Lattice-Strained Bimetallic Nanocatalysts: Fundamentals of Synthesis and Structure
Abstract
:1. Introduction
2. Fundamentals of Nanoparticle Catalysts
2.1. Overview of Synthesis Techniques
2.2. Morphologically Controlled Synthesis
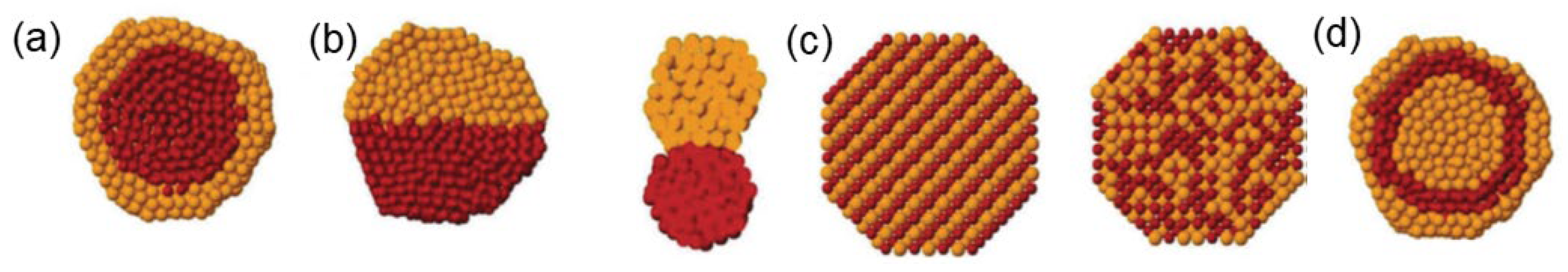
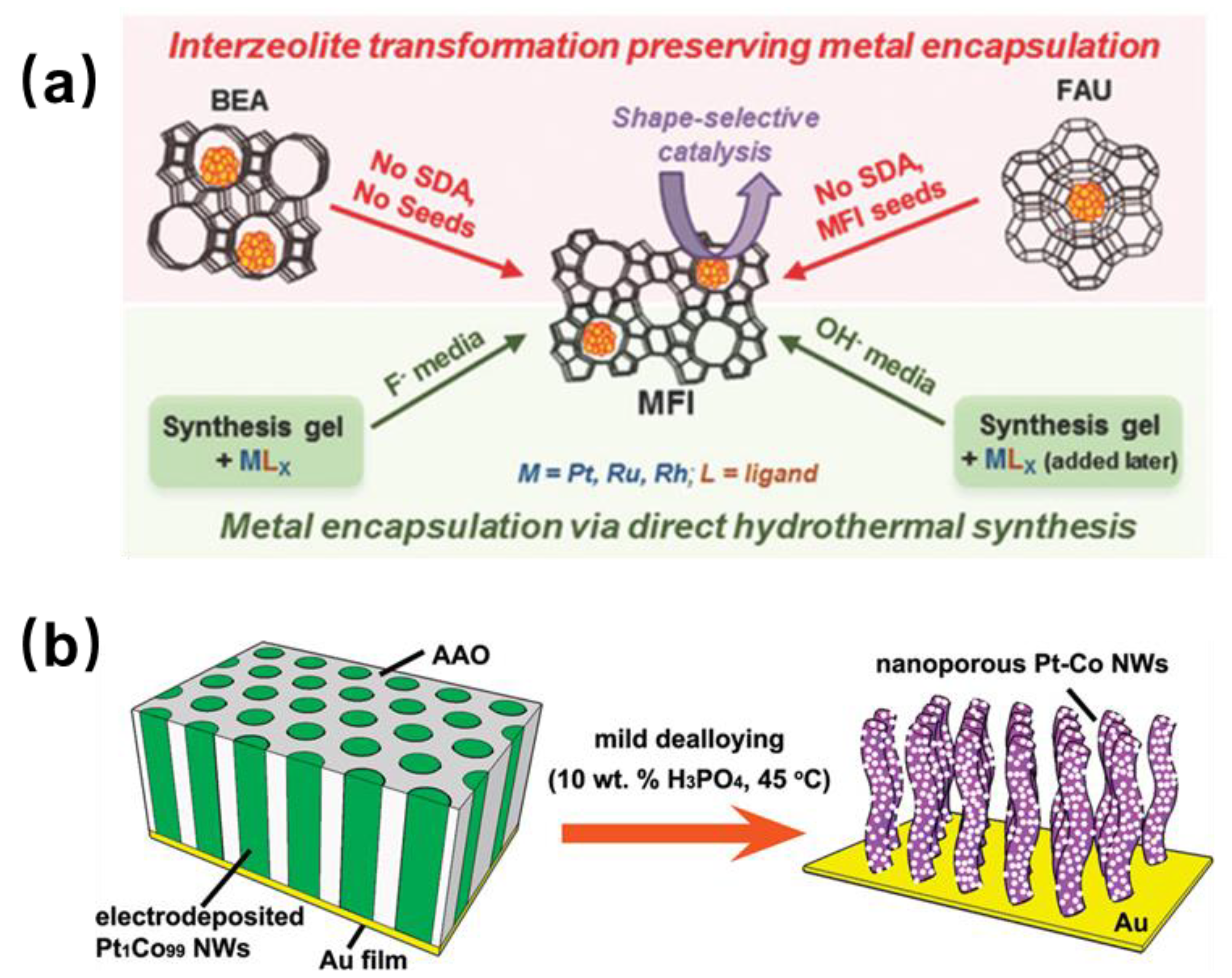
2.2.1. Encapsulation Method by the Confinement Effect
2.2.2. Electrochemical Deposition Method
2.2.3. Liquid-Phase Chemical Reduction Method
2.2.4. Ultrasound-/Microwave-Assisted Reduction Method
2.2.5. Microemulsion Method
3. Synthesis and Properties of Bimetallic Nanoparticles
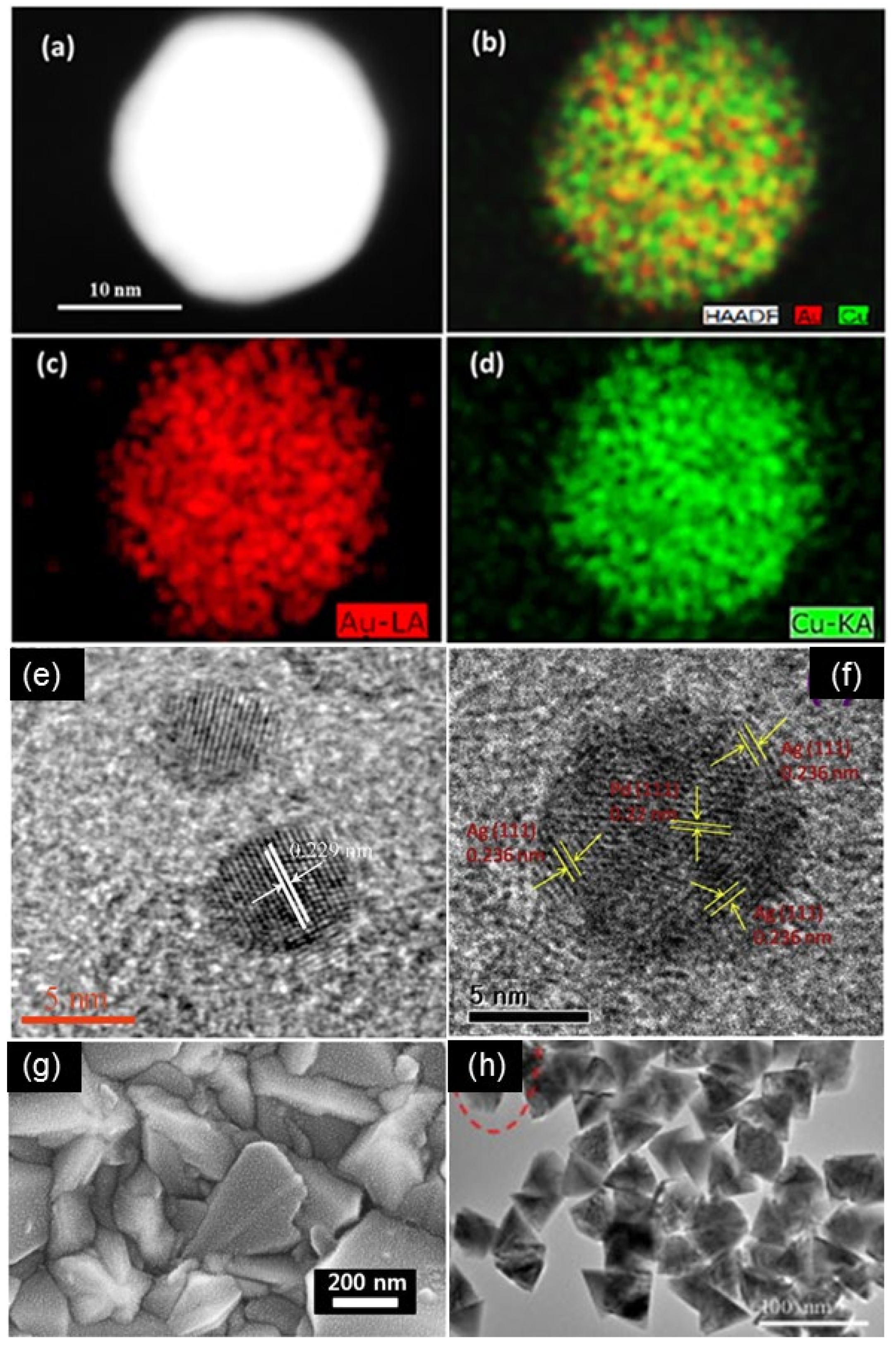
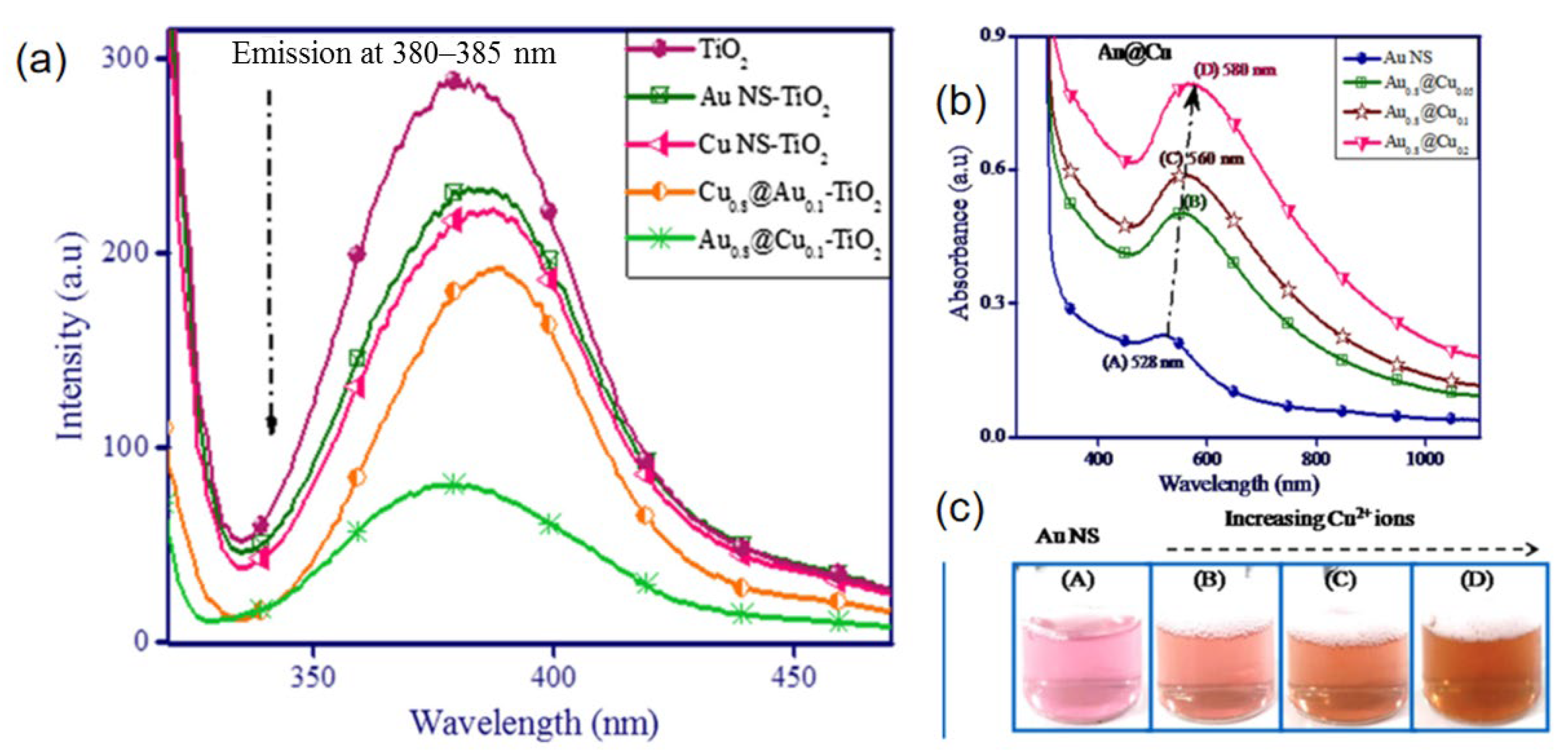
3.1. Au-Based Bimetallic Nanoparticles
| Name | Preparation | Morphology | Ref |
|---|---|---|---|
| AuPd/HOPG * | Ar, HOPG, UHV, 300 °C | “Au core–Pd shell” | [47] |
| AuPd/g-C3N4-N * | one-pot deposition reduction method, melamine, sodium borohydride | the lattice edge distance is 0.229 nm, in the range of 0.234 nm and 0.223 nm, respectively | [45] |
| PdCore–AuShell/rGO | two-step protocol, rGO *, MeAB, Ag | spherical, varying in size from 1.6 to 2.8 nm | [46] |
| AuPd/activated carbon cloth | impregnation method, nitric acid, activated carbon cloth, PdCl2, HAuCl4 | [58] | |
| AuPt/FTO glass | a new process for efficiently synthesizing supported metal (or metal oxide) NPs using dry plasma reduction at near room temperature under atmospheric | AuPtBNP sample shows a finer morphology, where Au sputtered particles produce uniform coverage FTO tight junctions of glass | [48] |
