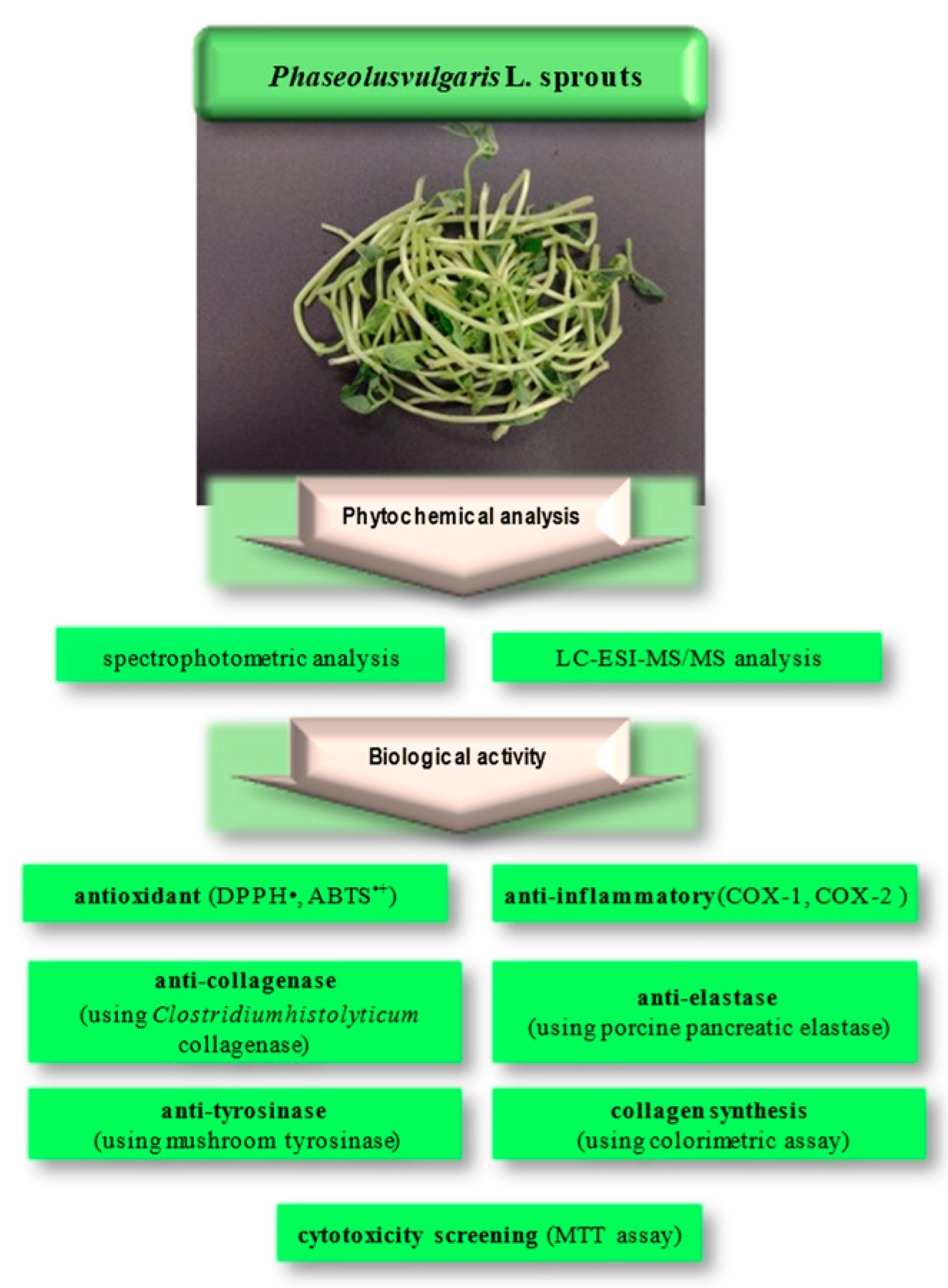
Novel Insights into Phaseolus vulgaris L. Sprouts: Phytochemical Analysis and Anti-Aging Properties
Abstract
:1. Introduction
2. Results and Discussion
