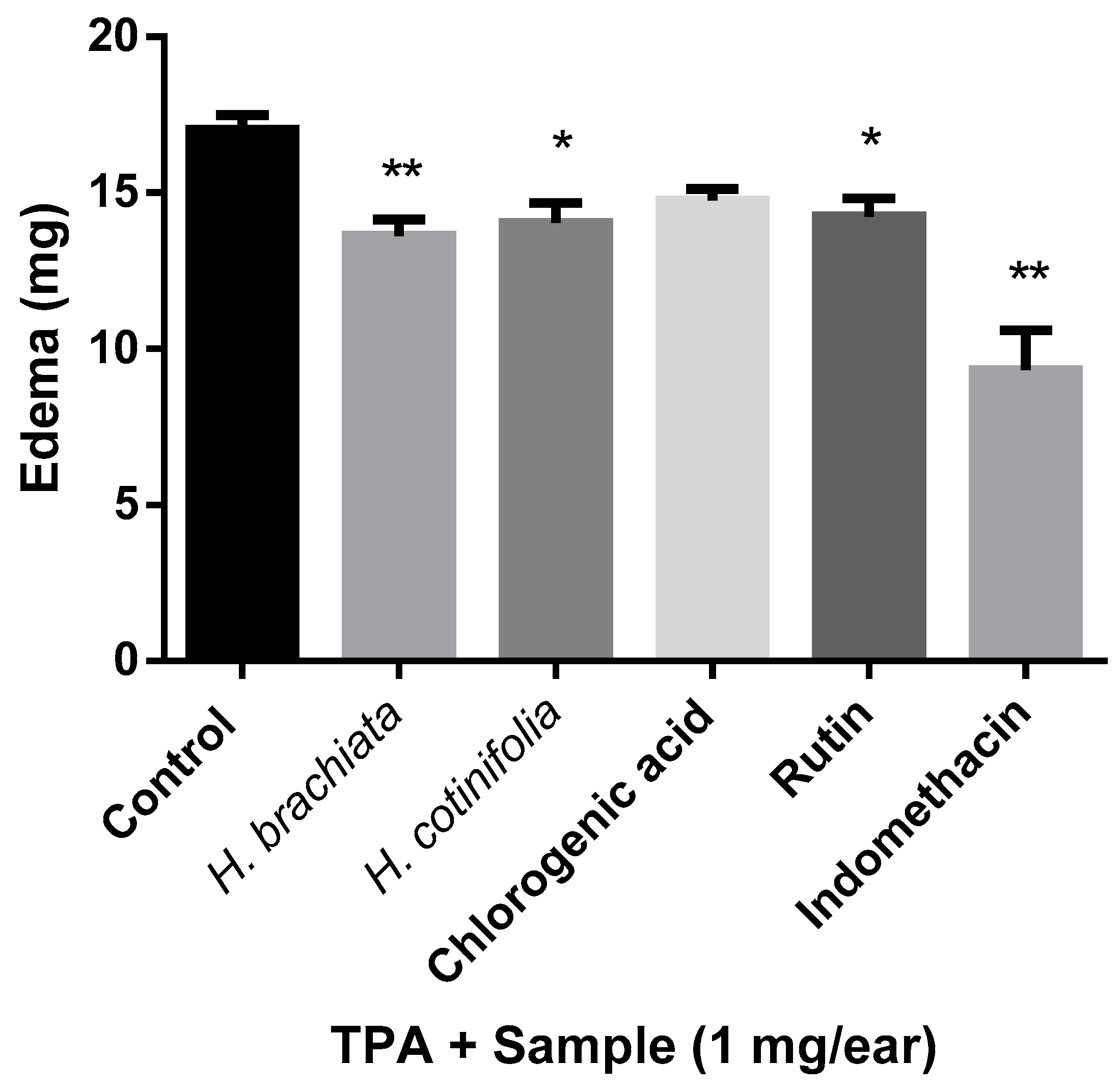
2.1. TPA-Induced Ear Edema Test
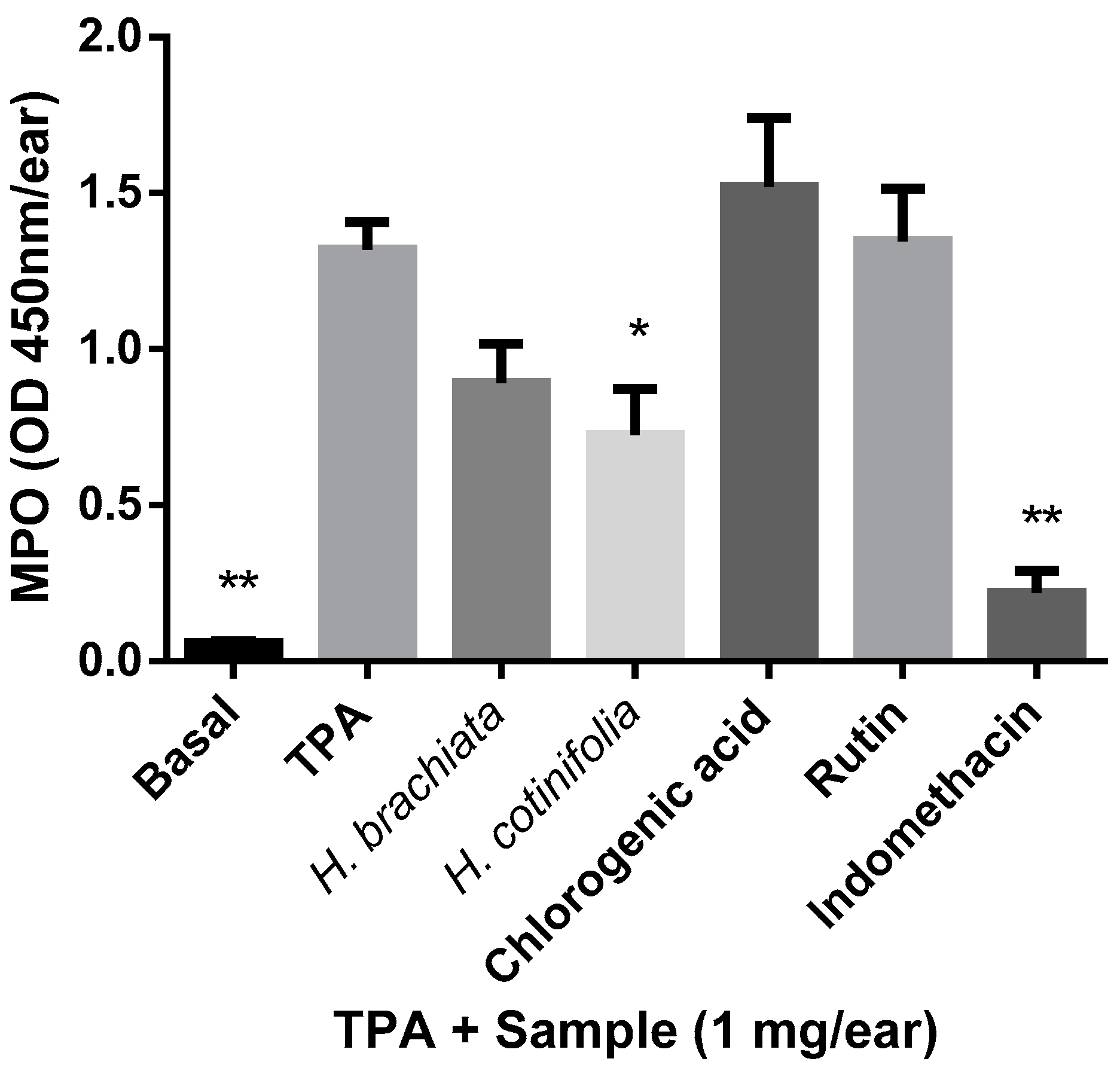
2.2. Myeloperoxidase (MPO) Assay
2.3. Inhibition of Acetylcholinesterase (AChE)
2.4. TBARS Assay
2.5. DPPH Assay
2.6. Ferrozine Assay
3. Materials and Methods
3.1. Obtaining the Methanolic Extracts of H. brachiata and H. cotinifolia
3.2. Animals
3.3. TPA-Induced Ear Edema Test
3.4. Myeloperoxidase (MPO) Assay
3.5. Inhibition of Acetylcholinesterase (AChE)
3.6. TBARS Assay
3.7. DPPH Assay
3.8. Ferrozine Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase Enzyme |
| TBARS | Thiobarbituric Acid Reactive Substances |
| DPPH | (2,2-Diphenyl-1-picrylhydrazyl) |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| MPO | Myeloperoxidase |
| CNS | Central Nervous System |
| TNF-α | Tumor Necrosis Factor-alpha |
| COX-2 | Cyclooxygenase-2 |
| IL-6 | Interleukin-6 |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| OD | Optical Density |
| HTAB | Hexadecyltrimethylammonium Bromide |
| TBA | Thiobarbituric Acid |
| TMP | Tetramethoxypropane 1,1,3,3 |
| SOD | Superoxide Dismutase |
| GPx | Glutathione Peroxidase |
References
- Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 12 April 2024).