| AuAg/TiO2 | sequential deposition method, NaOH, urea, hydrogen | 530 nm | [49] |
| AuAg/HES | graft copolymer reduced method, acrylamide-co-acrylic acid, NaOH, potassium bisulfate | [50] | |
| AuCu/rGO | deposition–precipitation method, NaBH4, natural graphite powder, HAuCl4·3H2O, Cu(NO3)2·3H2O | no agglomeration, average size 15 ± 1 nm, surface spacing 0.22 nm | [52] |
| AuCu-ZnO-Gr | CuAu-ZnO-Gr of two-step synthesis, OLA * | ellipsoid, size 18.0 ± 2.0 nm | [54] |
| AuRh | hydrogen sacrificial reduction method, alcohol reduction method, sodium borohydride reduction method, PVP *, MNPs, Rh/Au | Rh (0.22 nm), Au (0.24 nm) | [55] |
3.2. Pt-Based Bimetallic Nanoparticles
| Name | Preparation | Morphology | Ref |
|---|---|---|---|
| Pt-Fe/Al2O3 | H2PtCl6·6H2O, cetyltrimethylammonium bromide, butanol, cyclohexane, N2H4 H2O, Al2O3, ethanol, H2PtCl6, FeCl3 80 °C | Polycrystalline structure with high crystallinity | [32] |
| PtCu NPs | Platinum (IV) chloride, copper (II) acetylacetonate, oleylamine, polyvinylpyrrolidone, ethylene glycol 200 °C | Spherical, mean particle size (7.0 ± 0.7) nm | [85] |
| PtCu NWs | Platinum (II) acetylacetonate, cupricchloride, dihydrate, hexacarbonyltungsten, C6H12O6, dodecyl trimethyl ammonium bromide, oleylamine, 1-octadecene, C6H12, CH3COOH, CH3CH2OH, CH3OH 170 °C | Nanowire structure | [92] |
