Tritium-Labeled Nanodiamonds as an Instrument to Analyze Bioprosthetic Valve Coatings: A Case of Using a Nanodiamond Containing Coating on a Pork Aorta
Abstract
:1. Introduction
2. Results
2.1. Tritium Labeling of Nanodiamonds
2.2. Analysis of Nanodiamond-Containing Coatings
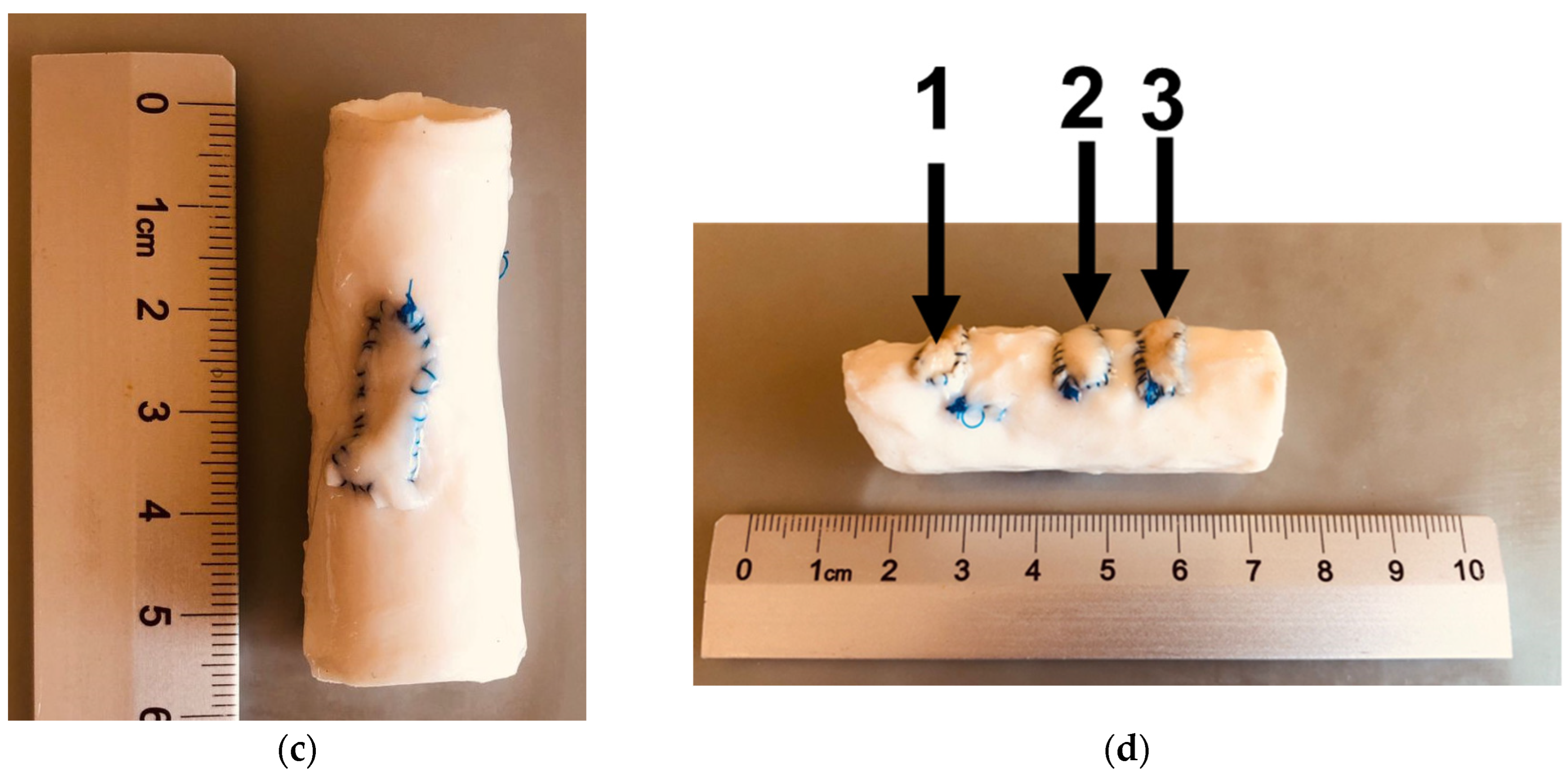
2.3. Histological Analysis Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Tritium Labeling of Nanodiamonds
4.3. Nanodiamond Coating Preparation and Animal Trial
4.4. Histological Analysis
4.5. Quantitative Analysis of the Coating for the Content of Nanodiamonds and Metals, Calcium in Particular
5. Conclusions
6. Ethical Conduct
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Z.; Tao, T.; Xu, H.; Chen, C.; Lee, I.; Chung, S.; Dong, Z.; Li, W.; Ma, L.; Bai, H.; et al. Recent Progress in Biomaterials for Heart Valve Replacement: Structure, Function, and Biomimetic Design. View 2021, 2, 20200142. [Google Scholar] [CrossRef]
- Ekser, B.; Cooper, D.K.C.; Tector, A.J. The Need for Xenotransplantation as a Source of Organs and Cells for Clinical Transplantation. Int. J. Surg. 2015, 23, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, K.; Morticelli, L.; Goecke, T.; Kolbeck, L.; Ramm, R.; Höffler, H.K.; Brandes, G.; Korossis, S.; Haverich, A.; Hilfiker, A. Toward Acellular Xenogeneic Heart Valve Prostheses: Histological and Biomechanical Characterization of Decellularized and Enzymatically Deglycosylated Porcine Pulmonary Heart Valve Matrices. Xenotransplantation 2020, 27, e12617. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.A.; Lee, W.; Cooper, D.K.C. Xenograft Bioprosthetic Heart Valves: Past, Present and Future. Int. J. Surg. 2015, 23, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Vaesken, A.; Heim, F.; Chakfe, N. Fiber Heart Valve Prosthesis: Influence of the Fabric Construction Parameters on the Valve Fatigue Performances. J. Mech. Behav. Biomed. Mater. 2014, 40, 69–74. [Google Scholar] [CrossRef]
- Gross, J.M. Calcification of Bioprosthetic Heart Valves and Its Assessment. J. Thorac. Cardiovasc. Surg. 2003, 125, S6–S8. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef] [PubMed]
- Abolhoda, A.; Yu, S.; Oyarzun, J.R.; McCormick, J.R.; Bogden, J.D.; Gabbay, S. Calcification of Bovine Pericardium: Glutaraldehyde versus No-React Biomodification. Ann. Thorac. Surg. 1996, 62, 169–174. [Google Scholar] [CrossRef]
- Levy, R.J.; Wolfrum, J.; Schoen, F.J.; Hawley, M.A.; Lund, S.A.; Langer, R. Inhibition of Calcification of Bioprosthetic Heart Valves by Local Controlled-Release Diphosphonate. Science 1985, 228, 190–192. [Google Scholar] [CrossRef]