- Hopwood, M. Anxiety symptoms in patients with Major Depressive Disorder: Commentary on prevalence and clinical implications. Neurol. Ther. 2023, 12, S5–S12. [Google Scholar] [CrossRef]
- COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Poweleit, E.A.; Ramsey, L.B.; Croarkin, P.E. Adverse effects of antidepressant medications and their management in children and adolescents. Pharmacotherapy 2023, 43, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Singewald, N.; Sartori, S.B.; Reif, A.; Holmes, A. Alleviating anxiety and taming trauma: Novel pharmacotherapeutics for anxiety disorders and posttraumatic stress disorder. Neuropharmacology 2023, 226, 109418. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Klabnik, J.J.; O’Donnell, J.M. Novel therapeutic targets in depression and anxiety: Antioxidants as a candidate treatment. Curr. Neuropharmacol. 2014, 12, 108–119. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Kappelmann, N.; Ye, Z.; Lamers, F.; Moser, S.; Jones, P.B.; Burgess, S.; Penninx, B.W.J.H.; Khandaker, G.M. Association of inflammation with depression and anxiety: Evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol. Psychiatry 2021, 26, 7393–7402. [Google Scholar] [CrossRef]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients 2022, 29, 597. [Google Scholar] [CrossRef]
- Malpighiaceae. Available online: https://webapps.lsa.umich.edu/herb/malpigh/index.html (accessed on 12 April 2024).
- Anderson, W.R. Origins of Mexican Malpighiaceae. Acta Bot. Mex. 2013, 104, 107–156. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; González-Cortazar, M.; Jiménez-Ferrer, E.; Zamilpa, A.; Álvarez, L.; Ramírez, G.; Tortoriello, J. Anxiolytic effect of natural galphimines from Galphimia glauca and their chemical derivatives. J. Nat. Prod. 2006, 69, 59–61. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Zamilpa, A.; González-Cortazar, M.; Reyes-Chilpa, R.; León, E.; García, M.P.; Tortoriello, J.; Huerta-Reyes, M. Antidepressant effect and pharmacological evaluation of standardized extract of flavonoids from Byrsonima crassifolia. Phytomedicine 2011, 18, 1255–1261. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Zamilpa, A.; Alvarez-Chimal, R.; Luna-Manzanares, J.A.; León-Velasco, M.E.; Aguilar-Rojas, A.; Jiménez-Estrada, M.; Campos-Lara, M.G. Heteropterys cotinifolia: A neuropharmacological and phytochemical approach with possible taxonomic implications. Sci. World J. 2013, 2013, 870468. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Reyes, M.; Herrera-Ruiz, M.; González-Cortazar, M.; Zamilpa, A.; León, E.; Reyes-Chilpa, R.; Aguilar-Rojas, A.; Tortoriello, J. Neuropharmacological in vivo effects and phytochemical profile of the extract from the aerial parts of Heteropterys brachiata (L.) DC. (Malpighiaceae). J. Ethnopharmacol. 2013, 146, 311–317. [Google Scholar] [CrossRef]
- Anderson, W.R. Notes on neotropical Malpighiaceae—IV. Contr. Univ. Michigan Herb. 1993, 19, 355–392. [Google Scholar]