| PtCu/TiO2 | Pt, Cu, Ar, 500 °C | Nanotube structure | [93] |
| PtNi/HSNs | H2PtCl6·6H2O, Ni(CH3COO)2·4H2O, oleylamine, cetyltrimethyl ammonium chloride, H2SO4 160 °C | hierarchical framework of multilayer structure, uniform octahedral shape, narrow particle size distribution, mean particle size nm 79.8 | [94] |
| Pt-Ni/CeO2 | Ce(NO3)3·6H2O, NaOH, NiCl2, H2PtCl6, H2/N2 600 °C | Average particle size 2 nm | [95] |
| Pt–Ni/ZnO-rod | Zn(NO3)2·6H2O, NaOH, Pt, Pt-acetylacetonate, acetone, Ni(NO3)2·6H2O, ethanol, Ni, H2 500 °C | Nano-rod structure 3–14 nm | [96] |
| Pt-Co/TiO2 | Pt(C5H7O2)2, CoC22H14O4, Ti(OCH2CH2CH2CH3)4 500 °C | Large spherical particles | [97] |
4. Conclusions and Outlooks
- (i)
- Removal of template agents. Despite beautiful and controllable morphologies owing to the addition of template agents, they are detrimental for catalysis, as residual species of polymers block active sites for surface reactions. Therefore, future efforts should be paid to develop efficient methods to effectively remove template agents, rather than merely focusing on morphological control.
- (ii)
- Scale-up synthesis is still missing. Although existing studies have revealed the unique kinetics of the formation of bimetallic nanoparticles, experimental studies on scale-up synthesis are still missing. This might mislead young researchers by an ill-defined yield of nanoparticles per precursor added, as such information is not available in most of the literature.