- Chanda, J. Anticalcification Treatment of Pericardial Prostheses. Biomaterials 1994, 15, 465–469. [Google Scholar] [CrossRef]
- Gallyamov, M.O.; Chaschin, I.S.; Khokhlova, M.A.; Grigorev, T.E.; Bakuleva, N.P.; Lyutova, I.G.; Kondratenko, J.E.; Badun, G.A.; Chernysheva, M.G.; Khokhlov, A.R. Collagen Tissue Treated with Chitosan Solutions in Carbonic Acid for Improved Biological Prosthetic Heart Valves. Mater. Sci. Eng. C 2014, 37, 127–140. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Chaschin, I.S.; Badun, G.A.; Vasil’ev, V.G.; Mikheev, I.V.; Shen, T.; Sinolits, M.A.; Bakuleva, N.P. Novel Nanodiamond Coatings for Durable Xenogenic Heart Valve Prostheses: Mechanical Properties and in Vivo Stability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130373. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Chaschin, I.S.; Sinolits, A.V.; Vasil’ev, V.G.; Popov, A.G.; Badun, G.A.; Bakuleva, N.P. Chitosan-Nanodiamond Composites for Improving Heart Valve Biological Prostheses Materials: Preparation and Mechanical Trial. Fuller. Nanotub. Carbon. Nanostructures 2020, 28, 256–261. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Shen, T.; Chaschin, I.S.; Badun, G.A.; Vasil’ev, V.G.; Mikheev, I.V.; Bakuleva, N.P. Nanodiamond–Drug Conjugates for Coating Xenogenic Heart Valve Prostheses. Mendeleev Commun. 2024, 34, 104–106. [Google Scholar] [CrossRef]
- Fusco, L.; Avitabile, E.; Armuzza, V.; Orecchioni, M.; Istif, A.; Bedognetti, D.; Da Ros, T.; Delogu, L.G. Impact of the Surface Functionalization on Nanodiamond Biocompatibility: A Comprehensive View on Human Blood Immune Cells. Carbon. N. Y. 2020, 160, 390–404. [Google Scholar] [CrossRef]
- Cui, J.-F.; Fang, X.-W.; Schmidt-Rohr, K. Quantification of C=C and C=O Surface Carbons in Detonation Nanodiamond by NMR. J. Phys. Chem. C 2014, 118, 9621–9627. [Google Scholar] [CrossRef]
- Kulakova, I.I. Surface Chemistry of Nanodiamonds. Phys. Solid. State 2004, 46, 636–643. [Google Scholar] [CrossRef]
- Osipov, V.Y.Y.; Aleksenskiy, A.E.E.; Shames, A.I.I.; Panich, A.M.M.; Shestakov, M.S.S.; Vul’, A.Y.; Vul, A.Y. Infrared Absorption Study of Surface Functional Groups Providing Chemical Modification of Nanodiamonds by Divalent Copper Ion Complexes. Diam. Relat. Mater. 2011, 20, 1234–1238. [Google Scholar] [CrossRef]
- Shenderova, O.A.; McGuire, G.E. Science and Engineering of Nanodiamond Particle Surfaces for Biological Applications (Review). Biointerphases 2015, 10, 030802. [Google Scholar] [CrossRef]