- Juárez, J.C. La Familia Malpighiaceae en el Estado de Morelos. Bachelor’s Thesis, Universidad Autónoma del Estado de Morelos, Cuernavaca, México, 1998. [Google Scholar]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Bralley, E.E.; Greenspan, P.; Hargrove, J.L.; Wicker, L.; Hartle, D.K. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J. Inflamm. 2008, 5, 1. [Google Scholar] [CrossRef]
- Huang, M.T.; Newmark, H.L.; Frenkel, K. Inhibitory effects of curcumin on tumorigenesis in mice. J. Cell Biochem. 1997, 27, 26–34. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Zamilpa, A.; Álvarez-Chimal, R.; Luna-Manzanares, J.Á.; León-Velasco, M.E.; Aguilar-Rojas, A.; Jiménez-Estrada, M.; Campos-Lara, M.G. Traditional Medicine Heteropterys cotinifolia: A neuropharmacological and phytochemical approach with possible taxonomic implications. In Emerging Issues in Science and Technology; Elangovan, P., Ed.; Book Publisher International: Tarkeshwar, India, 2020; Volume 4, pp. 33–47. [Google Scholar]
- Gałecki, P.; Talarowska, M. Inflammatory theory of depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.G.; Ben-Azu, B.; Ajayi, A.M.; Wopara, I.; Aduema, W.; Kolawole, T.A.; Umoren, E.B.; Onyeleonu, I.; Ebo, O.T.; Ajibo, D.N.; et al. Gingko biloba abrogate lead-induced neurodegeneration in mice hippocampus: Involvement of NF-κB expression, myeloperoxidase activity and pro-inflammatory mediators. Biol. Trace Elem. Res. 2022, 200, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Malle, E.; Furtmüller, P.G.; Sattler, W.; Obinger, C. Myeloperoxidase: A target for new drug development? Br. J. Pharmacol. 2007, 152, 838–854. [Google Scholar] [CrossRef]
- Pabuçcuoğlu, A.; Konyalioğlu, S.; Baş, M.; Meral, G.E. The in vitro effects of Hypericum species on human leukocyte myeloperoxidase activity. J. Ethnopharmacol. 2003, 87, 89–92. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Adewusi, E.A.; Moodley, N.; Steenkamp, V. Antioxidant and acetylcholinesterase inhibitory activity of selected southern African medicinal plants. S. Afr. J. Bot. 2011, 77, 638–644. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Andrione, D.G.; Ruiz, G.; Palacios, S.M. Screening for acetylcholinesterase inhibitory activity in plant extracts from Argentina. Phytother. Res. 2010, 24, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Suarez-Lopez, J.R.; Nguyen, A.; Klas, J.; Gahagan, S.; Checkoway, H.; Lopez-Paredes, D.; Noble, M. Associations of acetylcholinesterase inhibition between pesticide spray seasons with depression and anxiety symptoms in adolescents, and the role of sex and adrenal hormones on gender moderation. Expo. Health. 2021, 13, 51–64. [Google Scholar] [CrossRef]
- Jawaid, A.; Pawlowicz, E.; Schulz, P.E. Do acetylcholinesterase inhibitors increase anxiety and depression in elderly adults with dementia? J. Am. Geriatr. Soc. 2015, 63, 1702–1704. [Google Scholar] [CrossRef]
- Ghasemi, M.; Navidhamidi, M.; Rezaei, F.; Azizikia, A.; Mehranfard, N. Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms. Cogn. Affect. Behav. Neurosci. 2022, 22, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yao, X.; Zhao, F.; Zhao, H.; Cheng, Z.; Yang, W.; Cui, R.; Xu, S.; Li, B. Changes in hippocampal plasticity in depression and therapeutic approaches influencing these changes. Neural Plast. 2020, 2020, 8861903. [Google Scholar] [CrossRef]