Author Contributions
Funding
Conflicts of Interest
References
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar] [CrossRef]
- Goel, S.; Wu, Z.; Zones, S.I.; Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695. [Google Scholar] [CrossRef]
- Azadi, O.; Taheri, A.; Babaei, A. New hybrid approach for desulfurization of diesel fuel using an efficient heterogeneous polyoxometalate nanocatalyst. Mater. Chem. Phys. 2023, 297, 127400. [Google Scholar] [CrossRef]
- Bodaghifard, M.A.; Hamidinasab, M.; Soleimani, N. Heteropoly acid-based ionic liquid grafted on hybrid nanomaterial for deep oxidative desulfurization of diesel fuel. Res. Chem. Intermed. 2023, 49, 1563–1579. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Shaari, N.; Saharuddin, T.S.T. Progress and major BARRIERS of nanocatalyst development in direct methanol fuel cell: A review. Int. J. Hydrogen Energy 2022, 47, 22114–22146. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Ardeshiri, H.H.; Aghasadeghi, Z. Extractive–Oxidative Desulfurization of Real and Model Gasoline Using (gly)3H[SiW12O40]⊂CoFe2O4 as a Recoverable and Efficient Nanocatalyst. Energy Fuels 2023, 37, 2245–2254. [Google Scholar] [CrossRef]
- Yadav, D.; Datta, S.; Saha, S.; Pradhan, S.; Kumari, S.; Gupta, P.K.; Chauhan, V.; Saw, S.K.; Sahu, G. Heterogeneous Nanocatalyst for Biodiesel Synthesis. ChemistrySelect 2022, 7, e202201671. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. Engl. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, Z.; Liu, J.; Lei, X.; Chang, Z.; Luo, L.; Sun, X. Metal oxide and hydroxide nanoarrays: Hydrothermal synthesis and applications as supercapacitors and nanocatalysts. Prog. Nat. Sci. Mater. Int. 2013, 23, 351–366. [Google Scholar] [CrossRef]
- Dehghan Banadaki, A.; Kajbafvala, A. Recent Advances in Facile Synthesis of Bimetallic Nanostructures: An Overview. J. Nanomater. 2014, 2014, 985948. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X. Bimetallic nanoparticles: Kinetic control matters. Angew. Chem. Int. Ed. Engl. 2012, 51, 3311–3313. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Pan, Y.T.; Warren, S.; Yin, X.; Yang, H. Surface lattice-engineered bimetallic nanoparticles and their catalytic properties. Chem. Soc. Rev. 2012, 41, 8066–8074. [Google Scholar]
- An, K.; Somorjai, G.A. Nanocatalysis I: Synthesis of Metal and Bimetallic Nanoparticles and Porous Oxides and Their Catalytic Reaction Studies. Catal. Lett. 2014, 145, 233–248. [Google Scholar]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Shiju, N.R.; Guliants, V.V. Recent developments in catalysis using nanostructured materials. Appl. Catal. A Gen. 2009, 356, 1–17. [Google Scholar] [CrossRef]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2013, 42, 2880–2904. [Google Scholar] [CrossRef]
- Pal, J.; Pal, T. Faceted metal and metal oxide nanoparticles: Design, fabrication and catalysis. Nanoscale 2015, 7, 14159–14190. [Google Scholar]
- Tada, M.; Iwasawa, Y. Advanced chemical design with supported metal complexes for selective catalysis. Chem. Commun. 2006, 27, 2833–2844. [Google Scholar]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar]
- Roucoux, A.; Schulz, J.; Patin, H. Reduced Transition Metal Colloids: A Novel Family of Reusable Catalysts? Chem. Rev. 2002, 102, 3757–3778. [Google Scholar]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Graham, J.; Hutchings, C.J.K. Strategies for the Synthesis of Supported Gold Palladium Nanoparticles with Controlled Morphology and Composition. Acc. Chem. Res. 2013, 46, 1759–1772. [Google Scholar]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Veith, G.M.; Vindigni, F.; Prati, L. Sol immobilization technique: A delicate balance between activity, selectivity and stability of gold catalysts. Catal. Sci. Technol. 2013, 3, 3036–3041. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–905. [Google Scholar] [CrossRef]
- Helmut Bönnemann, R.M.R. Nanoscopic Metal ParticlesSynthetic Methods and Potential Applications. Eur. J. Inorg. Chem. 2001, 2001, 2455–2480. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.; Yu, J. Ultrasmall Metal Nanoparticles Confined within Crystalline Nanoporous Materials: A Fascinating Class of Nanocatalysts. Adv. Mater. 2019, 31, e1803966. [Google Scholar] [CrossRef]
- Laursen, A.B.; Hojholt, K.T.; Lundegaard, L.F.; Simonsen, S.B.; Helveg, S.; Schuth, F.; Paul, M.; Grunwaldt, J.D.; Kegnaes, S.; Christensen, C.H.; et al. Substrate size-selective catalysis with zeolite-encapsulated gold nanoparticles. Angew. Chem. Int. Ed. Engl. 2010, 49, 3504–3507. [Google Scholar] [CrossRef]