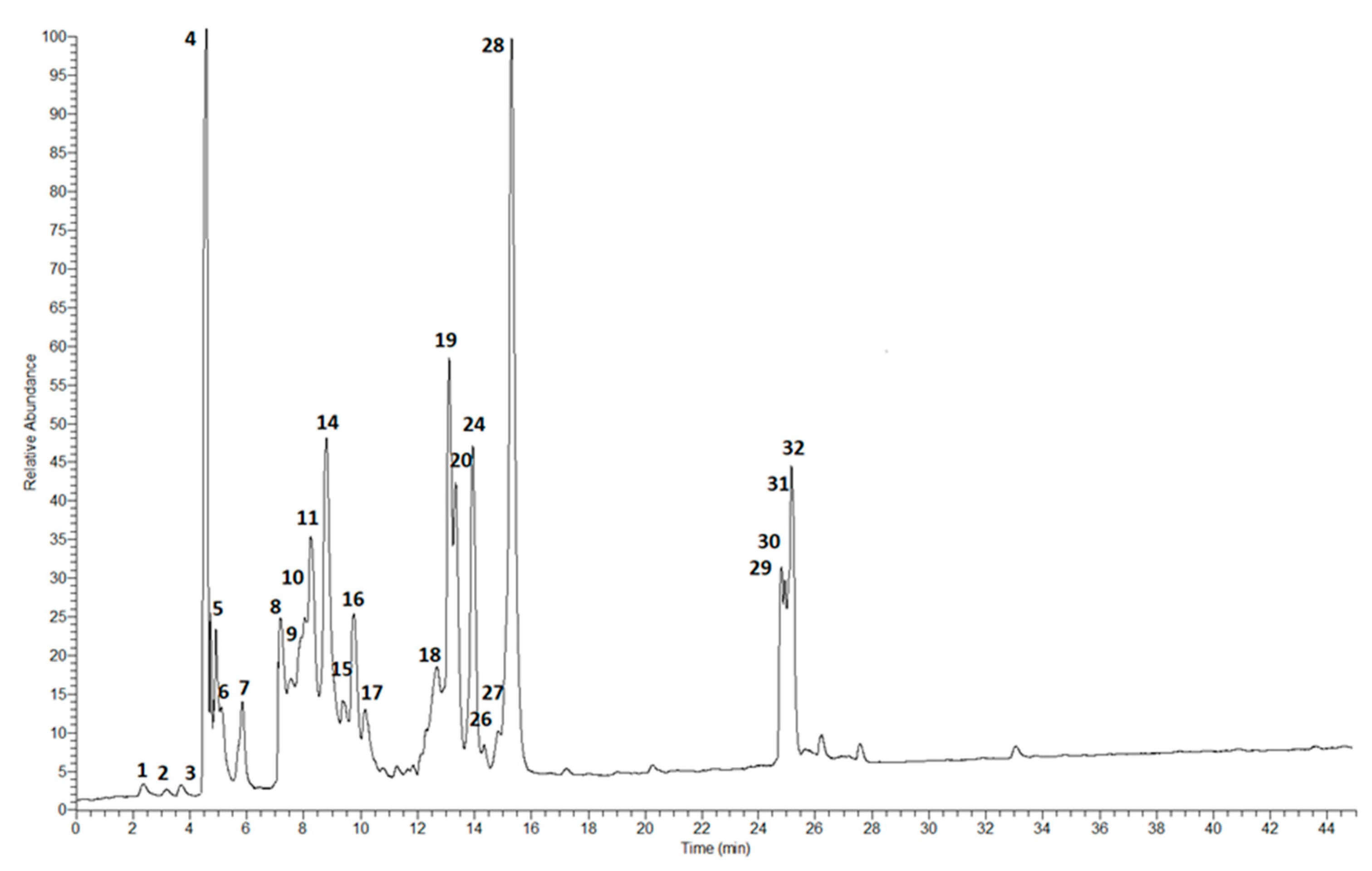
2.1. Phytochemical Analysis
2.2. Antioxidant Activity
2.3. Anti-Inflammatory Activity
2.4. Anti-Collagenase and Anti-Elastase Activities
2.5. Anti-Tyrosinase Activity
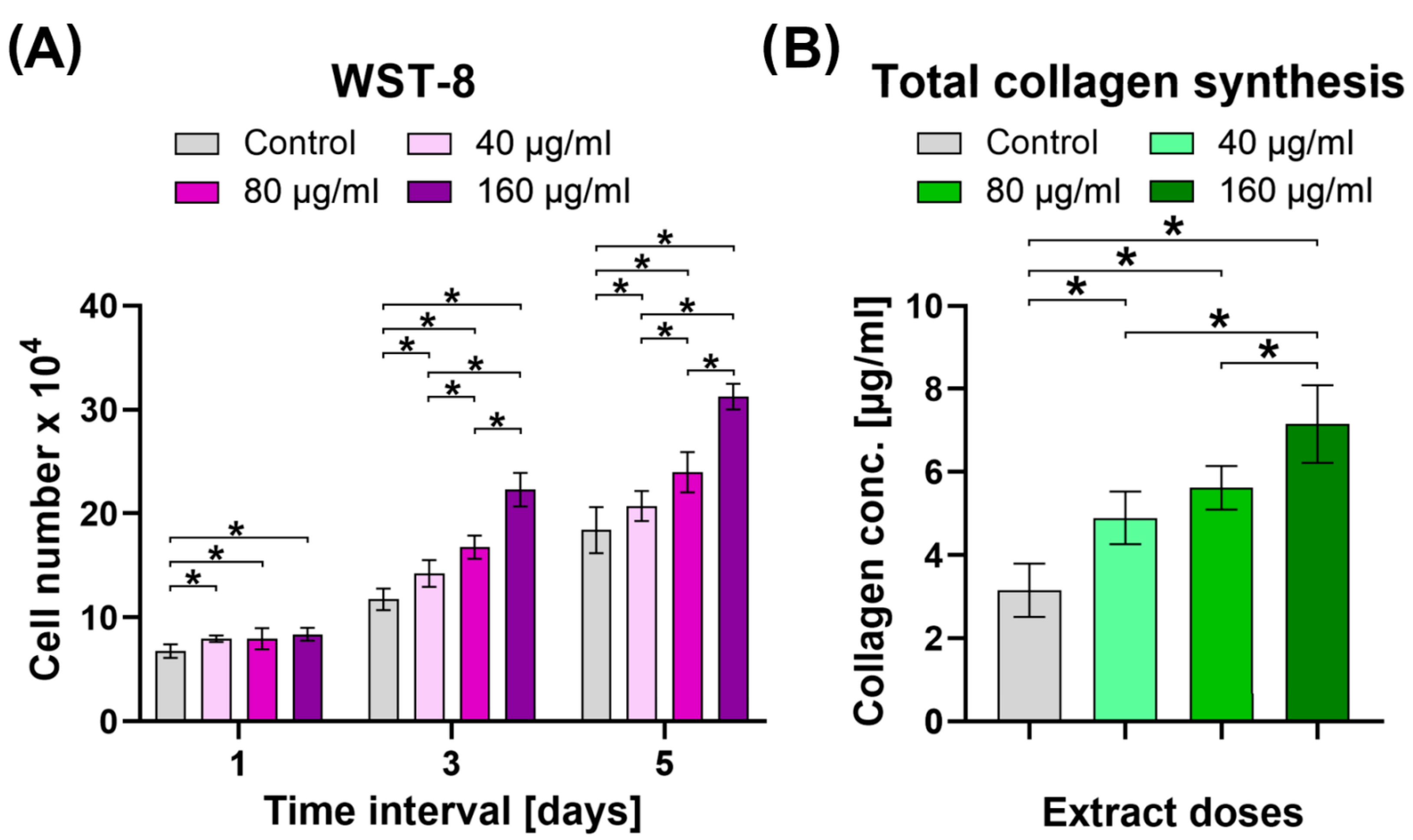
2.6. Cytotoxic Activity, Cell Proliferation Assessment, and Evaluation of Collagen Synthesis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Growing Sprouts from Seeds (Phaseolus vulgaris L.)