- Tottenham, N.; Galván, A. Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav. Rev. 2016, 70, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Pace, S.; Haskell, C.; Okello, E.J.; Milne, A.; Scholey, A.B. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology 2006, 31, 845–852. [Google Scholar] [CrossRef]
- Soodi, M.; Naghdi, N.; Hajimehdipoor, H.; Choopani, S.; Sahraei, E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res. Pharm. Sci. 2014, 9, 107–114. [Google Scholar] [PubMed]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-Khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar]
- Shah, A.S.; Ahmed, M.; Alkreathy, H.M.; Khan, M.R.; Khan, R.A.; Khan, S. Phytochemical screening and protective effects of Trifolium alexandrinum (L.) against free radical-induced stress in rats. Food Sci. Nutr. 2014, 2, 751–757. [Google Scholar] [CrossRef]
- Gomez-Vidales, V.; Granados-Oliveros, G.; Nieto-Camacho, A.; Reyes-Solís, M.; Jimenez-Estrada, M. Cacalol and cacalol acetate as photoproducers of singlet oxygen and as free radical scavengers, evaluated by EPR spectroscopy and TBARS. RSC Adv. 2014, 4, 1371–1377. [Google Scholar] [CrossRef]
- Aquib, M.; Najmi, A.K.; Akhtar, M. Antidepressant effect of Thymoquinone in animal models of depression. Drug Res. 2015, 65, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, G.; Farajdokht, F.; Mohaddes, G.; Babri, S.; Ebrahimi, V.; Ebrahimi, H. Garlic (Allium sativum) improves anxiety- and depressive-related behaviors and brain oxidative stress in diabetic rats. Arch. Physiol. Biochem. 2020, 126, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid. Med. Cell Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Şenol, F.S.; Yilmaz, G.; Şener, B.; Koyuncu, M.; Orhan, I. Preliminary screening of acetylcholinesterase inhibitory and antioxidant activities of Anatolian Heptaptera species. Pharm. Biol. 2010, 48, 337–341. [Google Scholar] [CrossRef]
- Venuprasad, M.P.; Kandikattu, H.K.; Razack, S.; Khanum, F. Phytochemical analysis of Ocimum gratissimum by LC-ESI–MS/MS and its antioxidant and anxiolytic effects. S. Afr. J. Bot. 2014, 92, 151–158. [Google Scholar] [CrossRef]
- Hassan, W.; Silva, C.E.; Mohammadzai, I.U.; da Rocha, J.B.; Landeira-Fernandez, J. Association of oxidative stress to the genesis of anxiety: Implications for possible therapeutic interventions. Curr. Neuropharmacol. 2014, 12, 120–139. [Google Scholar] [CrossRef]
- Salustri, C.; Squitti, R.; Zappasodi, F.; Ventriglia, M.; Bevacqua, M.G.; Fontana, M.; Tecchio, F. Oxidative stress and brain glutamate-mediated excitability in depressed patients. J. Affect. Disord. 2010, 127, 321–325. [Google Scholar] [CrossRef]
- Das, P.; Ashraf, G.J.; Baishya, T.; Dua, T.K.; Paul, P.; Nandi, G.; Singh, R.K.; Dutta, A.; Kumar, A.; Sahu, R. In vitro pharmacological evaluation, phytochemical profiling, and in silico molecular docking of Duabanga grandiflora leaves and flowers. Vegetos 2023, 1–13. [Google Scholar] [CrossRef]
- Fernández, S.P.; Wasowski, C.; Paladini, A.; Marder, M. Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur. J. Pharmacol. 2005, 512, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef]