- Liu, L.; Pippel, E.; Scholz, R.; Gosele, U. Nanoporous Pt-Co Alloy Nanowires: Fabrication, Characterization, and Electrocatalytic Properties. Nano Lett. 2009, 9, 4352–4358. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Bai, X.; Wu, W. Ultrasound-excited hydrogen radical from NiFe layered double hydroxide for preparation of ultrafine supported Ru nanocatalysts in hydrogen storage of N-ethylcarbazole. Ultrason. Sonochem. 2021, 81, 105840. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, B.; Li, C.; Pang, M.; Su, D.; Williams, C.T.; Liang, C. Microwave-assisted green synthesis of uniform Ru nanoparticles supported on non-functional carbon nanotubes for cinnamaldehyde hydrogenation. Catal. Commun. 2012, 24, 65–69. [Google Scholar] [CrossRef]
- Feng, L.; Liang, J.; Wang, K.; Cao, B.; Song, H. Fe-Promoted Pt-Fe/Al2O3 Catalyst Prepared by Microemulsion Technique for m-Chloronitrobenzene Hydrogenation. Russ. J. Phys. Chem. A 2018, 92, 1279–1284. [Google Scholar] [CrossRef]
- Tianimoghadam, S.; Salabat, A. A microemulsion method for preparation of thiol-functionalized gold nanoparticles. Particuology 2018, 37, 33–36. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Gómez, E.; Vallés, E.; Aldaz, A.; Feliu, J.M. Synthesis and structural, magnetic and electrochemical characterization of PtCo nanoparticles prepared by water-in-oil microemulsion. J. Nanoparticle Res. 2009, 12, 1149–1159. [Google Scholar] [CrossRef]
- Rajapantulu, A.; Bandyopadhyaya, R. Formation of Gold Nanoparticles in Water-in-Oil Microemulsions: Experiment, Mechanism, and Simulation. Langmuir 2021, 37, 6623–6631. [Google Scholar] [CrossRef] [PubMed]
- Pawlonka, J.; Gac, W.; Greluk, M.; Słowik, G. Application of microemulsion method for development of methanol steam reforming Pd/ZnO catalysts. J. Therm. Anal. Calorim. 2016, 125, 1265–1272. [Google Scholar] [CrossRef]
- Pal, A. Gold–platinum alloy nanoparticles through water-in-oil microemulsion. J. Nanostructure Chem. 2014, 5, 65–69. [Google Scholar] [CrossRef]
- Gnanakumar, E.S.; Ng, W.; Coskuner Filiz, B.; Rothenberg, G.; Wang, S.; Xu, H.; Pastor-Perez, L.; Pastor-Blas, M.M.; Sepulveda-Escribano, A.; Yan, N.; et al. Plasma-Assisted Synthesis of Monodispersed and Robust Ruthenium Ultrafine Nanocatalysts for Organosilane Oxidation and Oxygen Evolution Reactions. ChemCatChem 2017, 9, 4159–4163. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Zhang, J.; Zhang, X. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process. Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Djerdj, I.; Arčon, D.; Jagličić, Z.; Niederberger, M. Nonaqueous synthesis of metal oxide nanoparticles: Short review and doped titanium dioxide as case study for the preparation of transition metal-doped oxide nanoparticles. J. Solid State Chem. 2008, 181, 1571–1581. [Google Scholar] [CrossRef]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179–1218. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, Y.; He, X.; Chen, G.; Xie, C.; Huang, J. AuPd nanoporous dendrites: High electrocatalytic activity and surface plasmon-enhanced stability for ethanol electrooxidation. Chem. Eng. J. 2023, 453, 139962. [Google Scholar] [CrossRef]
- Dimitratos, N.; Villa, A.; Wang, D.; Porta, F.; Su, D.; Prati, L. Pd and Pt catalysts modified by alloying with Au in the selective oxidation of alcohols. J. Catal. 2006, 244, 113–121. [Google Scholar] [CrossRef]
- Fang, W.; Deng, Y.; Tang, L.; Zeng, G.; Zhou, Y.; Xie, X.; Wang, J.; Wang, Y.; Wang, J. Synthesis of Pd/Au bimetallic nanoparticle-loaded ultrathin graphitic carbon nitride nanosheets for highly efficientcatalytic reduction of p-nitrophenol. J. Colloid Interface Sci. 2017, 490, 834–843. [Google Scholar] [CrossRef]
- Raghavendra, P.; Vishwakshan Reddy, G.; Sivasubramanian, R.; Sri Chandana, P.; Subramanyam Sarma, L. Reduced graphene oxide-supported Pd@Au bimetallic nano electrocatalyst for enhanced oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2018, 43, 4125–4135. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Burueva, D.B.; Prosvirin, I.P.; Klyushin, A.Y.; Panafidin, M.A.; Kovtunov, K.V.; Bukhtiyarov, V.I.; Koptyug, I.V. Bimetallic Pd–Au/Highly Oriented Pyrolytic Graphite Catalysts: From Composition to Pairwise Parahydrogen Addition Selectivity. J. Phys. Chem. C 2018, 122, 18588–18595. [Google Scholar] [CrossRef]
- Dao, V.-D.; Choi, Y.; Yong, K.; Larina, L.L.; Shevaleevskiy, O.; Choi, H.-S. A facile synthesis of bimetallic AuPt nanoparticles as a new transparent counter electrode for quantum-dot-sensitized solar cells. J. Power Sources 2015, 274, 831–838. [Google Scholar] [CrossRef]