3.3. Preparation of the Extract
3.4. Total Flavonoid, Phenolic, and Phenolic Acid Content
3.5. LC-ESI-MS/MS Analysis
3.6. Antioxidant Activity
3.6.1. DPPH• Assay
3.6.2. ABTS•+ Assay
3.7. Anti-Inflammatory Activity
3.8. Enzyme Inhibitory Activity
3.8.1. Anti-Elastase Activity
3.8.2. Anti-Collagenase Activity
3.8.3. Anti-Tyrosinase Activity
3.9. Cell Culture Experiments
3.10. Screening Cytotoxicity Test
3.11. Cell Proliferation Assessment
3.12. Evaluation of Collagen Synthesis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pukalskienė, M.; Venskutonis, P.R.; Pukalskas, A. Phytochemical composition and antioxidant properties of Filipendula vulgaris as a source of healthy functional ingredients. J. Funct. Foods 2015, 15, 233–242. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Oyelude, E.O.; Gli, N.P.; Amafo, J. Proximate, mineral and anti-nutrient composition of Phaseolus vulgaris leaf. J. Sci. Innov. Dev. 2012, 1, 12–21. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Özer, Ö.; Mutlu, B.; Kıvçak, B. Antityrosinase activity of some plant extracts and formulations containing ellagic acid. Pharm. Biol. 2007, 45, 519–524. [Google Scholar] [CrossRef]
- Schmid, D.; Sacher, R.; Belser, E.; Zülli, F. Vegetable sprouts: A potent source for cosmetic actives. Househ. Pers. Care Today 2011, 1, 50–52. [Google Scholar]
- Chavan, J.K.; Kadam, S.S.; Beuchat, L.R. Nutritional improvement of cereals by sprouting. Crit. Rev. Food Sci. Nutr. 1989, 28, 401–437. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Nioi, P.; McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: Reassessment of the ARE consensus sequence. Biochem. J. 2003, 374, 337–348. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Hänggi, S.; Zülli, F. Extract of Cress Sprouts for a Broad Skin Protection to Prevent Wrinkle Formation; Mibelle Biochemistry: Buchs, Switzerland, 2007. [Google Scholar]
- Santos, E.; Marques, G.; Lino-Neto, T. Phaseolus vulgaris L. as a functional food for aging protection. Oxidative Stress and Dietary Antioxidants. In Aging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 289–295. [Google Scholar]
- Fonseca-Hernández, D.; Lugo-Cervantes, E.D.C.; Escobedo-Reyes, A.; Mojica, L. Black bean (Phaseolus vulgaris L.) polyphenolic extract exerts antioxidant and antiaging potential. Molecules 2021, 26, 6716. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shengdao, Z.; Minzhe, F.; Myeongju, K.; Arce Defeo, B.; Jeehaeng, J.; Tae-Hoo, Y. Anti-photoaging effect of (Phaseolus angularis). Extract on uvb-exposed hacat keratinocytes and possibilities as cosmetic materials. Molecules 2023, 28, 1407. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Usman, M.; Nawaz, H.A.; Rasheed, H.; Khan, R.R.; Anjum, S.M.M.; Khokhar, M.I.; Akhtar, N. Formulation of Phaseolus vulgaris L. cream and its characterization. Pak. J. Pharm. Sci. 2020, 33, 815–820. [Google Scholar] [PubMed]
- Lee, Y.L.; Choi, J.H.; Park, S.Y.; Jeong, M.Y.; Lee, H.C.; Song, J.H. The influences of Phaseolus radiatus L.‘s ethanol extracts and fractions on skin whitening and anti-inflammatory effects. JKOOD 2018, 31, 39–49. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chavez-Servia, J.L.; Carrillo-Rodríguez, J.C.; Vera-Guzmán, A.M.; Heredia-García, E. Anthocyanin, polyphenol, and flavonoid contents and antioxidant activity in Mexican common bean (Phaseolus vulgaris L.) landraces. Emir. J. Food Agric. 2016, 28, 581–588. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Polyphenols in common beans (Phaseolus vulgaris L.): Chemistry, analysis, and factors affecting composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Wimalasiri, K.M.S.; Chassy, A.W.; Mitchell, A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.S.C.; Tilbrook, D.; Pereira, G.; Dykes, G.A.; George, N.; Coorey, R. Antioxidant activities, phenolic compounds, and mineral composition of seed from Acacia retinodes, A. provincialis and A. tenuissima. Int. Food Res. 2023, 173, 113452. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Akillioglu, H.G.; Karakaya, S. Changes in total phenols, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking, and in vitro digestion process. Food Sci. Biotechnol. 2010, 19, 633–639. [Google Scholar] [CrossRef]
- Hernández-Saavedra, D.; Mendoza-Sánchez, M.; Hernández-Montiel, H.L.; Guzmán-Maldonado, H.S.; Loarca-Piña, G.F.; Salgado, L.M.; Reynoso-Camacho, R. Cooked common beans (Phaseolus vulgaris) protect against β-cell damage in streptozotocin-induced diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of southern Italy before and after cooking. Oxid. Med. Cell Longev. 2016, 1398298. [Google Scholar] [CrossRef]
- Valdés, S.T.; Medeiros Coelho, C.M.; Michelluti, D.J.; Cardoso García Tramonte, V.L. Association of genotype and preparation methods on the antioxidant activity, and antinutrients in common beans (Phaseolus vulgaris L.). LWT-Food Sci. Technol. 2011, 44, 2104–2111. [Google Scholar] [CrossRef]
- Doria, E.; Campion, B.; Sparvoli, F.; Tava, A.; Nielsen, E. Anti-nutrient components and metabolites with health implications in seeds of 10 common bean (Phaseolus vulgaris L. and Phaseolus lunatus L.) landraces cultivated in Southern Italy. J. Food Compos. Anal. 2012, 26, 72–80. [Google Scholar] [CrossRef]