- Rabanal, R.M.; Bonkanka, C.X.; Hernández-Pérez, M.; Sánchez-Mateo, C.C. Analgesic and topical anti-inflammatory activity of Hypericum canariense L. and Hypericum glandulosum Ait. J. Ethnopharmacol. 2005, 96, 591–596. [Google Scholar] [CrossRef]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F.; et al. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium). Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Michalak, D.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. The antiglycation potential of H1 receptor antagonists—In vitro studies in bovine serum albumin model and in silico molecular docking analyses. Biomed. Pharmacother. 2024, 175, 116632. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Michalak, D.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. Antioxidant and Anti-Glycation Potential of H2 Receptor Antagonists-In Vitro Studies and a Systematic Literature Review. Pharmaceuticals 2023, 16, 1273. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shen, C.; Zheng, H.; Xu, Y.; Xue, C.; Zhu, B.; Hu, J. Metabolomic analysis of acerola cherry (Malpighia emarginata) fruit during ripening development via UPLC-Q-TOF and contribution to the antioxidant activity. Food Res. Int. 2020, 130, 108915. [Google Scholar] [CrossRef] [PubMed]
- Ksiksi, T.; Hamza, A.A. Antioxidant, lipoxygenase and histone deacetylase inhibitory activities of Acridocarpus orientalis from Al Ain and Oman [corrected]. Molecules 2012, 24, 12521–12532. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, D.M.O.; Justino, A.B.; Sousa, R.M.F.; Munoz, R.A.A.; de Aquino, F.J.T.; Martins, M.M.; Goulart, L.R.; Pivatto, M.; Espindola, F.S.; de Oliveira, A. Antioxidant compounds from Banisteriopsis argyrophylla leaves as α-amylase, α-glucosidase, lipase, and glycation inhibitors. Bioorg. Chem. 2020, 105, 104335. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, A.A.; do Carmo, L.F.; do Nascimento, S.B.; de Matos, N.A.; de Carvalho Veloso, C.; Castro, A.H.; De Vos, R.C.; Klein, A.; de Siqueira, J.M.; Carollo, C.A.; et al. Chemical composition and anti-inflammatory activity of the leaves of Byrsonima verbascifolia. J. Nat. Med. 2016, 70, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Verdam, M.C.; Guilhon-Simplicio, F.; de Andrade, K.C.; Fernandes, K.L.; Machado, T.M.; da Silva, F.M.; de Souza, M.P.; Koolen, H.H.; Paula, C.D.; Hirota, B.C.; et al. Analgesic, anti-Inflammatory, and antioxidant activities of Byrsonima duckeana W. R. Anderson (Malpighiaceae). Sci. World J. 2017, 2017, 8367042. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Reyes, M.; Herrera-Ruiz, M.; Zamilpa-Álvarez, A.; González-Cortazar, M.; Tortoriello-García, J.; Aguilar-Rojas, A. Inventors: Extracto de Heteropterys brachiata, Método de Obtención y Uso para el Tratamiento de Ansiedad y Depresión. Patent 289104, 29 June 2011. [Google Scholar]