- Salaam, A.D.; Hwang, P.T.J.; Poonawalla, A.; Green, H.N.; Jun, H.; Dean, D. Nanodiamonds Enhance Therapeutic Efficacy of Doxorubicin in Treating Metastatic Hormone-Refractory Prostate Cancer. Nanotechnology 2014, 25, 425103. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Mochalin, V.N.; Leong, D.T. Tuning Endothelial Permeability with Functionalized Nanodiamonds. ACS Nano 2016, 10, 1170–1181. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Liu, M.; Hui, J.; Yang, B.; Tao, L.; Wei, Y. Surfactant-Dispersed Nanodiamond: Biocompatibility Evaluation and Drug Delivery Applications. Toxicol. Res. 2013, 2, 335–342. [Google Scholar] [CrossRef]
- Tinwala, H.; Wairkar, S. Production, Surface Modification and Biomedical Applications of Nanodiamonds: A Sparkling Tool for Theranostics. Mater. Sci. Eng. C 2019, 97, 913–931. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical Applications of Nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Lebedev, V.T.; Kulvelis, Y.V.; Soroka, M.A.; Kyzyma, O.A.; Vul, A.Y. Structures of Nanodiamonds with Photoactive Modifiers. J. Surf. Investig. X-ray Synchrotron Neutron. Tech. 2023, 17, 7–16. [Google Scholar] [CrossRef]
- Xu, J.; Chow, E.K. Biomedical Applications of Nanodiamonds: From Drug-Delivery to Diagnostics. SLAS Technol. 2023, 28, 214–222. [Google Scholar] [CrossRef]
- Vaijayanthimala, V.; Lee, D.K.; Kim, S.V.; Yen, A.; Tsai, N.; Ho, D.; Chang, H.-C.C.; Shenderova, O. Nanodiamond-Mediated Drug Delivery and Imaging: Challenges and Opportunities. Expert. Opin. Drug Deliv. 2015, 12, 735–749. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Pentecost, A.; Li, X.-M.; Neitzel, I.; Nelson, M.; Wei, C.; He, T.; Guo, F.; Gogotsi, Y. Adsorption of Drugs on Nanodiamond: Toward Development of a Drug Delivery Platform. Mol. Pharm. 2013, 10, 3728–3735. [Google Scholar] [CrossRef]
- Gao, G.; Guo, Q.; Zhi, J. Nanodiamond-Based Theranostic Platform for Drug Delivery and Bioimaging. Small 2019, 15, e1902238. [Google Scholar] [CrossRef]
- Jimenez, C.M.; Knezevic, N.Z.; Rubio, Y.G.; Szunerits, S.; Boukherroub, R.; Teodorescu, F.; Croissant, J.G.; Hocine, O.; Seric, M.; Raehm, L.; et al. Nanodiamond–PMO for Two-Photon PDT and Drug Delivery. J. Mater. Chem. B 2016, 4, 5803–5808. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Lin, Y.-C.; Cheng, C.-L. A Review of Recent Advances in Nanodiamond-Mediated Drug Delivery in Cancer. Expert. Opin. Drug Deliv. 2021, 18, 369–382. [Google Scholar] [CrossRef]
- Mengesha, A.E.; Youan, B.-B.C. Nanodiamonds for Drug Delivery Systems. In Diamond-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 186–205. ISBN 978-085-709-3516. [Google Scholar]