- Ariza-Nieto, M.; Blair, M.W.; Welch, R.M.; Glahn, R.P. Screening of iron bioavailability patterns in eight bean (Phaseolus vulgaris L.) genotypes using the Caco-2 cell in vitro model. J. Agric. Food Chem. 2007, 55, 7950–7956. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Flores, D.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem. 2013, 141, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Shohag, M.J.I.; Wei, Y.Y.; Yang, X.E. Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Wang, M.F.; Lui, W.Y.; Wu, K.; Corke, H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int. J. Food Sci. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic composition of the leaves of Pyrola rotundifolia L. and their antioxidant and cytotoxic activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Frias, J.; Zielinski, H.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Kinetic study of the antioxidant compounds and antioxidant capacity during germination of Vigna radiata cv. emmerald, Glycine max cv. jutro and Glycine max cv. merit. Food Chem. 2008, 111, 622–630. [Google Scholar] [CrossRef]
- Ampofo, J.O.; Ngadi, M. Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrason. Sonochem. 2020, 64, 104974. [Google Scholar] [CrossRef] [PubMed]
- Borges-Martínez, E.; Gallardo-Velázquez, T.; Cardador-Martínez, A.; Moguel-Concha, D.; Osorio-Revilla, G.; Ruiz-Ruiz, J.C.; Martínez, C.J. Phenolic compounds profile and antioxidant activity of pea (Pisum sativum L.) and black bean (Phaseolus vulgaris L.) sprouts. Food Sci. Technol. 2021, 42, e46920. [Google Scholar] [CrossRef]
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gómez-Serranillos, M.P.; Palomino, O.M.; Carretero, M.E. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Bozzo, G.G.; Freixas-Coutin, J.A.; Marcone, M.F.; Pauls, P.K.; Tang, Y.; Tsao, R. Free and conjugated phenolic compounds and their antioxidant activities in regular and non-darkening cranberry bean (Phaseolus vulgaris L.) seed coats. J. Funct. Foods 2015, 18, 1047–1056. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Sholihah, I.A.; Raif, M.A.; Kunardi, S.; Million, H.; Widowati, W. Antioxidant and antiaging activity of rutin and caffeic acid. Pharmaciana 2020, 10, 147–156. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential anti-skin aging effect of (-)-catechin isolated from the root bark of Ulmus davidiana var. japonica in tumor necrosis factor-α-stimulated normal human dermal fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Barone, E.; Calabrese, V.; Mancuso, C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009, 10, 97–108. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.-N.; Kim, K.; Joo, D.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Ehrich Lister, I.N.; Widowati, W.; Wibowo, S.H.B.; Perdana, F.S.; Rizal, R. In silico analysis of phytochemical compound found in snake fruit (Salacca zalacca) peel as anti-aging agent. Thai J. Pharm. Sci. 2019, 43, 105–109. [Google Scholar] [CrossRef]
- Li, T.; Chen, S.; Feng, T.; Dong, J.; Li, Y.; Li, H. Rutin protects against aging-related metabolic dysfunction. Food Funct. 2016, 7, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, M.; Faffa, C.; Sisani, M.; Petracci, A. Caffeic acid-layered double hydroxide hybrid: A new raw material for cosmetic applications. Cosmetics 2018, 5, 51. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Aslani, B.A.; Ghobadi, S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016, 146, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Baranowska, M.; Namieśnik, J.; Bartoszek, A. Determination of antioxidant activity of phytochemicals in cellular models by fluorescence/luminescence methods. Adv. Hyg. Exp. Med. 2017, 71, 602–617. [Google Scholar]
- Gautam, R.; Srivastava, A.; Jachak, S.M.; Saklani, A. Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory and antioxidant effects of Dysophylla stellate Benth. Fitoterapia 2010, 81, 45–49. [Google Scholar] [CrossRef]
- Oomah, B.D.; Corbé, A.; Balasubramanian, P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J. Agric. Food Chem. 2010, 58, 8225–8230. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; de Mejia, E.G.; Dia, V.P.; Amaya-Llano, S.L. Common bean (Phaseolus vulgaris L.) hydrolysates inhibit inflammation in LPS-induced macrophages through suppression of NF-κB pathways. Food Chem. 2011, 127, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 9, 146832. [Google Scholar] [CrossRef] [PubMed]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic potential of dietary phenolic acids. Adv. Pharmacol. Pharm. Sci. 2015, 10, 823539. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. Connective tissue biochemistry of the aging dermis: Age-associated alternations in collagen and elastin. Clin. Med. Geriatr. 1989, 5, 127–148. [Google Scholar] [CrossRef]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Polish Pharmacopoeia IX, PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150.