- Sandoval, A.; Delannoy, L.; Méthivier, C.; Louis, C.; Zanella, R. Synergetic effect in bimetallic Au–Ag/TiO2 catalysts for CO oxidation: New insights from in situ characterization. Appl. Catal. A Gen. 2015, 504, 287–294. [Google Scholar] [CrossRef]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M. Green synthesis of Ag-Au bimetallic nanocomposites using a biodegradable synthetic graft copolymer; hydroxyethyl starch-g-poly (acrylamide-co-acrylic acid) and evaluation of their catalytic activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar] [CrossRef]
- Bhukta, A.; Bagarti, T.; Guha, P.; Ravulapalli, S.; Satpati, B.; Rakshit, B.; Maiti, P.; Parlapalli, V.S. Study of Ag induced bimetallic (Au–Ag) nanowires on silicon (5 5 12) surfaces: Experiment and theoretical aspects. Surf. Sci. 2017, 664, 29–37. [Google Scholar] [CrossRef]
- Rout, L.; Kumar, A.; Dhaka, R.S.; Reddy, G.N.; Giri, S.; Dash, P. Bimetallic Au-Cu alloy nanoparticles on reduced graphene oxide support: Synthesis, catalytic activity and investigation of synergistic effect by DFT analysis. Appl. Catal. A Gen. 2017, 538, 107–122. [Google Scholar] [CrossRef]
- Monga, A.; Bathla, A.; Pal, B. A Cu-Au bimetallic co-catalysis for the improved photocatalytic activity of TiO2 under visible light radiation. Sol. Energy 2017, 155, 1403–1410. [Google Scholar] [CrossRef]
- Xie, H.; Ye, X.; Duan, K.; Xue, M.; Du, Y.; Ye, W.; Wang, C. CuAu–ZnO–graphene nanocomposite: A novel graphene-based bimetallic alloy-semiconductor catalyst with its enhanced photocatalytic degradation performance. J. Alloys Compd. 2015, 636, 40–47. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Jiao, C.; Lu, L.; Zhang, S. Preparation and catalytic activities for H2O2 decomposition of Rh/Au bimetallic nanoparticles. Mater. Res. Bull. 2016, 79, 29–35. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Yan, W.; Zeng, C.; Bobba, P.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Anisotropic growth of PtFe nanoclusters induced by lattice-mismatch: Efficient catalysts for oxidation of biopolyols to carboxylic acid derivatives. J. Catal. 2016, 337, 272–283. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, G.; Liu, Y.; He, Y.; Feng, J.; Li, D. Preparation of AuPd/ZnO–CuO for the directional oxidation of glycerol to DHA. Catal. Sci. Technol. 2020, 10, 6223–6234. [Google Scholar] [CrossRef]
- Gudarzi, D.; Ratchananusorn, W.; Turunen, I.; Heinonen, M.; Salmi, T. Promotional effects of Au in Pd–Au bimetallic catalysts supported on activated carbon cloth (ACC) for direct synthesis of H2O2 from H2 and O2. Catal. Today 2015, 248, 58–68. [Google Scholar] [CrossRef]
- Liu, M.; Lai, L.; Wang, Z.; Jin, X.; Shen, J.; Wang, Y.; Zhang, Q.; Zhang, D.; Sun, Y.; Ning, H.; et al. Catalytic Synthesis of Tartaric Acid from Glucose and Gluconic Acid over AuPt/TiO2 Catalysts: Studies on Catalyst Structure–Performance Dependency. Ind. Eng. Chem. Res. 2023, 62, 6052–6068. [Google Scholar] [CrossRef]
- Liu, M.; Jin, X.; Zhang, G.; Xia, Q.; Lai, L.; Wang, J.; Zhang, W.; Sun, Y.; Ding, J.; Yan, H.; et al. Bimetallic AuPt/TiO2 Catalysts for Direct Oxidation of Glucose and Gluconic Acid to Tartaric Acid in the Presence of Molecular O2. ACS Catal. 2020, 10, 10932–10945. [Google Scholar] [CrossRef]
- Lai, L.; Liu, M.; Liu, J.; Li, W.; Miao, W.; Sun, Z.; Wang, Z.; Wang, Y.; Shi, H.; Chen, C.; et al. Kinetic Modeling of Glucose Oxidation to Tartaric Acid over Monometallic Pt/TiO2 and Bimetallic AuPt/TiO2 Catalysts: Role of Bimetals on C–H and C–C Cleavages. ACS Sustain. Chem. Eng. 2023, 11, 15851–15864. [Google Scholar] [CrossRef]
- Müslehiddinoglu, J.; Vannice, M.A. CO adsorption on supported and promoted Ag epoxidation catalysts. J. Catal. 2003, 213, 305–320. [Google Scholar] [CrossRef]
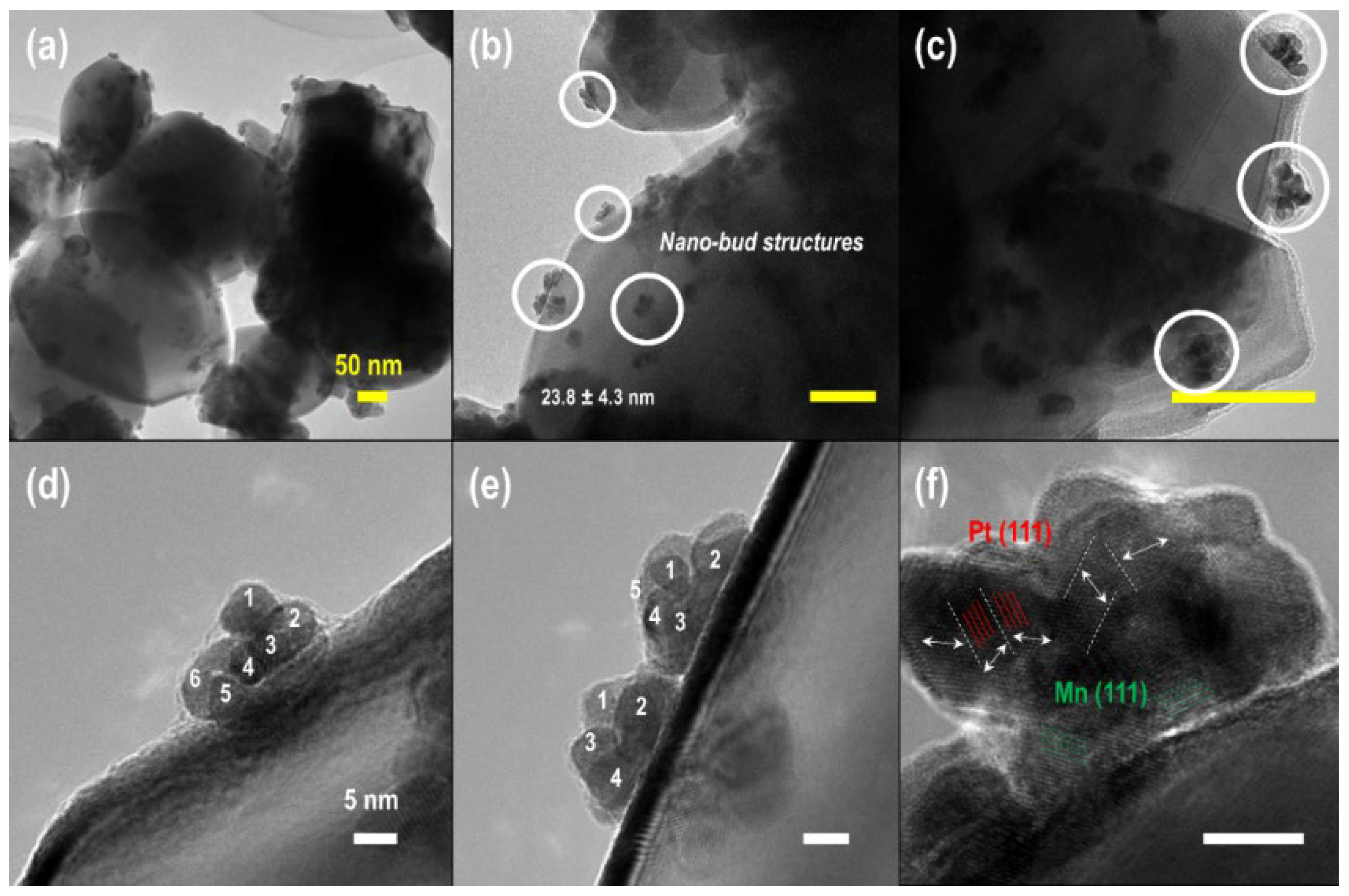
- Jin, X.; Zeng, C.; Yan, W.; Zhao, M.; Bobba, P.; Shi, H.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Lattice distortion induced electronic coupling results in exceptional enhancement in the activity of bimetallic PtMn nanocatalysts. Appl. Catal. A Gen. 2017, 534, 46–57. [Google Scholar] [CrossRef]
- Bossola, F.; Pereira-Hernández, X.I.; Evangelisti, C.; Wang, Y.; Dal Santo, V. Investigation of the promoting effect of Mn on a Pt/C catalyst for the steam and aqueous phase reforming of glycerol. J. Catal. 2017, 349, 75–83. [Google Scholar] [CrossRef]
- Nakano, A.; Manabe, S.; Higo, T.; Seki, H.; Nagatake, S.; Yabe, T.; Ogo, S.; Nagatsuka, T.; Sugiura, Y.; Iki, H.; et al. Effects of Mn addition on dehydrogenation of methylcyclohexane over Pt/Al2O3 catalyst. Appl. Catal. A Gen. 2017, 543, 75–81. [Google Scholar] [CrossRef]