- Oviedo-Chávez, I.; Ramírez-Apan, T.; Soto-Hernández, M.; Martínez-Vázquez, M. Principles of the bark of Amphipterygium adstringens (Julianaceae) with anti-inflammatory activity. Phytomedicine 2004, 11, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Guzmán-Gutiérrez, S.L.; Nieto-Camacho, A.; Castillo-Arellano, J.I.; Huerta-Salazar, E.; Hernández-Pasteur, G.; Silva-Miranda, M.; Argüello-Nájera, O.; Sepúlveda-Robles, O.; Espitia, C.I.; Reyes-Chilpa, R. Mexican Propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative. Molecules 2018, 23, 334. [Google Scholar] [CrossRef] [PubMed]
- Ganjewala, D.; Tomar, N.; Gupta, A.K. Phytochemical composition and antioxidant properties of methanol extracts of leaves and fruits of Neolamarckia cadamba (Roxb.). JBAPN 2013, 3, 232–240. [Google Scholar]

| Sample | Concentration (µg/mL) | O.D. (412 nm) | Inhibition (%) | IC50 (µg/mL) |
|---|---|---|---|---|
| H. cotinifolia a | 1 | 0.366 ± 0.020 | 9.16 ± 4.59 | 97.44 ± 4.88 |
| 3.16 | 0.356 ± 0.017 | 11.65 ± 4.00 | ||
| 10 | 0.322 ± 0.014 ** | 19.99 ± 3.32 ** | ||
| 31.62 | 0.275 ± 0.013 ** | 31.69 ± 3.01 ** | ||
| 100 | 0.197 ± 0.008 ** | 51.14 ± 1.89 ** | ||
| 316.23 | 0.081 ± 0.007 ** | 79.94 ± 1.89 ** | ||
| 1000 | 0.012 ± 0.006 ** | 96.97 ± 1.52 ** | ||
| Galantamine b | 0.00037 | 0.374 ± 0.014 * | 18.77 ± 3.30 * | 0.09 ± 0.03 |
| 0.0037 | 0.343 ± 0.022 ** | 25.26 ± 4.08 ** | ||
| 0.037 | 0.256 ± 0.022 ** | 43.94 ± 4.84 ** | ||
| 0.37 | 0.120 ± 0.022 ** | 74.12 ± 4.55 ** | ||
| 3.68 | 0.026 ± 0.007 ** | 94.43 ± 1.54 ** |
| Sample a | Concentration (µg/mL) | TBARS (nmol/mg prot) | Inhibition (%) | IC50 (µg/mL) |
|---|---|---|---|---|
| H. brachiata b | 5.62 | 6.15 ± 0.57 * | 21.60 ± 4.59 * | 13.80 ± 1.04 |
| 10 | 4.89 ± 0.55 ** | 37.77 ± 5.43 ** | ||
| 17.78 | 3.02 ± 0.44 ** | 61.81 ± 5.52 ** | ||
| 31.62 | 0.92 ± 0.17 ** | 88.35 ± 2.48 ** | ||
| 56.23 | 0.67 ± 0.03 ** | 95.33 ± 0.20 ** | ||
| 100 | 0.43 ± 0.05 ** | 94.41 ± 1.56 ** | ||
| H. cotinifolia c | 5.62 | 5.67 ± 0.45 ** | 27.29 ± 8.76 ** | 11.52 ± 0.46 |
| 10 | 4.51 ± 0.36 ** | 42.31 ± 4.55 ** | ||
| 17.78 | 1.69 ± 0.27 ** | 78.63 ± 3.58 ** | ||
| 31.62 | 0.54 ± 0.05 ** | 93.13 ± 0.49 ** | ||
| 56.23 | 0.39 ± 0.05 ** | 95.01 ± 1.00 ** | ||
| 100 | 0.35 ± 0.06 ** | 95.59 ± 1.11 ** | ||
| Chlorogenic acid d | 5.62 | 7.76 ± 0.40 | −0.10 ± 3.87 | 22.05 ± 1.55 |
| 10 | 6.96 ± 0.44 | 10.32 ± 3.72 | ||
| 17.78 | 4.74 ± 0.41 ** | 39.12 ± 4.31 ** | ||
| 31.62 | 1.83 ± 0.23 ** | 76.61 ± 3.44 ** | ||
| 56.23 | 0.66 ± 0.11 ** | 91.39 ± 2.81 ** | ||
| 100 | 0.31 ± 0.01 ** | 95.96 ± 0.11 ** | ||
| Rutin e | 5.62 | 7.24 ± 0.40 | 7.13 ± 4.35 | 14.20 ± 1.27 |
| 10 | 5.41 ± 0.46 ** | 28.34 ± 8.50 ** | ||
| 17.78 | 2.44 ± 0.31 ** | 66.24 ± 8.46 ** | ||
| 31.62 | 1.03 ± 0.10 ** | 86.20 ± 1.94 ** | ||
| 56.23 | 0.59 ± 0.05 ** | 92.50 ± 0.39 ** | ||
| 100 | 0.35 ± 0.02 ** | 95.72 ± 0.48 ** | ||
| BHT f | 0.17 | 5.56 ± 0.29 * | 23.92 ± 2.69 ** | 0.27 ± 0.10 |
| 0.22 | 4.46 ± 0.28 ** | 37.14 ± 7.44 ** | ||
| 0.29 | 3.23 ± 0.57 ** | 53.59 ± 8.93 ** | ||
| 0.39 | 1.32 ± 0.49 ** | 81.59 ± 6.89 ** | ||
| 0.52 | 0.49 ± 0.08 ** | 93.16 ± 1.16 ** | ||
| 0.70 | 0.53 ± 0.04 ** | 95.08 ± 0.68 ** |
| Sample | Concentration (µg/mL) | O.D. (515 nm) | DPPH Reduction (%) | IC50 (µg/mL) |
|---|---|---|---|---|
| H. brachiata | 3.16 | 0.614 ± 0.003 ** | 9.74 ± 1.25 ** | 25.65 ± 0.12 |
| 5.62 | 0.566 ± 0.007 ** | 16.77 ± 0.80 ** | ||
| 10 | 0.521 ± 0.002 ** | 23.52 ± 1.20 ** | ||