- Olech, M.; Łyko, L.; Nowak, R. Influence of accelerated solvent extraction conditions on the LC-ESI-MS/MS polyphenolic profile, triterpenoid content, and antioxidant and anti-lipoxygenase activity of Rhododendron luteum sweet leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Pieczykolan, A.; Pietrzak, W.; Dos Santos Szewczyk, K.; Gawlik-Dziki, U.; Nowak, R. LC-ESI-MS/MS polyphenolic profile and in vitro study of cosmetic potential of Aerva lanata (L.) Juss. herb extracts. Molecules 2022, 27, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular mechanisms of sanguinarine in cancer prevention and treatment. Anticancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Razzaq, A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Menaa, F.; Ullah, N.; Shehzadi, S.; Nawaz, T.; Iqbal, H. Sanguinarine attenuates lung cancer progression via oxidative stress-induced cell apoptosis. Curr. Mol. Pharmacol. 2024, 17, e18761429269383. [Google Scholar] [CrossRef] [PubMed]

| Sample | Total Phenolic Content [mg GAE/g DE] | Total Phenolic Acids [mg CAE/g DE] | Total Flavonoid Content [mg QE/g DE] |
|---|---|---|---|
| PVS | 192.85 ± 10.24 | 4.66 ± 0.29 | 178.73 ± 1.59 |
| Peak No. | Compound Name | [M-H]− | MS2 | Theoretical Mass [M-H]− (Da) | Experimental Mass [M-H]− (Da) | Δ mDa | Δ ppm | Elemental Composition |
|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 169 | 69, 79, 81, 97, 124, 125b | 169.01370 | 169.01373 | 0.03 | 0.18 | C7H6O5 |
| 2 | Chlorogenic acid | 353 | 179, 191b | 353.08726 | 353.08733 | 0.07 | 0.20 | C16H18O9 |
| 3 | p-Hydroxybenzoic acid | 137 | 92, 93, 136, 137b | 137.02387 | 137.02385 | −0.02 | 0.15 | C7H6O3 |
| 4 | p-Coumaric aldaric acid | 355 | 119, 163b, 311 | 355.06653 | 355.06650 | −0.03 | 0.08 | C15H16O10 |
| 5 | o-Coumaric aldaric acid | 355 | 119, 163b, 311 | 355.06653 | 355.06651 | −0.02 | 0.06 | C15H16O10 |
| 6 | Protocatechuic acid | 153 | 108, 109, 153b | 153.01879 | 153.01874 | −0.05 | 0.33 | C7H6O4 |
| 7 | Feruroyl aldaric acid | 385 | 73, 89, 106, 117, 133, 134, 179, 193b, 341 | 385.07709 | 385.07712 | 0.03 | 0.08 | C16H18O11 |
| 8 | Caffeic acid | 179 | 97, 107, 135b | 179.03444 | 179.03447 | 0.03 | 0.17 | C9H8O4 |
| 9 | p-Coumaric acid | 163 | 119, 162b | 163.03952 | 163.03949 | −0.03 | 0.18 | C9H8O3 |
| 10 | o-Coumaric acid | 163 | 119, 162b | 163.03952 | 163.03951 | −0.01 | 0.06 | C9H8O3 |
| 11 | Ferulic acid | 193 | 73, 89b, 106, 117, 133, 134, 179 | 193.05009 | 193.05015 | 0.06 | 0.31 | C10H10O4 |
| 12 | Vanillic acid | 178 | 108, 123, 152, 167b | 178.04774 | 178.04768 | 0.09 | 0.54 | C8H8O4 |
| 13 | Quercetin-3-O-xylosylglucoside | 595 | 151, 179, 192, 209, 301b, 421 | 595.12992 | 595.12995 | 0.04 | 0.06 | C26H28O16 |
| 14 | Rutin | 609 | 151, 179, 301b, 463, 609 | 609.18195 | 609.18192 | −0.03 | 0.05 | C27H30O16 |
| 15 | Quercetin-3-O-(6″-O-malonyl) glucoside | 549 | 151, 179, 192, 209, 301b, 375 | 549.08805 | 549.08809 | 0.04 | 0.07 | C24H22O15 |