- Osawa, E.; Ho, D. Nanodiamond and Its Application to Drug Delivery. J. Med. Allied Sci. 2012, 2, 31–40. [Google Scholar]
- Lam, R.; Ho, D. Nanodiamonds as Vehicles for Systemic and Localized Drug Delivery. Expert. Opin. Drug Deliv. 2009, 6, 883–895. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Arvind, O.R.; Sriram, G.; Losic, D.; Jung, H.-Y.; Kigga, M.; Kurkuri, M.D. Nanodiamonds and Their Surface Modification Strategies for Drug Delivery Applications. J. Drug Deliv. Sci. Technol. 2020, 60, 101993. [Google Scholar] [CrossRef]
- Kromka, A.; Jira, J.; Stenclova, P.; Kriha, V.; Kozak, H.; Beranova, J.; Vretenar, V.; Skakalova, V.; Rezek, B. Bacterial Response to Nanodiamonds and Graphene Oxide Sheets. Phys. Status Solidi B Basic. Res. 2016, 253, 2481–2485. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Costa, P.; Fernandes, L.; Carvalho, E.O.; Fernandes, M.M.; Carabineiro, S.A.C.; Buijnsters, J.G.; Tubio, C.R.; Lanceros-Mendez, S. Antimicrobial and Antibiofilm Properties of Fluorinated Polymers with Embedded Functionalized Nanodiamonds. ACS Appl. Polym. Mater. 2020, 2, 5014–5024. [Google Scholar] [CrossRef]
- Moskvitina, E.; Kuznetsov, V.; Moseenkov, S.; Serkova, A.; Zavorin, A. Antibacterial Effect of Carbon Nanomaterials: Nanotubes, Carbon Nanofibers, Nanodiamonds, and Onion-like Carbon. Materials 2023, 16, 957. [Google Scholar] [CrossRef]
- Shen, T.; Chernysheva, M.G.; Badun, G.A.; Popov, A.G.; Egorov, A.V.; Anuchina, N.M.; Chaschin, I.S.; Bakuleva, N.P. Levofloxacin and Amikacin Adsorption on Nanodiamonds: Mechanism and Application Prospects. Colloids Interfaces 2022, 6, 35. [Google Scholar] [CrossRef]
- Aversa, R.; Petrescu, R.V.V.; Petrescu, F.I.T.; Apicella, A. Nanodiamond for Structural Biomimetic Scaffolds. J. Mater. Sci. Chem. Eng. 2018, 6, 6–17. [Google Scholar] [CrossRef]
- Mangal, U.; Seo, J.Y.; Yu, J.; Kwon, J.S.; Choi, S.H. Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3d-Printed Resin-Based Biomedical Appliances. Nanomaterials 2020, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Aleksenskiy, A.E.; Eydelman, E.D.; Vul’, A.Y. Deagglomeration of Detonation Nanodiamonds. Nanosci. Nanotechnol. Lett. 2011, 3, 68–74. [Google Scholar] [CrossRef]
- Stehlik, S.; Miliaieva, D.; Varga, M.; Kromka, A.; Rezek, B. Size Decrease of Detonation Nanodiamonds by Air Annealing Investigated by AFM. MRS Adv. 2016, 1, 1067–1073. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Popov, A.G.; Dzianisik, M.G.; Egorov, A.V.; Egorova, T.B.; Gopin, A.V.; Mitrofanov, A.A.; Badun, G.A. Peculiarities of Atomic Hydrogen Interactions with Detonation Nanodiamonds. Mendeleev Commun. 2023, 33, 228–230. [Google Scholar] [CrossRef]