| 17.78 | 0.428 ± 0.006 ** | 37.15 ± 0.33 ** | ||
| 31.62 | 0.281 ± 0.005 ** | 58.75 ± 0.17 ** | ||
| 56.23 | 0.091 ± 0.002 ** | 86.65 ± 0.05 ** | ||
| H. cotinifolia | 3.16 | 0.531 ± 0.012 ** | 18.97 ± 0.66 ** | 9.53 ± 0.3 |
| 5.62 | 0.447 ± 0.008 ** | 31.87 ± 0.13 ** | ||
| 10 | 0.320 ± 0.009 ** | 51.15 ± 0.48 ** | ||
| 17.78 | 0.111 ± 0.005 ** | 83.04 ± 0.53 ** | ||
| 31.62 | 0.057 ± 0.001 ** | 91.22 ± 0.21 ** | ||
| 56.23 | 0.055 ± 0.001 ** | 91.63 ± 0.13 ** | ||
| Chlorogenic acid | 3.16 | 0.585 ± 0.004 ** | 10.69 ± 1.47 | 16.13 ± 0.22 |
| 5.62 | 0.535 ± 0.005 ** | 18.47 ± 1.09 | ||
| 10 | 0.451 ± 0.002 ** | 31.12 ± 1.22 | ||
| 17.78 | 0.303 ± 0.005 ** | 53.76 ± 0.88 | ||
| 31.62 | 0.094 ± 0.003 ** | 85.67 ± 0.13 | ||
| 56.23 | 0.044 ± 0.003 ** | 93.25 ± 0.33 | ||
| Rutin | 3.16 | 0.647 ± 0.010 | 1.29 ± 0.84 | 8.2 ± 0.15 |
| 5.62 | 0.620 ± 0.002 ** | 5.40 ± 1.46 ** | ||
| 10 | 0.528 ± 0.002 ** | 19.35 ± 1.39 ** | ||
| 17.78 | 0.271 ± 0.003 ** | 58.66 ± 0.45 ** | ||
| 31.62 | 0.074 ± 0.001 ** | 88.65 ± 0.05 ** | ||
| 56.23 | 0.069 ± 0.002 ** | 89.48 ± 0.07 ** | ||
| Quercetin | 1.07 | 0.560 ± 0.004 ** | 17.03 ± 0.79 ** | 3.67 ± 0.13 |
| 1.90 | 0.491 ± 0.007 ** | 27.14 ± 1.24 ** | ||
| 3.38 | 0.363 ± 0.008 ** | 46.21 ± 1.29 ** | ||
| 6.01 | 0.167 ± 0.016 ** | 75.17 ± 2.40 ** | ||
| 10.69 | 0.041 ± 0.001 ** | 93.87 ± 0.19 ** |
| Sample | O.D. (562 nm) | Chelation (%) |
|---|---|---|
| Control | 0.785 ± 0.013 | |
| H. brachiata | 0.729 ± 0.030 | 7.27 ± 2.83 |
| H. cotinifolia | 0.697 ± 0.012 * | 11.21 ± 0.93 * |
| Chlorogenic acid | 0.850 ± 0.015 | −8.28 ± 1.09 (NO ACTIVITY) |
| Rutin | 0.505 ± 0.014 ** | 35.68 ± 0.99 ** |
| Quercetin | 0.812 ± 0.020 | −3.44 ± 3.97 (NO ACTIVITY) |
| EDTA | 0.002 ± 0.001 ** | 99.73 ± 0.09 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto Camacho, A.; Baca Ibarra, I.I.; Huerta-Reyes, M. Antioxidant and Anti-Inflammatory Profiles of Two Mexican Heteropterys Species and Their Relevance for the Treatment of Mental Diseases: H. brachiata (L.) DC. and H. cotinifolia A. Juss. (Malpighiaceae). Molecules 2024, 29, 3053. https://doi.org/10.3390/molecules29133053
Nieto Camacho A, Baca Ibarra II, Huerta-Reyes M. Antioxidant and Anti-Inflammatory Profiles of Two Mexican Heteropterys Species and Their Relevance for the Treatment of Mental Diseases: H. brachiata (L.) DC. and H. cotinifolia A. Juss. (Malpighiaceae). Molecules. 2024; 29(13):3053. https://doi.org/10.3390/molecules29133053
Chicago/Turabian StyleNieto Camacho, Antonio, Itzel Isaura Baca Ibarra, and Maira Huerta-Reyes. 2024. "Antioxidant and Anti-Inflammatory Profiles of Two Mexican Heteropterys Species and Their Relevance for the Treatment of Mental Diseases: H. brachiata (L.) DC. and H. cotinifolia A. Juss. (Malpighiaceae)" Molecules 29, no. 13: 3053. https://doi.org/10.3390/molecules29133053
APA StyleNieto Camacho, A., Baca Ibarra, I. I., & Huerta-Reyes, M. (2024). Antioxidant and Anti-Inflammatory Profiles of Two Mexican Heteropterys Species and Their Relevance for the Treatment of Mental Diseases: H. brachiata (L.) DC. and H. cotinifolia A. Juss. (Malpighiaceae). Molecules, 29(13), 3053. https://doi.org/10.3390/molecules29133053