| 16 | Kaempferol-3-O-glucosylxylose | 579 | 93, 97, 119, 164, 285b, 435 | 579.13500 | 579.13507 | 0.07 | 0.12 | C26H28O15 |
| 17 | Kaempferol-3-O-(6″-O-malonyl) glucoside | 533 | 93, 97, 119, 164, 285b, 359 | 533.09314 | 533.09311 | −0.02 | 0.05 | C24H22O14 |
| 18 | Hyperoside | 463 | 151, 179, 255, 271, 301b | 463.08766 | 463.08769 | 0.03 | 0.06 | C21H20O12 |
| 19 | Isoquercitrin | 463 | 151, 179, 192, 301, 461, 463b | 463.08766 | 463.08762 | −0.04 | 0.09 | C21H20O12 |
| 20 | Quercitrin | 447 | 151, 243, 255, 271, 300b, 301, 447 | 447.09274 | 447.09278 | 0.04 | 0.09 | C21H20O11 |
| 21 | Sinapic aldaric acid | 415 | 141, 149, 164, 208, 223b, 371 | 415.08766 | 415.08761 | −0.05 | 0.11 | C17H20O12 |
| 22 | Myricetin-3-O-glucoside | 479 | 137, 153, 165, 229b, 257 | 479.08257 | 479.08252 | −0.05 | 0.10 | C21H20O13 |
| 23 | Prunin | 433 | 119, 151, 271b | 433.11348 | 433.11353 | 0.06 | 0.13 | C21H22O10 |
| 24 | Astragalin | 447 | 257, 285b, 447 | 447.09274 | 447.09281 | 0.07 | 0.16 | C21H20O11 |
| 25 | Myricetin | 317 | 107, 109, 137, 151, 179, 317b | 317.02975 | 317.02970 | −0.05 | 0.16 | C15H10O8 |
| 26 | Sinapic acid | 223 | 141, 149, 164b, 208, 223 | 223.06065 | 223.06068 | 0.03 | 0.13 | C11H12O5 |
| 27 | Quercetin | 301 | 151, 179b, 192, 209 | 301.03483 | 301.03481 | −0.02 | 0.07 | C15H10O7 |
| 28 | (+)-Catechin | 289 | 109, 123, 125, 137, 165, 179, 203, 205, 245, 289b | 289.07122 | 289.07129 | 0.07 | 0.24 | C15H14O6 |
| 29 | Kaempferol | 285 | 93, 97, 119b, 164, 285 | 285.03992 | 285.03980 | −0.12 | 0.42 | C15H10O6 |
| 30 | Naringenin | 271 | 107, 119, 151b, 177, 271 | 271.06065 | 271.06071 | 0.06 | 0.22 | C15H12O5 |
| 31 | Daidzein | 253 | 89, 117, 135b, 151, 169, 179, 227 | 253.05009 | 253.05013 | 0.05 | 0.18 | C15H10O4 |
| 32 | Glycitein | 283 | 240, 268b, 269, 283 | 283.06065 | 283.06060 | −0.05 | 0.18 | C16H12O5 |
| Peak No. | Compound Name | Calibration Standards | Amounts [μg/g DE] |
|---|---|---|---|
| 1 | Gallic acid | Gallic acid | 25.3 ± 1.3 |
| 2 | Chlorogenic acid | Chlorogenic acid | 467.8 ± 20.9 |
| 3 | p-Hydroxybenzoic acid | p-Hydroxybenzoic acid | 4693.5 ± 214.0 |
| 4 | p-Coumaryl aldaric acid | p-Coumaric acid | 58,668.2 ± 3056.6 |
| 5 | o-Coumaryl aldaric acid | p-Coumaric acid | 21,923.4 ± 1091.8 |
| 6 | Protocatechuic acid | Protocatechuic acid | 109.9 ± 5.2 |
| 7 | Feruroyl aldaric acid | Feruroyl acid | 10,266.9 ± 541.1 |
| 8 | Caffeic acid | Caffeic acid | 11,857.2 ± 550.2 |
| 9 | p-Coumaric acid | p-Coumaric acid | 1605.7 ± 85.3 |
| 10 | o-Coumaric acid | p-Coumaric acid | 1442.0 ± 65.9 |
| 11 | Ferulic acid | Ferulic acid | 11,208.7 ± 558.2 |
| 12 | Vanillic acid | Vanillic acid | 37.2 ± 1.9 |
| 13 | Quercetin-3-O-xylosylglucoside | Rutin | 1204.2 ± 60.5 |
| 14 | Rutin | Rutin | 10,637.5 ± 529.7 |
| 15 | Quercetin-3-O-(6″-O-malonyl) glucoside | Rutin | 1151.7 ± 52.6 |
| 16 | Kaempferol-3-O-glucosylxylose | Rutin | 2640.1 ± 133.6 |