- Badun, G.A.; Chernysheva, M.G.; Myasnikov, I.Y.; Gopin, A.V. Method for Production of Tritium-Labeled Nanodiamonds. Patent RU 2672741 С1, 10 August 2017. [Google Scholar]
- Badun, G.A.; Chernysheva, M.G.; Yakovlev, R.Y.; Leonidov, N.B.; Semenenko, M.N.; Lisichkin, G.V. A Novel Approach Radiolabeling Detonation Nanodiamonds through the Tritium Thermal Activation Method. Radiochim. Acta 2014, 102, 941–946. [Google Scholar] [CrossRef]
- Chaschin, I.S.; Sinolits, M.A.; Badun, G.A.; Chernysheva, M.G.; Anuchina, N.M.; Krasheninnikov, S.V.; Khugaev, G.A.; Petlenko, A.A.; Britikov, D.V.; Zubko, A.V.; et al. Chitosan/Hyaluronic Acid Polyanion Bilayer Applied from Carbon Acid as an Advanced Coating with Intelligent Antimicrobial Properties for Improved Biological Prosthetic Heart Valves. Int. J. Biol. Macromol. 2022, 222, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Chaschin, I.S.; Britikov, D.V.; Khugaev, G.A.; Salokhedinova, R.R.; Zubko, A.V.; Abramchuk, S.S.; Petlenko, A.A.; Muratov, R.M.; Bakuleva, N.P. Decellularization of the Human Donor Aortic Conduit by a New Hybrid Treatment in a Multicomponent System with Supercritical CO2 and Tween 80. J. Supercrit. Fluids 2022, 180, 105452. [Google Scholar] [CrossRef]
- Volkov, D.S.; Proskurnin, M.A.; Korobov, M.V. Elemental Analysis of Nanodiamonds by Inductively-Coupled Plasma Atomic Emission Spectroscopy. Carbon N. Y. 2014, 74, 1–13. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial Applications of Nanodiamonds. Int. J. Environ. Res. Public Health 2016, 13, 413. [Google Scholar] [CrossRef]
- Chatterjee, A.; Perevedentseva, E.; Jani, M.; Cheng, C.-Y.; Ye, Y.-S.; Chung, P.-H.; Cheng, C.-L. Antibacterial Effect of Ultrafine Nanodiamond against Gram-Negative Bacteria Escherichia coli. J. Biomed. Opt. 2014, 20, 051014. [Google Scholar] [CrossRef]
- Badun, G.A.; Chernysheva, M.G.; Gus’kov, A.V.; Sinolits, A.V.; Popov, A.G.; Egorov, A.V.; Egorova, T.B.; Kulakova, I.I.; Lisichkin, G.V. Adsorption of Alkyltrimethylammonium Bromides on Nanodiamonds. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 361–367. [Google Scholar] [CrossRef]
- Gallyamov, M.O.; Chaschin, I.S.; Bulat, M.V.; Bakuleva, N.P.; Badun, G.A.; Chernysheva, M.G.; Kiselyova, O.I.; Khokhlov, A.R. Chitosan Coatings with Enhanced Biostability In Vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 270–277. [Google Scholar] [CrossRef] [PubMed]
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). International Organization for Standardization: Geneva, Switzerland, 2007.