- Pazos Urrea, M.; Herold, F.; Chen, D.; Rønning, M. Nitrogen-containing carbon nanofibers as supports for bimetallic Pt-Mn catalysts in aqueous phase reforming of ethylene glycol. Catal. Today 2023, 418, 114066. [Google Scholar] [CrossRef]
- Roongcharoen, T.; Yang, X.; Han, S.; Sementa, L.; Vegge, T.; Hansen, H.A.; Fortunelli, A. Oxidation and de-alloying of PtMn particle models: A computational investigation. Faraday Discuss 2023, 242, 174–192. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, S.; Mu, R.; Fu, Q. Atmosphere-Dependent Structures of Pt–Mn Bimetallic Catalysts. J. Phys. Chem. C 2020, 124, 17548–17555. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, R.; Yang, L.; Yang, J.; Shan, C.; Liu, Q. Revealing opposite behaviors of catalyst for VOCs Oxidation: Modulating electronic structure of Pt nanoparticles by Mn doping. Chem. Eng. J. 2023, 465, 142807. [Google Scholar] [CrossRef]
- Huang, J.; Peng, B.; Stracensky, T.; Liu, Z.; Zhang, A.; Xu, M.; Liu, Y.; Zhao, Z.; Duan, X.; Jia, Q.; et al. 1D PtCo nanowires as catalysts for PEMFCs with low Pt loading. Sci. China Mater. 2021, 65, 704–711. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Yang, H.; Luo, Y.; Qun Tian, Z.; Kang Shen, P. Surface-structure tailoring of Dendritic PtCo nanowires for efficient oxygen reduction reaction. J. Colloid Interface Sci. 2023, 652 Pt B, 1597–1608. [Google Scholar] [CrossRef]
- Guo, S.; Li, D.; Zhu, H.; Zhang, S.; Markovic, N.M.; Stamenkovic, V.R.; Sun, S. FePt and CoPt nanowires as efficient catalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2013, 52, 3465–3468. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, N.; Chaubey, G.S.; Nandwana, V.; Rong, C.B.; Yano, K.; Liu, J.P. Synthesis of FePt nanorods and nanowires by a facile method. Nanotechnology 2008, 19, 355601. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, X.; Wei, Y.; Cao, Z.; Li, H.; Jiang, Y.; Xie, Z. PtCo-excavated rhombic dodecahedral nanocrystals for efficient electrocatalysis. Nanoscale Adv. 2020, 2, 4881–4886. [Google Scholar] [CrossRef]
- Liu, Z.; Senanayake, S.D.; Rodriguez, J.A. Elucidating the interaction between Ni and CeOx in ethanol steam reforming catalysts: A perspective of recent studies over model and powder systems. Appl. Catal. B Environ. 2016, 197, 184–197. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Tian, R.; Sun, C.; Dai, R.; Li, X.; Wu, X.; An, X.; Xie, X. Ni-hierarchical Beta zeolite catalysts were applied to ethanol steam reforming: Effect of sol gel method on loading Ni and the role of hierarchical structure. Mol. Catal. 2018, 453, 64–73. [Google Scholar] [CrossRef]
- Fan, A.; Qin, C.; Zhao, R.; Sun, H.; Sun, H.; Dai, X.; Ye, J.-Y.; Sun, S.-G.; Lu, Y.; Zhang, X. Phosphorus-doping-tuned PtNi concave nanocubes with high-index facets for enhanced methanol oxidation reaction. Nano Res. 2022, 15, 6961–6968. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, J.; Zhu, W.; Chen, Z.; Gao, X.; Du, X.; Wan, J.; Zhou, K.; Chen, C.; Li, Y. 50 ppm of Pd dispersed on Ni(OH)2 nanosheets catalyzing semi-hydrogenation of acetylene with high activity and selectivity. Nano Res. 2017, 11, 905–912. [Google Scholar] [CrossRef]
- Pei, G.; Liu, X.; Chai, M.; Wang, A.; Zhang, T. Isolation of Pd atoms by Cu for semi-hydrogenation of acetylene: Effects of Cu loading. Chin. J. Catal. 2017, 38, 1540–1548. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Krivoruchenko, D.S.; Medvedev, M.G.; Mashkovsky, I.S.; Stakheev, A.Y.; Svitanko, I.V. Diphenylacetylene hydrogenation on a PdAg /Al2O3 single-atom catalyst: An experimental and DFT study. Mendeleev Commun. 2017, 27, 615–617. [Google Scholar] [CrossRef]
- Jin, X.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Kinetic Modeling of Sorbitol Hydrogenolysis over Bimetallic RuRe/C Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 6037–6047. [Google Scholar] [CrossRef]
- Liu, W.J.; Xu, Z.; Zhao, D.; Pan, X.Q.; Li, H.C.; Hu, X.; Fan, Z.Y.; Wang, W.K.; Zhao, G.H.; Jin, S.; et al. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jiang, Y.; Zhang, J.; Zhang, L.; Chen, Q.; Xie, Z.; Zheng, L. Unique excavated rhombic dodecahedral PtCu3 alloy nanocrystals constructed with ultrathin nanosheets of high-energy 110 facets. J. Am. Chem. Soc. 2014, 136, 3748–3751. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ma, Y.; Lin, Z.; Xu, Q.; Yan, Y.; Zhang, H.; Wu, J.; Yang, D. Facile synthesis of PtCu3alloy hexapods and hollow nanoframes as highly active electrocatalysts for methanol oxidation. CrystEngComm 2016, 18, 7823–7830. [Google Scholar] [CrossRef]