| 17 | Kaempferol-3-O-(6″-O-malonyl) glucoside | Rutin | 1347.8 ± 59.8 |
| 18 | Hyperoside | Rutin | 3597.3 ± 173.4 |
| 19 | Isoquercitrin | Rutin | 290.3 ± 14.7 |
| 20 | Quercitrin | Rutin | 2037.9 ± 106.2 |
| 21 | Sinapyl aldaric acid | Sinapic acid | 378.3 ± 17.1 |
| 22 | Myricetin-3-O-glucoside | Rutin | 217.7 ± 10.1 |
| 23 | Prunin | Rutin | 140.2 ± 7.1 |
| 24 | Astragalin | Astragalin | 3504.7 ± 174.5 |
| 25 | Myricetin | Myricetin | 54.7 ± 2.8 |
| 26 | Sinapic acid | Sinapic acid | 1442.0 ± 74.6 |
| 27 | Quercetin | Quercetin | 683.9 ± 32.0 |
| 28 | (+)-Catechin | (+)-Catechin | 41,530.9 ± 2126.4 |
| 29 | Kaempferol | Kaempferol | 2809.9 ± 128.4 |
| 30 | Naringenin | Kaempferol | 1215.0 ± 55.3 |
| 31 | Daidzein | Kaempferol | 1165.6 ± 59.3 |
| 32 | Glycitein | Kaempferol | 1133.2 ± 54.1 |
| DPPH [mgTE/g] | ABTS [mgTE/g] | |
|---|---|---|
| Micellar extract | 246.24 ± 0.05 | 74.38 ± 0.01 |
| Micellar Extract [μg/mL] | COX-1 Inhibition [%] ± SD | COX-2 Inhibition [%] ± SD |
|---|---|---|
| 10 | 6.90 ± 0.14 | 6.25 ± 0.42 |
| 25 | 16.79 ± 0.44 | 11.31 ± 0.25 |
| 50 | 60.80 ± 0.28 | 52.57 ± 0.43 |
| 100 | 57.22 ± 0.31 | 48.86 ± 0.35 |
| Indomethacin | 61.15 ± 0.26 | 55.29 ± 0.17 |
| Micellar Extract [μg/mL] | Collagenase Inhibition | Elastase Inhibition | Tyrosinase Inhibition |
|---|---|---|---|
| 5 | 8.40 ± 0.41 | 10.62 ± 0.36 | 18.35 ± 0.43 |
| 10 | 14.22 ± 0.17 | 18.10 ± 0.17 | 26.13 ± 0.12 |
| 25 | 31.72 ± 0.29 | 42.71 ± 0.42 | 35.70 ± 0.54 |
| 50 | 69.69 ± 0.38 | 67.18 ± 0.30 | 49.84 ± 0.26 |
| 100 | 61.27 ± 0.15 | 86.29 ± 0.35 | 58.33 ± 0.46 |
| EGCG | 83.29 ± 0.36 | 90.35 ± 0.43 | nt |
| Kojic acid | nt | nt | 65.49 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostkowska, E.; Poleszak, E.; Przekora, A.; Wójcik, M.; Typek, R.; Wojciechowska, K.; Dos Santos Szewczyk, K. Novel Insights into Phaseolus vulgaris L. Sprouts: Phytochemical Analysis and Anti-Aging Properties. Molecules 2024, 29, 3058. https://doi.org/10.3390/molecules29133058
Rostkowska E, Poleszak E, Przekora A, Wójcik M, Typek R, Wojciechowska K, Dos Santos Szewczyk K. Novel Insights into Phaseolus vulgaris L. Sprouts: Phytochemical Analysis and Anti-Aging Properties. Molecules. 2024; 29(13):3058. https://doi.org/10.3390/molecules29133058
Chicago/Turabian StyleRostkowska, Ewelina, Ewa Poleszak, Agata Przekora, Michał Wójcik, Rafał Typek, Katarzyna Wojciechowska, and Katarzyna Dos Santos Szewczyk. 2024. "Novel Insights into Phaseolus vulgaris L. Sprouts: Phytochemical Analysis and Anti-Aging Properties" Molecules 29, no. 13: 3058. https://doi.org/10.3390/molecules29133058
APA StyleRostkowska, E., Poleszak, E., Przekora, A., Wójcik, M., Typek, R., Wojciechowska, K., & Dos Santos Szewczyk, K. (2024). Novel Insights into Phaseolus vulgaris L. Sprouts: Phytochemical Analysis and Anti-Aging Properties. Molecules, 29(13), 3058. https://doi.org/10.3390/molecules29133058