| Type of Nanodiamonds | Radioactivity, mCi | |
|---|---|---|
| Initial Radioactivity (Directly after the Reaction with Atomic Tritium) | After Purification from the Labile Tritium | |
| DND | 8.54 | 3.8 |
| DND-O2 | 20.0 | 5.0 |
| SDND | 17.1 | 5.4 |
| Type of Nanodiamonds | mg of Nanodiamonds per 1 g of Aorta Tissue | |
|---|---|---|
| After Preparation | After Animal Exploitation | |
| DND | 2.00 ± 0.14 | 0.13 ± 0.05 |
| DND-O2 | 3.00 ± 0.20 | 0.40 ± 0.10 |
| SDND | 5.50 ± 0.25 | 0.80 ± 0.20 |
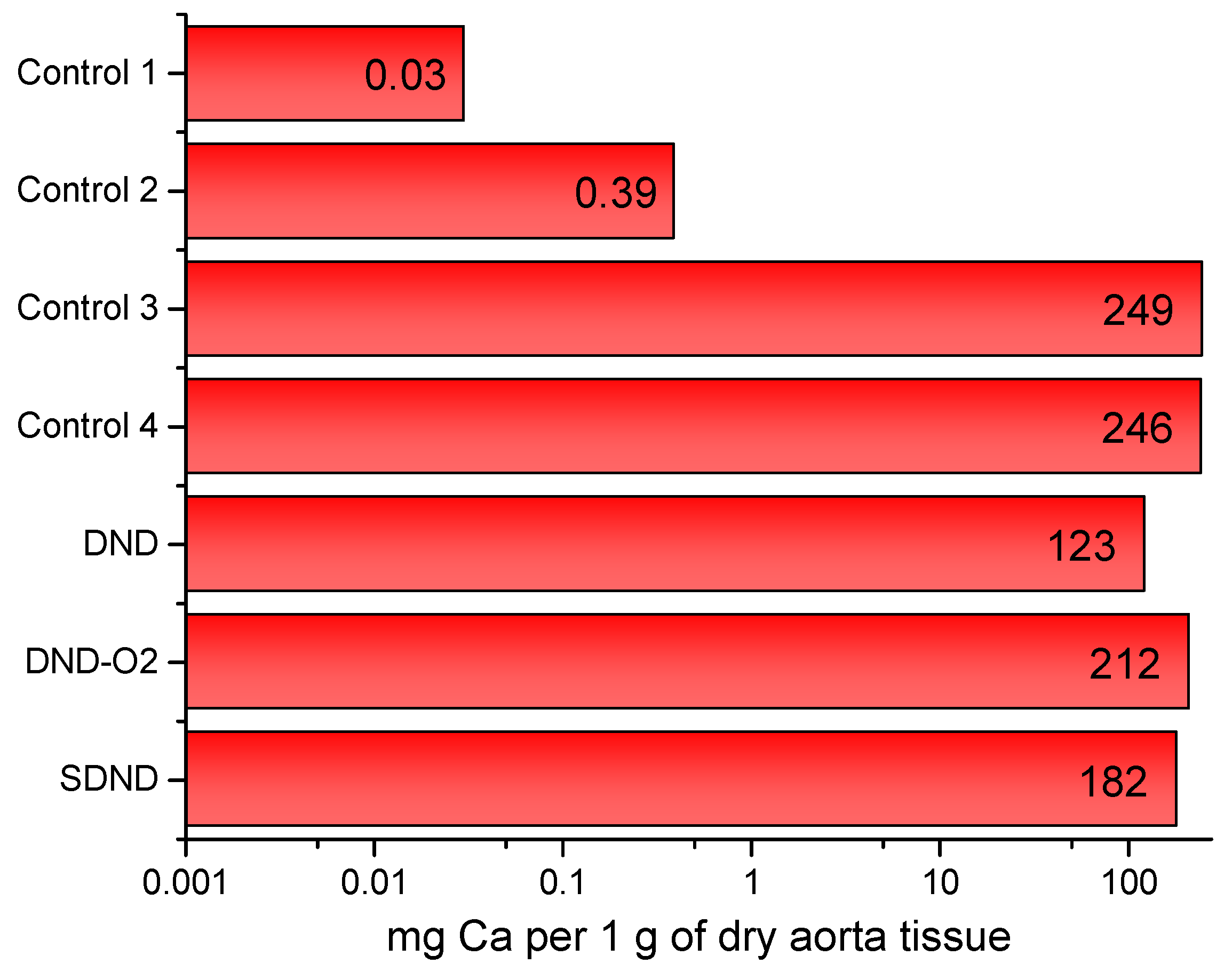
| Sample Index | Metal Content, mg per 1 g of Dry Aorta Tissue | ||||
|---|---|---|---|---|---|
| Ca | K | Mg | Na | Fe | |
| Control sample of the native pork aorta of the pig (far away from the allograft) | 0.03 | 0.004 | 0.01 | 1.66 | 9 × 10−4 |
| Control sample of the native pork aorta of the pig (near the allograft) | 0.39 | 0.23 | 0.13 | 22 | 0.02 |
| Control sample inside the conduit (#4) | 246 | 0.22 | 2.57 | 36 | 0.13 |
| Control sample of the donor pork aorta devitalized by a hybrid approach | 249 | 0.24 | 4.53 | 36 | 0.04 |
| DND | 123 | 0.30 | 1.13 | 36 | 0.13 |
| DND-O2 | 212 | 0.22 | 3.11 | 39 | 0.11 |
| SDND | 182 | 0.28 | 1.86 | 42 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernysheva, M.G.; Shen, T.; Badun, G.A.; Mikheev, I.V.; Chaschin, I.S.; Tsygankov, Y.M.; Britikov, D.V.; Hugaev, G.A.; Bakuleva, N.P. Tritium-Labeled