- Kalyva, M.; Sunding, M.F.; Gunnæs, A.E.; Diplas, S.; Redekop, E.A. Correlation between surface chemistry and morphology of PtCu and Pt nanoparticles during oxidation-reduction cycle. Appl. Surf. Sci. 2020, 532, 147369. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Synthesis and electrocatalytic oxygen reduction properties of truncated octahedral Pt3Ni nanoparticles. Nano Res. 2010, 4, 72–82. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Fang, J.; Zou, S. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 2010, 10, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Radtke, M.; Ignaszak, A. Classical group theory adapted to the mechanism of Pt3Ni nanoparticle growth: The role of W(CO)6 as the “shape-controlling” agent. Phys. Chem. Chem. Phys. 2016, 18, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Shin, Y.-J.; Kim, M.-S.; Kwon, J.A.; Lim, D.-H. Density functional theory–based design of a Pt-skinned PtNi catalyst for the oxygen reduction reaction in fuel cells. Appl. Surf. Sci. 2021, 565, 150518. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tian, C.; Geng, D.; Wang, D.; Bai, S. One-Pot Synthesis of Highly Efficient Carbon-Supported Polyhedral Pt3Ni Alloy Nanoparticles for Oxygen Reduction Reaction. Electrocatalysis 2019, 10, 613–620. [Google Scholar] [CrossRef]
- Debe, M.K.; Steinbach, A.J.; Vernstrom, G.D.; Hendricks, S.M.; Kurkowski, M.J.; Atanasoski, R.T.; Kadera, P.; Stevens, D.A.; Sanderson, R.J.; Marvel, E.; et al. Extraordinary Oxygen Reduction Activity of Pt3Ni7. J. Electrochem. Soc. 2011, 158, B910. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, W.; Zhong, Y.; Li, P.; Xiang, D.; Uddin, W.; Li, X.; Wang, Y.-G.; Yuan, X.; Wang, D.; et al. Surface-structure tailoring of ultrafine PtCu nanowires for enhanced electrooxidation of alcohols. Sci. China Mater. 2020, 64, 601–610. [Google Scholar] [CrossRef]
- Shahvaranfard, F.; Ghigna, P.; Minguzzi, A.; Wierzbicka, E.; Schmuki, P.; Altomare, M. Dewetting of PtCu Nanoalloys on TiO2 Nanocavities Provides a Synergistic Photocatalytic Enhancement for Efficient H2 Evolution. ACS Appl. Mater. Interfaces 2020, 12, 38211–38221. [Google Scholar] [CrossRef]
- Li, S.; Tian, Z.Q.; Liu, Y.; Jang, Z.; Hasan, S.W.; Chen, X.; Tsiakaras, P.; Shen, P.K. Hierarchically skeletal multi-layered Pt-Ni nanocrystals for highly efficient oxygen reduction and methanol oxidation reactions. Chin. J. Catal. 2021, 42, 648–657. [Google Scholar] [CrossRef]
- Araiza, D.G.; Arcos, D.G.; Gómez-Cortés, A.; Díaz, G. Dry reforming of methane over Pt-Ni/CeO2 catalysts: Effect of the metal composition on the stability. Catal. Today 2021, 360, 46–54. [Google Scholar] [CrossRef]
- Lugo, V.R.; Mondragon-Galicia, G.; Gutierrez-Martinez, A.; Gutierrez-Wing, C.; Rosales Gonzalez, O.; Lopez, P.; Salinas-Hernandez, P.; Tzompantzi, F.; Reyes Valderrama, M.I.; Perez-Hernandez, R. Pt-Ni/ZnO-rod catalysts for hydrogen production by steam reforming of methanol with oxygen. RSC Adv. 2020, 10, 41315–41323. [Google Scholar] [CrossRef]
- Tolek, W.; Khruechao, K.; Pongthawornsakun, B.; Mekasuwandumrong, O.; Cadete Santos Aires, F.J.; Weerachawanasak, P.; Panpranot, J. Flame spray-synthesized Pt-Co/TiO2 catalysts for the selective hydrogenation of furfural to furfuryl alcohol. Catal. Commun. 2021, 149, 106246. [Google Scholar] [CrossRef]
- Wang, L.L.; Johnson, D.D. Predicted Trends of Core-Shell Preferences for 132 Late Transition-Metal Binary-Alloy Nanoparticles. J. Am. Chem. Soc. 2009, 131, 14023–14029. [Google Scholar] [CrossRef]
- Serkov, A.A.; Vol’f, L.A. Oxidation of hemicellulose during the continuous mercerization process. Plenum Publ. Corp. 1985, 16, 329–331. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Abareshi, N.; Kamrani, M. Role of the middle-shell in the stability of three-shell nanoparticles: A molecular dynamics study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132163. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, H.; Zhao, D.; Zhang, D.; Yan, W.; Jin, X. Lattice-Strained Bimetallic Nanocatalysts: Fundamentals of Synthesis and Structure. Molecules 2024, 29, 3062. https://doi.org/10.3390/molecules29133062
Wang Y, Shi H, Zhao D, Zhang D, Yan W, Jin X. Lattice-Strained Bimetallic Nanocatalysts: Fundamentals of Synthesis and Structure. Molecules. 2024; 29(13):3062. https://doi.org/10.3390/molecules29133062
Chicago/Turabian StyleWang, Yaowei, Huibing Shi, Deming Zhao, Dongpei Zhang, Wenjuan Yan, and Xin Jin. 2024. "Lattice-Strained Bimetallic Nanocatalysts: Fundamentals of Synthesis and Structure" Molecules 29, no. 13: 3062. https://doi.org/10.3390/molecules29133062