Nanodiamonds as an Instrument to Analyze Bioprosthetic Valve Coatings: A Case of Using a Nanodiamond Containing Coating on a Pork Aorta. Molecules 2024, 29, 3078. https://doi.org/10.3390/molecules29133078
Chernysheva MG, Shen T, Badun GA, Mikheev IV, Chaschin IS, Tsygankov YM, Britikov DV, Hugaev GA, Bakuleva NP. Tritium-Labeled Nanodiamonds as an Instrument to Analyze Bioprosthetic Valve Coatings: A Case of Using a Nanodiamond Containing Coating on a Pork Aorta. Molecules. 2024; 29(13):3078. https://doi.org/10.3390/molecules29133078
Chicago/Turabian StyleChernysheva, Maria G., Tianyi Shen, Gennadii A. Badun, Ivan V. Mikheev, Ivan S. Chaschin, Yuriy M. Tsygankov, Dmitrii V. Britikov, Georgii A. Hugaev, and Natalia P. Bakuleva. 2024. "Tritium-Labeled Nanodiamonds as an Instrument to Analyze Bioprosthetic Valve Coatings: A Case of Using a Nanodiamond Containing Coating on a Pork Aorta" Molecules 29, no. 13: 3078. https://doi.org/10.3390/molecules29133078
APA StyleChernysheva, M. G., Shen, T., Badun, G. A., Mikheev, I. V., Chaschin, I. S., Tsygankov, Y. M., Britikov, D. V., Hugaev, G. A., & Bakuleva, N. P. (2024). Tritium-Labeled Nanodiamonds as an Instrument to Analyze Bioprosthetic Valve Coatings: A Case of Using a Nanodiamond Containing Coating on a Pork Aorta. Molecules, 29(13), 3078. https://doi.org/10.3390/molecules29133078







